Abstract
Introduction
Optic pathway gliomas (OPGs), also known as Visual Pathway Gliomas, are insidious, debilitating tumours. They are most commonly WHO grade 1 pilocytic astrocytomas and frequently occur in patients with neurofibromatosis type 1. The location of OPGs within the optic pathway typically precludes complete resection or optimal radiation dosing, hence outcomes remain poor compared to many other low-grade gliomas. The aim of this systematic review was to formulate a comprehensive list of all current ongoing clinical trials that are specifically looking at clinical care of OPGs in order to identify trends in current research and provide an overview to guide future research efforts.
Methods
This systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The Cochrane Controlled Register of Trials (CENTRAL) and ClinicalTrials.gov were searched. Inclusion and exclusion criteria were applied and final results were reviewed.
Results
501 clinical trials were identified with the search strategy. All were screened and eligible studies extracted and reviewed. This yielded 36 ongoing clinical trials, 27 of which were pharmacological agents in phase I-III. The remaining trials were a mixture of biological agents, radiation optimisation, diagnostic imaging, surgical intervention, and a social function analysis.
Conclusion
OPG is a complex multifaceted disease, and advances in care require ongoing research efforts across a spectrum of different research fields. This review provides an update on the current state of research in OPG and summarises ongoing trials.
Keywords: Optic, Visual, Pathway, Glioma, Trial, Systematic review
Introduction
Optic pathway gliomas (OPGs), also known as visual pathway gliomas, are insidious, debilitating tumours that account for 3–5% of all paediatric brain tumours. They are a subtype of low-grade glioma (LGG), most often World Health Organization (WHO) grade 1 juvenile pilocytic astrocytomas (JPAs), with a smaller proportion being pilomyxoid astrocytomas (PXAs) [1]. They are common in patients with neurofibromatosis type 1 (NF1), with up to 20% developing an OPG at a mean age of 4.5–5 years old [2]. OPGs most commonly arise at the chiasmatic-hypothalamic region but can arise anywhere along the optic pathway [3]. Their intimate relationship to the optic apparatus, hypothalamus, ventricular system and brain parenchyma can result in a variety of clinical sequelae including visual loss, endocrinopathies and hypothalamic dysfunction, developmental/neuropsychological disorders, hydrocephalus and focal neurological deficits. This complexity of symptomatology, and the close relationship to key structures make the treatment of OPG challenging. Unlike other JPAs, the location typically precludes complete surgical resection or optimum radiation dosing without incurring an often-unacceptable neurological cost. Furthermore, key aspects of their behaviour including their natural history and optimal management are incompletely understood. Tumour stabilisation, progression or regression can all occur unpredictably. OPG management is highly individualised. Many undergo a period of observation with serial imaging unless there is progressive growth or visual symptoms. Chemotherapy with a carboplatin/vincristine ‘Packer’ regimen is often the first line, although alternatives such as the TPCV (thioguanine, procarbazine, lomustine, vincristine) are sometimes used, except in NF1 patients due to the risk of secondary leukaemia [4]. Radiotherapy is typically avoided in younger children due to risks of toxicity wherever possible [5], although it remains an option in the setting of refractory, progressive disease or where visual loss is occurring despite systemic therapy [6, 7]. The role of surgical biopsy and debulking surgery remains controversial [8, 9]. OPGs require multidisciplinary care by neurosurgeons, neuro-oncologists, radiation oncologists, endocrinologists, ophthalmologists, pathology, neuropsychology, paediatricians, geneticists and a host of allied health professionals. This multifaceted care underlies the complexity of the disease. Advances in the understanding and treatment of OPG could come from a variety of sources, including novel or repurposed pharmacological agents, emerging biological agents and tumour vaccines, refinement of surgical approaches, diagnostic and therapeutic radiological developments or quality of life–focussed research [10–16]. The aim of this systematic review was to formulate a comprehensive list of all current ongoing clinical trials that were specifically looking at a clinical care of OPGs in order to identify trends in current research and provide an overview of the field to guide future research efforts. This review includes trials that are registered, and in any stage of recruitment or analysis, but not yet published. A summary of each of the ongoing clinical trials will be presented to facilitate a rapid review of the field.
Methods
This systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines on April 10th, 2020 [17, 18]. Two clinical trial databases, the Cochrane Controlled Register of Trials (CENTRAL) and ClinicalTrials.gov, were searched. The search terms used to identify clinical trials were as follows: ‘(optic OR pathway OR visual OR opg OR chiasm OR midline OR hypothalamic OR hypothalamus OR neurofibromatosis OR NF1 OR pediatric OR paediatric) AND (glioma OR pilocytic OR astrocytoma OR pilomyxoid)’. Trials older than 2010 were excluded. Trial titles and abstracts were reviewed by two of the authors. Titles with no English language translation available, and duplicates, were removed. Exclusion criteria were applied; these included titles that were not related to OPG or to trials that would not include OPG as a subgroup based on their inclusion/exclusion criteria. Already-published studies were excluded; these articles were reviewed to ensure that the publications were final and complete, and not an interim analysis or partial publication, with further publication of trial results expected. Studies that had been withdrawn, abandoned or not updated in 3 years or more were also excluded. For identified trials where there had been no recent clinical trial update (in print or online) for > 3 years, contact was made with the principal investigator of the trial to establish progress and assess if the trial was still running. Two authors (CSH and AB) performed the search strategy independently and reviewed the final papers separately. Any conflicting findings were reviewed (by CSH, AB and WI) and a consensus agreed.
Results
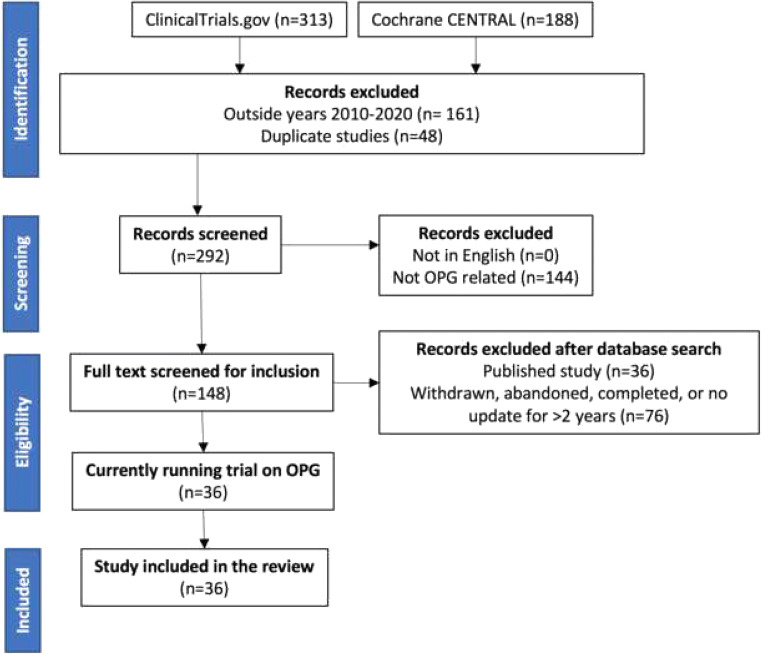
Five hundred one clinical trials were identified with the search strategy described. After initial exclusions, 292 were screened. Thirty-six eligible studies were included in the final analysis. The identification, screening, eligibility and inclusion were conducted according to PRISMA guidelines. The strategy is summarised in Fig. 1.
Fig. 1.
PRISMA process summary. This figure demonstrates article inclusion and exclusion across the various stages of review
After application of the PRISMA search strategy, we included 36 clinical trials in our final analysis.
A detailed summary of the stratified clinical trials included in the review is presented in Table 1.
Table 1.
Clinical trials involving optic pathway glioma that are currently in progress
| Trial name Registration number |
Primary objective | Trial design | Estimated study completion | Sample size | Patient age | Intervention | Comparison | Primary outcome | Country (primary institution) |
|---|---|---|---|---|---|---|---|---|---|
| Phase 0/I pharmacological trials | |||||||||
|
Pediatric Neuro-Oncology Consortium (PNOC)-002: Safety, Phase 0, and Pilot Efficacy Study of Vemurafenib, an Oral Inhibitor of BRAFV600E, in Children and Young Adults with Recurrent/Refractory BRAFV600E- or BRAF Ins T Mutant Brain Tumors |
Assess safety and establish maximum tolerated and recommended phase II dose of vemurafenib in recurrent or refractory gliomas containing the BRAFV600E or BRAF Ins T mutation |
Open label Non-randomised Single group Phase 0/I |
December 2020 | 40 | 0–25 years | Drug: vemurafenib | Dose escalation | Maximum tolerated dose, toxicity, pharmacokinetics and objective response. Then intratumoural drug concentration | USA (UCSF) |
| Phase I pharmacological trials | |||||||||
|
A Phase I Study of TAK-580 (MLN2480) for Children with Low-Grade Gliomas and other RAS/RAF/MEK/ERK Pathway Activated Tumors |
Determine safety of treatment and appropriate dosage of TAK-580 in children and adolescent participants with LGG |
Open label Non-randomised Single group Phase I |
December 2024 | 53 | 1–25 years | Drug: TAK-580 (MLN2480) | N/A: safety analysis | Dose-limiting toxicity and progression-free survival | USA (Dana-Farber) |
|
A Phase I Trial of Pomalidomide for Children with Recurrent, Progressive, or Refractory CNS Tumors |
Determine best dosage of pomalidomide in treating younger patients with recurrent, progressive or refractory CNS tumours |
Open label Non-randomised Single group Phase I |
March 2020 | 42 | 3–20 years | Drug: pomalidomide | N/A: safety analysis | Maximum tolerated dose and recommended phase II dose, toxicity, pharmacokinetics | USA (NCI) |
|
Sirolimus in Combination with Metronomic Therapy in Children with Recurrent and Refractory Solid Tumors: A Phase I Study |
Investigate anti-tumour activity, optimal dosing and toxicity of sirolimus in combination with other chemotherapy in recurrent or refractory solid tumours |
Open label Non-randomised Single group Phase I |
April 2021 | 24 | 0–30 years | Drug: sirolimus | Dose escalation | Maximum tolerated dose and recommended phase II dose | USA (Emory) |
|
Anti-Angiogenic Therapy After Autologous Stem Cell Rescue (ASCR) for Pediatric Solid Tumors |
Assess feasibility and safety of using anti-angiogenic drugs after autologous stem cell transplant |
Open label Non-randomised Sequential assignment Phase I |
February 2020 | 12 | 6 months–21 years | Drugs: cyclophosphamide and thalidomide | Control versus cyclophosphamide versus thalidomide | Toxicity and best overall response | USA (WUSM) |
|
Phase I/Ib Trial of Combined 5’Azacytidine and Carboplatin for Recurrent/Refractory Pediatric Brain and Solid Tumors |
Determine maximum tolerated dose and initial efficacy of 5′azacytidine in combination with carboplatin |
Open label Non-randomised Parallel assignment Phase I |
July 2021 | 54 | 1–18 years | Drug: 5′azacytidine | Dose escalation then expansion | Maximum tolerated dose and feasibility of treatment | Canada (SickKids) |
|
A Phase Ib Dose Escalation Study of Abemaciclib in Combination with Temozolomide and Irinotecan (Part A) and Abemaciclib in Combination with Temozolomide (Part B) in Pediatric and Young Adult Patients with Relapsed/Refractory Solid Tumors |
Determine safety and effectivity of abemaciclib in combination with irinotecan and/or temozolomide |
Open label Non-randomised Single group Phase I |
February 2022 | 60 | 0–18 years | Drug: abemaciclib | Dose escalation then dose expansion | Toxicity, pharmacokinetics and response rate | USA (Eli Lilly) |
|
A Phase 1 Study of Entinostat, an Oral Histone Deacetylase Inhibitor, in Pediatric Patients with Recurrent or Refractory Solid Tumors, Including CNS Tumors and Lymphoma |
Determine the maximum tolerated and recommended dose of entinostat in children with recurrent or refractory solid tumours |
Open label Non-randomised Single group Phase I |
April 2022 | 36 | 1–21 years | Drug: entinostat | Dose escalation then dose expansion | Maximum tolerated dose, toxicity and pharmacokinetics | USA (NCI) |
|
A Phase I Study of Mebendazole for the Treatment of Pediatric Gliomas |
Determine maximally tolerated dose of mebendazole in combination with vincristine, carboplatin and temozolomide (for LGG) OR bevacizumab and irinotecan (for HGG) |
Open label Non-randomised Parallel assignment Phase I |
April 2020 |
36 | 1–21 years | Drug: mebendazole | Dose escalation then dose expansion | Toxicity and response rate of participants | USA (Janssen) |
| Phase I/II pharmacological trials | |||||||||
|
A Phase 1 and Phase II and Re-Treatment Study of AZD6244 for Recurrent or Refractory Pediatric Low Grade Glioma |
Phase I: maximum tolerated dose and recommended phase II dose of selumetinib. Phase II: objective response and disease stabilisation rates with selumetinib treatment |
Open label Non-randomised Single group Phase I/II |
December 2020 | 180 | 3–21 years | Drug: selumetinib (AZD6244) | Dose escalation then dose expansion | Toxicity and response rate of participants | USA (NCI) |
|
Phase I/II Trial of Dabrafenib, Trametinib, and Hydroxychloroquine (HCQ) for BRAF V600E-mutant or Trametinib and HCQ for BRAF Fusion/Duplication Positive or NF1-associated Recurrent or Progressive Gliomas in Children and Young Adults |
Phase I: maximum tolerated and recommended phase II dose of HCQ with dabrafenib and/or trametinib in young patients with recurrent or progressive glioma Phase II: response rate |
Open label Non-randomised Parallel assignment Phase I/II |
February 2025 | 75 | 1–30 years | Drug: hydroxychloroquine | Dose escalation then dose expansion | Maximum tolerated dose and response rate | USA (PBTC) |
|
A Phase 2 Study of Trametinib for Patients with Pediatric Glioma or Plexiform Neurofibroma With Refractory Tumor and Activation of the MAPK/ERK Pathway. |
Asses response rate of paediatric glioma and plexiform neurofibroma (PN) to trametinib |
Open label Non-randomised Parallel assignment Phase I/II |
June 2026 | 150 | 1 month–25 years | Drug: trametinib | N/A | Objective response rate | Canada (St Justine’s Hospital) |
|
An Open-Label, Dose-Escalation, Phase I/II Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics and Clinical Activity of the MEK Inhibitor Trametinib in Children and Adolescents Subjects with Cancer or Plexiform Neurofibromas and Trametinib in Combination with Dabrafenib in Children and Adolescents with Cancers Harboring V600 Mutations |
Establish safe dose and effectivity of trametinib monotherapy and in combination with dabrafenib in young patients |
Open label Non-randomised Sequential assignment Phase I/II |
December 2020 | 142 | 1 month–17 years | Drug: trametinib | Dose escalation | Safe dose, toxicity and response rate | USA (Novartis) |
|
A Phase 1/2 Study of Lenvatinib in Combination with Everolimus in Recurrent and Refractory Pediatric Solid Tumors, Including CNS Tumors |
Determine safety, optimal dose and response rate of lenvatinib combined with everolimus in paediatric patients with recurrent/refractory solid tumours |
Open label Non-randomised Parallel assignment Phase I/II |
May 2022 | 120 | 2–21 years | Drug: lenvatinib | Dose escalation then dose expansion | Maximum tolerated dose, recommended phase II dose, toxicity and response rate | USA (Eisai Inc) |
|
A Paediatric Phase I/II Study of Intermittent Dosing of The Mek-1 Inhibitor Selumetinib In Children with Neurofibromatosis Type-1 And Inoperable Plexiform Neurofibroma and/or Progressive Optic Pathway Glioma Intermittent Dosing of Selumetinib in Childhood NF1 Associated Tumours (INSPECT) |
Phase I: evaluate maximum tolerated dose of selumetinib Phase II: response rate to selumetinib in NF1 inoperable plexiform neurofibroma and OPG |
Open label Non-randomised Single group Phase I/II |
December 2023 | 30 | 3–18 years | Drug: selumetinib | Dose escalation | Response rate of participants measured with 3D volumetric analysis/2D assessment of tumour size | UK (GOSH) |
|
Phase I Study of MEK162 for Children with Progressive or Recurrent Cancer and a Phase II Study for Children with Low-Grade Gliomas and other Ras/Raf/MAP Pathway Activated Tumors |
Phase I: determine best dose and safety of MEK162 in children and adolescents Phase II: define response rate |
Open label Non-randomised Parallel assignment Phase I/II |
January 2021 | 120 | 1–18 years | Drug: MEK162 | Dose escalation | Maximum tolerated dose and response rate | USA (CHLA) |
| Phase II pharmacological trials | |||||||||
|
A Phase II, Open-Labeled, Multi-Center, Randomized Controlled Trial of Vinblastine +/- Bevacizumab for the Treatment of Chemotherapy-Naïve Children with Unresectable or Progressive Low-Grade Glioma (LGG) |
Determine efficacy of adding bevacizumab to vinblastine in chemotherapy-naïve paediatric patients with progressive/unresectable LGG |
Open label Randomised Parallel assignment Phase II |
August 2026 | 150 | 6 months–18 years | Drug: bevacizumab | Vinblastine versus vinblastine plus bevacizumab | Response rate | Canada (SickKids) |
|
Phase II Open-label Global Study to Evaluate the Effect of Dabrafenib in Combination with Trametinib in Children and Adolescent Patients with BRAF V600 Mutation Positive Low Grade Glioma (LGG) or Relapsed or Refractory High Grade Glioma (HGG) |
Investigate effect of dabrafenib combined with trametinib in young patients with LGG BRAF V600 positive or relapsed or refractory HGG |
Open label Non-randomised Single group Phase II |
November 2021 | 142 | 1–17 years | Drug: dabrafenib | Dabrafenib and trametinib versus carboplatin and vincristine | Overall response rate | USA (Novartis) |
|
NCI-COG Pediatric MATCH (Molecular Analysis for Therapy Choice) - Phase 2 Subprotocol of BVD-523FB (Ulixertinib) in Patients with Tumors Harboring Activating MAPK Pathway Mutations |
Assess efficacy of ulixertinib in patients with mutations in the MAPK signalling pathway |
Open label Non-randomised Single group Phase II |
December 2025 | 49 | 1–21 years | Drug: ulixertinib | N/A | Objective response rate | USA (NCI) |
|
Memantine for Prevention of Cognitive Late Effects in Pediatric Patients Receiving Cranial Radiation Therapy for Localized Brain Tumors: A Pilot Study |
Estimate participation rate in a study of memantine as a neuroprotective agent, rate of medication adherence and completion of cognitive assessments |
Double blind Randomised Placebo controlled Parallel assignment Phase II |
January 2021 | 50 | 6–21 years | Drug: memantine | Memantine versus placebo | Percentage who participate, complete 12 weeks of therapy and a minimum of 3 cognitive assessments | USA (St. Jude) |
|
Phase II Study of Everolimus for Recurrent or Progressive Low-grade Gliomas in Children |
Determine efficacy of everolimus in children with recurrent or progressive LGG |
Open label Non-randomised Single group Phase II |
January 2028 | 66 | 3–21 years | Drug: everolimus | N/A | Progression-free survival | USA (UCSF) |
|
A Phase II Trial of Poly-ICLC in the Management of Recurrent Pediatric Low Grade Gliomas |
Determine safety and effectivity of poly-ICLC in young patients with LGG |
Open label Non-randomised Single group Phase II |
July 2019 | 23 | 0–21 years | Drug: poly-ICLC | N/A | Overall response rate | USA (UCSD) |
|
Pilot Study of the MEK1/2 Inhibitor Selumetinib (AZD6244 Hydrogen Sulfate) for Adults with Neurofibromatosis Type 1 (NF1) and Cutaneous Neurofibromas (CNF) |
Establish if selumetinib can result in volume decrease of cutaneous neurofibromas |
Open label Non-randomised Single group Phase II |
December 2021 | 24 | 18+ years | Drug: selumetinib | N/A | Change in tumour size | USA (NCI) |
|
A Phase II Study of Pegylated Interferon alfa-2b in Children with Recurrent or Refractory and Radiographically or Clinically Progressive Juvenile Pilocytic Astrocytomas & Optic Pathway Gliomas |
Response rate to pegylated interferon |
Open label Non-randomised Single group Phase II |
December 2021 | 20 | 3–25 years | Drug: pegylated interferon alfa-2b | N/A | Response rate of participants from baseline to 2 years | USA (Emory) |
|
A Phase II Randomized Trial of Lenalidomide (NSC # 703813) in Pediatric Patients with Recurrent, Refractory or Progressive Juvenile Pilocytic Astrocytomas and Optic Pathway Gliomas |
Response rate (complete or partial) to low- or high-dose lenalidomide |
Open label Randomised Parallel assignment Phase II |
June 2020 | 80 | 0–21 years | Drug: lenalidomide | Low-dose or high-dose lenalidomide | Objective best response rate | USA (NCI) |
| Phase III pharmacological trials | |||||||||
|
A Phase 3 Randomized Study of Selumetinib Versus Carboplatin/Vincristine in Newly Diagnosed or Previously Untreated Neurofibromatosis Type 1 (NF1) Associated Low-Grade Glioma |
Assess selumetinib compared with treatment with carboplatin/vincristine (CV) for participants with NF1-associated low-grade glioma, and to assess if selumetinib is better than CV in improving vision in participants with LGG of the optic pathway |
Open label Randomised Parallel assignment Phase III |
May 2027 | 290 | 2–21 years | Drug: selumetinib | Selumetinib versus carboplatin and vincristine | Event-free survival and number of participants with visual improvement | USA (NCI) |
|
A Phase 3 Randomized Non-Inferiority Study of Carboplatin and Vincristine Versus Selumetinib (NSC# 748727) in Newly Diagnosed or Previously Untreated Low-Grade Glioma (LGG) Not Associated with BRAFV600E Mutations or Systemic Neurofibromatosis |
Determine efficacy of treatment with selumetinib compared with carboplatin and vincristine in untreated LGG (not NF1 or BRAFV600E associated) |
Open label Randomised Parallel assignment Phase III |
December 2026 | 200 | 2–12 years | Drug: selumetinib | Selumetinib versus carboplatin and vincristine | Event-free survival | USA (NCI) |
| Biological therapy trials | |||||||||
|
A Phase I Study of Ad-RTS-hIL-12, an Inducible Adenoviral Vector Engineered to Express hIL-12 in the Presence of the Activator Ligand Veledimex in Pediatric Brain Tumor Subjects |
Assess safety and tolerability of intratumoural Ad-RTS-hIL-12 injection in combination with oral veledimex |
Open label Non-randomised Sequential single groups Phase I |
March 2021 | 24 | 0–21 years | Biological: Ad-RTS-hIL-12 | N/A: safety analysis | Dose-limiting toxicity and safety of treatment | USA (Ziopharm) |
|
Phase 1 Study of B7-H3-Specific CAR T Cell Locoregional Immunotherapy for Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma and Recurrent or Refractory Pediatric Central Nervous System Tumors |
Assess safety and feasibility of B7H3-specific CAR T cell administration via indwelling catheter into the tumour resection cavity or ventricular system in DIPG, DMG and recurrent or refractory CNS tumours |
Open label Non-randomised Parallel assignment Phase I |
May 2041 | 70 | 1–26 years | Biological: B7H3-specific CAR T cell | N/A: safety analysis | Successful delivery, toxicity and response rate | USA (Seattle Children’s) |
| Radiotherapy-based trials | |||||||||
|
A Phase II Study of Proton Radiotherapy for Pediatric Brain Tumors Requiring Partial Brain Irradiation: An Assessment of Long Term Neurocognitive, Neuroendocrine an Ototoxicity Outcomes |
Determine if proton radiotherapy has reduced long-term neuroendocrine and neurocognitive impact compared with standard radiation |
Open label Non-randomised Single group Phase II |
September 2022 | 100 | 1–25 years | Radiation: proton radiotherapy | Standard radiation versus proton radiotherapy | Endocrine dysfunction and neurocognitive sequelae | USA (MGH) |
|
A Phase II Study of Hippocampal-Avoidance Using Proton Therapy in Low-Grade Glioma |
Determine feasibility of reducing radiation therapy doses to the hippocampi using proton therapy |
Open label Non-randomised Single group Phase II |
May 2023 | 74 | 6–21 years | Radiation: hippocampal-avoidance proton therapy | N/A | Percentage of plans meeting first or second dose constraints | USA (St. Jude) |
| Diagnostic imaging trials | |||||||||
|
Developing Fast Pediatric Imaging |
Development of improved MRI technologies to better measure brain tumour tissue volume |
Open label Non-randomised Parallel assignment |
September 2021 | 300 | All | Other: Wave-CAIPI (controlled aliasing in parallel imaging) | N/A | Validation of Wave-CAIPI as a diagnostic tool | USA (Dana-Farber) |
|
Pilot Study of [18F] Fluorodeoxyglucose Positron Emission Tomography- Magnetic Resonance Imaging (FDG-PET-MRI) in CNS and Extra-CNS Tumors of Patients with Neurofibromatosis-1 (NF1) |
Acquire preliminary data and report differences in FDG-avidity in patients with NF1-associated optic glioma and plexiform neurofibroma |
Observational Prospective cohort study (pilot) |
December 2019 | 30 | 6+ years | Device: FDG-PET-MRI | Progressive versus non-progressive disease | Comparison of FDG-avidity between progressive and non-progressive lesion/disease | USA (Lineberger Centre) |
| Miscellaneous trials (genetic, social function, image-guided thermotherapy) | |||||||||
|
Frameshift Peptides of Children with Neurofibromatosis Type 1 (NF1) and Either Low-Grade Gliomas or Plexiform Neurofibromas |
Establish if children and young adults with NF1 and LGGs or plexiform neurofibroma have a specific frameshift peptide profile for potential development of a disease-specific vaccine |
Observational Prospective cohort study |
April 2021 | 60 | 0–30 years | Genetic: frameshift array blood sample test | Active LGG versus plexiform neurofibroma, versus neither | Frameshift peptide protein profile in patients with NF1 and those who develop LGGs versus plexiform neurofibromas | USA (CNRI) |
|
Components of Social Functioning in Survivors of Pediatric Brain Tumors |
Observe social and neurocognitive functioning in paediatric survivors of brain tumours |
Observational Prospective cohort study |
February 2021 | 80 | 8–12 years | N/A | N/A | Predictors of social, cognitive and neurocognitive abilities on social interaction | USA (St. Jude) |
|
A Pilot Study of Using MRI-Guided Laser Heat Ablation to Induce Disruption of the Peritumoral Blood Brain Barrier to Enhance Delivery and Efficacy of Treatment of Pediatric Brain Tumors |
Assess and establish the window of maximal blood–brain barrier disruption post laser ablation |
Open label Non-randomised Parallel assignment Phase I |
October 2020 | 12 | 3–21 years | Device: MRI-guided laser ablation | MRI-guided laser ablation versus MRI-guided laser ablation plus doxorubicin and etoposide | Progression-free survival, overall survival and quality of life | USA (Washington University) |
Out of the 36 clinical trials assessed, 27 were pharmacological studies. There was one phase 0 trial, 8 phase 0/I drug trials, 7 mixed phase I and phase II, 9 phase II, and 2 phase III trials. There were 2 trials focussed on biological agents, 2 based on optimising radiation therapy, 2 on diagnostic neuroimaging, one on imaging-guided thermotherapy, one on genetic testing and one on social functioning.
Discussion
Systematically reviewing the ongoing clinical trials is important to update clinicians on potential new therapies, avoid duplication of research and identify research trends and developing areas of study to stimulate new investigation. As might be expected, the main focus of ongoing research studies in OPG is related to pharmacological agents. Twenty-seven out of the 36 identified research trials were drug trials. Such investigations are necessary to establish safety and efficacy of all new drugs and are essential to determine if these agents should be adopted or discarded. Many of the new agents were related to the BRAF/MEK/ERK or RAS pathways. A summary of the pharmacological agents under investigation is presented in Table 2.
Table 2.
Pharmacological agents under investigation as potential therapies in optic pathway gliomas
| Drug/intervention | Proposed mechanism of action |
|---|---|
|
BRAF/MEK/ERK pathway targeting agents The MAPK/ERK signalling pathway is a key regulator of biological growth, proliferation and differentiation. The MAPK/ERK pathway is often upregulated in a variety of tumour types including glioma. The BRAFV600E mutation leads to constitutive overactivation of BRAF which is a driver of tumourigenesis in BRAFV600E mutant low-grade gliomas [27–29]. | |
| Vemurafenib/dabrafenib | Selective type 1 B-Raf competitive small molecule enzyme inhibitors. Selectively recognises and binds the ATP-binding domain of BRAFV600E mutants. This interrupts the B-Raf/MEK step on the B-Raf/MEK/ERK pathway that is a driver of tumourigenesis in BRAFV600E mutant LGGs. Vemurafenib has shown some promise in the treatment for BRAFV600E mutant LGGs [30]. |
| Tak580 | Oral pan-Raf kinase inhibitor. A single-patient study demonstrated apparently good tolerance in a patient with refractory LGG ahead of a larger planned clinical trial [31]. |
| Ulixertinib | An ERK1/2 inhibitor. Blocking ERK-mediated signal transduction may inhibit ERK-dependent tumour cell proliferation and survival. |
|
RAS pathway targeting agents The RAS/MAPK pathway is important in the control of cancer cell growth and proliferation. The NF1 product neurofibromin functions as a negative regulator of RAS activity. RAS overactivity in patients with NF1 drives tumourigenesis. The MEK pathway is hyperactivated in NF1-deficient astrocytes and this drives NF1-associated optic glioma growth. Hence, the rationale for sustained MEK pharmacologic blockade in attenuating NF1-deficient astrocytes and NF1 optic glioma volume and proliferation [32]. The development of agents that target RAS signalling and associated pathways implicated in the pathogenesis of neurofibromas has led to clinical trials of various new pharmacological agents. | |
| Selumetinib/trametinib | Selective MEK inhibitor. A phase I study assessing selumetinib dose tolerance and pharmacokinetics in NF1-related plexiform neurofibromas was conducted by Dombi et al. who demonstrated benefit in inoperable plexiform neurofibromas without excess toxicity [33]. A further phase II study in relapsed LLG that included OPG showed sustained partial therapeutic response [34]. |
| MEK162 (binimetinib) | Orally available inhibitor of MAPK2. |
| Anti-angiogenic agents | |
| Bevacizumab | A humanised monoclonal antibody directed against VEGF, this inhibition leads to a reduction in microvascular growth of tumour blood vessels and thus limits the blood supply to tumour tissues. There are several reports of the benefit of anti-VEGF drugs in uncontrolled case series [35–38]. |
| Lenvatinib | Multireceptor TK inhibitor with preferential anti-angiogenic capacity. It inhibits VEGF receptor family 1-3, FGFR family 1-4, PDGFRa, TK receptor and RET. As a result, it may modulate the tumour microenvironment and anti-tumour responses [39]. |
| Thalidomide | An anti-angiogenic agent. The mechanism of anti-angiogenic action is not fully known [40]. Its use has been investigated in the care of high-grade glioma [41]. |
| Lenalidomide | An analogue of the anti-angiogenic agent thalidomide. It has demonstrated a significant anti-tumour activity in patients with multiple myeloma and myelodysplastic syndrome. Phase I studies in adults and children demonstrated tolerability but was associated with thromboembolic events and myelosuppression [42–44]. Antagonistically, the use of lenalidomide has been associated with secondary primary high-grade astrocytoma development in multiple myeloma [45]. |
| Pomalidomide | An analogue of the anti-angiogenic agent thalidomide. It failed to demonstrate benefit in a phase II clinical study as monotherapy for children and young adults with recurrent or progressive primary brain tumours [46]. |
| Miscellaneous pharmacological agents | |
| Mebendazole | Methyl N-[6-(benzoyl)-1H-benzimidazol-2-yl] carbamate. A benzimidazole anti-helminthic used to treat a variety of worm infections and for echinococcosis (hydatid disease). Mebendazole binds to tubulin subunits preventing polymerisation. Tubulin is a key molecule in cell division and has been proposed as an anti-cancer target. Other potential targets of mebendazole include the Hh signalling pathway, and angiogenesis through VEGF inhibition [47]. Repurposing of mebendazole has been suggested in various tumours and it has been previously trialled in preclinical models of glioblastoma with some success [48–50]. |
| Entinostat | Entinostat is a synthetic benzamide HDAC type 1 and III inhibitor. It is orally bioactive. Its proposed mode of action is to regulate chromatin structure and through histone deacetylation control epigenetic gene transcription. Other mechanisms of effect may include induction of reactive oxygen species leading to mitochondrial damage and inducing cell cycle arrest [51, 52]. |
| Pegylated interferon alfa-2b | Pegylated interferon α-2b is a member of the interferon family; these are glycoproteins with anti-cytokine effects that have documented immune-modulating and anti-proliferative effects. One phase II study is examining the effect of pegylated interferon alfa-2b in a child with recurrent or refractory/progressive JPA and OPG in children. Studies have shown that interferon alfa-2b is tolerable and may delay progression in DIPG [53]. |
| Abemaciclib | Reversible small molecular CDK inhibitor. Some gliomas have been shown to overexpresses cyclin D1, which in turn increases activity of CDK4 and CDK6; these enzymes phosphorylate (and therefore deactivate) retinoblastoma protein. Deactivating retinoblastoma protein leads to excess cellular growth by loss of cell cycle control at the G1 to S phases. Abemaciclib has demonstrated some effect in slowing growth in preclinical glioblastoma models [54]. |
| Hydroxychloroquine | Various possibilities including modulation of autophagy, cellular metabolism or direct chemotoxic effects [55]. |
| Sirolimus/everolimus | First- and second-generation mTOR inhibitors. The mTOR pathway is important in control of cellular growth. These drugs have been trialled in giant cell astrocytomas as part of tuberous sclerosis complex, and everolimus is currently used in some cases that are not amenable to surgical resection [56, 57]. |
| Memantine | An NMDAR1 antagonist thought to have anti-tumour and neuroprotective effects. It has been studied in glioblastoma. |
| Poly-ICLC | A synthetic double-stranded RNA complex. Poly-ICLC is a ligand for toll-like receptor-3 and MDA-5 that can activate immune cells (including dendritic cells and natural killer cells) and therefore act as a stimulating immunomodulating agent to induce tumour cell killing [58]. |
| Cyclophosphamide | A chemotherapeutic agent. Cyclophosphamide forms an active metabolite phosphoramide mustard in cells with low levels of ALDH, as is the case in some tumour cells. The phosphoramide mustard forms irreversible DNA interstrand cross-links that trigger tumour cell apoptosis. It has been shown to enhance glioma virotherapy by inhibiting innate immune responses [59–61]. |
| 5′Azacytidine | An inhibitor of DNA methylation. This may be beneficial in LGGs with hypermethylation as part of their genetic phenotype [62]. |
BRAF rapidly accelerated fibrosarcoma gene B (B-Raf = protein), CDK cyclin-dependent kinase, DIPG diffuse intrinsic pontine glioma, ERK extracellular signal–regulated kinases, FGFR fibroblast growth factor receptor, HDAC histone deacetylase, Hh hedgehog, LGG low-grade glioma, MAPK selective mitogen-activated protein kinase, MEK selective mitogen-activated protein kinase, mTOR mammalian target of rapamycin, NF1 neurofibromatosis 1, NMDAR N-methyl-d-aspartate receptor, OPG optic pathway glioma, PDGFRa platelet-derived growth factor receptor alpha, RET rearranged during transfection receptor, Poly-ICLC polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose, TK tyrosine kinase, VEGF vascular endothelial growth factor
There were 5 ongoing trials into the same pharmacological agent selumetinib, and 2 looking at trametinib; this raises a question as to whether a collaborative approach would allow data sharing and ensure a common data element and consensus reporting. The included trials were dominated by North American centres and pharmaceutical companies. We did not identify any trials run from a low- or middle-income country (LMIC) despite reports that up to 80% of all paediatric cancer patients occur in these countries. Potential reasons for the lack of clinical trials in LMIC include a lack of specialised centres, equipment and staff, and a lower priority of cancer for healthcare planning strategies [19–21]. There were a small number of trials looking at non-pharmacological diagnostics, therapeutics and assessment social functioning relating to OPG. These are summarised in Table 3.
Table 3.
Ongoing non-pharmacological trials related to optic pathway glioma
| Object of investigation | Explanation |
|---|---|
| Non-pharmacological therapeutics | |
| Ad-RTS-hIL-12 | An inducible adenoviral (Ad) vector encoding human pro-inflammatory cytokine interleukin-12 (hIL-12) p70 transgene. This is under the transcriptional control of the RheoSwitch Therapeutic System (RTS) and can be activated by administration of the proprietary diacylhydrazine-based activator ligand veledimex. Activation and transcription of IL-12 is proposed to have immunomodulating and anti-neoplastic activities [63]. |
| B7-H3-specific CAR T cell | Chimeric antigen receptor (CAR) T cell therapy has emerged as a potential treatment in a range of cancers. CAR T cells are synthetic molecules composed of antibody binding domains connected to T cell activating domains and co-stimulatory domains. This allows highly specific tumour antigen recognition and subsequent T cell activation. The B7-H3 antigen is a transmembrane protein that is overexpressed in a range of tumours, and so manufacture of CAR T cells targeted to B7-H3 may be effective in a range of solid cancers including glioma [64, 65]. |
| Proton radiotherapy |
There were two studies identified that relate to proton radiotherapy. Protons are high-energy subatomic particles that when accelerated can be used to deliver focussed ionising radiation to a tumour in order to damage its DNA—killing tumour cells and inhibiting growth. One study is focussed on assessing long-term neurocognitive, neuroendocrine and ototoxicity outcomes. The other is assessing the capacity of proton beam therapy to successfully avoid hippocampal irradiation in LGG. |
| MRI-guided laser heat ablation | MRI-guided laser ablation (MLA) is a minimally invasive laser surgery technique that involves a scalp incision and a small burr hole through which a laser probe is inserted into the centre of a brain tumour under MRI guidance. The probe delivers hyperthermic ablation to the tumour which can destroy tumour tissue and also disrupts the tumour blood–brain barrier potentially enhancing the delivery of chemotherapeutic agents [66]. |
| Frameshift peptides | A frameshift mutation is caused by insertion of deletion of a nucleotide sequence into DNA that creates a ‘frameshift’ as it is not a multiple of 3 (the number of nucleotides in a codon). This frameshift alters the whole DNA sequence and results in an altered gene and subsequent protein product. These ‘frameshift peptides’ can act as new epitopes that are potential targets for tumour vaccines. The study NCT04212351 proposes to determine if NF1-associated LGGs have a specific frameshift peptide protein profile that could be targeted with a specific vaccine [67]. |
| Imaging diagnostics | |
| Fast paediatric imaging with Wave-CAIPI | Wave-CAIPI (controlled aliasing in parallel imaging) is an MRI-based technology that is faster than conventional MRI imaging. Parallel imaging works by acquiring a reduced amount of k-space data with an array of receiver coils. Research is underway to see if it can provide equivalent information to the current gold-standard MRI [68, 69]. |
| Positron emission tomography | 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) is a nuclear imaging modality that identifies increased glucose uptake in tissues. FDG-PET can be fused with MRI to improve the identification of high-grade features in gliomas [70]. |
| Psychological assessment | |
| Social functioning | Children with brain tumours are at risk for a number of late psychological effects, including neurocognitive and social deficits. These can be assessed with psychological questionnaires [16]. |
Pending trials
The authors are aware of a further relevant clinical trial that is yet to start, the LOw Grade Glioma In Children (LOGGIC) study. LOGGIC will compare the effect of standard chemotherapy versus MEK inhibitor (trametinib) as first-line agents in paediatric low-grade glioma. The outcome measures will include a child’s quality of life, including visual and neurological function. The LOGGIC trial will be run by the European Society for Paediatric Oncology (SIOPE). As with any emerging field, this is likely to be just one of several trials not yet registered on public databases.
Unanswered questions
This systematic review highlights the dominant pharmacologic-centric nature of current OPG clinical trial research. There are several important areas of OPG care that remain clouded in uncertainty and controversy and are not currently being addressed by the ongoing clinical trials we identified in this study. A key outstanding question is regarding the natural history of OPG and how we can predict outcomes. We have a paucity of understanding of the natural history of OPGs, and we lack tools to predict their clinical course and long-term outcomes. Furthermore, it is controversial which outcome measure should be used. Commonly used oncological outcome measures such as overall survival and (radiological) progression-free survival may not be the most appropriate for the study of OPGs; alternatives include visual function, endocrine/hypothalamic dysfunction and quality-of-life measures. While one trial identified in this review relates to social functioning, this remains a poorly examined area. Hypothalamic dysfunction is a key component of the OPG disease that is often neglected in clinical studies and can be challenging to quantify. The hypothalamic consequences of existing and emerging therapies need examination. Questions remain regarding the optimal therapeutic management. Although general consensus statements exist, there remains uncertainty around the optimal surgical strategy. The timing and nature of the surgical approaches to OPGs have not been subjected to rigorous trial data. Surgical options include biopsy, partial/subtotal resection and radical resection [13]. Furthermore, the optimal timing of chemotherapy/radiotherapy initiation and the influence this has on overall outcomes are not fully known. The focus of current research, including all trials identified in this study, is on recurrent/refractory OPGs rather than new diagnosis. The long-term efficacy of various treatments for newly diagnosed OPGs is unknown. Optic pathway gliomas are typically treated as a single disease. However, it may be important to stratify and subclassify based on factors such as age (infantile versus juvenile) and based on molecular/genetic subtyping. An example of this is stratification into syndromic/NF1-related and non-syndromic/non-NF1-related OPGs. These patients have different anatomical predilections, their natural histories can vary and the optimal therapies may be different [22–24].
Limitations
We found few trials that were specifically focussed on OPGs as a distinct tumour subtype. As OPGs are a subset of LGG, they may be bundled into trials under umbrella terms like ‘LGG’ or ‘glioma’ or ‘JPA/PXA’. We not only developed a search strategy aimed at capturing trials that had specific reference to OPG (with the search terms; optic/visual/pathway/chiasm/midline/hypothalamus/hypothalamic) but also included terms to capture trials that may include OPGs without specific reference to them, e.g. by using terms (Neurofibromatosis/NF1/paediatric/paediatric) combined with generic blanket terms (glioma/astrocytoma/pilocytic/pilomyxoid). Where appropriate, we screened the trial protocols and inclusion/exclusion criteria to determine if OPGs might be included in the trials. As with any systematic review, there is always a risk that our search strategy missed a relevant trial. This risk is higher with a disease like OPG that is a subcategory of a wider disease. In order to try and minimise this danger, we used a reproducible search strategy that conformed to PRISMA guidelines. We elected to include all trials that could recruit OPGs; however, the vast majority are not focussed on OPGs but on LGG in general. We ensured that all included trials would allow OPGs as part of their criteria, but we cannot be sure that they will eventually recruit patients with this pathology in representative numbers, or if they will examine the data adequately to allow subgroup analysis of this pathology.
Conclusion
OPG are a debilitating childhood cancer that causes a significant burden of suffering. Our understanding of this disease is limited and we lack effective treatments and clear management consensus [1, 25]. This manuscript details a systematic review of current ongoing trials in OPG. We find that from an initial yield of 501 hits, 36 ongoing trials fulfilled criteria for inclusion. The majority of these are studies or pharmacological agents, mostly phase I or II. OPG is a complex multifaceted disease, and advances in care are likely to require ongoing research efforts across a spectrum of different research fields [26]. Studies investigating surgical interventions and quality of life were notably absent. This review provides an update on the current state of research in OPG and highlights the main agents under investigation. We hope that this updates and stimulates clinicians and research scientists to engage with this important topic.
Acknowledgements
Ciaran S. Hill is supported by a National Institute for Health Research Academic Clinical Lectureship, an Academy of Medical Science Grant, and a Cancer Research UK Pioneer Award.
Abbreviations
- CENTRAL
Cochrane Controlled Register of Trials
- FDA
Food and Drug Administration
- GOSH
Great Ormond Street Hospital
- HDAC
Histone deacetylase
- HGG
High-grade glioma
- JPAs
Juvenile pilocytic astrocytomas
- LGG
Low-grade glioma
- LMIC
Low- or middle-income country
- MRI
Magnetic resonance imaging
- NCI
National Cancer Institute
- NF
Neurofibromatosis
- OPG
Optic pathway glioma
- PXAs
Pilomyxoid astrocytomas
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCT
Randomised controlled trial
- WHO
World Health Organization
- WHO ICTRP
World Health Organization International Clinical Trials Registry Platform
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Binning MJ, Liu JK, Kestle JRW, Brockmeyer DL, Walker ML. Optic pathway gliomas: a review. Neurosurg Focus. 2007;23:E2. doi: 10.3171/FOC-07/11/E2. [DOI] [PubMed] [Google Scholar]
- 2.Helfferich J, Nijmeijer R, Brouwer OF et al. Neurofibromatosis type 1 associated low grade gliomas: a comparison with sporadic low grade gliomas. Crit Rev Oncol Hematol. 2016;104:30–41. doi: 10.1016/j.critrevonc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Aquilina K, Daniels DJ, Spoudeas H, Phipps K, Gan HW, Boop FA. Optic pathway glioma in children: does visual deficit correlate with radiology in focal exophytic lesions. Childs Nerv Syst. 2015;31:2041–2049. doi: 10.1007/s00381-015-2855-7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RP, Gibbs IC, Xu LW, et al. Treatment options for optic pathway gliomas. Curr Treat Options Neurol. 2015;17:333. doi: 10.1007/s11940-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 5.Calixto NC, Simão GN, Dos Santos AC, et al. Monitoring optic chiasmatic-hypothalamic glioma volumetric changes by MRI in children under clinical surveillance or chemotherapy. Childs Nerv Syst. 2019;35:63–72. doi: 10.1007/s00381-018-3904-9. [DOI] [PubMed] [Google Scholar]
- 6.Awdeh RM, Kiehna EN, Drewry RD, Kerr NC, Haik BG, Wu S, Xiong X, Merchant TE. Visual outcomes in pediatric optic pathway glioma after conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:46–51. doi: 10.1016/j.ijrobp.2011.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang DS, Murphy ES, Merchant TE. Radiation therapy for optic pathway and hypothalamic low-grade gliomas in children. Int J Radiat Oncol Biol Phys. 2017;99:642–651. doi: 10.1016/j.ijrobp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Sawamura Y, Kamada K, Kamoshima Y, Yamaguchi S, Tajima T, Tsubaki J, Fujimaki T. Role of surgery for optic pathway/hypothalamic astrocytomas in children. Neuro-Oncology. 2008;10:725–733. doi: 10.1215/15228517-2008-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodden J, Pizer B, Pettorini B, Williams D, Blair J, Didi M, Thorp N, Mallucci C. The role of surgery in optic pathway/hypothalamic gliomas in children. J Neurosurg Pediatr. 2014;13:1–12. doi: 10.3171/2013.8.PEDS12546. [DOI] [PubMed] [Google Scholar]
- 10.Gan H-W, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100:3787–3799. doi: 10.1210/jc.2015-2028. [DOI] [PubMed] [Google Scholar]
- 11.Robert-Boire V, Rosca L, Samson Y, Ospina LH, Perreault S. Clinical presentation and outcome of patients with optic pathway glioma. Pediatr Neurol. 2017;75:55–60. doi: 10.1016/j.pediatrneurol.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Rasool N, Odel JG, Kazim M. Optic pathway glioma of childhood. Curr Opin Ophthalmol. 2017;28:289–295. doi: 10.1097/ICU.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo ET, Kvint S, Orillac C, et al. Long-term clinical and visual outcomes after surgical resection of pediatric pilocytic/pilomyxoid optic pathway gliomas. J Neurosurg Pediatr. 2019;1:1–8. doi: 10.3171/2019.2.PEDS18529. [DOI] [PubMed] [Google Scholar]
- 14.Y-y L, Y-t L, Hu Q-l, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:152. doi: 10.1186/s12957-019-1686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taddei M, Erbetta A, Esposito S, et al. Brain tumors in NF1 children: influence on neurocognitive and behavioral outcome. Cancers (Basel) 2019;11(11):1772. doi: 10.3390/cancers11111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papini C, Dineen RA, Walker DA, et al. Neuropsychological outcomes of children with optic pathway glioma. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-59896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 19.Zaghloul MS. Single pediatric neuro-oncology center may make difference in low/middle-income countries. Childs Nerv Syst. 2016;32:241–242. doi: 10.1007/s00381-015-2987-9. [DOI] [PubMed] [Google Scholar]
- 20.Chan MH, Boop F, Qaddoumi I. Challenges and opportunities to advance pediatric neuro-oncology care in the developing world. Childs Nerv Syst. 2015;31:1227–1237. doi: 10.1007/s00381-015-2771-x. [DOI] [PubMed] [Google Scholar]
- 21.Hessissen L, Parkes J, Amayiri N, Mushtaq N, Sirachainan N, Anacak Y, Mitra D, Figaji A, Schouten-van Meeteren A, Sullivan M, Burger H, Davidson A, Bouffet E, Bailey S. SIOP PODC adapted treatment guidelines for low grade gliomas in low and middle income settings. Pediatr Blood Cancer. 2017;64:e26737. doi: 10.1002/pbc.26737. [DOI] [PubMed] [Google Scholar]
- 22.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamji MF, Benoit BG. Syndromic and sporadic pediatric optic pathway gliomas: review of clinical and histopathological differences and treatment implications. Neurosurg Focus. 2007;23:E3. doi: 10.3171/foc.2007.23.5.4. [DOI] [PubMed] [Google Scholar]
- 24.de Blank PMK, Fisher MJ, Liu GT, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol. 2017;37(Suppl 1):S23–S32. doi: 10.1097/WNO.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan M-Y, Foong AP, Heisey DM, Harkness W, Hayward R, Michalski A. Potential prognostic factors of relapse-free survival in childhood optic pathway glioma: a multivariate analysis. Pediatr Neurosurg. 1998;29:23–28. doi: 10.1159/000028680. [DOI] [PubMed] [Google Scholar]
- 26.Laithier V, Grill J, Le Deley M-C, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan RJ, Infante JR, Janku F, Wong DJL, Sosman JA, Keedy V, Patel MR, Shapiro GI, Mier JW, Tolcher AW, Wang-Gillam A, Sznol M, Flaherty K, Buchbinder E, Carvajal RD, Varghese AM, Lacouture ME, Ribas A, Patel SP, DeCrescenzo GA, Emery CM, Groover AL, Saha S, Varterasian M, Welsch DJ, Hyman DM, Li BT. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8:184–195. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 28.Schreck KC, Grossman SA, Pratilas CA. BRAF mutations and the utility of RAF and MEK inhibitors in primary brain tumors. Cancers. 2019;11:1262. doi: 10.3390/cancers11091262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez JN, Wang T, Cohen MS. BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs. 2018;78:549–566. doi: 10.1007/s40265-018-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Bufalo F, Ceglie G, Cacchione A, et al. BRAF V600E inhibitor (Vemurafenib) for BRAF V600E mutated low grade gliomas. Front Oncol. 2018;8:526. doi: 10.3389/fonc.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright JD, Zimmerman MA, Fine E, Aspri T, et al. Type 2 II BRAF inhibitor TAK-580 shows promise for upcoming clinical trial as evidenced by single patient IND study. Neuro-oncology. 2018;20:i110. [Google Scholar]
- 32.Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro-Oncology. 2015;17:843–853. doi: 10.1093/neuonc/nou329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–2560. doi: 10.1056/NEJMoa1605943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Goldman S, Pollack IF, Qaddoumi I, Jakacki RI, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Jones DTW, Pfister SM, Vezina G, Stern JS, Panigrahy A, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20:1011–1022. doi: 10.1016/S1470-2045(19)30277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132:111–114. doi: 10.1001/jamaophthalmol.2013.5819. [DOI] [PubMed] [Google Scholar]
- 36.Wu WS, Liu JJ, Sun YL, et al. Effect of bevacizumab in treatment of children with optic pathway glioma. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21:1193. doi: 10.7499/j.issn.1008-8830.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhukova N, Rajagopal R, Lam A, Coleman L, Shipman P, Walwyn T, Williams M, Sullivan M, Campbell M, Bhatia K, Gottardo NG, Hansford JR. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019;8:40–50. doi: 10.1002/cam4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki F, Takano M, Yonezawa U, Taguchi A, Kolakshyapati M, Okumichi H, Kiuchi Y, Kurisu K. Bevacizumab for optic pathway glioma with worsening visual field in absence of imaging progression: 2 case reports and literature review. Childs Nerv Syst. 2020;36:635–639. doi: 10.1007/s00381-019-04407-6. [DOI] [PubMed] [Google Scholar]
- 39.Capozzi M, De Divitiis C, Ottaiano A, et al. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag Res. 2019;11:3847–3860. doi: 10.2147/CMAR.S188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajkumar SV, Witzig TE. A review of angiogenesis and antiangiogenic therapy with thalidomide in multiple myeloma. Cancer Treat Rev. 2000;26:351–362. doi: 10.1053/ctrv.2000.0188. [DOI] [PubMed] [Google Scholar]
- 41.Hassler MR, Sax C, Flechl B, Ackerl M, Preusser M, Hainfellner JA, Woehrer A, Dieckmann KU, Rössler K, Prayer D, Marosi C. Thalidomide as palliative treatment in patients with advanced secondary glioblastoma. Oncology. 2015;88:173–179. doi: 10.1159/000368903. [DOI] [PubMed] [Google Scholar]
- 42.Fine HA, Kim L, Albert PS, Duic JP, Ma H, Zhang W, Tohnya T, Figg WD, Royce C. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13:7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 43.Drappatz J, Wong ET, Schiff D, et al. A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:222–227. doi: 10.1016/j.ijrobp.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Warren KE, Goldman S, Pollack IF, Fangusaro J, Schaiquevich P, Stewart CF, Wallace D, Blaney SM, Packer R, MacDonald T, Jakacki R, Boyett JM, Kun LE. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC-018. J Clin Oncol. 2011;29:324–329. doi: 10.1200/JCO.2010.31.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore CA, Ibrahim M, Kapila A, et al. Glioblastoma multiforme in a patient with multiple myeloma: a case report and literature review. Perm J. 2018;22:17–125. doi: 10.7812/TPP/17-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fangusaro JR, Locatelli F, Garré ML et al (2019) A phase II clinical study of pomalidomide (CC-4047) monotherapy for children and young adults with recurrent or progressive primary brain tumors [DOI] [PMC free article] [PubMed]
- 47.Mukhopadhyay T, Sasaki J, Ramesh R, et al. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2002;8:2963–2969. [PubMed] [Google Scholar]
- 48.Bai RY, Staedtke V, Aprhys CM, Gallia GL, Riggins GJ. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro-Oncology. 2011;13:974–982. doi: 10.1093/neuonc/nor077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purow B. Repurposing existing agents as adjunct therapies for glioblastoma. Neuro-Oncol Pract. 2016;3:154–163. doi: 10.1093/nop/npv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerini AE, Triggiani L, Maddalo M, et al. Mebendazole as a candidate for drug repurposing in oncology: an extensive review of current literature. Cancers (Basel) 2019;11(9):1284. doi: 10.3390/cancers11091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eyüpoglu IY, Hahnen E, Tränkle C, Savaskan NE, Siebzehnrübl FA, Buslei R, Lemke D, Wick W, Fahlbusch R, Blümcke I. Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol Cancer Ther. 2006;5:1248–1255. doi: 10.1158/1535-7163.MCT-05-0533. [DOI] [PubMed] [Google Scholar]
- 52.Lee P, Murphy B, Miller R, et al. Mechanisms and clinical significance of histone deacetylase inhibitors: epigenetic glioblastoma therapy. Anticancer Res. 2015;35:615–625. [PMC free article] [PubMed] [Google Scholar]
- 53.Warren K, Bent R, Wolters PL, Prager A, Hanson R, Packer R, Shih J, Camphausen K. A phase 2 study of pegylated interferon α-2b (PEG-Intron®) in children with diffuse intrinsic pontine glioma. Cancer. 2012;118:3607–3613. doi: 10.1002/cncr.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lubanska D, Porter L. Revisiting CDK inhibitors for treatment of glioblastoma multiforme. Drugs R D. 2017;17:255–263. doi: 10.1007/s40268-017-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weyerhäuser P, Kantelhardt SR, Kim EL. Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front Oncol. 2018;8:335. doi: 10.3389/fonc.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 57.MacKeigan JP, Krueger DA. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro-Oncology. 2015;17:1550–1559. doi: 10.1093/neuonc/nov152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kyi C, Roudko V, Sabado R, et al. Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: a pilot trial. Clin Cancer Res. 2018;24:4937–4948. doi: 10.1158/1078-0432.CCR-17-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, Chiocca EA. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolff JE, Wagner S, Reinert C, et al. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J Neuro-Oncol. 2006;79:315–321. doi: 10.1007/s11060-006-9147-8. [DOI] [PubMed] [Google Scholar]
- 61.Du B, Waxman DJ. Medium dose intermittent cyclophosphamide induces immunogenic cell death and cancer cell autonomous type I interferon production in glioma models. Cancer Lett. 2020;470:170–180. doi: 10.1016/j.canlet.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamashita AS, da Costa RM, Borodovsky A, et al. Demethylation and epigenetic modification with 5-azacytidine reduces IDH1 mutant glioma growth in combination with temozolomide. Neuro-Oncology. 2019;21:189–200. doi: 10.1093/neuonc/noy146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505):eaaw5680. doi: 10.1126/scitranslmed.aaw5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Majzner RG, Theruvath JL, Nellan A, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang B, Luo L, Wang J, He B, Feng R, Xian N, Zhang Q, Chen L, Huang G. B7-H3 specific T cells with chimeric antigen receptor and decoy PD-1 receptors eradicate established solid human tumors in mouse models. Oncoimmunology. 2020;9:1684127. doi: 10.1080/2162402X.2019.1684127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salem U, Kumar VA, Madewell JE, Schomer DF, de Almeida Bastos DC, Zinn PO, Weinberg JS, Rao G, Prabhu SS, Colen RR. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT) Cancer Imaging. 2019;19:65. doi: 10.1186/s40644-019-0250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L, Zhang J, Lee H, Batista MT, Johnston SA. RNA transcription and splicing errors as a source of cancer frameshift neoantigens for vaccines. Sci Rep. 2019;9:14184. doi: 10.1038/s41598-019-50738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bilgic B, Gagoski BA, Cauley SF, et al. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med. 2015;73:2152–2162. doi: 10.1002/mrm.25347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conklin J, Longo MGF, Cauley SF, et al. Validation of highly accelerated wave-CAIPI SWI compared with conventional SWI and T2*-weighted gradient recalled-echo for routine clinical brain MRI at 3T. AJNR Am J Neuroradiol. 2019;40:2073–2080. doi: 10.3174/ajnr.A6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw TB, Jeffree RL, Thomas P, Goodman S, Debowski M, Lwin Z, Chua B. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of glioma. J Med Imaging Radiat Oncol. 2019;63:650–656. doi: 10.1111/1754-9485.12929. [DOI] [PubMed] [Google Scholar]