Abstract
The advent of immune checkpoint inhibitors (ICIs) caused a paradigm shift both in drug development and clinical practice; however, by virtue of their mechanism of action, the excessively activated immune system results in a multitude of off-target toxicities, the so-called immune-related adverse events (irAEs), requiring new skills for timely diagnosis and a multidisciplinary approach to successfully manage the patients. In the recent past, a plethora of large-scale pharmacovigilance analyses have characterized various irAEs in terms of spectrum and clinical features in the real world. This review aims to summarize and critically appraise the current landscape of pharmacovigilance studies, thus deriving take-home messages for oncologists. A brief primer to study design, conduction, and data interpretation is also offered. As of February 2020, 30 real-world postmarketing studies have characterized multiple irAEs through international spontaneous reporting systems, namely WHO Vigibase and the US FDA Adverse Event Reporting System. The majority of studies investigated a single irAE and provided new epidemiological evidence about class-specific patterns of irAEs (i.e. anti-cytotoxic T-lymphocyte antigen 4 [CTLA-4] versus anti-programmed cell death 1 [PD-1] receptor, and its ligand [PD-L1]), kinetics of appearance, co-occurrences (overlap) among irAEs, and fatality rate. Oncologists should be aware of both strengths and limitations of these pharmacovigilance analyses, especially in terms of data interpretation. Optimal management (including rechallenge), predictivity of irAEs (as potential biomarkers of effectiveness), and comparative safety of ICIs (also in terms of combination regimens) represent key research priorities for next-generation real-world studies.
Electronic supplementary material
The online version of this article (10.1007/s11523-020-00738-6) contains supplementary material, which is available to authorized users.
Key Points
| Safety of immune checkpoint inhibitors (ICIs) is a currently unsettled issue in clinical practice, requiring multidisciplinary expertise and heterogeneous skills of oncologists to early diagnose and timely manage immune-related adverse events (irAEs). |
| In the recent past, a plethora of real-world pharmacovigilance studies have characterized epidemiological features of irAEs, namely spectrum, kinetics, co-reporting, and fatality rate. |
| Considering the agnostic approval and evolving uses of different ICIs, including drug combination with targeted therapy, pharmacovigilance still plays a pivotal role in the postmarketing assessment of irAEs, thus supporting oncologists in optimal management. |
Introduction
The advent of immune checkpoint inhibitors (ICIs) caused a paradigm shift both in drug development and in the treatment and prognosis of a wide range of different cancers, including melanoma and non-small cell lung cancer (NSCLC), with sustained long-term remission and prolonged survival [1]. However, by virtue of their mechanism of action, namely blocking either cytotoxic T-lymphocyte antigen 4 (CTLA-4) or programmed cell death 1 (PD-1), or its ligand (PD-L1), the excessively activated immune system results in off-target toxicities characterized by a unique and distinct spectrum of adverse effects, the so-called immune-related adverse events (irAEs) [2], with a different mechanistic basis depending on the damaged organ [3].
As a consequence of the rapid extension of therapeutic indications in different tumor types (the agnostic approval of pembrolizumab is a paradigmatic example) and settings, clinicians are increasingly facing both common and rare irAEs, which can virtually affect any organ or system in the body, requiring new skills to timely diagnosis, and a multidisciplinary approach to successful patient management [4–8].
Although most common irAEs were identified during preapproval clinical development, their assessment and clinical characterization in terms of spectrum, timing and outcomes were only recently investigated through real-world, large-scale pharmacovigilance analyses. In particular, the analysis of spontaneous reporting systems (SRSs) allows real-time long-term monitoring of the safety profile of medicines, and also timely identification of rare irAEs worldwide [9].
In the recent past, pharmacovigilance studies have attracted considerable interest in the medical literature and a number of articles have been published: a search in MEDLINE using a combination of simple keywords (e.g. adverse event reporting system, spontaneous reporting system, pharmacovigilance database, FAERS, Vigibase, Eudravigilance) yielded 3283 articles (search performed on 15 January 2020), of which 1445 were published in the last 5 years (390 in 2019). This scenario may be attributable to multifold reasons, including the increased awareness by clinicians regarding the importance of pharmacovigilance (namely postmarketing surveillance) and relevant commitment of submitting AEs for regulatory purposes, public availability of large SRSs, and open-access tools to independently analyze international databases [10].
In particular, the so-called disproportionality analyses (DAs) are now the most frequently epidemiological approach for postmarketing safety assessment, and the term ‘association’ is increasingly recorded in major specialized journals. When properly conducted, the performance of these studies is noteworthy for signal detection (i.e. the capacity to discriminate true from false positive drug–event associations) [11]. However, consolidated expertise is required to minimize bias not only during study conception and design but also in the proper interpretation of study results to avoid common mistakes by clinicians: incorrect use of SRSs to infer causality, assess the incidence of AEs, or provide risk stratification, which may ultimately result in unjustified alarm. Therefore, the debate regarding the proper use of these studies and the benefit of their publication, which arose in 2011 [12, 13], is now pressing [14, 15].
The case of oncology, and in particular immunotherapy, is not an exception, and a plethora of postmarketing DAs have documented the occurrence of various toxicities with ICIs in the real world [16, 17]. Therefore, clinicians may wonder whether and how these data will impact their routine practice, especially in the era of real-world evidence and relevant debate on their complementary role with randomized controlled trials (RCTs).
In this critical appraisal, we summarized the current landscape of pharmacovigilance studies in order to derive take-home messages for clinical practice, in particular what these studies add to current knowledge, what is still missing, and how to address knowledge gap by future pharmacovigilance approaches. To this aim, a brief primer to DAs is first offered to guide clinicians towards better understanding of their clinical and research implications.
Pharmacovigilance in Oncology: Challenges and Opportunities
Pharmacovigilance in oncology is not straightforward compared with other medical areas. Variegate clinically significant toxicities have been described both for old-fashioned chemotherapy and for targeted therapies, including biological agents and immunotherapy [18–20]. Frequent use of multiple therapeutic regimens makes it difficult to disentangle the adverse effects of individual drugs versus drug–drug interactions versus ‘innocent bystander’ effects [21, 22]. Moreover, complexity of patient histories results in high potential for confounding and effect modification (i.e. drug–disease interactions). Finally, the unique benefit–risk consideration may result in a higher threshold for recognizing and reporting AEs.
In the field of immunotherapy, the term ‘immunovigilance’ was also proposed [23], considering the documented increasing uptake and expectations of ICIs in clinical practice, as well as suboptimal knowledge about their actual safety profile due to accelerated approval and inherent limitations of RCTs in detecting rare toxicities. Therefore, immunotherapy likely represents a privileged setting to perform fourth-generation pharmacovigilance studies, namely big data, to assess emerging toxicities in real life, thus promoting safe prescribing.
Disproportionality Analysis: A Primer for Oncologists
As a general note, DAs alone cannot be used per se to assess a drug-related risk and cannot replace clinical judgment in the individual patient; however, their role is undisputable and unreplaceable for rapid detection of rare and unpredictable AEs with a strong drug-attributable component, especially when combined with a case-by-case analysis (individual inspection of reports for causality assessment) and detailed descriptive data (reporting trend, demographic data, concomitant drugs, indications, outcome). Until now, there has been no recognized gold standard among disproportionality measures (frequentist or Bayesian approaches). In particular, frequentist methods such as the reporting odds ratio (ROR), proportional reporting ratio (PRR), and relative reporting ratio (RRR) are based on the case/non-case approach: if the proportion of adverse events of interest is greater in patients exposed to a specific drug (cases) than in patients not exposed to this drug (non-cases), a disproportionality signal emerges and subsequent analytical investigations are usually required before taking regulatory actions [24].
While there are common thresholds for defining statistical significance (using a 95% confidence interval [CI]) and signaling criteria to perform and claim disproportionality (usually three or more cases are necessary), there is debate on relevant interpretation and clinical implications. Although, as a general rule, the higher the ROR/PRR/RRR value, the stronger the disproportion, disproportionality measures are only an indicator of risk (not a measure of risk) and express the extent of reporting (a proxy of actual occurrence). Only for a limited set of 15 known drug–event associations, disproportionality measures significantly correlated with the relative risks of analytical studies, thus providing an initial indication of the likely clinical importance of an adverse event [25].
Considering limitations of both spontaneous reports and pharmacovigilance databases (lack of exposure data, inability to firmly infer causality, the general under- and overreporting), incidence, reporting rate, risk quantification, and risk ranking cannot be claimed. The term ‘association’, usually found in several articles, should be used carefully to indicate that statistically significant disproportionality exists (referred to as a signal of disproportionate reporting or disproportionality signal). Notably, there is no consensus on whether the lack of significant disproportionality is actually a true negative finding (i.e. absence of risk) and, more importantly, on the controversial interpretation of negative disproportionality as a potential inverse causality (i.e. a signal of protective effect potentially indicative of a ‘beneficial drug reaction’) [26, 27]. In fact, negative findings from the disproportionality approach may be the result of comparator choice (see below) or the consequence of a ‘dilution effect’ by other frequently reported toxicities (disproportionality measures are interdependent).
Only under stringent criteria (no major distortions in reporting patterns, similar marketing approval, comparable penetration and utilization), can adverse event rates be compared, and disproportionality by therapeutic area may also be considered (i.e. comparing a drug with other agents within the same therapeutic class). Notably, the choice of comparator is a critical issue in pharmacovigilance because it may substantially impact on results. Disproportionality by therapeutic area can be viewed as an intraclass analysis to provide a clinical perspective and reduce the so-called indication bias by selecting a dataset where only anticancer therapies are represented, using relevant reports as a proxy of exposure; this allows to identify a real-world subpopulation that presumably shares at least a set of common risk factors. It is anticipated that, in the majority of DAs published in the literature, the analysis by therapeutic area was not performed: ICIs were compared with all other drugs in the database, but direct comparison between anti-PD-1/PD-L1 and anti-CTLA-4 monotherapy was carried out. The latter approach is likely influenced by disease-specific (rather than treatment-specific) factors, considering that melanoma patients largely comprise the anti-CTLA-4 group, and may have distinct demographic and toxicity proclivities, compared with the more pan-tumor population treated with anti-PD-1/PD-L1.
The existence of biases represents another technical issue when planning and designing DAs. In the oncological area, there are some peculiarities that warrant discussion. The so-called channeling bias (selective prescription of newer drugs to patients with more severe disease) is unlikely to be fully accounted for even through analysis by therapeutic area, considering the existence of first-, second-, and third-line therapies. Moreover, a non-negligible proportion of reports describe drug indication as an adverse event, a phenomenon known as indication bias. Finally, there is still no consensus on the interpretation of the outcome ‘death’ and reports of ‘drug ineffective’ [28, 29].
Therefore, when approaching DA in oncology, the existence of specific biases and relevant minimization strategies should be considered (Table 1). For a systematic discussion of all technical aspects in study concept, design, conduction, presentation, and discussion of results, the reader may refer to dedicated book chapters [10, 24] and expert documents such as the article on Good Signal Detection Practices [30].
Table 1.
Key conceptual and technical challenges when performing disproportionality analysis in oncology
| Domain of clinical relevance | Concepts and proposal | Points to be considered | Specific issues for immunotherapy |
|---|---|---|---|
| Scientific rationale | The scientific basis must be clearly described (see text) | Timeliness is a requisite: hypothesis raised by DA to be tested by subsequent analytical pharmacoepidemiological studies | Avoid overlapping studies (redundant publication) on the same topic, unless there are key differences in the analyses (e.g. updated information in terms of events and drugs of interest) |
| Choice of denominator | Usually, the entire database is used as a screening strategy to map the safety profile | Additional control drugs may be used to support the robustness of disproportionality, but the choice is arbitrary and debated | Disproportionality by therapeutic area or versus specific drug class (anti-CTLA-4 vs. anti-PD-1/PD-L1) provides clues on intraclass comparison, but distortions remain (channeling bias or confounding by disease-specific factors) |
| Case definition | This aspect should be clearly defined by providing a dedicated material with search strategy for case identification | Usually, analyses are performed in terms of PTs, which can be combined to obtain the so-called SMQs | No specific definition (and reporting) in pharmacovigilance of irAEs or dedicated SMQ. Therefore, possibility of reporting false positives (e.g. pneumonitis) |
| Use of case-by-case analysis | It may per se be used for standalone signal detection or may complement DA | No single method is universally accepted for causality assessment and there is no gold standard | A key added value, by characterizing spectrum, timing, and outcomes of irAEs |
| Bias identification and minimization | The potential existence of biases must be a priori conceived (depending on the drug and events under investigation) | There are three sources of bias: (1) the drug-event reporting; (2) the nature of SRS; and (3) the high variability of event reporting | Indication and channeling bias are likely to occur and relevant minimization strategies should be considered; notoriety and competition bias are unlikely to occur (no specific warnings or media attention on a single irAE) |
| Interpretation | Disproportion per se is not an estimate of risk; disproportionality may approach the relative risk estimate only under stringent criteria (no reporting biases and confounders) |
Disproportionalities are interdependent (consider existence of competition bias and other sources of confounding) Inverse causality is highly debated |
Avoid terms such as ‘association’, ‘risk’, and ‘incidence’. Avoid specific clinical recommendations in terms of risk rankings and identification of safe drugs. Higher/increased reporting can be used |
DA disproportionality analysis, irAEs immune-related adverse events, SRS spontaneous reporting system, CTLA-4 cytotoxic T-lymphocyte antigen 4, PD-1 programmed cell death 1, PD-L1 programmed cell death-ligand 1, PTs preferred terms, SMQs standardized MedDRA queries, MedDRA Medical Dictionary for Regulatory Activities
Methods
To summarize the current landscape of pharmacovigilance studies, a search strategy was performed in MEDLINE to extract relevant articles (performed as of 25 February 2020). This overview was not intended to be comprehensive, and eligible studies were postmarketing analyses performed on international SRSs, aiming to specifically investigate the safety/toxicities of ICIs, with minimum information to describe the epidemiology of irAEs. Notwithstanding the potential contribution and value of national databases, these studies were excluded after considering (1) the aim of the review (to provide a global perspective); (2) the limited catchment area of national archives and relevant influence of reporting pattern by local prescription features; and (3) better suitability and performance for drugs with well-established use [31]. Conversely, all types of pharmacovigilance analysis were included, using both a disproportionality and descriptive approach, considering the importance of a case-by-case assessment.
Therefore, a combination of the following keywords was used: spontaneous reporting system, spontaneous reporting database, pharmacovigilance database, FDA Adverse Event Reporting System, FAERS, Vigibase, Vigilyze, Eudravigilance, disproportionality analysis, disproportionality study, real-world study, toxicities, immune-related adverse events, immune checkpoint inhibitors, checkpoint inhibitors, anti-PD-1, anti-PD-L1, anti-CTLA-4, nivolumab, ipilimumab, pembrolizumab, atezolizumab, and durvalumab. Snowballing of retained articles was performed. For each included study, the following information was extracted: database used, time window of the analysis, type of analysis (descriptive or disproportionality), disproportionality measure (e.g. ROR), strategies for minimization of bias, irAEs of interest, relative frequency, fatality rate, time to onset and other key findings (combinations vs. monotherapy, anti-PD-1/PD-L1 vs. anti-CTLA-4). The relative frequency was derived from presented data by dividing the number of cases of a given irAE with the total number of events recorded for ICIs; the fatality rate (i.e. the proportion of death reports of the total reported events) was extracted from original studies.
Results
After full-text analysis of 36 potentially eligible studies, 30 met the inclusion criteria and were finally retained [9, 16, 17, 32–58]. Of these 30 studies, 14 performed DAs (Table 2) and 16 were only based on a descriptive design (Table 3), mainly due to the rarity of irAEs under investigation. It is anticipated that a direct comparison among studies, even those analyzing the same topic, cannot be formally carried out because of technical issues, including the time window of the analysis, removal of duplicates, drug codification, and calculation of disproportionality (disproportionality measure, comparator and adjustment).
Table 2.
Overview of disproportionality studies
| Authors (year); database | Toxicity (irAEs) | Main disproportionality findings | Relative frequency (reporting proportion)a | Fatality rate | Comments/notes |
|---|---|---|---|---|---|
| Zhai et al. (2019); FAERS [36] | Endocrinopathies | Higher reporting with anti-CTLA-4 (vs. anti-PD-1/PD-L1), combo vs. mono, hypothyroidism and hyperthyroidism with anti-PD-1, ipilimumab and hypophysitis, combo with adrenal insufficiency | 8.6% (overall), 1.2% (hypothyroidism), 1% (adrenal insufficiency), 0.9% (hypophysitis), 0.7% (hyperthyroidism), 0.5% (diabetes mellitus) | 9.6% | Overreporting with anti-CTLA-4 monotherapy, males, NSCLC, combination; hypo/hyperthyroidism especially with anti-PD-1 and combination; adrenal insufficiency and hypophysitis with ipilimumab and combination |
| Bai et al. (2020); Vigibase [37] | Thyroid dysfunction | Higher reporting of hypothyroidism, hyperthyroidism, and thyroiditis (anti-PD-1 and combo), thyrotoxic crisis with combo | 3.8% (overall), 1.8% (hypothyroidism), 1.5% (hyperthyroidism) | 0.15% (hyperthyroidism), 0.62% (thyroiditis), 0.93% (hypothyroidism), 20% for thyrotoxic crisis) | Hypo-/hyperthyroidism, and thyroiditis overreported for anti-PD-1 drug monotherapy. TTO = 46, 63, 92 combination, anti-CTLA-4, anti-PD-1, respectively). Recovered/resolved 60–78% |
| Johnson et al. (2019); Vigibase [38] | Neurologic toxicity | Higher reporting of myasthenia gravis, non-infectious encephalitis/myelitis with anti-PD-1/PD-L1; Guillain–Barre syndrome and non-infectious meningitis with anti-CTLA-4. Higher reporting with combo (except myasthenia gravis) | 1.16% (peripheral neuropathy); 0.51% (noninfectious encephalitis and/or myelitis; 0.47% (myasthenia gravis); 0.15% (noninfectious meningitis) | 19.3% (myasthenia gravis); 13.2% (noninfectious encephalitis and/or myelitis) | TTO = 29 for myasthenia gravis, 61–80 for other events. Myasthenia gravis frequently overlapped with myocarditis/myositis, with a 62.5% mortality rate |
| Salem et al. (2018); Vigibase [34] | Cardiovascular toxicity | Higher reporting of myocarditis, pericardial diseases, supraventricular arrhythmias, vasculitis (temporal arteritis, polymyalgia rheumatic) | 0.4% (myocarditis), 0.3% (pericardial diseases, vasculitis) | 50% (myocarditis), 21% (pericardial disease), 6% (vasculitis) | TTO = 30 (myocarditis, pericardial disease), 55 (vasculitis). Myocarditis overreported with anti-PD-1/PD-L1, and combo; pericardial diseases with anti-PD-1/PD-L1; vasculitis with anti-CTLA-4 |
| Fan et al. (2019); FAERS [39] | Myocarditis | Higher reporting for all ICI monotherapies and two ICI combos | 0.7% | 51% (66% in combination) | TTO = 23, earlier for combination vs. monotherapy (16.5 vs. 32) |
| Ederhy et al. (2019); Vigibase [55] | TTS | Higher reporting of TTS, especially with ipilimumab and pembrolizumab | 0.03% | 13% (1 patient) | 1333 cases of stress cardiomyopathy; TTO = 6 (median) and 76 (mean); no cases in combo; use of positive (venlafaxine/adrenaline) and negative (paracetamol) controls; 3 patients did not recover |
| Nguyễn et al. (2020); Vigibase [56] | Myositis | Higher reporting with anti-PD-1/PD-L1 (vs. anti-CTLA-4) and combo; no myasthenia cases with anti-CTLA-4 | 0.6% (345 cases) | 22.3% (51.3% with myocarditis) | Median TTO = 33; 95% serious; myocarditis and myasthenia in 11.3% and 11.9%, respectively |
| Oshima et al. (2018); FAERS [43] | IP | Higher proportion of IP in nivolumab + EGFR TKIs | 20,516 patients with NSCLC; 985 IP cases (18 exposed to nivolumab + EGFR TKIs) | NA | The statistically significant interaction effect supported the existence of the interaction |
| Aggarwal (2019); FAERS [40] | BP | Consistent signal of BP across analyses | 37 cases with pembrolizumab, 81 with nivolumab | 7.4% (nivolumab), 2.7% (pembrolizumab) |
Also literature review of case reports. Drug withdrawal in > 50% Heat map confirming and detecting signals of SJS, TEN (pembrolizumab), TEN (nivolumab) |
| Jimenez et al. (2020); FAERS [57] | BDs | Signals of BDs for anti-PD-1/PD-L1, except avelumab | 99 cases with nivolumab, 43 with pembrolizumab | NA | Pemphigoid, pemphigus, and bullous dermatitis were searched linked to serious outcome |
| Fang et al. (2019); FAERS [41] | Ocular AEs | Uveitis with ipilimumab, nivolumab, pembrolizumab; ocular myasthenia with nivolumab (three cases) | NA | NA | 113 ocular AEs; 68 with nivolumab |
| Ali and Watson (2017); FAERS [42] | irAEs | Colitis and pneumonitis (ipilimumab and nivolumab), pneumonitis and hepatitis (pembrolizumab), endocrinopathies (all ICIs) | 2.6% (1.5% colitis, 0.5% endocrinopathies, 0.3% pneumonitis, hepatitis, 0.1% nephritis) | 18.8% (26% pembrolizumab) | TTO = 76 days (180 for nivolumab); 45.5% no concomitant drugs, except nivolumab (74% ≥ 4 drugs); 46% monotherapy; on average, irAEs persisted for 26 days after stopping ICI. 77% hospitalization |
| Ji et al. (2019); FAERS [16] | irAEs | Pneumonitis, hypothyroidism, myocarditis, autoimmune hemolytic anemia for anti-PD-1; colitis, hypophysitis, adrenal insufficiency with anti-CTLA-4 | 1.05% (hypothyroidism), 0.92% (pneumonitis), 0.62% (colitis), 0.20% (hepatitis), 0.15% (myocarditis, encephalitis, myasthenia gravis), 0.14% hypophysitis | 29% (overall) | Analyses at PT and SMQ levels. Rash and hepatitis at similar rate; combo higher rate vs. monotherapy |
| Raschi et al. (2019); FAERS [17] | irAEs | Pneumonitis, hypothyroidism, cholangitis, tumor pseudoprogression, and inappropriate schedule of drug administration for anti-PD-1; hypophysitis, adrenal insufficiency, hypopituitarism, and prescribed overdose with anti-CTLA-4 | 2.7% (pneumonitis), 1.6% (hypothyroidism), 1.3% (hypophysitis), 1.0% adrenal insufficiency), 0.9% (hepatitis) | 29% (overall) | Also overview of systematic review to identify toxicity priorities. No increased reporting with combination regimens. Overlap in co-reporting of endocrine, hepatobiliary, and respiratory irAEs. Also signal of potential lack of efficacy |
NA not available (i.e. not provided or not calculated based on the information provided), TTO time to onset in days (expressed as mean or median, depending on the study), IC information component, ICIs immune checkpoint inhibitors, EGFR TKIs epidermal growth factor receptor tyrosine kinase inhibitors, NSCLC non-small cell lung cancer, SJS Stevens–Johnson Syndrome, TEN toxic epidermal necrolysis, BP bullous pemphigoid, BDs bullous disorders, IP interstitial pneumonitis, TTS Takotsubo syndrome, combo combination regimen, mono monotherapy, irAEs immune-related adverse events, CTLA-4 cytotoxic T-lymphocyte antigen 4, PD-1 programmed cell death 1, PD-L1 programmed cell death-ligand 1, PT preferred term, SMQ standardized MedDRA query, MedDRA Medical Dictionary for Regulatory Activities, AEs adverse events, FAERS US FDA Adverse Event Reporting System
aOf the total reported AEs
Table 3.
Descriptive pharmacovigilance studies
| Authors (year); database | Toxicity (irAEs) | Fatality rate | Onset (days) | Comments/notes |
|---|---|---|---|---|
| Wang et al. (2018); Vigibase [33] | Fatal irAEs |
2% (613/31,059) 39.7% (myocarditis); 5% (colitis); 3.7% (adrenal insufficiency); 2% (hypophysitis) |
40; 40; 14.5 (ipilimumab, anti-PD-1/PD-L1, combination; Vigibase + multicenter data) |
Colitis/diarrhea (70%) for anti-CTLA-4; pneumonitis (35%), hepatitis (22%) for anti-PD-1/PD-L1 monotherapy; colitis (37%), myocarditis (25%) hepatitis (22%) for combination Fatality rates: 17% (myositis), ≈ 15% (pneumonitis, hepatitis, hematological), ≈ 10% (neurological, nephritis) Myositis and myocarditis frequently co-occurred |
| Raschi et al. (2019); FAERS [9] | SCARs | 2.6% (1 case), 18%, and 36% (DRESS, SJS and TEN, respectively; > 70% for TEN in subjects aged 40–59 years | Mean TTO = 46 (47 for SJS, 48 for TEN, and 40 for DRESS), 41 for combo, 45 for anti-PD-1/PD-L1, and 65 for anti-CTLA-4 |
191 SCAR cases (4618 cutaneous; SJS = 96, TEN = 66, DRESS = 39). Allopurinol and antiepileptics in 17% (10% for other anticancer drugs) Longest recorded latencies were 251, 197, and 164 (SJS, TEN, and DRESS, respectively) Also DA on cutaneous events (bullous dermatoses, especially pemphigoid, mainly reported with anti-PD-1/PD-L1) |
| Anquetil et al. (2018); Vigibase [44] | Myositis | 21%; Higher with combo vs. monotherapy: 38.5% vs. 18.1%; higher in patients with concomitant myocarditis: 51.7% vs. 14.9% | Median TTO = 26 (data for 61 patients) |
180 cases (myocarditis = 29; myasthenia-like disorder = 28), 85% monotherapy Overlap with myocarditis and myasthenia gravis-like symptoms in 16% |
| Wright et al. (2018); Vigibase [45] | DM | 2.8% | Median TTO = 116 (69% developed DM while receiving therapy or within 1 month after cessation, 22% developed DM between 1 and 3 months later, and 9% developed DM more than 3 months after stopping drug |
283 cases of new-onset DM (52.7% nivolumab); 17% combination; 50% with DKA; 5.6%; on corticosteroids at diagnosis; death 2.8% Maximum duration from cessation of therapy to DM onset was 247 days Co-occurrence in 21% (endocrine 8.5%) |
| Vozy et al. (2019); Vigibase [46] | Hepatitis | 19% | Median TTO = 46 (34 with anti-CTLA-4; 48 with anti-PD-1/PD-L1; 41 for fatal hepatitis; no differences between monotherapies and monotherapy vs. combo) |
531 cases (85% had no co-reported drugs) Concurrent irAEs occurred in 31% (most commonly thyroiditis and dermatitis) Age > 65 years as the only independent risk factor for reporting fatal hepatitis |
| Guerrero et al. (2019); Vigibase [47] | Hypophysitis | 3.3% | Median TTO = 76 |
689 cases (ipilimumab 57.5%; combination 18%); commonly reported after three doses; melanoma 81% Overlap with thyroiditis (31%), enterocolitis (19%), dermatologic manifestations (13.7%) |
| Moey et al. (2020); Vigibase [58] | Pneumonitis | 17.5% (22% with pembrolizumab; 60% in lung cancer) | Median TTO = 43 (24 vs. 53, fatal and non-fatal, respectively) | 1694 cases (73% anti-PD-1 monotherapy in fatal cases). Overlap with colitis (8.1% in fatal cases) and hematotoxicity (7.4%). Fatal cases with more respiratory failure, thromboembolism and tumor progression |
| Arnaud et al. (2019); Vigibase [48] | Arthritis | 4.1% | Median TTO = 81 (2.7 months) | 86 cases (82.6% anti-PD-1 monotherapy), rheumatoid arthritis (68%), autoimmune arthritis (16%) or lupus (15%). Recovered in 71% (data for 36 patients). Overlap with colitis (5.8%), thyroid diseases (4.6%) |
| Raschi et al. (2019); FAERS [32] | SLE | 0.02% (among rheumatic events) | Median TTO = 196 |
4870 rheumatic events (arthralgia, n = 711), 18 cases of SLE (plus 7 cases of cutaneous SLE), only with anti-PD-1/PD-L1 No anti-TNF drugs/procainamide/hydralazine were recorded among concomitant drugs; overlap in two cases with arthralgia, arthritis and other rheumatic events |
| Davis et al. (2019); Vigibase [49] | Hematological toxicities | 23% (HLH), 15% (HH), 11% (ITP) | Median TTO = 40 for HLH (23 for anti-CTLA-4, 47.5 for anti-PD-1/PD-L1), 50 for HA, 41 for ITP |
168 cases (HA = 68, ITP = 57, HLH = 26); HLH mainly with anti-CTLA-4, earlier onset (26), and higher fatality (23%) Overlap only with non-hematologic irAEs (23%) |
| Noseda et al. (2019); Vigibase [50] | HLH | 26% | Median TTO = 46.9 | 38 cases (0.08% of total events), 90% ICIs as the solely suspected drug), 58% with anti-PD-1/PD-L1. Recovered in 61%. Overlap with autoimmune hepatitis and interstitial lung disease in 5% |
| Tanios et al. (2019); FAERS [51] | Autoimmune hemolytic anemia | NA | NA (literature data: median TTO = 70) | 68 cases (0.21% of total events), 43 with nivolumab, 32 cases with melanoma. 26.5% with other irAEs (idiopathic thrombocytopenic purpura). Disproportionality analysis stated but not shown |
| Moslehi et al. (2018); Vigibase [35] | Myocarditis | 46% (higher in combo vs. monotherapies) | Median TTO = 27 (data for 33 patients) | 101 cases (57% with anti-PD-1). Concurrent irAEs in 42% (myositis, myasthenia gravis) |
| Dolladille et al. (2020); Vigibase [52] | Cardiac events | 30–27% (early and late events) | Median TTO = 21 (early cases)—178 (late cases) for Vigibase; median TTO = 14 (early cases)—104 (late cases) in three French cardio-oncology units | 437 early cases (onset < 90 days), 159 late cases (onset ≥ 90 cases); almost exclusively with anti-PD-1/PD-L1. Late cases had significantly more frequent HF (21.1% vs. 31.4%). Late cases (19) vs. early cases (19) from three French cardio-oncology units had significantly more LVSD and HF, and less frequent supraventricular arrhythmias; similar mortality (40–42%; early and late events) |
| Garcia et al. (2019); FAERS [53] | Multiple sclerosis | 15.4% (two deaths) | Median TTO = 29 (30 with anti-CTLA-4, 23 with anti-PD-1/PD-L1); median time for symptom resolution was 8 weeks | 13 cases (0.03% of total events) and one additional case from a research center. History of MS was confirmed in eight patients. Three patients did not receive MS drugs while receiving ICIs. Resolution in five cases. Outcomes did not vary by comparing anti-CTLA-4 and anti-PD-1/PD-L1. All cases with other irAEs |
| Zhou et al. (2019); FAERS [54] | Inflammatory bowel disease | 10% (of 38 cases) | NA | Nine cases (of 38 total cases with ICIs and metformin); 60 cases of 36,815 with anti-PD-1/PD-L1 without metformin; 11 cases of 23,572 with metformin without anti-PD-1/PD-L1 diagnosed with DM |
irAEs immune-related adverse events, DM diabetes mellitus, TTO time to onset, combo combination regimen, SLE systemic lupus erythematosus, SCARs serious cutaneous adverse reactions, SJS Stevens–Johnson Syndrome, TEN toxic epidermal necrolysis, DRESS drug reaction with eosinophilia and systemic symptoms, HLH hemophagocytic lymphohistiocytosis, HF heart failure, HH hemolytic anemia, ITP immune thrombocytopenia purpura, LVSD left ventricular systolic dysfunction, CTLA-4 cytotoxic T-lymphocyte antigen 4, PD-1 programmed cell death 1, PD-L1 programmed cell death-ligand 1, FAERS US FDA Adverse Event Reporting System, TNF tumor necrosis factor, ICIs immune checkpoint inhibitors, DKA diabetic ketoacidosis, DA disproportionality analysis, MS multiple sclerosis, NA not available
The US FDA Adverse Event Reporting System (FAERS) and Vigibase (the largest worldwide databases collecting spontaneous reports) were exploited to perform DAs (using both frequentist and Bayesian approaches with traditional signaling criteria); no specific techniques were implemented to minimize potential bias, with the exception of dynamic disproportionality (i.e. calculation of disproportionality over time). The vast majority of studies compared ICIs with all other drugs in the database (only one study calculated disproportionality by therapeutic area and compared ICIs with anticancer agents) and also compared reporting of anti-CTLA-4 with anti-PD-1/PD-L1 medicines.
Only four studies (three disproportionality approaches) investigated the overall safety profile aiming to describe the spectrum of reporting and relevant differences between anti-PD-1/PD-L1 and anti-CTLA-4 monoclonal antibodies, while the vast majority focused on specific irAEs with relevant characterization: 11 articles described a single rare irAE (e.g. myocarditis, hypophysitis, hepatitis, systemic lupus erythematosus, multiple sclerosis, inflammatory bowel disease, hemophagocytic lymphohistiocytosis), while seven addressed a larger spectrum of events, including cardiovascular, neurological, hematological, ocular, skin, and endocrine toxicities. With few exceptions (myocarditis, hematological events), studies were not overlapping.
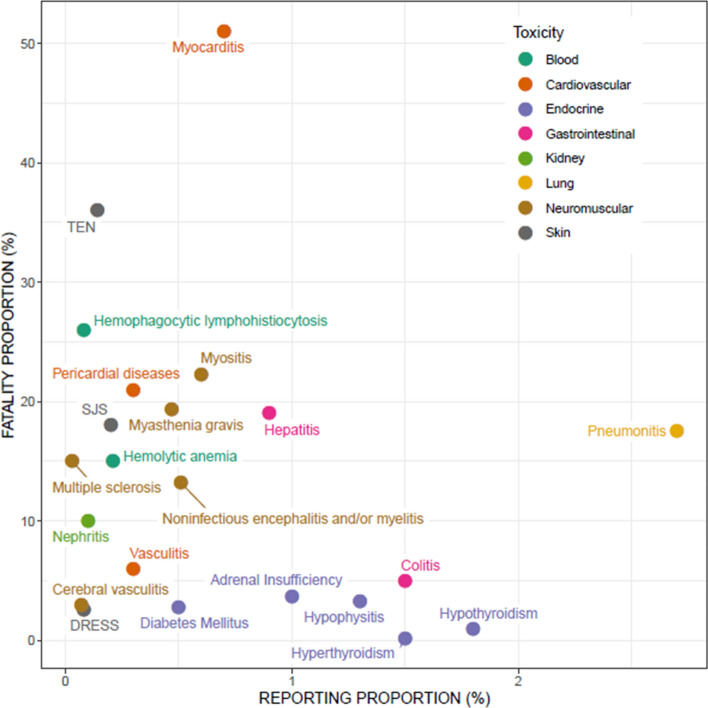
The relative frequency (for simplicity, referred to as reporting proportion) varied slightly depending on the event of interest, with endocrinopathies representing up to 8.6% (3.8% thyroid dysfunction), followed by pneumonitis (2.7%), hypothyroidism (1.8%), hyperthyroidism and colitis (1.5% each), and hypophysitis (1.3%). The vast majority of other irAEs have a reporting frequency of < 1%. The median fatality rate was 20.4% across all studies providing data, and ranged considerably across irAEs: from hyperthyroidism and hypothyroidism (0.15–0.93%) to myocarditis (consistent estimates among studies, ranging from 39.7 to 62.5% when co-occurring with myositis and myasthenia gravis) (Fig. 1). Median time to onset was 46 days, with earlier occurrence for combination regimens, compared with monotherapies, especially in the case of fatality (14.5 days vs. 40 days) [33].
Fig. 1.
Scatter plot showing reporting proportion (i.e. rates of a given irAE compared with total reports) and fatality proportion (i.e. rates of reports where death was recorded as the outcome compared with total number of reports) of the different toxicities with immune checkpoint inhibitors. Data were extracted from published studies. In cases where more than one estimate was available, the highest value was used (worst-case scenario). DRESS drug reaction with eosinophilia and systemic symptoms, SJS Stevens–Johnson Syndrome, TEN toxic epidermal necrolysis
Discussion
Key Messages from Pharmacovigilance Studies
Overall, the collected body of evidence from postmarketing real-world settings underline the urgent need to assess the actual epidemiological impact and risk–benefit profile of ICIs in clinical practice: a remarkable exponential increase in reporting consistently emerged in all studies. This may be viewed not only as a general phenomenon of pharmacovigilance (i.e. a direct consequence of latest European legislation, and increased awareness of clinicians regarding the culture of reporting) but also as a specific issue of immunotherapy: perceived expectations, rapid approval of drugs, and extension of therapeutic indications in different oncological settings. The case of pembrolizumab (first agnostic approval by the FDA) is noteworthy, and the drug has also been tested as a potential treatment option for progressive multifocal leukoencephalopathy [59]. Moreover, newer anti-PD-L1 agents such as atezolizumab have recently been approved in small-cell lung cancer and triple-negative breast cancer, with promising results [60, 61]. This scenario supports the need for continuing monitoring, especially for newer anti-PD-L1 agents.
What’s New: Spectrum and Epidemiology of Immune-Related Adverse Events (irAEs)
Collectively, ICIs cannot be considered as an homogeneous class, not only from a pharmacological viewpoint (different pharmacokinetic features) but also therapeutically, especially from a safety perspective. There is consensus that anti-CTLA-4 and anti-PD-1/PD-L1 drugs have different spectrums of toxicities, and, overall, patients receiving anti-PD-1/PD-L1 antibodies have a lower incidence of any grade irAEs than those under anti-CTLA-4 agents, with combinations increasing the incidence, severity, and onset of irAEs [62, 63].
Data from pharmacovigilance, especially a disproportionality approach, corroborated and extended this knowledge regarding class-specific patterns of irAEs. Based on DAs, gastrointestinal (colitis) and endocrine disorders (hypophysitis, adrenal insufficiency, hypopituitarism) were more frequently reported with anti-CTLA-4 drugs, whereas thyroid dysfunction, pneumonitis and myocarditis were preferentially recorded with anti-PD-1/PD-L1 agents. Although autoimmune hepatitis appears to have a similar reporting frequency, in both anti-CTLA-4 and anti-PD-1/PD-L1 drugs, cholangitis showed higher reporting with anti-PD-1/PD-L1 monoclonal antibodies. This is corroborated by recent case series, and can manifest as ‘large duct or small duct cholangitis’, and may have different clinical presentations, biochemical evolution, and outcome, including secondary sclerosing cholangitis and vanishing bile duct syndrome, even after discontinuation [64–70].
From an epidemiological perspective, the reporting proportion of irAEs (compared with all other events) appears very low, and only a minority of toxicities reached a relative frequency higher than 2% (thyroid dysfunction and pneumonitis). The first pharmacovigilance study aiming at characterizing immune-related signals found 1018 irAEs, corresponding to 2.6% of all reports with ICIs. These data underline that nonimmune-related reactions are substantially reported with ICIs, such as fatigue, pruritus, nausea, vomiting, diarrhea, and musculoskeletal complaints, in line with evidence from RCTs [71, 72].
Although increased reporting over recent years was documented for serious events in the FAERS [73], the phenomenon of underreporting is well documented in pharmacovigilance but may vary considerably depending on the various drugs and the type of event; a recent analysis using FAERS reports and US sales estimated that only 20% of serious events were actually reported with biologics (i.e. underreporting can reach 80%) [74].
What’s New: Kinetics of irAEs
According to RCTs, the frequency of irAEs is dependent not only on the agents used, exposure time and the administered dose but also on the patient’s intrinsic risk factors. Conversely, the timing (kinetics) of appearance is often dictated by the affected organ system [75]. Variable onsets have been described for the different toxicities, from early occurrence (first week) to delayed events (up to 26 weeks for acute kidney injury), with 4–12 weeks as the main window of onset.
Based on data from pharmacovigilance, the first 2 months can be considered as the ‘critical pharmacovigilance window’ because the median onset was ∼ 40 days and the vast majority of events reported to international SRSs occurred in this period. Delayed events were described, including rheumatic diseases (median onset of 81 days for arthritis and mean onset of 196 for systemic lupus erythematosus), diabetes mellitus (mean onset 116 days), serious cutaneous reactions (mean onset of 65 days for anti-CTLA-4 drugs), and thyroid dysfunction (median of 92 days with anti-PD-1/PD-L1 agents). Notably, for myocarditis, both rapid (median 16.5 days in combination regimens) and late events are described (median 178 days).
What’s New: Fatality Rate, Concurrent irAEs
The lower than expected reporting registered for some irAEs, such as hypothyroidism (compared with estimates from RCTs), likely suggests that multiple irAEs are reported in the same patient. In fact, overlap was recorded among irAEs by several studies, not only for the most frequently reported irAEs (endocrine, respiratory, and hepatobiliary disorders) but also among rare toxicities, including myocarditis, myositis, myasthenia gravis, neurological disorders, pneumonitis, and hepatitis.
Several pharmacovigilance studies also characterized the reporting of ICIs in terms of fatality. Collectively, these data pointed out that one-third of reported events have a fatal outcome (i.e. death was recorded as the final outcome of the reaction), and special attention should be paid to combination therapies and myocarditis events. Substantial fatality rates also emerged for toxic epidermal necrolysis (36%), hemophagocytic lymphohistiocytosis (26%), myositis (21%), hepatitis and myasthenia gravis (19% each), and pneumonitis (17.5%). The spectrum of fatal irAEs differs between agents: colitis was the most frequent cause of death in patients receiving anti-CTLA-4 drugs, whereas pneumonitis and hepatitis fatalities were mainly attributed to anti-PD-1/PD-L1, and myocarditis has the highest fatality rate overall [33]. These data underline the importance of timely recognition and early management of irAEs in order to limit the need for treatment interruption and minimize fatality.
What’s Missing: Management
Disappointingly, there are no clues on management strategies such as ICI discontinuation and treatment with corticosteroids. Several guidelines on the management of irAEs have been published, providing comprehensive general treatment algorithms for most of the frequently occurring irAEs, with clear recommendations on immunosuppressive treatment, including duration and tapering based on the severity of the irAE [76–79].
The reader should refer to dedicated guidelines for specific toxicities, especially for severe and/or treatment refractory irAEs [80]. In this evolving field, only expert opinions are available, and a personalized algorithm was recently proposed based on the immune-pathological pattern of each patient [4]. With few exceptions, ICIs should be continued with close monitoring for grade 1 toxicities, whereas grade 2 toxicity requires drug interruption, with permanent discontinuation advocated for high-grade toxicities (except for thyroid dysfunction) [see the Electronic Supplementary Material]. Overall, the decision to reintroduce the drug(s) should be made on an individual basis. As the use of ICIs becomes more common, the number of rare irAEs will increase, thus making suspicion and differential diagnosis vital for treatment and tailored management strategy [81].
Among the various toxicities, work-up and management of liver injury is challenging for different reasons [82], including the use of ICIs in hepatocellular carcinoma [83] and chronic hepatitis B infection, as well as the common presence of hepatic metastasis [84, 85]. Moreover, the type of liver injury does not affect work-up, whereas thresholds of liver function tests represent the main criteria for diagnosis and relevant management. Data from a French pharmacovigilance register of 536 patients with grade 3 hepatitis highlighted the importance of liver biopsy for a patient-guided approach to avoid corticosteroids [86], whereas analysis of 31 case reports of sclerosing cholangitis, besides highlighting clinical and pathological features, documented poor response to corticosteroid therapy [87]. A retrospective study conducted at MD Anderson Cancer Center on 5762 patients receiving ICIs found that 2% developed hepatotoxicity (especially in combination treatment), of whom 69% permanently discontinued relevant ICIs; 10 of 67 patients receiving corticosteroids had recurrent hepatotoxicity after the corticosteroid taper, and 31 patients resumed ICIs after transaminases improvement, 8 of whom (26%) developed recurrent hepatotoxicity. Notably, no differences emerged in terms of characteristics of liver injury, response to corticosteroids, and outcomes between individuals with and without possible pre-existing liver diseases [88].
What’s Next: An Evolving Research Agenda
Although specific knowledge gaps can be identified for each irAE, there are also common unsettled issues representing a research priority for next real-world pharmacovigilance studies. In particular, we can identify three main grey areas, with additional uncertainty in special populations and patients with pre-existing autoimmune diseases [89]. First, the role of rechallenge in patients’ management, and the relevant impact on the outcome, is unclear. The re-introduction of ICIs in the same patients with previous irAEs poses ethical and scientific issues, especially for anti-CTLA-4, which are contraindicated in cases of severe colitis due to the high risk of relapse and/or bowel perforation. Conversely, there are preliminary data for which rechallenge appears feasible and acceptable with anti-PD-1/PD-L1 agents. In a cohort of 93 patients, 40 were rechallenged with the same anti-PD-1/PD-L1 agent; the same or a different irAE occurred in 55% of patients, with similar severity but shorter time linked to the occurrence of a second irAE (9 vs. 15 weeks) [90]. The latest Vigibase survey found recurrences in 28.8% of patients with irAEs (of 452 informative rechallenges), with higher rates for colitis, hepatitis, and pneumonitis [91].
Second, the predictive value of irAEs, namely actual correlation between occurrence of irAEs and effectiveness of ICIs, is a promising area of investigation, with preliminary prospective data [92]. While earlier studies in patients with melanoma suggested no association between irAE onset and anti-CLTA4 efficacy, several lines of evidence suggest that irAE onset is predictive of anti-PD-1/PD-L1 response, namely progression-free survival, overall survival, and overall response rate, across a variety of solid tumors. However, before adopting clinically irAE onset as a predictive biomarker for ICI response, larger prospective studies are needed to better understand the nuances between irAE characteristics (severity, site, management, timing of onset) and ICI effectiveness [93]. In fact, a potential confounding factor in this relationship was identified: the notion that patients who experience irAEs are those who remain on ICIs for longer time periods and thus have a better prognosis than those who do not, by virtue of their disease biology, could be a source of guarantee-time bias [94]. Recently, a mechanistic link was documented: a prospective cohort study on 73 patients with NSCLC exposed to anti-PD-1 drugs suggested that T cells recognizing shared antigens in skin and lung tumor infiltrated both sites, thus supporting the conclusion that the toxic effect mechanism was related to overlapping antigens in lung tumor and skin [95].
Third, comparative safety among ICIs is an unresolved debated topic. Apart from the general notion that anti-CTLA-4 agents are considered more toxic than anti-PD-1/PD-L1 monoclonal antibodies, a reliable risk comparison is difficult owing to different therapeutic uses. Some authors have speculated that anti-PD-L1 drugs might be theoretically less toxic than anti-PD-1 agents due to preservation of PD-L2 signaling [62], although peculiarities do exist: avelumab (an anti-PD-L1 agent) causes infusion-related reactions in approximately one-quarter of patients, generally occurring during or just after the first four infusions. Reducing the rate of infusion, temporarily suspending the infusion, and administering premedications or corticosteroids, if needed, are all effective therapeutic measures. A network meta-analysis of 36 head-to-head RCTs provided a comparative assessment of ICIs. Atezolizumab (an anti-PD-L1) emerged with the most favorable safety profile in general, as well as nivolumab in the subgroup with lung cancer (probabilistic analysis). Among five ICIs, atezolizumab had the highest risk of hypothyroidism, nausea, and vomiting. The predominant treatment-related adverse events for pembrolizumab were arthralgia, pneumonitis, and hepatic toxicities. The main treatment-related adverse events for ipilimumab were skin, gastrointestinal, and renal toxicities. Nivolumab had a narrow and mild toxicity spectrum, mainly causing endocrine toxicities [96].
All major recently published systematic reviews consistently documented that patients treated with PD-1 inhibitors had an increased rate of any grade or high-grade irAEs compared with patients receiving PD-L1 inhibitors, with pneumonitis emerging as a key serious and frequently fatal event [97, 98]. A recent systematic review of 125 RCTs applied Bayesian multilevel regression models and estimated incidences using both fully reported and censored data (simulating individual patient-level meta-analysis): 66% of patients exposed to anti-PD-1/PD-L1 drugs developed at least one adverse event of any grade, and 14% developed at least one adverse event of grade 3 or higher severity, with hypothyroidism (6.07%) and hyperthyroidism (2.82%) being the most common [72]. Of note, 24% of pneumonitis cases were grade 3 or higher in severity, and pneumonitis was the most common cause of treatment-related death. In addition, hepatitis was found most likely to be serious, with 51% being grade 3 or higher. Nivolumab appeared to have a higher mean incidence of all-grade and grade 3 or higher adverse events, compared with pembrolizumab, but the mechanism and clinical significance are unclear. In all major systematic reviews recently published, PD-1 inhibitors were associated with a higher mean incidence of grade 3 or higher adverse events compared with PD-L1 inhibitors (odds ratio 1.58, 95% CI 1.00–2.54).
As anticipated, pharmacovigilance studies through DAs cannot directly compare ICIs for safety, but continuous vigilance is needed to intercept early the rare irAEs that may be unique to a given pharmacological class and better characterize their safety profile. In particular, the impact of concomitant medications represents an emerging area to be explored, also in the light of evolving immunotherapy combinations [99, 100].
Conclusion and Perspectives
A number of pharmacovigilance studies, using descriptive and disproportionality approaches, were conducted in 2019 to systematically characterize the variegate and multifaceted spectrum of toxicities with ICIs. A concerted effort is required by all stakeholders (regulatory agencies, patients, manufacturers, healthcare professionals) considering the fast-track approval of ICIs, their evolving clinical use due to rapid extension of therapeutic indications in different oncological settings with multiple anticancer combinations, and our imperfect prediction and characterization of irAEs. The recent description of solid organ transplant rejection [101] strengthened the role of pharmacovigilance as a tool for real-time safety monitoring of immunotherapy, especially for rare but potentially fatal complications. A direct comparison with data from RCTs is misleading, considering that incidence cannot be calculated through spontaneous reporting. Furthermore, the unexpectedly (and apparently) low reporting proportion of irAEs extracted from original studies have multifold interpretations, including the high reporting of fatigue, asthenia, nausea, and vomiting (although to a lesser extent compared with chemotherapy), and the co-occurrence of multiple irAEs in the same patient.
Data from pharmacovigilance, especially a disproportionality approach, consistently confirmed the existence of a class-specific pattern of irAEs in the real world; colitis, hypophysitis, adrenal insufficiency, and hypopituitarism were more frequently reported with anti-CTLA-4 drugs, whereas thyroid dysfunction, pneumonitis, cholangitis and myocarditis were preferentially recorded with anti-PD-1/PD-L1 agents. As regards the kinetics of occurrence, the median onset of ∼ 40 days should remind clinicians about the importance of stringent monitoring during this initial 2-month window, especially when combination regimens are used. During this critical vulnerable period, fatal events are reported, and particular attention should be devoted to myocarditis, hepatitis, and pneumonitis for anti-PD-1/PD-L1 drugs, and colitis for anti-CTLA-4 agents.
We put forward some advice and suggestions for next-generation pharmacovigilance studies.
Implement disproportionality techniques, including machine learning algorithms, to investigate potential drug interactions and predictors of irAE occurrence, especially considering multiple combinations.
Link adverse event reporting to drug consumption (prescription/dispensation data) in order to calculate a reporting rate. This measure, accounting for empirical estimation of the underreporting, might allow to calculate a minimum incidence rate from postmarketing real-world evidence, and may also provide a safety comparison [10].
Address missing information on management (including outcomes after drug interruption and rechallenge) and describe the long-term consequences of irAEs.
Prospectively characterize the exposure–response relationships (including actual assessment of the value of therapeutic drug monitoring) and the role of irAEs as potential biomarkers of effectiveness.
We endorse the concept of a global registry trial, combined with artificial intelligence techniques, and welcome the new reporting of adverse event outcomes proposed by the Side Effect Reporting Immuno-Oncology (SERIO) recommendations [102], to open the new era of pharmacovigilance 4.0 in immuno-oncology.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. The authors are supported by Institutional Research Funds (Ricerca Fondamentale Orientata). They thank Michele Fusaroli (MD) for assistance in the editing of Fig. 1.
Author Contributions
Conception/design: ER; Collection and/or assembly of data: ER; Data analysis and interpretation: All authors; Manuscript writing: ER; Critical revision: All authors; Final approval of the manuscript: All authors.
Declaration
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Andrea Ardizzoni reports grants and personal fees from BMS, personal fees from MSD, Eli-Lilly, Boehringer, and Pfizer, and grants from Celgene, outside the submitted work. Emanuel Raschi reports personal fees from Novartis, outside the submitted work. Milo Gatti, Francesco Gelsomino, Elisabetta Poluzzi, and Fabrizio De Ponti declare no potential conflicts of interest relevant to the contents of this article.
References
- 1.Hoos A. Development of immuno-oncology drugs—from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 3.Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42:281–294. doi: 10.1007/s40264-018-0774-8. [DOI] [PubMed] [Google Scholar]
- 4.Martins F, Sykiotis GP, Maillard M, Fraga M, Ribi C, Kuntzer T, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20:e54–e64. doi: 10.1016/S1470-2045(18)30828-3. [DOI] [PubMed] [Google Scholar]
- 5.Barquìn-Garcìa A, Molina-Cerrillo J, Garrido P, Garcia-Palos D, Carrato A, Onso-Gordoa T. New oncologic emergencies: what is there to know about inmunotherapy and its potential side effects? Eur J Intern Med. 2019;66:1–8. doi: 10.1016/j.ejim.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Kottschade LA. Incidence and management of immune-related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep. 2018;20(3):24. doi: 10.1007/s11912-018-0671-4. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri DJ, Carlino MS. Immune checkpoint inhibitor toxicity. Curr Oncol Rep. 2018;20(9):72. doi: 10.1007/s11912-018-0718-6. [DOI] [PubMed] [Google Scholar]
- 8.Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-related adverse events (irAEs): diagnosis, management, and clinical Pearls. Curr Oncol Rep. 2020;22(4):39. doi: 10.1007/s11912-020-0897-9. [DOI] [PubMed] [Google Scholar]
- 9.Raschi E, Antonazzo IC, La Placa M, Ardizzoni A, Poluzzi E, De Ponti F. Serious cutaneous toxicities with immune checkpoint inhibitors in the US Food and Drug Administration Adverse Event Reporting System. Oncologist. 2019;24:e1228–e1231. doi: 10.1634/theoncologist.2019-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raschi E, Moretti U, Salvo F, Pariente A, Antonazzo IC, et al. Evolving roles of spontaneous reporting systems to assess and monitor drug safety. In: Kothari C, Shah M, Patel R, et al., editors. Pharmacovigilance. London: InTech; 2018. [Google Scholar]
- 11.Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93:539–546. doi: 10.1038/clpt.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer A. When to publish measures of disproportionality derived from spontaneous reporting databases? Br J Clin Pharmacol. 2011;72:909–911. doi: 10.1111/j.1365-2125.2011.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72:905–908. doi: 10.1111/j.1365-2125.2011.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel C, Scosyrev E, Petrin M, Schmouder R. Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin Drug Investig. 2017;37:415–422. doi: 10.1007/s40261-017-0503-6. [DOI] [PubMed] [Google Scholar]
- 15.Raschi E, Poluzzi E, Salvo F, Pariente A, De Ponti F, Marchesini G, et al. Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: what a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis. 2018;28:533–542. doi: 10.1016/j.numecd.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Ji HH, Tang XW, Dong Z, Song L, Jia YT. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: analysis of spontaneous reports submitted to FAERS. Clin Drug Investig. 2019;39:319–330. doi: 10.1007/s40261-018-0735-0. [DOI] [PubMed] [Google Scholar]
- 17.Raschi E, Mazzarella A, Antonazzo IC, Bendinelli N, Forcesi E, Tuccori M, et al. Toxicities with immune checkpoint inhibitors: emerging priorities from disproportionality analysis of the FDA adverse event reporting system. Target Oncol. 2019;14:205–221. doi: 10.1007/s11523-019-00632-w. [DOI] [PubMed] [Google Scholar]
- 18.Giezen TJ, Mantel-Teeuwisse AK, Meyboom RH, Straus SM, Leufkens HG, Egberts TC. Mapping the safety profile of biologicals: a disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Drug Saf. 2010;33:865–878. doi: 10.2165/11538330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Cutroneo PM, Isgro V, Russo A, Ientile V, Sottosanti L, Pimpinella G, et al. Safety profile of biological medicines as compared with non-biologicals: an analysis of the italian spontaneous reporting system database. Drug Saf. 2014;37:961–970. doi: 10.1007/s40264-014-0224-1. [DOI] [PubMed] [Google Scholar]
- 20.Gouverneur A, Claraz P, Rousset M, Arnaud M, Fourrier-Reglat A, Pariente A, et al. Comparative safety of targeted therapies for metastatic colorectal cancer between elderly and younger patients: a study using the international pharmacovigilance database. Target Oncol. 2017;12:805–814. doi: 10.1007/s11523-017-0529-y. [DOI] [PubMed] [Google Scholar]
- 21.Tuccori M, Montagnani S, Capogrosso-Sansone A, Mantarro S, Antonioli L, Fornai M, et al. Adverse reactions to oncologic drugs: spontaneous reporting and signal detection. Expert Rev Clin Pharmacol. 2015;8:61–75. doi: 10.1586/17512433.2015.974555. [DOI] [PubMed] [Google Scholar]
- 22.Hauben M, Hung E, Wood J, Soitkar A, Reshef D. The impact of database restriction on pharmacovigilance signal detection of selected cancer therapies. Ther Adv Drug Saf. 2017;8:145–156. doi: 10.1177/2042098616685010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raschi E, Diemberger I, Cosmi B, De Ponti F. ESC position paper on cardiovascular toxicity of cancer treatments: challenges and expectations. Intern Emerg Med. 2018;13:1–9. doi: 10.1007/s11739-017-1755-0. [DOI] [PubMed] [Google Scholar]
- 24.Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS) In: Karahoca A, editor. Data mining applications in engineering and medicine. London: InTech Open; 2012. [Google Scholar]
- 25.Macia-Martinez MA, de Abajo FJ, Roberts G, Slattery J, Thakrar B, Wisniewski AF. An empirical approach to explore the relationship between measures of disproportionate reporting and relative risks from analytical studies. Drug Saf. 2016;39:29–43. doi: 10.1007/s40264-015-0351-3. [DOI] [PubMed] [Google Scholar]
- 26.Antonazzo IC, Poluzzi E, Forcesi E, Salvo F, Pariente A, Marchesini G, et al. Signal of potentially protective drug–drug interactions from spontaneous reporting systems: proceed with caution. Acta Diabetol. 2020;57:115–116. doi: 10.1007/s00592-019-01441-3. [DOI] [PubMed] [Google Scholar]
- 27.Raschi E, Poluzzi E, De Ponti F. Reduced neuropsychiatric events as “beneficial reactions” to drugs: seek associations with caution. Brain Behav Immun. 2020;84:275–276. doi: 10.1016/j.bbi.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Marwitz K, Jones SC, Kortepeter CM, Dal Pan GJ, Munoz MA. An evaluation of postmarketing reports with an outcome of death in the US FDA adverse event reporting system. Drug Saf. 2020;43(5):457–465. doi: 10.1007/s40264-020-00908-5. [DOI] [PubMed] [Google Scholar]
- 29.Misu T, Kortepeter CM, Munoz MA, Wu E, Dal Pan GJ. An evaluation of “drug ineffective” postmarketing reports in drug safety surveillance. Drugs Real World Outcomes. 2018;5:91–99. doi: 10.1007/s40801-018-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisniewski AF, Bate A, Bousquet C, Brueckner A, Candore G, Juhlin K, et al. Good signal detection practices: evidence from IMI PROTECT. Drug Saf. 2016;39:469–490. doi: 10.1007/s40264-016-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raschi E, Poluzzi E, Salvo F, Koci A, Suling M, Antoniazzi S, et al. The contribution of national spontaneous reporting systems to detect signals of torsadogenicity: issues emerging from the ARITMO Project. Drug Saf. 2016;39:59–68. doi: 10.1007/s40264-015-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raschi E, Antonazzo IC, Poluzzi E, De Ponti F. Drug-induced systemic lupus erythematosus: should immune checkpoint inhibitors be added to the evolving list? Ann Rheum Dis. 2019 doi: 10.1136/annrheumdis-2019-215819. [DOI] [PubMed] [Google Scholar]
- 33.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Y, Ye X, Hu F, Xu J, Guo X, Zhuang Y, He J. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7:286. doi: 10.1186/s40425-019-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai X, Chen X, Wu X, Huang Y, Zhuang Y, Lin X. Immune checkpoint inhibitor-associated thyroid dysfunction: a disproportionality analysis using the WHO Adverse Drug Reaction Database, VigiBase. Eur J Endocrinol. 2020;182:1–9. doi: 10.1530/EJE-19-0535. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7:134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan Q, Hu Y, Yang C, Zhao B. Myocarditis following the use of different immune checkpoint inhibitor regimens: a real-world analysis of post-marketing surveillance data. Int Immunopharmacol. 2019;76:105866. doi: 10.1016/j.intimp.2019.105866. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal P. Disproportionality analysis of bullous pemphigoid adverse events with PD-1 inhibitors in the FDA adverse event reporting system. Expert Opin Drug Saf. 2019;18:623–633. doi: 10.1080/14740338.2019.1619693. [DOI] [PubMed] [Google Scholar]
- 41.Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31:319–322. doi: 10.1016/j.joco.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali AK, Watson DE. Pharmacovigilance assessment of immune-mediated reactions reported for checkpoint inhibitor cancer immunotherapies. Pharmacotherapy. 2017;37:1383–1390. doi: 10.1002/phar.2035. [DOI] [PubMed] [Google Scholar]
- 43.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4:1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune checkpoint inhibitor-associated myositis. Circulation. 2018;138:743–745. doi: 10.1161/CIRCULATIONAHA.118.035898. [DOI] [PubMed] [Google Scholar]
- 45.Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care. 2018;41:e150–e151. doi: 10.2337/dc18-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vozy A, De Martin E, Johnson DB, Lebrun-Vignes B, Moslehi JJ, Salem JE. Increased reporting of fatal hepatitis associated with immune checkpoint inhibitors. Eur J Cancer. 2019;123:112–115. doi: 10.1016/j.ejca.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Guerrero E, Johnson DB, Bachelot A, Lebrun-Vignes B, Moslehi JJ, Salem JE. Immune checkpoint inhibitor-associated hypophysitis-World Health Organisation VigiBase report analysis. Eur J Cancer. 2019;113:10–13. doi: 10.1016/j.ejca.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Arnaud L, Lebrun-Vignes B, Salem JE. Checkpoint inhibitor-associated immune arthritis. Ann Rheum Dis. 2019;78:e68. doi: 10.1136/annrheumdis-2018-213470. [DOI] [PubMed] [Google Scholar]
- 49.Davis EJ, Salem JE, Young A, Green JR, Ferrell PB, Ancell KK, et al. Hematologic complications of immune checkpoint inhibitors. Oncologist. 2019;24:584–588. doi: 10.1634/theoncologist.2018-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noseda R, Bertoli R, Muller L, Ceschi A. Haemophagocytic lymphohistiocytosis in patients treated with immune checkpoint inhibitors: analysis of WHO global database of individual case safety reports. J Immunother Cancer. 2019;7:117. doi: 10.1186/s40425-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol. 2019;102:157–162. doi: 10.1111/ejh.13187. [DOI] [PubMed] [Google Scholar]
- 52.Dolladille C, Ederhy S, Allouche SP, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8:e000261. doi: 10.1136/jitc-2019-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia CR, Jayswal R, Adams V, Anthony LB, Villano JL. Multiple sclerosis outcomes after cancer immunotherapy. Clin Transl Oncol. 2019;21:1336–1342. doi: 10.1007/s12094-019-02060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Liu J, Zhang Y, Zhang L. Inflammatory bowel disease associated with the combination treatment of nivolumab and metformin: data from the FDA adverse event reporting system. Cancer Chemother Pharmacol. 2019;83:599–601. doi: 10.1007/s00280-018-03763-5. [DOI] [PubMed] [Google Scholar]
- 55.Ederhy S, Dolladille C, Thuny F, Alexandre J, Cohen A. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur J Heart Fail. 2019;21:945–947. doi: 10.1002/ejhf.1497. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen T, Maria ATJ, Ladhari C, Palassin P, Quantin X, Lesage C, et al. Rheumatic disorders associated with immune checkpoint inhibitors: what about myositis? An analysis of the WHO’s adverse drug reactions database. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217018. [DOI] [PubMed] [Google Scholar]
- 57.Jimenez J, Gwillim EC, Kosche C, Figueiredo A, Rauck C, Rangel SM, et al. Bullous disorders associated with PD-1 and PD-L1 inhibitors: pharmacovigilance analysis of the United States Food and Drug Administration Adverse Event Reporting System from the Research on Adverse Drug Events And Reports Program. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moey MYY, Gougis PA, Goldschmidt VA, Johnson DB, Lebrun-Vignes B, Moslehi J, et al. Increased reporting of fatal pneumonitis associated with immune checkpoint inhibitors—a WHO pharmacovigilance database analysis. Eur Respir J. 2020;55(6):2000038. doi: 10.1183/13993003.00038-2020. [DOI] [PubMed] [Google Scholar]
- 59.Cortese I, Muranski P, Enose-Akahata Y, Ha SK, Smith B, Monaco M, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380:1597–1605. doi: 10.1056/NEJMoa1815039. [DOI] [PubMed] [Google Scholar]
- 60.Horn L, Mansfield AS, Szcz-Ösna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 61.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 62.Khoja L, Day D, Wei-Wu CT, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 63.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 64.Gelsomino F, Vitale G, D’Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28:671–672. doi: 10.1093/annonc/mdw649. [DOI] [PubMed] [Google Scholar]
- 65.Gelsomino F, Vitale G, Ardizzoni A. Immune-mediated cholangitis: is it always nivolumab’s fault? Cancer Immunol Immunother. 2018;67:1325–1327. doi: 10.1007/s00262-018-2159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gelsomino F, Vitale G, Ardizzoni A. A case of nivolumab-related cholangitis and literature review: how to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Investig New Drugs. 2018;36:144–146. doi: 10.1007/s10637-017-0484-6. [DOI] [PubMed] [Google Scholar]
- 67.Kashima J, Okuma Y, Shimizuguchi R, Chiba K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother. 2018;67:61–65. doi: 10.1007/s00262-017-2062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Investig New Drugs. 2017;35:529–536. doi: 10.1007/s10637-017-0453-0. [DOI] [PubMed] [Google Scholar]
- 69.Ogawa K, Kamimura K, Terai S. Anti-programmed cell death-1 immunotherapy-related secondary sclerosing cholangitis. Hepatology. 2019;69:914–916. doi: 10.1002/hep.30189. [DOI] [PubMed] [Google Scholar]
- 70.Noda-Narita S, Mizuno S, Noguchi S, Watanabe K, Nakai Y, Koike K, et al. Development of mild drug-induced sclerosing cholangitis after discontinuation of nivolumab. Eur J Cancer. 2019;107:93–96. doi: 10.1016/j.ejca.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 71.Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonawane KB, Cheng N, Hansen RA. Serious adverse drug events reported to the FDA: analysis of the FDA Adverse Event Reporting System 2006–2014 database. J Manag Care Spec Pharm. 2018;24:682–690. doi: 10.18553/jmcp.2018.24.7.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alatawi YM, Hansen RA. Empirical estimation of under-reporting in the US Food and Drug Administration Adverse Event Reporting System (FAERS) Expert Opin Drug Saf. 2017;16:761–767. doi: 10.1080/14740338.2017.1323867. [DOI] [PubMed] [Google Scholar]
- 75.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 76.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw. 2019;17:255–289. doi: 10.6004/jnccn.2019.0013. [DOI] [PubMed] [Google Scholar]
- 77.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puzanov I, Diab A, Abdallah K, Bingham CO, III, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:4119–4142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 80.Esfahani K, Meti N, Miller WH, Jr, Hudson M. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ. 2019;191:E40–E46. doi: 10.1503/cmaj.180870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schoenfeld SR, Aronow ME, Leaf RK, Dougan M, Reynolds KL. Diagnosis and management of rare immune-related adverse events. Oncologist. 2020;25:6–14. doi: 10.1634/theoncologist.2019-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu C, Marshall JL, He AR. Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. Oncologist. 2020;25:105–111. doi: 10.1634/theoncologist.2018-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72:320–341. doi: 10.1016/j.jhep.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lombardi A, Mondelli MU. Review article: immune checkpoint inhibitors and the liver, from therapeutic efficacy to side effects. Aliment Pharmacol Ther. 2019;50:872–884. doi: 10.1111/apt.15449. [DOI] [PubMed] [Google Scholar]
- 85.Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah-Korang A, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther. 2019;50:800–808. doi: 10.1111/apt.15413. [DOI] [PubMed] [Google Scholar]
- 86.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 87.Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J Gastroenterol. 2020;26:353–365. doi: 10.3748/wjg.v26.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller ED, Bu-Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A, et al. Clinical characteristics and adverse impact of hepatotoxicity due to immune checkpoint inhibitors. Am J Gastroenterol. 2020;115:251–261. doi: 10.14309/ajg.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 89.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168:121–130. doi: 10.7326/M17-2073. [DOI] [PubMed] [Google Scholar]
- 90.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310–1317. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:1–7. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akamatsu H, Murakami E, Oyanagi J, Shibaki R, Kaki T, Takase E, et al. Immune-related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non-small cell lung cancer. Oncologist. 2020;25(4):e679–e683. doi: 10.1634/theoncologist.2019-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 98.Sun X, Roudi R, Dai T, Chen S, Fan B, Li H, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19:558. doi: 10.1186/s12885-019-5701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gandhi S, Pandey M, Ammannagari N, Wang C, Bucsek MJ, Hamad L, et al. Impact of concomitant medication use and immune-related adverse events on response to immune checkpoint inhibitors. Immunotherapy. 2020;12:141–149. doi: 10.2217/imt-2019-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bashir B, Wilson MA. Novel immunotherapy combinations. Curr Oncol Rep. 2019;21(11):96. doi: 10.1007/s11912-019-0851-x. [DOI] [PubMed] [Google Scholar]
- 101.Saberianfar S, Nguyen LS, Manouchehri A, Lebrun-Vignes B, Moslehi JJ, Johnson DB, et al. Solid organ transplant rejection associated with immune-checkpoint inhibitors. Ann Oncol. 2020;31:543–544. doi: 10.1016/j.annonc.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Heinzerling L, Ascierto PA, Dummer R, Gogas H, Grob JJ, Lebbe C, et al. Adverse events 2.0-Let us get SERIOs: new reporting for adverse event outcomes needed in the era of immuno-oncology. Eur J Cancer. 2019;112:29–31. doi: 10.1016/j.ejca.2019.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.