Abstract
Background
Polycystic ovary syndrome (PCOS) is one of the most common disorders of endocrinology in reproductive-age women. In this study, we reviewed data on the effects and underlying mechanisms of herbal medicines used in the treatment of PCOS in laboratory studies.
Methods
Articles published in English up to June 30, 2018 were searched in Medline and EMBASE. We extracted data regarding herbal intervention; target cell (or animal model) usage; method of herbal extraction; route of administration; dosage and periods; and outcomes of the compounds isolated from herbs, individual herbal extracts, and herbal formula decoctions. We summarized the actions and the mechanisms underlying the beneficial effects of herbal medicines on PCOS.
Results
A total of 27 studies involving 22 herbal medicines reported their efficacy on PCOS. The herbal interventions in the 27 studies comprised four compounds isolated from herbs (6 studies), nine individual herbal extracts (11 studies), and nine herbal formula decoctions (10 studies). Herbal medicines normalized female hormones, diminished male hormones, recovered the estrous cycle, ameliorated insulin resistance, and improved lipid metabolism in PCOS. The mechanisms underlying the beneficial effects of herbal medicines on PCOS were found to be associated with anti-inflammation, anti-oxidative stress, inhibition of autophagy and/or apoptosis, and ovarian nerve growth factor reduction.
Conclusions
Herbal medicines are thought to be promising resources in the development of effective therapeutic agents for PCOS. Further studies that include methodological quality assessment and quantitative synthesis of outcomes are recommended.
Keywords: polycystic ovary syndrome, review, inflammation, oxidative stress, autophagy, apoptosis, nerve growth factor
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common disorders of endocrinology in women of reproductive age. PCOS is diagnosed by confirming the presence of two of the following: oligo- and/or anovulation, clinical and/or biochemical hyperandrogenism, and ultrasound features of polycystic ovaries, with the exclusion of other etiologies (Fr and Tarlatzis, 2004). The prevalence rates of PCOS depend on the diagnostic criteria used, but they can be up to 18% when using the Rotterdam diagnostic criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Hyperandrogenism is found in 60–80% of women with PCOS (Azziz et al., 2006). The major clinical or biochemical features of hyperandrogenism are acne, hirsutism, alopecia, and seborrheic dermatitis; elevated testosterone, androstenedione, and dehydroepiandrosterone sulfate levels; and decreased sex hormone binding globulin (SHBG) levels. The syndrome not only presents with reproductive manifestations but also has metabolic implications including insulin resistance, dyslipidemia, obesity, type 2 diabetes, and systemic inflammation (Deugarte et al., 2005; Sartor and Dickey, 2005; Escobar-Morreale et al., 2011).
While the first-line treatment for ovulation induction in women with PCOS is clomiphene citrate administration, the antiestrogenic effects of clomiphene citrate on the endometrium and cervical mucus are thought to cause a low conception rate of 20% (Gonen and Casper, 1990). Clomiphene citrate is also associated with a number of side effects including hot flushes, breast discomfort, abdominal distention, nausea, vomiting, nervousness, headache, hair loss, and disturbed vision (Legro et al., 2007a). Recent studies have investigated the role of metformin as an insulin-sensitizing agent, and although its use is increasing, the understanding of its mechanism is incomplete (Legro et al., 2007b). Moreover, it can cause the development of multiple follicles, along with a risk of ovarian hyperstimulation, multiple pregnancies, and congenital malformations such as neural tube defects, thereby leading to potentially unsatisfactory treatment outcomes.
Since PCOS is defined as a multifactorial metabolic-endocrine disorder (Laven et al., 2002), lifestyle and diet, and the Mediterranean diet in particular, play a relevant role, alongside pharmacological treatment (Barrea et al., 2019). Recent studies have suggested that complementary and alternative treatments, including herbal medicines and acupuncture, may alleviate PCOS symptoms, but evidence of their efficacy and safety is insufficient. Therefore, novel treatment strategies incorporating complementary and alternative therapies need to be investigated to optimize the treatment of PCOS. In this study, we reviewed data on the effects and underlying mechanisms of herbal medicines used in the treatment of PCOS model in laboratory studies.
Methods
Articles published in English up to June 30, 2018 were searched in Medline (via PubMed) and EMBASE (via Elsevier). The search terms were a combination of medical subject heading (MeSH) terms and their synonyms. The search terms used were as follows: [herb* (Title/Abstract) OR Chinese herbal medicine (Title/Abstract) OR Chinese traditional medicine (MeSH) OR Korean medicine (Title/Abstract) OR Kampo medicine (MeSH)] AND [polycystic ovary syndrome (MeSH) OR polycystic ovarian syndrome (Title/Abstract)].
The inclusion criteria of our review included the following:
In vitro and in vivo studies that assessed the potential effects of herbal medicines on PCOS model
Research on the compounds isolated from herbs, individual herbal extracts, or herbal formula decoctions
Articles written in English
The exclusion criteria were as follows:
Clinical trials of herbal medicines for PCOS
Review articles
Articles that did not describe the components of the herbal medicine; however, this was allowed in the case of patented herbal medicines
Among the retrieved studies, after removing the duplicates, the titles and abstracts were reviewed to find potentially relevant articles. Then, the full-texts of screened articles were reviewed to confirm that they met our inclusion criteria.
We extracted data regarding herbal intervention; target cell (or animal model) usage; method of herbal extraction: route of administration; dosage and periods; and outcomes of the compounds isolated from herbs, individual herbal extracts, and herbal formula decoctions. Based on those data, we summarized the actions and the mechanisms underlying the beneficial effects of herbal medicines on PCOS model.
Results
Study Characteristics
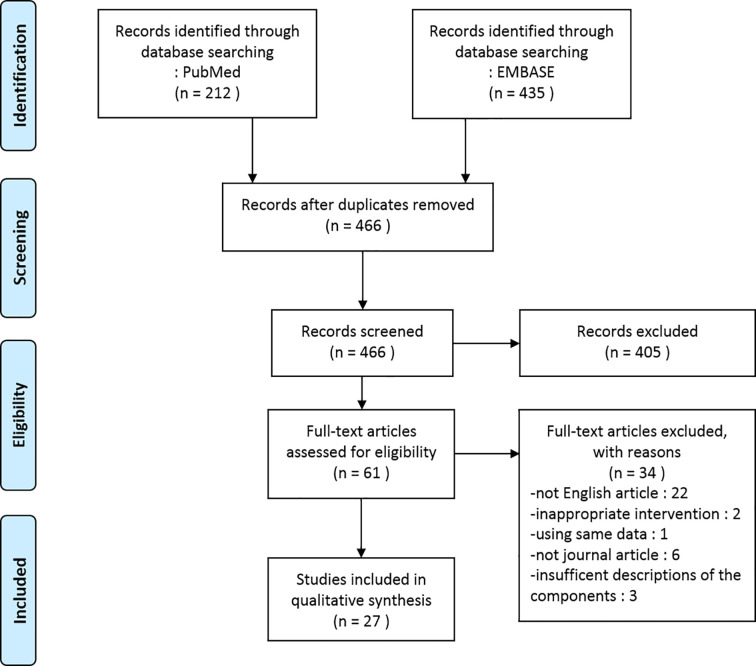
In the present review, we included a total of 27 studies involving 22 herbal medicines that reported their efficacy on PCOS model. We identified two in vitro studies, 22 in vivo studies, and three studies with both in vitro and in vivo experiments. The herbal interventions in the 27 studies comprised four compounds isolated from herbs (6 studies), nine individual herbal extracts (11 studies), and nine herbal formula decoctions (10 studies). A flow diagram of the article selection process is shown in Figure 1 .
Figure 1.
Flow diagram of the study selection process.
Herbal Interventions and Their Laboratory Outcomes
Compounds Isolated From Herbs
Three studies revealed the effects of Cryptotanshinone: it reduced ovarian weight and body weight (Yang et al., 2011; Yu et al., 2014; Xia et al., 2017), the level of luteinising hormone (LH) and the LH/follicle-stimulating hormone (FSH) ratio (Yu et al., 2014; Xia et al., 2017), and the serum insulin and glucose levels (Yang et al., 2011; Yu et al., 2014). Iridoid (genipin, geniposide, and geniposidic acid) reduced the messenger RNA (mRNA) expressions of interleukin (IL)-1β, IL-6, IL-10, and inducible nitric oxide synthase (iNOS), and the over-secretion of nitrite (Zuo et al., 2017). Total saponins from Korean red ginseng (Panax ginseng C. A. Meyer) reduced the number of cystic follicles and the protein expression of nerve growth factor (NGF) in ovaries of Sprague Dawley (SD) female rats (Pak et al., 2005). Quercetin-treated female Wistar rats showed decreased levels of IL-1β, IL-6, and tumor necrosis factor (TNF)- α, and decreased insulin resistance (Wang et al., 2017). The data are summarized in Table 1 .
Table 1.
In vitro and in vivo studies of compounds isolated from herbs (n=6).
| Interventions | Target cells or animal models | Herbal extraction | Route of administration | Dosage | Results | Author (year) |
|---|---|---|---|---|---|---|
| Cryptotanshinone | <in vivo>(1)
Adult SD female rats (aged 21 days, 35–40 g) |
Purity > 98% | Oral | 27 mg/kg/d For 3 weeks |
1) Ovarian weight ↓, body weight ↓ 2) Ovarian quotiety ↓ 3) Normalization of estrous cycle 4) P (-), T ↓, E2 ↓ 5) LH ↓, FSH ↑, LH/FSH ratio ↓ 4) Activin A ↑, inhibin B ↓, follistatin ↓ 5) mRNA expressions of inhibin B and follistatin in ovaries ↓, mRNA expression of activin A in ovaries ↑ 6) Protein expressions of inhibin B and follistatin in ovaries ↓, protein expression of activin A in ovaries ↑ |
Xia et al. (2017) |
| <in vivo>(1)
SPF grade female Wistar rats (aged 21 days, 35–40 g) |
Purity = 99% | Oral | 0.027 mg/g/d For 4 weeks |
1) Recovery of estrous cycle 2) Body weight ↓ 3) Ovaries quotiety (= ovarian weight/body weight) ↓ 4) T ↓, androstenedione ↓, E2 (-), LH ↓, FSH (-), LH/FSH ↓, SHBG ↑ 5) TC (-), TG (-), LDL-C ↓, HDL-C (-), FPG (-), fasting insulin ↓ 6) Morphology: recovery of the color of ovarian tissue and number of granulosa cell layer, number of cystic follicles ↓, number of corpora lutea ↑ 7) mRNA expression of CYP17 in ovaries ↓, mRNA expressions of CYP19, AR, iGF-1, 3β-HSD and GDF-9 in ovaries (-) 8) Protein expressions of CYP17 and AR in ovaries ↓, protein expressions of CYP19, IGF-1, 3β-HSD and GDF-9 in ovaries (-) |
Yu et al. (2014) | |
| <in vivo>(2)
Adult Wistar female rats (aged 12–14 weeks, 250–300 g) |
Purity = 98% | Oral | 0.027 mg/g/d For 14 days |
1) Body weight ↓, ovarian weight ↓ 2) Improved OGTT profile 3) Serum insulin ↓, glucose ↓, insulin resistance ↓ 4) Recovery of estrous cycle 5) Number of atretic follicles ↓, number of corpora lutea ↑ 5) Serum levels of FSH, LH, and E2 (-), serum levels of 17-OH, androstenedione, and T ↓ 6) Protein expressions of IRS-1, IRS-2, PI3K p85α, and GLUT4 in ovaries ↑ 7) Protein expressions of ERK and CYP17 in ovaries ↓ |
Yang et al. (2011) | |
| Iridoid (genipin, geniposide, and geniposidic acid) | <in vitro> RAW 264.7 and KGN cell lines |
Purity ≥ 98% | – | 1) genipin: 10, 50, 100 μM 2) geniposide: 10, 50, 100 μM 3) geinposidic acid: 10, 50, 100 μM |
1) mRNA expression of IL-1β, IL-6, IL-10 and iNOS ↓ 2) Over-secretion of nitrite ↓ 3) Phosphorylation and degradation of IκB ↓ 4) NF-κB P65 ↑ 5) Nuclear entry of NF-κB P65 ↓ 6) Scavenge O2- (only genipin) 7) SOD activity ↓, HO-1 mRNA expression ↓, catalase activity ↑ 8) MiR-15b expression ↓ |
Zuo et al. (2017) |
| Korean red ginseng total saponins | <in vivo>(3)
SD female rats (190–210 g) |
not reported | Intraperitoneally | 100 mg/kg/2d For 60 days |
1) Morphology: numbers of corpora lutea and corpora albicantia ↑, the number of cystic follicles ↓, the number of growing secondary follicle ↑ 2) Protein expression of NGF in ovaries ↓, protein expression of NGF in pituitary and hippocampus (-) 3) Ovarian weight ↓, body weight (-) |
Pak et al. (2005) |
| Quercetin | <in vivo>(1)
Female Wistar rats (aged 21 days) |
not reported | Gavage | 100 mg/kg/d For 28 days |
1) Recovery of estrous cycle 2) Body weight ↓ 3) Ovarian weight (-), ovary coefficients (-) 4) Morphology: follicles at various stages and more lutea, increased layers of granulosa cells within the follicle 5) Insulin ↓, IAUC ↓, FBG ↓ 6) Insulin resistance ↓ 7) IL-1β ↓, IL-6 ↓, TNF- α ↓ 8) Phosphorylation of IRS-1 ↑ 9) Nuclear translocation of NF-κB P65 ↓ 10) mRNA expressions of p22phox, OX-LDL, and TLR-4 in ovaries ↓ 11) Protein expressions of p22phox, OX-LDL, and TLR-4 in ovaries ↓ |
Wang et al. (2017) |
17-OH, 17-Hydroxyprogesterone; AR, Androgen receptor; CYP17, Cytochrome P450 17alpha hydroxylase/17,20 lyase; CYP19, Cytochrome P450 aromatase; E2, Estradiol; ERK, Extracellular signal–regulated kinase; FBG, Fasting blood glucose; FPG, Fasting plasma glucose; FSH, Follicle-stimulating hormone; GDF-9, Growth differentiation factor-9; GULT4, Glucose transporter type 4; HDL-C, High density lipoprotein cholesterol; HO-1, Heme oxygenase-1; HSD, Hydroxysteroid dehydrogenase; IAUC, Area under the curve of blood insulin release; IGF-1, Insulin-like growth factor 1; IL, Interleukin; iNOS, Inducible nitric oxide synthase; IRS, Insulin receptor substrate; LDL-C, Low density lipoprotein cholesterol; LH, Luteinising hormone; NF-κB, Nuclear factor kappa B; NGF, Nerve growth factor; OGTT, Oral glucose tolerance test; OX-LDL, Oxidized low-density lipoprotein; P, Progesterone; PI3K, Phosphatidylinositol 3-kinase; SD, Sprague-Dawley; SHBG, Sex hormone-binding globulin; SOD, Superoxide dismutase; SPF, Specific-pathogen-free; T, Testosterone; TC, Total cholesterol; TG, Triglyceride; TLR, Toll-like receptor; TNF, Tumor necrosis factor.
In vivo polycystic ovary model by using (1) dehydroepiandrosterone; (2) testosterone propionate; (3) estradiol valerate; (4) letrozole; (5) dihydrotestosterone; (6) sodium prasterone sulfate; and (7) chronic unpredictable mild stress.
Individual Herbal Extracts
Aloe vera (L.) Burm.f. reduced plasma levels of total cholesterol (TC), triglyceride (TG), and low density lipoprotein cholesterol (LDL-C); enhanced high density lipoprotein cholesterol (HDL-C) levels (Desai et al., 2012); normalized follicular growth; and recovered the estrous cycle (Maharjan et al., 2010). Atractylodes macrocephala Koidz. induced the recovery of the estrous cycle and the reduction of testosterone levels, androstenedione levels, the free androgen index, LH levels, the LH/FSH ratio, and anti-Müllerian hormone levels (Zhou et al., 2016). Matricaria chamomilla L.-treated female Wistar rats showed decreased cysts in ovarian tissue and an increased number of dominant follicles (Zangeneh et al., 2010). Cocos nucifera L. recovered the estrous cycle; reduced TC, very low density cholesterol and TG levels; and increased HDL-C levels (Soumya et al., 2014). Linum usitatissimum L.-treated female SD rats showed increased antral follicles and corpus luteum, a decreased number of cystic follicles, and reduced diameter of antral follicles (Jelodar et al., 2018). Foeniculum vulgare Mill. induced normal glomerulus, normal basement membrane, and capillaries (Sadrefozalayi and Farokhi, 2014). Zingiber officinale Roscoe lowered the levels of LH and estrogen, and increased the levels of FSH and progesterone in neonatal female SD rats (Atashpour et al., 2017). Korean red ginseng (Panax ginseng C.A.Mey.)-treated SD female rats showed fewer cystic follicles and mRNA expression of NGF in ovaries (Pak et al., 2009; Jung et al., 2011). Labisia pumila var. alata enhanced the glucose infusion rate and reduced TC and TG levels (Mannerås et al., 2010). The data are summarized in Table 2 .
Table 2.
In vitro and in vivo studies of individual herbal extracts (n=11).
| Interventions | Target cells or animal models | Herbal extraction | Route of administration | Dosage | Results | Author (year) |
|---|---|---|---|---|---|---|
| Aloe vera gel | <in vivo>(4)
Adult virgin female Charles Foster rats (aged 3–4 months, 200–225 g) |
not reported | Oral | 1 ml (10 mg)/d For 30 days |
1) Ovarian weight (-) 2) 3β HSD activity ↓, 17β HSD activity (-) 3) Improved OGTT profile 4) Body weight ↓ 5) Plasma levels of TC ↓, TG ↓, HDL-C ↑, and LDL-C ↓ 6) Liver cholesterol ↓ 7) Activity of HMG-CoA reductase ↓ 8) Activity of LCAT (-) |
Desai et al. (2012) |
| <in vivo>(4)
[1] Adult virgin Charles Foster female rats (200–225 g) <in vitro> [2] Ovarian protein (30–35 g) |
not reported | Oral | [1] 1 ml (10 mg)/d For 45 days [2] 1 mg For 1 h |
[1] 1) Body weight ↓, ovarian weight ↓ 2) Recovery of estrous cycle 3) Improved OGTT profile 4) Histology: ovary atretic cysts ↓, normalization of follicular growth 5) Activities of ovarian 3β HSD and 17β HSD ↓ 6) Serum glutamate pyruvate (-), transaminase (-), creatinine (-) [2] 1) Activities of ovarian 3β HSD and 17β HSD ↓ |
Maharjan et al. (2010) | |
| Atractylodes macrocephala koidz | <in vivo>(2)
[1] Female SD rats <in vitro> [2] Human ovarian granulosa-like KGN cells |
Solvent: ethanol (70%) Time: three times for 0.5 h |
Oral | [1] 0.1, 0.3, 0.9 g/kg/d For 8 weeks [2] 50, 200, 800 μg/mL For 24 h |
[1] 1) Recovery of estrous cycle 2) Body weight ↓(only 0.3, 0.9 g/kg/d), ovarian weight ↓(only 0.9 g/kg/d), uterus weight (-) 3) T ↓, SHBG (-), free androgen index ↓, androstenedione ↓, LH ↓(only 0.3, 0.9 g/kg/d), FSH ↑, LH/FSH ↓, AMH ↓ 4) mRNA expression of FSHR in ovaries ↓, mRNA expression of AQP-9 in ovaries ↑ 5) Protein expression of FSHR in ovaries ↓, protein expression of AQP-9 in ovaries ↑ 6) ALT (-), AST (-), GGT (-) [2] 1) Protein expression of FSHR ↓, protein expression of AQP-9 ↑ |
Zhou et al. (2016) |
| Chamomile | <in vivo>(3)
Virgin adult cycling Wistar rats (aged 8 weeks, 200–220 g) |
Solvent: ethanol (70%) | Intraperitoneally | 25, 50, 75 mg/kg/d For 10 days |
1) Morphology: cysts in ovarian tissue ↓(only 50 mg/kg/d), number of dominant follicles ↑(only 50 mg/kg/d), better endometrial tissue arrangements (only 50 mg/kg/d) 2) Serum levels of E2, LH, and FSH ↓ |
Zangeneh et al. (2010) |
| Cocus nucifera flower | <in vivo>(4)
Wistar female rats (100–150 g) |
Solvent: aqueous alchhol (75%) Time: 12 h |
Oral | 100, 200 mg/kg/d For 4 weeks |
1) Recovery of estrous cycle 2) Blood sugar level ↓ 3) TC ↓, VLDL-C ↓, HDL-C ↑, TG ↓ 4) Uterus weight ↑, ovary weight ↓ 5) Histophthology: number and diameter of cysts ↓, normal developing primary follicles ↑(only 200 mg/kg/d) |
Soumya et al. (2014) |
| Flaxseed | <in vivo>(3)
Adult female SD rats (200 ± 20 g) |
Solvent: ethanol (70%) | Gavage | 200 mg/kg/d For 30 days |
1) P ↑, T ↓, E2 (-), dehydroepiandrosterone (-) 2) Histomorphometric study: number of preantral follicles, antral follicles and corpora lutea ↑, number of cystic follicles and diameter of antral follicles ↓, number of primary follicle (-), thickness of granulosa layer ↑, thickness of theca layer and tunica albuginea ↓ |
Jelodar et al. (2018) |
| Foeniculum vulgare seeds | <in vivo>(3)
Adult female Wistar rats (200 ± 20 g) |
Solvent: 400 ml of distilled water Time: 24 h |
Gavage | 100, 150 mg/kg/d For 4 weeks |
1) Serum creatinine (-), serum urea ↓(only 150 mg/kg/d) 2) Histopathology: normal glomerulus, normal basement membrane, and capillaries; Bowman’s space (urinary space) and acute tubular necrosis were improved towards normal |
Sadrefozalayi and Farokhi (2014) |
| Ginger | <in vivo>(3)
Neonatal female SD rats (aged 7–8 weeks, 170–200 g) |
Solvent: ethanol (70%) Time: 48 h |
Oral | 4, 350 mg/kg/d For 88 weeks |
1) LH ↓, FSH ↑, E ↓, P ↑ | Atashpour et al. (2017) |
| Korean red ginseng | <in vivo>(3)
SD female rats (190–210 g) |
not reported | Oral | 200 mg/kg/d For 60 days |
1) Histology: numbers of corpora lutea and corpora albicantia ↑, number of cystic follicles ↓ 2) mRNA expression of NGF in ovaries ↓, protein expression of NGF in ovaries ↓ |
Jung et al. (2011) |
| <in vivo>(3)
SD female rats (190-210 g) |
not reported | Oral | 200 mg/kg/d For 60 days |
1) Histology: numbers of corpora lutea and corpora albicantia ↑, number of cystic follicles ↓ 2) mRNA expression of NGF in ovaries ↓, protein expression of NGF in ovaries ↓ |
Pak et al. (2009) | |
| Labisia pumila var. alata | <in vivo>(5)
Wistar female rats (aged 21 days) |
Solvent: hot distilled water (80˚C) Time: 3 h |
Oral | 50 mg/kg/d For 4–5 weeks |
1) Body weight (-), body composition (-) 2) Uterine weight ↑, weights of the inguinal, parametrial, retroperitoneal and mesenteric adipose tissue depots (-), weights of different hind limb muscles and the liver (-) 3) Size of adipocytes in mesenteric adipose tissue (-) 4) Glucose infusion rate ↑, plasma glucose (-) 5) TC ↓, TG ↓, HDL-C (-), LDL-C (-), resistin ↑, adiponectin (-), leptin (-) 6) mRNA expression of leptin in mesenteric adipose tissue ↓, mRNA expressions of resistin and adiponectin in mesenteric adipose tissue (-) |
Mannerås et al. (2010) |
ALT, Alanine aminotransferase; AMH, Anti-Müllerian hormone; AQP, Aquaporin; AST, Aspartate aminotransferase; E, Estrogen; FSH, Follicle-stimulating hormone; FSHR, Follicle-stimulating hormone receptor; GGT, Gamma glutamyltransferase; HDL-C, High density lipoprotein cholesterol; HMG-CoA, β-Hydroxy β-methylglutaryl-CoA; HSD, Hydroxysteroid dehydrogenase; LCAT, Lecithin–cholesterol acyltransferase; LDL-C, Low density lipoprotein cholesterol; LH, Luteinising hormone; NGF, Nerve growth factor; OGTT, Oral glucose tolerance test; P, Progesterone; SD, Sprague-Dawley; SHBG, Sex hormone-binding globulin; T, Testosterone; TC, Total cholesterol; TG, Triglyceride; VLDL-C, Very low density cholesterol.
In vivo polycystic ovary model by using (1) dehydroepiandrosterone; (2) testosterone propionate; (3) estradiol valerate; (4) letrozole; (5) dihydrotestosterone; (6) sodium prasterone sulfate; and (7) chronic unpredictable mild stress.
Herbal Formula Decoctions
Bushen Tongmai recipe (composed of Astragalus membranaceus Bge, Radix Polygoni Multiflori, Herba Cistanches, Radix Salviae Miltiorrhizae, Radix Notoginseng, Radix Puerariae, Herba Epimedii, Rhizoma Chuanxiong, and Radix Rehmanniae) reduced serum fasting insulin and enhanced protein expression of Protein kinase Bα in thecal and granulosa cells of antral follicles (Li et al., 2010). SD rats treated with Changbudodam-Tang (Rhizoma atractylodis, Rhizoma cyperi, Fructus ponciri, Pericarpium citri nobilis, Poria, Arisaematis Rhizoma, Radix glycyrrhizae, Massa Medicata Fermentata, and Zingiberis Rhizoma) and Yongdamsagan-Tang (Radix gentianae, Bupleuri Radix, Alimatis Rhizoma, Lignum akebiae, Plantaginis Semen, Hoelen, Rehmanniae Radix, Angelicae Gigantis Radix, Gardeniae Fructus, Scutellariae Radix, and Radix glycyrrhizae) showed a reduced number of cystic follicles, a higher number of growing secondary follicles, and reduced expression of NGF in ovaries (Lee et al., 2003). Chinese herbal medicine (CHM) 1 (Radix Astragali, Radix Rehmanniae Preparata, Cuscuta chinensis Lam, Fructus Ligustri Lucidi, Fructus Psoraleae, Radix Salviae Miltiorrhizae, and Rubus idaeus Linn) and CHM 2 (Cuscuta chinensis Lam, Fructus Ligustri Lucidi, and Rubus idaeus Linn) induced recovery of the estrous cycle and the LH/FSH ratio, and reduction of androstenedione levels and the free androgen index (Gu et al., 2015). Gui Zhu Yi Kun formula (GZYKF) (Semen Cuscutae, Rhizoma Atractylodis Macrocephalae, Angelica sinensis, Adenophora tetraphylla, Plantago asiatica, Rubia cordifolia, and Luffa cylindrical) reduced mRNA expressions of Beclin 1, light chain 3, and tumor suppressor p53 (Xing et al., 2017). HemoHIM (Angelica Radix, Cnidii Rhizoma, and Paeonia Radix)-treated SD female rats showed increased number and size of corpora lutea and a decreased level of NGF protein in ovaries (Kim et al., 2009). Heqi san [Curculigo orchioides Gaertn., Schisandra chinensis (Turcz.) Baill., Cynanchum otophyllum C. K. Schneid., Citrus medica L. var. sarcodactylis Swingle, Crataegus pinnatifida Bunge, Rhus chinensis Mill., Clinopodium megalanthum (Diels) C. Y. Wu & Hsuan ex H. W. Li, Cuscuta chinensis Lam., Poncirus trifoliata (L.) Raf., Hordeum vulgare L., Polygala tenuifolia Willd., and Epimedium davidii Franch.] was found to reduce LH and testosterone levels, and to mitigate insulin resistance (Zhao et al., 2017). A novel herbal immunomodulator drug (IMOD) (Rosa canina, Urtica dioica, and Tanacetum vulgare)-treated female albino Wistar rats showed fewer incidences of ovarian atretic and cystic follicles, and a higher number of corpora lutea (Rezvanfar et al., 2012). Kyung-Ok-Ko (KOK) [Rehmannia glutinosa Liboschitz var. purpurae Makino (Scrophulariaceae), Lycium chinense Miller (Solanaceae), Aquillaria agallocha Roxburgh (Thymelaeaceae), Poria cocos Wolf (Polyporaceae), Panax ginseng C.A. Meyer (Araliaceae), and honey] administration induced the recovery of the estrous cycle and cluster of differentiation (CD)11b, CD3, IL-1β, IL-6, TNF-α, IL-8, monocyte chemoattractant protein (MCP)-1, and iNOS mRNA expression reduction in ovaries of female SD rats (Jang et al., 2014; Lee et al., 2016). Female SD rats treated with Xiao-Yao-San (XYS) (radix bupleuri, angelica, radix paeoniae alba, rhizoma atractylodis macrocephalae, poria, ginger, mint, and glycyrrhizae) showed a reduced number of cystic follicles and lowered terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) positive cells in the antral follicles (Sun et al., 2017). The data are summarized in Table 3 .
Table 3.
In vitro and in vivo studies of herbal formula decoctions (n=10).
| Interventions | Components | Target cells or animal models | Herbal extraction | Route of administration | Dosage | Results | Author (year) |
|---|---|---|---|---|---|---|---|
| Bushen Tongmai Recipe | Astragalus membranaceus Bge, Radix Polygoni Multiflori, Herba Cistanches, Radix Salviae Miltiorrhizae, Radix Notoginseng, Radix Puerariae, Herba Epimedii, Rhizoma Chuanxiong, and Radix Rehmanniae | <in vivo>(6)
Female SD rats (aged 22 days) |
not reported | Gastric perfusion | 1.5 ml For 14 days |
1) Morphology: stratum granulosum of ovarian follicles ↑ 2) FBG (-), serum fasting insulin ↓, insulin sensitive index ↑ 3) mRNA expressions of PKBα in the hepatic, adipose, and skeletal muscle tissues ↑, protein expressions of PKBα in the hepatic and adipose tissues ↑, protein expressions of PKBα in thecal and granulosa cells of antral follicles ↑ |
Li et al. (2010) |
| Changbudodam-Tang & Yongdamsagan-Tang |
Rhizoma atractylodis, Rhizoma cyperi, Fructus ponciri, Pericarpium citri nobilis, Poria, Arisaematis Rhizoma, Radix glycyrrhizae, Massa Medicata Fermentata, and Zingiberis Rhizoma & Radix gentianae, Bupleuri Radix, Alimatis Rhizoma, Lignum akebiae, Plantaginis Semen, Hoelen, Rehmanniae Radix, Angelicae Gigantis Radix, Gardeniae Fructus, Scutellariae Radix, and Radix glycyrrhizae |
<in vivo>(3)
Virgin adult cycling SD rats (190–210 g) |
Solvent: 1,000 ml of boiling distilled water Time: 43 h |
Oral (zonde needle) | 50 mg/kg/2d for Changbudodam-Tang, & 40 mg/kg/2d for Yongdamsagan-Tang For 60 days |
1) Morphology: numbers of corpora lutea and corpora albicantia ↑, number of cystic follicles ↓, number of growing secondary follicle ↑ 2) Expression of NGF in ovaries ↓, expression of NGF in pituitary and hippocampus (-) 3) Ovarian weight ↑, body weight (-) |
Lee et al. (2003) |
| CHM1 & CHM2 |
Radix Astragali, Radix Rehmanniae Preparata, Cuscuta chinensis Lam, Fructus Ligustri Lucidi, Fructus Psoraleae, Radix Salviae Miltiorrhizae, Rubus idaeus Linn & Cuscuta chinensis Lam, Fructus Ligustri Lucidi, Rubus idaeus Linn |
<in vivo>(2)
Neonatal female SD rats (aged 9 days) |
Solvent: boiling water Time: twice for 60 min |
Oral | 0.54(CHM1), 0.24(CHM2) g/kg/d For 12 weeks |
1) Recovery of estrous cycle 2) LH/FSH ↓, free androgen index ↓, androstenedione ↓ 3) mRNA expression of PPARG1 in ovaries ↑, mRNA expression of HDAC3 in ovaries ↓ |
Gu et al. (2015) |
| GZYKF | Semen Cuscutae, Rhizoma Atractylodis Macrocephalae, Angelica sinensis, Adenophora tetraphylla, Plantago asiatica, Rubia cordifolia, and Luffa cylindrica | <in vitro> Granulosa cells from female SD rats (aged 23–25 days, 60 ± 10 g) |
Solvent: water Time: room temperature for 1 h and 100˚C for 1 h |
– | 13% rat serum containing GZYKF (administration of 5, 10, 20 g/kg/d for 4 days in low, medium, high-dose rat groups) For 72 h |
1) mRNA expression of Beclin-1 ↓ 2) mRNA expression of LC3 ↓ (only from high-dose group) 3) protein expression of Beclin-1 ↓ 4) Protein expression of LC3 (-) 5) mRNA expression of tumor suppressor p53 ↓, mRNA expression of sestrin2 ↓(only from low and medium-dose groups) 6) mRNA expressions of TSC1, TSC2 and mTOR (-) 7) mRNA expression of AMPK ↑(only from high-dose group) 8) Protein expressions of mTOR, p-mTOR, tumor suppressor p53, AMPKα and sestrin ↑ |
Xing et al. (2017) |
| HemoHIM | Angelica Radix, Cnidii Rhizoma, and Paeonia Radix | <in vivo>(3)
SD female rats (210–230 g) |
Solvent: boiling water Time: 4 h |
[1] Oral [2] Intraperitoneally |
[1] 100 mg/kg/d For 35 days [2] 50 mg/kg/2d For 35 days |
1) Body weight (-), ovary weight ↑(only oral 100 mg/kg/d) 2) Morphology: number and size of corpora lutea ↑, cystically dilated atretic follicles ↑ 3) Level of NGF protein in ovaries ↓ |
Kim et al. (2009)
Pak et al. (2009) |
| Heqi san | Curculigo orchioides Gaertn., Schisandra chinensis (Turcz.) Baill., Cynanchum otophyllum C. K. Schneid., Citrus medica L. var. sarcodactylis Swingle, Crataegus pinnatifida Bunge, Rhus chinensis Mill., Clinopodium megalanthum (Diels) C. Y. Wu & Hsuan ex H. W. Li, Cuscuta chinensis Lam., Poncirus trifoliata (L.) Raf., Hordeum vulgare L., Polygala tenuifolia Willd., and Epimedium davidii Franch. | <in vivo>(1)
Female SD rats (aged 3 months, 300 ± 20 g) |
Solvent: ddH2O Time: twice for 1.5 h |
Oral cannula | 8.1 g/kg/d For 30 days |
1) LH ↓, T ↓, E2 (-) 2) Insulin resistance ↓ 3) Histology: ovarian volume ↓, organ coefficient ↑, cystic dilatation in the ovarian follicles ↓, the oocytes in the follicles ↑, the number of granule cell layers ↑ 4) mRNA expressions of GLUT4 and PTEN in ovaries ↑ 5) Protein expressions of IRS-1 and PTEN in ovaries ↓ 6) Protein expressions of GLUT4 and p-IRS-1 in ovaries (-) 7) Expression of rno-miR-144-3p in ovaries ↓ 8) Expressions of rno-miR-30c-2-3p, rno-miR-486, and rno-miR-3586-3p in ovaries ↓ 10) Expression of rno-miR-146b-5p in ovaries ↑ |
Zhao et al. (2017) |
| IMOD | Rosa canina, Urtica dioica, and Tanacetum vulgare | <in vivo>(4)
Adult female albino Wistar rats (200 ± 10 g) |
Solvent: ethanol (96%) Time: 30 days |
Intraperitoneally | 30 mg/kg/d For 21 days |
1) Body weight ↓, ovarian weight ↓ 2) Recovery of estrous cycle 3) Histomorphology: total populations of ovarian atretic and cystic follicles ↓, number of corpora lutea ↑ 4) Serum levels of lipid peroxidation, peroxynitrite, TNF-α, and T ↓; serum levels of SOD, catalase, GPx, P, and E2 ↑ 5) Ovarian levels of lipid peroxidation, peroxynitrite, PGE ↓; ovarian levels of SOD, catalase, and GPx ↑ |
Rezvanfar et al. (2012) |
| Kyung-Ok-Ko | Rehmannia glutinosa Liboschitz var. purpurae Makino (Scrophulariaceae), Lycium chinense Miller (Solanaceae), Aquillaria agallocha Roxburgh (Thymelaeaceae), Poria cocos Wolf (Polyporaceae), Panax ginseng C.A. Meyer (Araliaceae), and honey | <in vivo>(1)
Female SD rats (aged 23 days) |
Solvent: hot water (80˚C) Time: 72 h |
Oral | 0.5, 1.0, 2.0 g/kg/d For 40 days |
1) Body weight ↓ 2) Ovary weight ↓(only 2.0 g/kg/d) 3) Number of follicular cysts ↓(only 2.0 g/kg/d), size of follicular cysts ↓(only 2.0 g/kg/d) 4) Insulin (-), insulin resistance (-) 5) E2 ↓(only 2.0 g/kg/d), P (-) 6) Recovery of estrous cycle (only 2.0 g/kg/d) 7) CD8 (+) in lymph node ↓(only 2.0 g/kg/d), CD4 (+) in lymph node (-) 8) CD8 (+) in ovary ↓(only 2.0 g/kg/d) 9) mRNA expressions of CD11b and CD3 in ovaries ↓(only 2.0 g/kg/d), Iba-1 immunoreactivity ↓(only 2.0 g/kg/d) 10) mRNA expressions of IL-1β, IL-6, TNF-α, IL-8, MCP-1, and iNOS in the ovaries ↓(only 2.0 g/kg/d) 11) mRNA expressions of EGF and TGF-β in the ovaries ↑(only 2.0 g/kg/d) |
Jang et al. (2014) |
| Rehmannia glutinosa, Liboschitz var. purpurae, Lycium chinense, Aquillaria agallocha, Poria cocos, Panax ginseng, and honey | <in vivo>(1)
Female SD rats (aged 23 days) |
not reported | Oral | 2.0 g/kg/d For 20 days |
1) Body weight ↓ 2) Histomorphometric study: size and number of follicular cysts in the ovaries ↓, sizes of uteri ↓, outer diameters of uteri ↓, normalization of endometrium, myometrium, epimetrium, and cystic glands 3) Recovery of estrous cycle 4) Uterine weight ↓ 5) TUNEL positive cells ↓ 6) Iba-1+ macrophages ↓, CD4+ T cells ↓, CD8+ T cells ↓ 7) mRNA expressions of IL-1β, IL-6, IL-8, and MMP-3 in uteri ↓ 8) mRNA expressions of IGF-β, TGF-β, TGF-β1, and VEGF in uteri ↑ |
Lee et al. (2016) | |
| XYS | Radix Bupleuri, Angelica, Radix Paeoniae Alba, Rhizoma Atractylodis Macrocephalae, Poria, Ginger, Mint, and Glycyrrhizae | <in vivo>(7)
[1] Female SD rats (aged 8 weeks, 220 ± 20 g) <in vitro> [2] Granulosa cells from female SD rats (aged 21 days) |
not reported | Gavage | [1] 0.505, 1.01 g/kg/d For 4 weeks [2] 15% rat serum containing XYS (administration of 1.01 g/kg/d for 3 days in female SD rats) For 24 h |
[1] 1) Recovery of estrous cycle 2) Morphology: number of cystic follicles ↓, recovery of cystic follicles formation and follicle development abnormalities 3) Noradrenaline in serum ↓(only 0.505 g/kg), noradrenaline in ovary ↑, LH ↓(only 1.01 g/kg), E2 (-), P ↑(only 1.01 g/kg) 4) Expression of β2R in the primordial and primary follicles ↓ 5) TUNEL positive cells in the antral follicles ↓ 6) Bax/Bcl-2 (-), cleaved caspase-3/GAPDH ↓(only 1.01 g/kg) 7) Expression of LC3A in the granulosa cells of the antral and cystic follicles ↓ 8) Conversion of LC3A-I to LC3A-II ↓(only 1.01 g/kg), conversion of LC3BI to LC3B-II ↓ 9) Phosphorylation of S6K I and Akt in ovarian tissues ↑ 10) Expression of DβH and c-fos in locus coeruleus ↓(only 1.01 g/kg) [2] 1) Expression of β2R ↓ 2) Expression of LC3 ↓(only 1.01 g/kg) 3) Conversion of LC3A-I to LC3A-II ↓(only 1.01 g/kg), conversion of LC3BI to LC3B-II ↓(only 1.01 g/kg) 4) Phosphorylation of S6K I and Akt ↑(only 1.01 g/kg) |
Sun et al. (2017) |
Akt, protein kinase B; AMPK, Adenosine monophosphate-activated protein kinase; Bax, B-cell lymphoma-2 associated X protein; Bcl-2, B-cell lymphoma-2; CD, Cluster of differentiation; CHM, Chinese herbal medicine; Cleaved caspase-3, Cleaved cysteinly aspartate specific proteinase-3; DβH, Dopamine beta hydroxylase; EGF, Epidermal growth factor; FBG, Fasting blood glucose; FSH, Follicle-stimulating hormone; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; GPx, Glutathione peroxidase; GULT4, Glucose transporter type 4; GZYKF, Gui Zhu Yi Kun formula; HDAC, Histone deacetylase; Iba1, Ionized calcium-binding adapter molecule 1; IL, Interleukin; iNOS, Inducible nitric oxide synthase; IRS, Insulin receptor substrate; LC, Light chain; LH, Luteinising hormone; MCP-1, Monocyte chemoattractant protein-1; mTOR, Mammalian target of rapamycin; NGF, Nerve growth factor; PGE, Prostaglandin E; PKBα, Protein kinase Bα; PPARG, Peroxisome proliferator-activated receptor gamma; PTEN, Phosphatase and tensin homolog; S6K I, Ribosomal protein S6 kinase polypeptide I; SD, Sprague-Dawley; SOD, Superoxide dismutase; TNF, Tumor necrosis factor; TSC, Tuberous sclerosis protein; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; VEGF, Vascular endothelial growth factor; XYS, Xiao-Yao-San; β2R, Beta 2 adrenergic receptor.
In vivo polycystic ovary model by using (1) dehydroepiandrosterone; (2) testosterone propionate; (3) estradiol valerate; (4) letrozole; (5) dihydrotestosterone; (6) sodium prasterone sulfate; and (7) chronic unpredictable mild stress.
The Actions of Herbal Medicines for Treating PCOS
Herbal medicines were found to normalize female hormones, (Zangeneh et al., 2010; Rezvanfar et al., 2012; Jang et al., 2014; Yu et al., 2014; Gu et al., 2015; Zhou et al., 2016; Atashpour et al., 2017; Sun et al., 2017; Xia et al., 2017; Zhao et al., 2017; Jelodar et al., 2018), diminish male hormones (Yang et al., 2011; Rezvanfar et al., 2012; Yu et al., 2014; Gu et al., 2015; Zhou et al., 2016; Xia et al., 2017; Zhao et al., 2017; Jelodar et al., 2018), recover the estrous cycle (Mohamadin et al., 2010; Yang et al., 2011; Rezvanfar et al., 2012; Jang et al., 2014; Soumya et al., 2014; Yu et al., 2014; Gu et al., 2015; Lee et al., 2016; Zhou et al., 2016; Sun et al., 2017; Wang et al., 2017; Xia et al., 2017), ameliorate insulin resistance (Li et al., 2010; Mannerås et al., 2010; Yang et al., 2011; Desai et al., 2012; Wang et al., 2017; Zhao et al., 2017), and improve lipid metabolism in PCOS model (Mannerås et al., 2010; Desai et al., 2012; Soumya et al., 2014). The actions of herbal medicines and relevant outcomes are shown in Table 4 .
Table 4.
The actions of herbal medicines for treating PCOS.
| Actions | Interventions | Outcomes | Author (year) |
| Normalizing female hormone | <in vitro, in vivo> Atractylodes macrocephala koidz |
LH ↓, FSH ↑, LH/FSH ↓ AMH ↓ |
Zhou et al. (2016) |
| <in vivo> Chamomile |
E2, LH, FSH ↓ | Zangeneh et al. (2010) | |
| <in vivo> CHM1 & CHM2 |
LH/FSH ↓ | Gu et al. (2015) | |
| <in vivo> Cryptotanshinone |
LH ↓, FSH ↑, LH/FSH ratio ↓, P (-), E2 ↓ | Xia et al. (2017) | |
| E2 (-), LH ↓, FSH (-), LH/FSH ↓ | Yu Jang et al. (2014) | ||
| <in vivo> Flaxseed |
P ↑, E2 (-) | Jelodar et al. (2018) | |
| <in vivo> Ginger |
LH ↓, FSH ↑, E ↓, P ↑ | Atashpour et al. (2017) | |
| <in vivo> Heqi san |
LH ↓, E2 (-) | Zhao et al. (2017) | |
| <in vivo> IMOD |
P ↑, E2 ↑ | Rezvanfar et al. (2012) | |
| <in vivo> Kyung-Ok-Ko |
E2 ↓, P (-) | Jang Jang et al. (2014) | |
| <in vitro, in vivo> XYS |
LH ↓, E2 (-), P ↑ | Sun et al. (2017) | |
| Diminishing male hormone | <in vitro, in vivo> Atractylodes macrocephala koidz |
T ↓, SHBG (-), free androgen index ↓, androstenedione ↓, | Zhou et al. (2016) |
| <in vivo> CHM1 & CHM2 |
Free androgen index ↓, androstenedione ↓ | Gu et al. (2015) | |
| <in vivo> Cryptotanshinone |
T ↓ | Xia et al. (2017) | |
| T ↓, androstenedione ↓, SHBG ↑ | Jang et al. (2014) | ||
| Androstenedione ↓, T ↓ | Yang et al. (2011) | ||
| <in vivo> Flaxseed |
T ↓, Dehydroepiandrosterone (-) | Jelodar et al. (2018) | |
| <in vivo> Heqi san |
T ↓ | Zhao et al. (2017) | |
| <in vivo> IMOD |
T ↓ | Rezvanfar et al. (2012) | |
| Recovery of the estrous cycle | <in vitro, in vivo> Aloe vera gel |
Recovery of estrous cycle | Maharjan et al. (2010) |
| <in vitro, in vivo> Atractylodes macrocephala koidz |
Recovery of estrous cycle | Zhou et al. (2016) | |
| <in vivo> CHM1 & CHM2 |
Recovery of estrous cycle | Gu et al. (2015) | |
| <in vivo> Cocus nucifera flower |
Recovery of estrous cycle | Soumya et al. (2014) | |
| <in vivo> Cryptotanshinone |
Normalization of estrous cycle | Xia et al. (2017) | |
| Recovery of estrous cycle |
Yu et al. (2014)
(Jang et al., 2014) |
||
| Recovery of estrous cycle | Yang et al. (2011) | ||
| <in vivo> IMOD |
Recovery of the estrous cycle | Rezvanfar et al. (2012) | |
| <in vivo> Kyung-Ok-Ko |
Recovery of estrous cycle | Lee et al. (2016) | |
| <in vivo> Kyung-Ok-Ko |
Recovery of estrous cycle | Jang et al. (2014) | |
| <in vivo> Quercetin |
Recovery of estrous cycle | Wang et al. (2017) | |
| <in vitro, in vivo> XYS |
Recovery of estrous cycle | Sun et al. (2017) | |
| Ameliorating insulin resistance | <in vivo> Aloe vera gel |
OGTT profile improvement | Desai et al. (2012) |
| <in vivo> Bushen Tongmai Recipe |
Fasting blood glucose (-) Serum fasting insulin ↓ Insulin sensitive index ↑ |
Li et al. (2010) | |
| <in vivo> Cryptotanshinone |
OGTT profile improvement | Yang et al. (2011) | |
| <in vivo> Heqi san |
Insulin resistance ↓ | Zhao et al. (2017) | |
| <in vivo> Labisia pumila var. alata |
Insulin sensitivity ↑ (glucose infusion rate ↑) Plasma glucose (-) |
Mannerås et al. (2010) | |
| <in vivo> Quercetin |
Insulin ↓, IAUC ↓, FBG ↓ Insulin resistance ↓ |
Wang et al. (2017) | |
| Lipid metabolism improvement | <in vivo> Aloe vera gel |
Plasma levels of TC ↓, TG ↓, HDL-C ↑, LDL-C ↓ Liver cholesterol ↓ Activity of HMG-CoA reductase ↓ Activity of LCAT (-) |
Desai et al. (2012) |
| <in vivo> Cocus nucifera flower |
TC ↓, VLDL-C ↓, HDL-C ↑, TG ↓ | Soumya et al. (2014) | |
| <in vivo> Labisia pumila var. alata |
TC ↓, TG ↓, HDL-C (-), LDL-C (-) Resistin ↑, adiponectin (-), leptin (-) mRNA expression of leptin in mesenteric adipose tissue ↓ mRNA expression of resistin and adiponectin in mesenteric adipose tissue (-) |
Mannerås et al. (2010) |
AMH, Anti-Müllerian hormone; CHM, Chinese herbal medicine; E, Estrogen; E2, Estradiol; FBG, Fasting blood glucose; FSH, Follicle-stimulating hormone; HDL-C, High density lipoprotein cholesterol; HMG-CoA, β-Hydroxy β-methylglutaryl-CoA; IAUC, Area under the curve of blood insulin release; LCAT, Lecithin–cholesterol acyltransferase; LDL-C, Low density lipoprotein cholesterol; LH, Luteinising hormone; OGTT, Oral glucose tolerance test; P, Progesterone; SHBG, Sex hormone-binding globulin; TC, Total cholesterol; TG, Triglyceride; VLDL-C, Very low density cholesterol; XYS, Xiao-Yao-San.
The Mechanisms of Action for PCOS
The mechanisms underlying the beneficial effects of herbal medicines on PCOS model were as follows: the alleviation of inflammation (Rezvanfar et al., 2012; Jang et al., 2014; Lee et al., 2016; Wang et al., 2017; Zuo et al., 2017) and/or oxidative stress, (Rezvanfar et al., 2012; Zuo et al., 2017), the inhibition of autophagy and/or apoptosis (Lee et al., 2016; Sun et al., 2017; Xing et al., 2017), and the reduction of the ovarian NGF (Lee et al., 2003; Pak et al., 2005; Kim et al., 2009; Pak et al., 2009; Jung et al., 2011). The mechanisms of action of each in PCOS model and relevant outcomes are shown in Table 5 .
Table 5.
The mechanisms of action of herbal medicines on PCOS.
| Mechanisms | Interventions | Outcomes | Author (year) |
|---|---|---|---|
| Anti-inflammation or immunomodulatory | <in vitro> Iridoid (genipin, geniposide, and geniposidic acid) |
1) mRNA expressions of IL-1β, IL-6, IL-10 and iNOS ↓ 2) Over-secretion of nitrite ↓ 3) MiR-15b expression ↓ |
Zuo et al. (2017) |
| <in vivo> Quercetin |
1) IL-1β ↓, IL-6 ↓, TNF-α ↓ 2) Phosphorylation of IRS-1 ↑ 3) Nuclear translocation of NF-κB P65 ↓ 4) mRNA expressions of p22phox, OX-LDL, and TLR-4 in ovaries ↓ 5) Protein expressions of p22phox, OX-LDL, and TLR-4 in ovaries ↓ |
Wang et al. (2017) | |
| <in vivo> Kyung-Ok-Ko |
1) Iba-1+ macrophages ↓, CD4+ T cells ↓, CD8+ T cells ↓ 2) mRNA expressions of IL-1β, IL-6, IL-8, and MMP-3 in uteri ↓ 3) mRNA expressions of IGF-β, TGF-β, TGF-β1, and VEGF in uteri ↑ |
Lee et al. (2016) | |
| <in vivo> Kyung-Ok-Ko |
1) CD8 (+) in lymph node ↓, CD4 (+) in lymph node (-) 2) CD8 (+) in ovary ↓ 3) mRNA expressions of CD11b and CD3 in ovaries ↓, Iba-1 immunoreactivity ↓ 4) mRNA expressions of IL-1β, IL-6, TNF-α, IL-8, MCP-1, and iNOS in ovaries ↓ 5) mRNA expressions of EGF and TGF-β in ovaries ↑ |
Jang et al. (2014) | |
| <in vivo> IMOD |
1) Serum TNF-α ↓ 2) Ovarian PGE ↓ |
Rezvanfar et al. (2012) | |
| Anti-oxidative stress | <in vitro> Iridoid (genipin, geniposide, and geniposidic acid) |
1) Phosphorylation and degradation of IκB ↓ 2) NF-κB P65 ↑ 3) Nuclear entry of NF-κB P65 ↓ 4) Scavenge O2- 5) SOD activity ↓, HO-1 mRNA expression ↓, catalase activity ↑ |
Zuo et al. (2017) |
| <in vivo> IMOD |
Ovarian and serum SOD, catalase, GPx ↓ | Rezvanfar et al. (2012) | |
| Autophagy or apoptosis inhibition | <in vitro> GZYKF |
1) mRNA expression of Beclin-1 ↓ 2) mRNA expression of LC3 ↓ 3) Protein expression of Beclin-1 ↓ 4) Protein expression of LC3 (-) 5) mRNA expressions of tumor suppressor p53 and sestrin2 ↓ 6) mRNA expressions of TSC1, TSC2 and mTOR (-) 7) mRNA expression of AMPK ↑ 8) Protein expressions of mTOR, p-mTOR, tumor suppressor p53, AMPKα and sestrin ↑ |
Xing et al. (2017) |
| <in vitro, in vivo> XYS |
<in vivo> 1) Expression of β2R in the primordial and primary follicles ↓ 2) TUNEL positive cells in the antral follicles ↓ 3) Bax/Bcl-2 (-), cleaved caspase-3/GAPDH ↓ 4) Expression of LC3A in the granulosa cells of the antral and cystic follicles ↓ 5) Conversion of LC3A-I to LC3A-II ↓, conversion of LC3BI to LC3B-II ↓ 6) Phosphorylation of S6K I and Akt in ovarian tissues ↑ 7) Expression of DβH and c-fos in locus coeruleus ↓ <in vitro> 1) Expression of β2R ↓ 2) Expression of LC3 ↓ 3) Conversion of LC3A-I to LC3A-II ↓, conversion of LC3BI to LC3B-II ↓ 4) Phosphorylation of S6K I and Akt ↑ |
Sun et al. (2017) | |
| <in vivo> Kyung-Ok-Ko |
TUNEL positive cells ↓ | Lee et al. (2016) | |
| Ovarian NGF reduction | <in vivo> Korean red ginseng |
1) mRNA expression of NGF in ovaries ↓ 2) Protein expression of NGF in ovaries ↓ |
Jung et al. (2011) |
| <in vivo> Korean red ginseng |
1) mRNA expression of NGF in ovaries ↓ 2) Protein expression of NGF in ovaries ↓ |
Pak et al. (2009) | |
| <in vivo> HemoHIM |
Protein expression of NGF in ovaries ↓ | Pak et al. (2009) | |
| <in vivo> Korean red ginseng total saponins |
1) Protein expression of NGF in ovaries ↓ 2) Protein expression of NGF in pituitary and hippocampus (-) |
Pak et al. (2005) | |
| <in vivo> Changbudodam-Tang & Yongdamsagan-Tang |
1) Expression of NGF in ovaries ↓ 2) Expression of NGF in pituitary and hippocampus (-) |
Lee et al. (2003) |
Akt, protein kinase B; AMPK, Adenosine monophosphate-activated protein kinase; Bax, B-cell lymphoma-2 associated X protein; Bcl-2, B-cell lymphoma-2; CD, Cluster of differentiation; Cleaved caspase-3, Cleaved cysteinly aspartate specific proteinase-3; DβH, dopamine beta hydroxylase; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; GPx, Glutathione peroxidase; GZYKF, Gui Zhu Yi Kun formula; HO-1, Heme oxygenase-1; Iba1, Ionized calcium-binding adapter molecule 1; IL, Interleukin; iNOS, Inducible nitric oxide synthase; IRS, Insulin receptor substrate; LC, Light chain; MCP-1, Monocyte chemoattractant protein-1; MMP, Matrix metalloproteinase; mTOR, Mammalian target of rapamycin; NF-κB, Nuclear factor kappa B; NGF, Nerve growth factor; OX-LDL, Oxidized low-density lipoprotein; PGE, Prostaglandin E; S6K I, Ribosomal protein S6 kinase polypeptide I; SOD, Superoxide dismutase; TLR, Toll-like receptor; TNF, Tumor necrosis factor; TSC, Tuberous sclerosis protein; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling; XYS, Xiao-Yao-San; β2R, Beta 2 adrenergic receptor.
Discussion
Our review of the literature published up to June 30, 2018 summarized the findings of in vitro and in vivo studies on the efficacy of herbal medicines for the treatment of PCOS model. A total of four compounds isolated from herbs (6 studies), nine individual herbal extracts (11 studies), and nine herbal formula decoctions (10 studies) were found to have inhibitory effects on PCOS. According to the results reported, herbal medicines normalized female hormones, diminished male hormones, recovered the estrous cycle, ameliorated insulin resistance, and improved lipid metabolism. We found that the potential inhibitory activity of herbal medicines could influence different aspects of PCOS, with the beneficial effects of herbal medicines arising mainly through anti-inflammation, anti-oxidative stress, inhibition of autophagy or apoptosis, and ovarian NGF reduction.
Herbal Medicines Can Inhibit the Inflammatory Conditions of PCOS
Recent studies have further explored the etiology and pathology of PCOS. Scientists found that in the microenvironment of patients with PCOS, mild chronic inflammation is a hallmark of the syndrome (Nestler, 2000). PCOS has been relevant in chronic inflammation (Benson et al., 2008), and macrophages have been its major pathogenesis (Wu et al., 2004). Ovarian macrophages produce cytokines, chemokines, and growth factors in both the normal and the inflammatory processes of the ovary. The macrophages can orchestrate apoptosis and tissue remodeling, which are involved in folliculogenesis, ovulation, and formation of the corpus luteum (Benson et al., 2008). Given the critical role of macrophages in PCOS (Benson et al., 2008), numerous studies have compared cytokine levels in serum and in follicular fluids in PCOS patients. TNF-α and IL-6 levels in serum and in follicular fluids were elevated in non-obese/non-diabetic PCOS patients treated with gonadotrophins (Amato et al., 2003). Macrophage inflammatory protein-1α and MCP-1 were increased in PCOS patients and associated with adiposity (Glintborg et al., 2009). iNOS, cyclooxygenase-2 (COX-2), and transforming growth factor (TGF)-β activity were increased in the ovaries of PCOS patients (Elia et al., 2006; Hatzirodos et al., 2011), and iNOS and COX-2 activity were prevented by metformin administration (Elia et al., 2006). These results suggest that the immune system is relevant to the pathogenesis of PCOS. Therefore new remedies targeting this inflammatory process can be a therapeutic alternative to the current treatment.
In this review, iridoids significantly inhibited IL-1β, IL-6, IL-10, and iNOS expression, thereby inhibiting inflammatory conditions (Zuo et al., 2017). Quercetin also significantly reduced the levels of IL-1β, IL-6, and TNF-α, and decreased nuclear translocation of nuclear factor kappa B (NF-κB) in an insulin-resistant PCOS rat model (Wang et al., 2017). Pre-administration of KOK diminished the increased expression of ionized calcium-binding adapter molecule -1 (+) macrophages in the theca cell layer of cysts and the stroma. KOK also increased mRNA expression of CD11b and CD3 in PCOS ovarian tissue. Pre-administration of KOK significantly decreased the increased levels of IL-1β, IL-6, IL-8, TNF-α, MCP-1, and iNOS; and increased the reduced mRNA expression of epidermal growth factor and TGF-β in PCOS ovaries. These results demonstrated that KOK regulates the expression of inflammatory mediators in the dehydroepiandrosterone (DHEA)-induced PCOS model (Jang et al., 2014). Inflammatory mediators were also regulated in the endometrium of the uterus by KOK administration, which has been shown to prevent endometrial hyperplasia in PCOS models (Lee et al., 2016). It was also shown that TNF-α in serum and prostaglandin E (PGE) in the ovary were decreased by IMODs (Rezvanfar et al., 2012).
Herbal Medicines Can Attenuate Oxidative Stress in PCOS
Reactive oxygen species are important signal molecules in the regulation of physiological functions in female reproduction, including steroidogenesis, folliculogenesis, oocyte maturation, corpus luteum function, and luteolysis (Agarwal et al., 2005). In addition, they play a key role in the pathological processes of female reproduction (Agarwal et al., 2003; Agarwal et al., 2008). Oxidative stress is a condition in which the equilibrium between antioxidant capacity of the body and toxic oxygen- and/or nitrogen-derived products is impaired. Consequently, free radicals are insufficiently detoxified by cellular antioxidants. Oxidative stress plays an important role in the female reproduction (Ruder et al., 2008; Ruder et al., 2009; Vakilian et al., 2009), and there is increasing literature on the effects of increased oxidative stress markers in infertile females, and on their involvement in the pathophysiology of PCOS (Joo et al., 2010; Mohamadin et al., 2010). PCOS is characterized by chronic inflammation, oxidative stress, and abnormal microRNA expression (Zhao et al., 2015; Zuo et al., 2016). Since it is known that inflammation and oxidative stress are closely linked, elevated oxidative stress usually results from, and leads to, an inflammatory condition (Zuo et al., 2016). It is difficult to separate inflammation from oxidative stress, and it has been proposed in recent years that inflammation and oxidative stress comprise the main foundation of disease occurrence (Duleba and Dokras, 2012).
In this review, IMODs (Rezvanfar et al., 2012) and iridoids (Zuo et al., 2017) showed positive effects on oxidative/nitrosative stress, either directly or indirectly, mainly by reducing free radicals and inhibiting inflammatory cytokines in PCOS rats. Administration of IMODs significantly reduced lipid peroxidation (a marker of oxidative stress) and increased superoxide dismutase, catalase and glutathione peroxidase (markers of antioxidant potential) levels in hyperandrogenism-induced PCOS. In addition, peroxynitrite (a marker of nitrosative stress), TNF-α, and PGE levels were significantly reduced by IMODs. Furthermore, these effects of IMODs were consistent with histologic evidence, which showed significant improvement in the microscopic characteristics of folliculogenesis compared with those in the control group (Rezvanfar et al., 2012). The NF-κB signaling system is known as a dominant paradigm for specific signal transduction molecules, gene activation, and regulatory proteins in response to inflammation (Ivanenkov et al., 2011). The iridoids efficiently attenuated the lipopolysaccharide induced elevation of IκB phosphorylation levels, decreased IκB expression levels, and decreased NF-κB P65, indicating that the iridoids exert their antioxidant effects via the NF-κB pathway (Zuo et al., 2017).
Herbal Medicines Can Regulate Apoptosis and/or Autophagy in PCOS
The survival or death of granulosa cells is recognized as a critical factor impacting the fate of follicles (Matsuda et al., 2012). Apoptosis and autophagy are two forms of programmed cell death. Autophagy is the process by which an autophagosome, which is a double-membrane vesicle, carries cytoplasmic material to the lysosome (Mizushima and Komatsu, 2011). It has been reported that both apoptosis and autophagy can be induced in granulosa cells, and that they are involved in the control of follicular development (Choi et al., 2010; Choi et al., 2013). Granulosa cells are recognized as critical players in follicle development. They produce estradiol, insulin-like growth factors, and other cytokines in the ovary and express the receptors for estradiol, LH, and FSH, (Juengel et al., 2006), all of which participate in the regulation of follicle development. Thus, any impairment of the granulosa cells may results in disordered development of follicles.
In the last decade, autophagy-related signaling pathways and their major protein regulators have been identified. The rat microtubule-associated protein 1 light chain 3 is associated with autophagosome membrane processing (Kabeya et al., 2000). Beclin-1 has also been demonstrated to have a critical role in autophagosome formation (Von Hoven et al., 2012). Furthermore, previous studies have identified tumor suppressor p53 (p53) as a dual modulator of autophagy in regulating cell death or survival (Vousden and Ryan, 2009; Zhang et al., 2010). At low energy levels, adenosine monophosphate-activated protein kinase (AMPK), which is activated by p53, is able to activate tuberous sclerosis complex 2, and therefore inhibit the mammalian target of rapamycin (mTOR) activity and increase autophagy (Feng et al., 2007). In this review, p53, which is activated by GZYKF in the nucleus, in turn activated AMPK and sestrin, acting as a feedback in mTOR inhibition, thereby activating autophagy. Autophagy is also modulated by phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathways (Pyo et al., 2012). In this review, XYS alleviated the reduction of phosphorylation of ribosomal protein S6 kinase polypeptide I and Akt, as well as the increase of microtubule-associated protein light chain 3-I to microtubule-associated protein light chain 3-II conversion both in vivo and in vitro (Sun et al., 2017).
The endometria of most PCOS patients are thick and exhibit simple, complicated, or atypical hyperplasia or malignant transformation that may be correlated with endometrial cell apoptosis (Villavicencio et al., 2007). Hyperandrogenism induced by DHEA is associated with a greater number of apoptotic cells in the endometria, and metformin (an insulinomimetic or insulin-sensitizing agent) is able to reduce the increased number of apoptotic cells (Elia et al., 2009). In this review, apoptotic cell death was evaluated by TUNEL staining. Apoptotic cells were rarely detected by TUNEL staining in the uterine tissue of the sham or the KOK-alone group. However, the number of TUNEL-positive cells was increased in the endometria of DHEA group. The increased number of apoptotic cells was significantly reduced after administration of KOK. These results indicate that pre-administration of KOK inhibited DHEA-induced endometrial malformation by reducing endometrial apoptosis (Lee et al., 2016).
Herbal Medicines Can Reduce the Level of NGF in PCOS
Previous studies have shown that PCOS is associated with abnormal activation of the sympathetic nervous system, resulting in increased catecholaminergic nerves (Semenova, 1969), impaired norepinephrine metabolism (Garcia-Rudaz et al., 1998), and increased activity of sympathetic nerves via the superior ovarian nerve (Lara et al., 1993). The development and function of ovarian sympathetic innervation depend on the ovary for the production of NGF, a target-derived neurotrophin required for peripheral sympathetic system development (Levi-Montalcini, 1987). The neurotrophin family, implicates the NGF receptor and NGF mRNA in ovulation and in the pathophysiology of PCOS (Lara et al., 2000; Stener-Victorin et al., 2003). In rat ovaries, NGF is principally synthesized in the cells of the follicular wall (Dissen et al., 1996), and in PCOS, the activation of NGF may be a factor involved in enhancing norepinephrine outflow to the gland, which is induced by estradiol valerate (EV) (Lara et al., 2000) PCOS exhibits a high intraovarian nerve fiber density that is associated with sympathetic hyperresponsiveness (Stener-Victorin et al., 2003).
In this review, the administration of the herbal formulas Changbudodam-Tang and Yongdamsagan-Tang significantly decreased elevated NGF in the ovaries with little effect on brain tissue (Lee et al., 2003). HemoHIM also normalized NGF, lowered the high number of antral follicles, and increased the number of corpora lutea in PCOS. These results are consistent with those of previous studies on the beneficial effects of HemoHIM in the prevention and treatment of PCOS (Pak et al., 2009). In addition, administration of Korean red ginseng extract (Pak et al., 2009; Jung et al., 2011) and Korean red ginseng total saponins (Pak et al., 2005) significantly decreased the expression of NGF protein and NGF mRNA, compared with those in EV-treated ovaries.
This study reviewed the evidence for herbal medicines that may be used to treat PCOS and its associated symptoms, and the findings are intended to add to clinicians’ understanding of the mechanisms of action of herbal medicines in PCOS treatment. The main limitation of our study is the heterogeneity of interventions. Furthermore, our study did not perform a quality assessment of each study or a quantitative synthesis of the outcomes. Further studies that include methodological quality assessment and quantitative synthesis of outcomes are warranted.
Conclusion
In this review, a total of 27 studies involving 22 herbal medicines exhibited beneficial effects on PCOS. Herbal interventions in the 27 studies comprised four compounds isolated from herbs (6 studies), nine individual herbal extracts (11 studies), and nine herbal formula decoctions (10 studies). Herbal medicines were shown to normalize female hormones, diminish male hormones, recover the estrous cycle, ameliorate insulin resistance, and improve lipid metabolism in PCOS. The mechanisms underlying the beneficial effects of herbal medicines on PCOS are associated with anti-inflammation, anti-oxidative stress, inhibition of autophagy and/or apoptosis, and ovarian NGF reduction. Herbal medicines can be considered as promising resources in the development of effective therapeutic agents for PCOS.
Author Contributions
I-HC and KP designed the study. C-YK and KP searched the articles and analyzed the data. KP wrote the manuscript and I-HC revised it. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No.NRF-2017R1C1B1006387) and the Korean Society of Ginseng (2019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Akt, protein kinase B; AMH, Anti-Müllerian hormone; AMPK, adenosine monophosphate-activated protein kinase; CD, cluster of differentiation; CHM, Chinese herbal medicine; COX, cyclooxygenase; DHEA, dehydroepiandrosterone; EV, estradiol valerate; FSH, Follicle-stimulating hormone; GZYKF, Gui Zhu Yi Kun formula; HDL-C, High density lipoprotein cholesterol; IL, interleukin; IMOD, immunomodulator drug; iNOS, inducible nitric oxide synthase; KOK, Kyung-Ok-Ko; LDL-C, Low density lipoprotein cholesterol; LH, Luteinising hormone; MCP, monocyte chemoattractant protein; MeSH, medical subject heading; mTOR, inhibit mammalian target of rapamycin; NF-κb, nuclear factor kappa B; NGF, nerve growth factor; p53, tumor suppressor p53; PCOS, polycystic ovary syndrome; PGE, prostaglandin E; PI3K, phosphoinositide3-kinase; SD, Sprague Dawley; SHBG, sex hormone binding globulin; TC, Total cholesterol; TG, Triglyceride; TGF, transforming growth factor; TNF, tumor necrosis factor; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VLDL-C, Very low density cholesterol; XYS, Xiao-Yao-San.
References
- Agarwal A., Saleh R. A., Bedaiwy M. A. (2003). Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility Sterility 79 (4), 829–843. 10.1016/S0015-0282(02)04948-8 [DOI] [PubMed] [Google Scholar]
- Agarwal A., Gupta S., Sharma R. K. (2005). Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3 (1), 28. 10.1186/1477-7827-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Gupta S., Sekhon L., Shah R. (2008). Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signaling 10 (8), 1375–1404. 10.1089/ars.2007.1964 [DOI] [PubMed] [Google Scholar]
- Amato G., Conte M., Mazziotti G., Lalli E., Vitolo G., Tucker A. T., et al. (2003). Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstetrics Gynecol. 101 (6), 1177–1182. 10.1016/s0029-7844(03)00233-3 [DOI] [PubMed] [Google Scholar]
- Atashpour S., Jahromi H. K., Jahromi Z. K., Maleknasab M. (2017). Comparison of the effects of Ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. Int. J. Reprod. BioMed. 15 (9), 561. 10.29252/ijrm.15.9.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H. F., Futterweit W., et al. (2006). Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J. Clin. Endocrinol. Metab. 91 (11), 4237–4245. 10.1210/jc.2006-0178 [DOI] [PubMed] [Google Scholar]
- Barrea L., Arnone A., Annunziata G., Muscogiuri G., Laudisio D., Salzano C., et al. (2019). Adherence to the mediterranean diet, dietary patterns and body composition in women with polycystic ovary syndrome (PCOS). Nutrients 11 (10), 2278. 10.3390/nu11102278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Janssen O., Hahn S., Tan S., Dietz T., Mann K., et al. (2008). Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav. Immun. 22 (2), 177–184. 10.1016/j.bbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Choi J. Y., Jo M. W., Lee E. Y., Yoon B. K., Choi D. S. (2010). The role of autophagy in follicular development and atresia in rat granulosa cells. Fertility Sterility 93 (8), 2532–2537. 10.1016/j.fertnstert.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Choi J., Jo M., Lee E., Choi D. (2013). AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 147, 73–80. 10.1530/REP-13-0386 [DOI] [PubMed] [Google Scholar]
- Desai B. N., Maharjan R. H., Nampoothiri L. P. (2012). Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Pharmacogn. Res. 4 (2), 109. 10.4103/0974-8490.94736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugarte C. M., Bartolucci A. A., Azziz R. (2005). Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertility Sterility 83 (5), 1454–1460. 10.1016/j.fertnstert.2004.11.070 [DOI] [PubMed] [Google Scholar]
- Dissen G. A., Hill D. F., Costa M. E., Les Dees C., Lara H. E., Ojeda S. R. (1996). A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 137 (1), 198–209. 10.1210/endo.137.1.8536613 [DOI] [PubMed] [Google Scholar]
- Duleba A. J., Dokras A. (2012). Is PCOS an inflammatory process? Fertility Sterility 97 (1), 7–12. 10.1016/j.fertnstert.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia E., Sander V., Luchetti C., Solano M., Di Girolamo G., Gonzalez C., et al. (2006). The mechanisms involved in the action of metformin in regulating ovarian function in hyperandrogenized mice. MHR: Basic Sci. Reprod. Med. 12 (8), 475–481. 10.1093/molehr/gal057 [DOI] [PubMed] [Google Scholar]
- Elia E. M., Belgorosky D., Faut M., Vighi S., Pustovrh C., Luigi D., et al. (2009). The effects of metformin on uterine tissue of hyperandrogenized BALB/c mice. Mol. Hum. Reprod. 15 (7), 421–432. 10.1093/molehr/gap033 [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale H. F., Luque-Ramírez M., González F. (2011). Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertility Sterility 95 (3), 1048–1058. e1042. 10.1016/j.fertnstert.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Hu W., De Stanchina E., Teresky A. K., Jin S., Lowe S., et al. (2007). The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 67 (7), 3043–3053. 10.1158/0008-5472.CAN-06-4149 [DOI] [PubMed] [Google Scholar]
- Fr D. D., Tarlatzis R. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility Sterility 81 (1), 19–25. 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Garcia-Rudaz C., Armando I., Levin G., Escobar M. E., Barontini M. (1998). Peripheral catecholamine alterations in adolescents with polycystic ovary syndrome. Clin. Endocrinol. 49 (2), 221–228. 10.1046/j.1365-2265.1998.00511.x [DOI] [PubMed] [Google Scholar]
- Glintborg D., Andersen M., Richelsen B., Bruun J. M. (2009). Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin. Endocrinol. 71 (5), 652–658. 10.1111/j.1365-2265.2009.03523.x [DOI] [PubMed] [Google Scholar]
- Gonen Y., Casper R. F. (1990). Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum. Reprod. 5 (6), 670–674. 10.1093/oxfordjournals.humrep.a137165 [DOI] [PubMed] [Google Scholar]
- Gu Y.-E., Wang F.-F., Yang D.-X., Zhou J., Ye Y.-H., Zhu Y.-H., et al. (2015). Chinese herbal medicine alleviating hyperandrogenism of PCOS rats through regulating PPARG1 and HDAC3 expression in the ovaries. Afr. J. Tradit. Complementary Altern. Medicines 12 (2), 6–11. 10.4314/ajtcam.v12i2.2 [DOI] [Google Scholar]
- Hatzirodos N., Bayne R. A., Irving-Rodgers H. F., Hummitzsch K., Sabatier L., Lee S., et al. (2011). Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 25 (7), 2256–2265. 10.1096/fj.11-181099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenkov Y. A., Balakin K. V., Lavrovsky Y. (2011). Small Molecule Inhibitors of NF-B and JAK/STAT Signal Transduction Pathways as Promising Anti-Inflammatory Therapeutics. Mini Rev. Med. Chem. 11 (1), 55–78. 10.2174/138955711793564079 [DOI] [PubMed] [Google Scholar]
- Jang M., Lee M. J., Lee J. M., Bae C.-S., Kim S.-H., Ryu J. H., et al. (2014). Oriental medicine Kyung-Ok-Ko prevents and alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats. PloS One 9 (2), e87623. 10.1371/journal.pone.0087623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelodar G., Masoomi S., Rahmanifar F. (2018). Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iranian J. Basic Med. Sci. 21 (6), 645–650. 10.22038/IJBMS.2018.25778.6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Y. L., Baw C. K., Gupta S., Aziz N., Agarwal A. (2010). Role of oxidative stress in polycystic ovary syndrome. Curr. Women’s Health Rev. 6 (2), 96–107. 10.2174/157340410791321336 [DOI] [Google Scholar]
- Juengel J. L., Heath D. A., Quirke L. D., McNatty K. P. (2006). Oestrogen receptor α and β, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction 131 (1), 81–92. 10.1530/rep.1.00704 [DOI] [PubMed] [Google Scholar]
- Jung J. H., Park H. T., Kim T., Jeong M. J., Lim S. C., Nah S. Y., et al. (2011). Therapeutic effect of korean red ginseng extract on infertility caused by polycystic ovaries. J. Ginseng Res. 35 (2), 250. 10.5142/jgr.2011.35.2.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., et al. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19 (21), 5720–5728. 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Lee H. J., Kim J. S., Moon C., Kim J. C., Bae C. S., et al. (2009). HemoHIM improves ovarian morphology and decreases expression of nerve growth factor in rats with steroid-induced polycystic ovaries. J. Med. Food 12 (6), 1348–1352. 10.1089/jmf.2008.1323 [DOI] [PubMed] [Google Scholar]
- Lara H., Ferruz J., Luza S., Bustamante D., Borges Y., Ojeda S. (1993). Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 133 (6), 2690–2695. 10.1210/endo.133.6.7902268 [DOI] [PubMed] [Google Scholar]
- Lara H., Dissen G., Leyton V., Paredes A., Fuenzalida H., Fiedler J., et al. (2000). An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 141 (3), 1059–1072. 10.1210/endo.141.3.7395 [DOI] [PubMed] [Google Scholar]
- Laven J. S., Imani B., Eijkemans M. J., Fauser B. C. (2002). New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstetrical Gynecol. Survey 57 (11), 755–767. 10.1097/00006254-200211000-00022 [DOI] [PubMed] [Google Scholar]
- Lee J. C., Pak S. C., Lee S. H., Lim S. C., Bai Y. H., Jin C. S., et al. (2003). The effect of herbal medicine on nerve growth factor in estradiol valerate-induced polycystic ovaries in rats. Am. J. Chin. Med. 31 (06), 885–895. 10.1142/S0192415X03001636 [DOI] [PubMed] [Google Scholar]
- Lee M. J., Jang M., Bae C. S., Park K.-S., Kim H. J., Lee S., et al. (2016). Effects of oriental medicine Kyung-Ok-Ko on uterine abnormality in hyperandrogenized rats. Rejuvenation Res. 19 (6), 456–466. 10.1089/rej.2015.1787 [DOI] [PubMed] [Google Scholar]
- Legro R. S., Barnhart H. X., Schlaff W. D., Carr B. R., Diamond M. P., Carson S. A., et al. (2007. a). Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. New Engl. J. Med. 356 (6), 551–566. 10.1056/NEJMoa063971 [DOI] [PubMed] [Google Scholar]
- Legro R. S., Zaino R. J., Demers L. M., Kunselman A. R., Gnatuk C. L., Williams N. I., et al. (2007. b). The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am. J. Obstetrics Gynecol. 196 (4), 402. e401–402. e411. 10.1016/j.ajog.2006.12.025 [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. (1987). The nerve growth factor 35 years later. Science 237 (4819), 1154–1162. 10.1126/science.3306916 [DOI] [PubMed] [Google Scholar]
- Li Q., Huang D.-m., Lu F.-e., Xie Y., Xu L.-j., Zou X., et al. (2010). Effects of Bushen Tongmai Recipe on protein kinase Bα expression in polycystic ovary rats with insulin resistance. Chin. J. Integr. Med. 16 (4), 324–330. 10.1007/s11655-010-0515-z [DOI] [PubMed] [Google Scholar]
- Maharjan R., Nagar P. S., Nampoothiri L. (2010). Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J. Ayurveda Integr. Med. 1 (4), 273. 10.4103/0975-9476.74090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannerås L., Fazliana M., Nazaimoon W. W., Lönn M., Gu H., Östenson C., et al. (2010). Beneficial metabolic effects of the Malaysian herb Labisia pumila var. alata in a rat model of polycystic ovary syndrome. J. Ethnopharmacol. 127 (2), 346–351. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Inoue N., Manabe N., Ohkura S. (2012). Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J. Reprod. Dev. 58 (1), 44–50. 10.1262/jrd.2011-012 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Komatsu M. (2011). Autophagy: renovation of cells and tissues. Cell 147 (4), 728–741. 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- Mohamadin A. M., Habib F. A., Elahi T. F. (2010). Serum paraoxonase 1 activity and oxidant/antioxidant status in Saudi women with polycystic ovary syndrome. Pathophysiology 17 (3), 189–196. 10.1016/j.pathophys.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Nestler J. E. (2000). Insulin resistance and the polycystic ovary syndrome: recent advances. Curr. Opin. Endocrinol. Diabetes Obes. 7 (6), 345–349. 10.1097/00060793-200012000-00009 [DOI] [Google Scholar]
- Pak S. C., Lim S. C., Nah S. Y., Lee J., Hill J. A., Bae C. S. (2005). Role of Korean red ginseng total saponins in rat infertility induced by polycystic ovaries. Fertility Sterility 84, 1139–1143. 10.1016/j.fertnstert.2005.04.042 [DOI] [PubMed] [Google Scholar]
- Pak S. C., Kim S. E., Oh D. M., Shim K. M., Jeong M. J., Lim S. C., et al. (2009). Effect of Korean red ginseng extract in a steroid-induced polycystic ovary murine model. Arch. Pharmacal. Res. 32 (3), 347–352. 10.1007/s12272-009-1306-y [DOI] [PubMed] [Google Scholar]
- Pyo J. O., Nah J., Jung Y. K. (2012). Molecules and their functions in autophagy. Exp. Mol. Med. 44 (2), 73. 10.3858/emm.2012.44.2.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvanfar M., Ahmadi A., Shojaei-Saadi H., Baeeri M., Abdollahi M. (2012). Molecular mechanisms of a novel selenium-based complementary medicine which confers protection against hyperandrogenism-induced polycystic ovary. Theriogenology 78 (3), 620–631. 10.1016/j.theriogenology.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility Sterility 81 (1), 19–25. 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Ruder E. H., Hartman T. J., Blumberg J., Goldman M. B. (2008). Oxidative stress and antioxidants: exposure and impact on female fertility. Hum. Reprod. Update 14 (4), 345–357. 10.1093/humupd/dmn011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder E. H., Hartman T. J., Goldman M. B. (2009). Impact of oxidative stress on female fertility. Curr. Opin. Obstetr. Gynecol. 21 (3), 219. 10.1097/GCO.0b013e32832924ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrefozalayi S., Farokhi F. (2014). Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J. Phytomed. 4 (2), 110. 10.1016/j.jep.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor B. M., Dickey R. P. (2005). Polycystic ovarian syndrome and the metabolic syndrome. Am. J. Med. Sci. 330 (6), 336–342. 10.1097/00000441-200512000-00012 [DOI] [PubMed] [Google Scholar]
- Semenova I. (1969). Adrenergic innervation of ovaries in Stein-Leventhal syndrome. Vestnik Akademii Meditsinskikh Nauk SSSR 24 (9), 58. [PubMed] [Google Scholar]
- Soumya V., Muzib Y. I., Venkatesh P., Hariprasath K. (2014). GC-MS analysis of Cocus nucifera flower extract and its effects on heterogeneous symptoms of polycystic ovarian disease in female Wistar rats. Chin. J. Natural Medicines 12 (9), 677–684. 10.1016/S1875-5364(14)60103-5 [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E., Lundeberg T., Cajander S., Aloe L., Manni L., Waldenström U., et al. (2003). Steroid-induced polycystic ovaries in rats: effect of electro-acupuncture on concentrations of endothelin-1 and nerve growth factor (NGF), and expression of NGF mRNA in the ovaries, the adrenal glands, and the central nervous system. Reprod. Biol. Endocrinol. 1 (1), 33. 10.1186/1477-7827-1-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. Y., Li Q., Liu Y. Y., Wei X. H., Pan C. S., Fan J. Y., et al. (2017). Xiao-Yao-San, a Chinese Medicine Formula, Ameliorates Chronic Unpredictable Mild Stress Induced Polycystic Ovary in Rat. Front. Physiol. 8, 729. 10.3389/fphys.2017.00729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakilian K., Ranjbar A., Zarganjfard A., Mortazavi M., Vosough-Ghanbari S., Mashaiee S., et al. (2009). On the relation of oxidative stress in delivery mode in pregnant women; a toxicological concern. Toxicol. Mech. Methods 19 (2), 94–99. 10.1080/15376510802232134 [DOI] [PubMed] [Google Scholar]
- Villavicencio A., Bacallao K., Gabler F., Fuentes A., Albornoz J., Casals A., et al. (2007). Deregulation of tissue homeostasis in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol. Oncol. 104 (2), 290–295. 10.1016/j.ygyno.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Von Hoven G., Kloft N., Neukirch C., Ebinger S., Bobkiewicz W., Weis S., et al. (2012). Modulation of translation and induction of autophagy by bacterial exoproducts. Med. Microbiol. Immunol. 201 (4), 409–418. 10.1007/s00430-012-0271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. H., Ryan K. M. (2009). p53 and metabolism. Nat. Rev. Cancer 9 (10), 691–700. 10.1038/nrc2715 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhai D., Zhang D., Bai L., Yao R., Yu J., et al. (2017). Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod. Sci. 24 (5), 682–690. 10.1177/1933719116667218 [DOI] [PubMed] [Google Scholar]
- Wu R., Van der Hoek K. H., Ryan N. K., Norman R. J., Robker R. L. (2004). Macrophage contributions to ovarian function. Hum. Reprod. Update 10 (2), 119–133. 10.1093/humupd/dmh011 [DOI] [PubMed] [Google Scholar]
- Xia Y., Zhao P., Huang H., Xie Y., Lu R., Dong L. (2017). Cryptotanshinone reverses reproductive disturbances in rats with dehydroepiandrosterone-induced polycystic ovary syndrome. Am. J. Trans. Res. 9 (5), 2447. [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Liu Y. X., Liu X., Wang S. L., Li P., Lin X. H., et al. (2017). Effects of Gui Zhu Yi Kun formula on the P53/AMPK pathway of autophagy in granulosa cells of rats with polycystic ovary syndrome. Exp. Ther. Med. 13 (6), 3567–3573. 10.3892/etm.2017.4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhang Y., Wu X., Bae C. S., Hou L., Kuang H., et al. (2011). Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of ovarian signaling mechanisms and androgen synthesis. Am. J. Physiol. Regulatory Integr. Comp. Physiol. 300 (4), R869–R875. 10.1152/ajpregu.00334.2010 [DOI] [PubMed] [Google Scholar]
- Yu J., Zhai D., Hao L., Zhang D., Bai L., Cai Z., et al. (2014). Cryptotanshinone reverses reproductive and metabolic disturbances in PCOS model rats via regulating the expression of CYP17 and AR. Evidence-Based Complementary Altern. Med. 2014, 1–10. 10.1155/2014/670743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangeneh F. Z., Minaee B., Amirzargar A., Ahangarpour A., Mousavizadeh K. (2010). Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertility 11 (3), 169. [PMC free article] [PubMed] [Google Scholar]
- Zhang X. D., Qin Z. H., Wang J. (2010). The role of p53 in cell metabolism. Acta Pharmacol. Sin. 31 (9), 1208–1212. 10.1038/aps.2010.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang C., Huang Y., Yu Y., Li R., Li M., et al. (2015). Up-regulated expression of WNT5a increases inflammation and oxidative stress via PI3K/AKT/NF-κB signaling in the granulosa cells of PCOS patients. J. Clin. Endocrinol. Metab. 100 (1), 201–211. 10.1210/jc.2014-2419 [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhou D., Chen Y., Liu D., Chu S., Zhang S. (2017). Beneficial effects of Heqi san on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. DARU J. Pharm. Sci. 25 (1), 21. 10.1186/s40199-017-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Qu F., Barry J. A., Pan J.-X., Wang F.-F., Fu Z.-Z., et al. (2016). An atractylodes macrocephala koidz extract alleviates hyperandrogenism of polycystic ovarian syndrome. Int. J. Clin. Exp. Med. 9 (2), 2758–2767. [Google Scholar]
- Zuo T., Zhu M., Xu W. (2016). Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longevity 2016, 1–14. 10.1155/2016/8589318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Zhu M., Xu W., Wang Z., Song H. (2017). Iridoids with Genipin Stem Nucleus Inhibit Lipopolysaccharide-Induced Inflammation and Oxidative Stress by Blocking the NF-κB Pathway in Polycystic Ovary Syndrome. Cell. Physiol. Biochem. 43 (5), 1855–1865. 10.1159/000484074 [DOI] [PubMed] [Google Scholar]