Abstract
Nanopore sequencing is regarded as one of the most promising third-generation sequencing (TGS) technologies. Since 2014, Oxford Nanopore Technologies (ONT) has developed a series of devices based on nanopore sequencing to produce very long reads, with an expected impact on genomics. However, the nanopore sequencing reads are susceptible to a fairly high error rate owing to the difficulty in identifying the DNA bases from the complex electrical signals. Although several basecalling tools have been developed for nanopore sequencing over the past years, it is still challenging to correct the sequences after applying the basecalling procedure. In this study, we developed an open-source DNA basecalling reviser, NanoReviser, based on a deep learning algorithm to correct the basecalling errors introduced by current basecallers provided by default. In our module, we re-segmented the raw electrical signals based on the basecalled sequences provided by the default basecallers. By employing convolution neural networks (CNNs) and bidirectional long short-term memory (Bi-LSTM) networks, we took advantage of the information from the raw electrical signals and the basecalled sequences from the basecallers. Our results showed NanoReviser, as a post-basecalling reviser, significantly improving the basecalling quality. After being trained on standard ONT sequencing reads from public E. coli and human NA12878 datasets, NanoReviser reduced the sequencing error rate by over 5% for both the E. coli dataset and the human dataset. The performance of NanoReviser was found to be better than those of all current basecalling tools. Furthermore, we analyzed the modified bases of the E. coli dataset and added the methylation information to train our module. With the methylation annotation, NanoReviser reduced the error rate by 7% for the E. coli dataset and specifically reduced the error rate by over 10% for the regions of the sequence rich in methylated bases. To the best of our knowledge, NanoReviser is the first post-processing tool after basecalling to accurately correct the nanopore sequences without the time-consuming procedure of building the consensus sequence. The NanoReviser package is freely available at https://github.com/pkubioinformatics/NanoReviser.
Keywords: nanopore sequencing, deep learning, sequencing revising, convolution neural network, long short-term memory networks, DNA methylation
Introduction
Oxford Nanopore Technologies (ONT) has recently shown the ability to use nanopores to achieve long-read sequencing, which has a tremendous impact on genomics (Brown and Clarke, 2016). The small-sized MinION device introduced by ONT is able to translate long sequencing reads from electrical signals produced by a single-stranded molecule of DNA passing through a nanopore (Lu et al., 2016). MinION is much cheaper than the other available sequencers and allows for sequencing to be carried out in real time, and hence has great prospects for use in many areas of genomics analysis such as variation detection and genome assembly (Leggett and Clark, 2017; Ameur et al., 2018; Pollard et al., 2018).
Although it is possible to produce long sequencing reads when using the MinION device with large arrays of nanopores, nanopore sequencing reads do show a fairly high error rate which could be introduced by abnormal fluctuations of the current or by the improper translation from raw electrical signals into DNA sequence, which has been sought to be corrected by the basecalling procedure developed by ONT (Rang et al., 2018). Several basecallers have been proposed since MinION was launched. Albacore (Loman et al., 2015), Guppy and Scrappie are basecallers provided by ONT. Notably, both Albacore and Guppy are only available to ONT’s commercial customers, while Albacore is the default basecaller. Scrappie, a technology demonstrator provided by ONT, actually combined with two basecallers in one: scrappie events and scrappie raw, which can only provide fasta output and was not trainable. Nanocall (David et al., 2017) predicted the DNA sequences using hidden Markov models (HMMs), but could not detect long homopolymer repeats. DeepNano (Boža et al., 2017), the first basecaller based on deep learning and one applying recurrent neural networks (RNNs) to predict DNA bases, showed that a deep learning model could make a crucial contribution to the basecalling performance. However, DeepNano was developed mainly based on the datasets sequenced using R7.3 and R9.0 flow cells, which are no longer used. Later on, BasecRAWller (Stoiber and James, 2017), designed to use the raw outputs of nanopores with unidirectional RNNs to basecall DNA bases, was shown to be able to process the electrical signals in a streaming fashion but at the expense of basecalling accuracy. Chiron (Teng et al., 2018), designed to adopt a combination of convolution neural network (CNN) and RNN methodologies together with a connectionist temporal classification decoder (CTC decoder) that was successfully used in machine translation, achieved a high basecalling accuracy. Despite this high accuracy, Chiron, however, required a high much computer time to get these accurate results. Notably, all of these tools were designed to focus on basecalling but not sequencing error correction. The only tool proposed previously for MinION sequencing error correction, nanoCORR (James, 2016), requires additional next-generation sequencing short read sequences, and thus is not a practical tool for error correction in its true sense.
In this study, we developed NanoReviser, the first error-correction tool for a MinION nanopore sequencing platform. To correct errors introduced by the default basecallers provided by the manufacturer, our tool was designed to first take both the original electrical signals and base sequences as the inputs. In the module designed for the tool, we used a CNN (Kalchbrenner et al., 2014) to extract the local patterns of the raw signals, and a highly powerful RNN (Pascanu et al., 2013) and bidirectional long short-term memory networks (Bi-LSTMs) (Hochreiter and Schmidhuber, 1997; Schuster and Paliwal, 1997) to determine the long-term dependence of the bidirectional variation of the raw electronical signals on DNA strand passing through the nanopore hidden in the basecalled sequences. Although the module was trained on a small dataset from Escherichia coli K12 MG1655 (Jain et al., 2017) and human NA12878 (Jain et al., 2018) sequences, it was shown to be able to correct errors of sequencing for many other genomes as long as corresponding training sets were provided. Furthermore, since studies have shown that DNA modification information could be detected by analyzing the electrical signals and since both the DNA sequences and the DNA modifications could be determined at the same time using the Oxford Nanopore sequencing (Stoiber et al., 2017), we analyzed the basecalling results for the methylated bases and trained our module with the modified data. We believe that NanoReviser will find significant use in genome analysis when ONT sequencing becomes, as expected, widely used in the future.
Materials and Methods
Data Sets
We performed this study with data from two public datasets: human data from the Nanopore WGS Consortium (Stoiber et al., 2017) and E. coli data from the MinION Analysis and Reference Consortium (MARC) (Jain et al., 2017). The Nanopore WGS Consortium sequenced the genome of the human sample CEPH1463 (NA12878/GM12878, Ceph/Utah pedigree) using 1D ligation kits with R9.4 chemistry. The sequencing of this data was basecalled using Albacore release v2.0.2. We trained our module on the chromosome 20 data and validated our module on the rel3 NA12878 genome data. MARC provided the whole genome sequencing of E. coli K12 MG1655 using 1D ligation kits and Rapid kits with R9.0 chemistry, and the basecaller used for these samples was Albacore release v0.8.4. A summary of the two datasets is listed in Table 1.
TABLE 1.
Summary of the experimental datasets for training NanoReviser.
| E. coli | Human chromosome 20 | |
| Numbers of reads | 560,000 | 395,514 |
| Numbers of mapped reads | 550,234 | 259,768 |
| Average length of mapped reads | 8198.05 | 6691.98 |
The sequence of the Escherichia coli K12 MG1655 genome may be accessed at the NCBI under Refseq accession number NC_000913.3 and the sequence of the Homo sapiens NA12878 genome was downloaded from the NCBI GenBank assembly accession GCA_002077035.3. The annotated file of the modifications in the E. coli genome was downloaded from the MethSMRT database (Ye et al., 2017).
Preprocessing
Re-segmentation of Events During Sequencing
The raw signals generated by the process of DNA passing through the nanopores were basecalled into DNA sequences immediately by default. The raw signals, the files used during the basecalling procedure, and the output file in fastq format were packed together into a fast5 file as the final output of the sequencing. Based on the files used during the basecalling procedure, which were the event files, we re-segmented the events provided by the default Albacore basecaller.
A period of raw signals that can be translated into a particular length of DNA was defined when using ONT procedures as an event, and a series of parameters were used to describe this event (David et al., 2017). Although the parameters in an event defined by the different basecallers differed, the common parameters were mean, start, stdv, length, model_state, move, weight, and p_model_state – with model_state referring to the corresponding predicted DNA sequence of the event, start being the starting time point of the event, length being the length of the raw signals corresponding to the event, mean and stdv being the basic statistics of this period of raw signals, move describing the movement of the DNA in nanopores and having only three values (0 standing for the DNA strand staying in the nanopore and 1 and 2 standing for the DNA strand moving, respectively, one base or two bases in the nanopore), weight and p_model_state being essential parameters for basecalling. Based on move, we re-segmented the events and calculated the mean and stdv as the input of our module. The re-segmentation process was crucial for basecalling in the following process.
Labeling and Training
We aligned the re-segmented reads against the E. coli K12 MG1655 reference genome using GraphMap (Sović et al., 2016), which was designed specifically for the alignment of the nanopore reads, with the default parameters and we labeled the sequencing errors and basecalling errors based on the alignment results. As for the methylation labeling, we aligned the re-segmented reads against the E. coli K12 MG1655 reference genome to get the positions of events. Then, we labeled the methylated bases according to the annotation from the MethSMRT database (Ye et al., 2017), which defined three types of methylated areas: m6A stands for N6-methyladenosine, m4C stands for N4-methylcytosine and modified bases.
Notably, the two dataset projects (Nanopore WGS Consortium and MARC) were quite different in many of their sequencing procedures such as library preparation (Rapid kit vs. 1D ligation kit), flow cell version (R9.4 chemistry vs. R9.0 chemistry) and basecaller tools (Albacore v2.0.2 vs. Albacore v0.8.4). Thus, we trained NanoReviser on the E. coli data and human data individually. In order to build the training set and validation set, we first divided the reference genome into 5,000 bp intervals. Then we aligned all of the reads and separated them into different intervals based on the alignment results. For the E. coli training process, we selected two reads from every interval, which was 2x depth data, to build up the low-coverage training set and we selected the reads located on the genome length of 2,225,000–2,300,000 bp to build up the local training set: There were 1,854 and 2,412 reads in, respectively, the low-coverage and local E. coli training sets.
We used 1x depth reads, which means the reads contained in our selection could cover the genome one times, and the reads located on 28,750,000–43,750,000 bp of the NA12878 chromosome 20 to build, respectively, the low-coverage training data and local training data for the training process using the human data. The low-coverage training data contained 4,820 reads and the local data contained 4,593 reads.
We used the Adam (adaptive moment estimation) algorithm with the default parameters (Kingma and Ba, 2014) in the training process to perform optimization. NanoReviser iterated through 50 epochs with the low-coverage data, the local data, and the combination of the low-coverage data and local data, which we denoted as NanoReviser (low coverage), NanoReviser (local) and NanoReviser respectively. We trained our models on a high-performance computer (Dell R930, 2019) with four CPU processors (2.1 GHz Intel Xeon E7-4809 v4), four GPU processors (Nvidia Tesla T4) and 18 DDR4 memories (18 × 32G, 2133 MHz). Additionally, we applied multiprocessing implementation in the training procedure of NanoReviser to reduce training time, and the corresponding training time is presented in Table 2.
TABLE 2.
Summary of the training time.
| E. coli | Human chromosome 20 | |
| Low-coverage data | 2.66 h/epoch | 9.02 h/epoch |
| 132.29 h/50 epochs | 451.22 h/50 epochs | |
| Local data | 3.05 h/epoch | 7.10 h/epoch |
| 152.61 h/50 epochs | 355.11 h/epochs | |
| Low-coverage + Local data | 6.69 h/epoch | 16.54 h/epoch |
| 334.50 h/50 epochs | 827.32 h/50 epochs |
Model Architecture
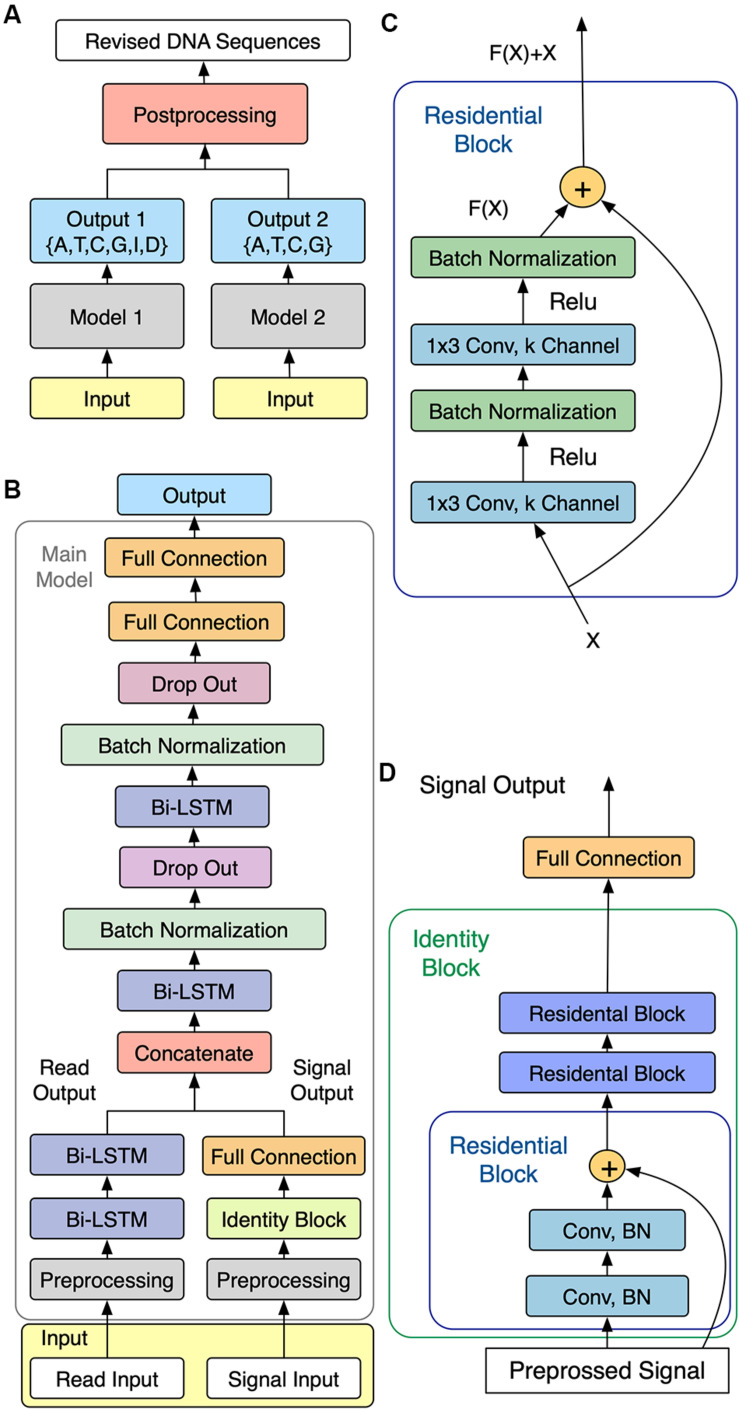
As shown in Figure 1A, NanoReviser was designed to be composed of two main models, named model 1 and model 2, respectively, with the main task for model 1 being the learning of the error types from the combination of the reads and electrical signal input and the main goal of model 2 being identification of the bases. Models 1 and 2 were designed to share a similar model structure, called the main model, which is shown in Figure 1B.
FIGURE 1.
Schematics of NanoReviser. (A) Structure of NanoReviser model building. (B) Structure of the main model. The preprocessed raw electrical signals (on the right) were passed through Identity Block and joined with the results of the preprocessed read input (on the left), passing through two bidirectional long short-term memory (Bi-LSTM) layers, and then the combination of the raw electrical signal features and read features were fed into the following Bi-LSTM layers. Finally, after the formation of two fully connected layers, the model gave a probability distribution of called bases. (C) Structure of Residential Block. Residential Block consisted of two convolutional layers and two batch normalization layers, which were used to accelerate the training speed. Conv stands for a convolutional layer and 1 × 3 was the size of the kernel used by the convolutional layer. (D) Structure of Identity Block. Identity Block consisted of three Residential Blocks. Conv stands for a convolutional layer and BN is the abbreviation for Batch Normalization.
Main Model Architecture
A simple multi-layer RNN cannot precisely capture the fine-scale changes of the electrical signals caused by DNA polymers passing through nanopores. In order to increase the model capacity, we designed the main model to be similar to a speech recognition deep learning model (Rosenberg et al., 2017) that used multiple CNN layers (Kalchbrenner et al., 2014) and RNN layers (Pascanu et al., 2013). In order to make our module perform well with extremely imbalanced datasets, we also exploited the center loss function (Wen et al., 2016) in our module. Figure 1B shows the architecture of our sequencing error correction system.
In our definition, the event of moment t defined as et was labeled as yt1 ∈{A,T,C,G,I,D} in model 1 (with I and D representing, respectively, an insertion and deletion in the CIGAR record of the SAM format file) and labeled as yt2 ∈{A,T,C,G} in model 2. As for the event of moment t, we defined the first input, i.e., the raw electrical signal, as s(t) – with s(t) corresponding to a time-series of length T(t), and with every time slice represented as a vector of normalized raw signal features in the form , i = 0,…,T(t)−1. We defined the second input as x(t), which consisted of the basecalled bases as well as the mean, standard deviation and length of the event of moment t. Besides, the second inputs we used for NanoReviser methylations contained the features mentioned above, as well as the methylated information of the events. These two inputs composed the input as shown in Figure 1B.
To be specific, for model 1 or model 2, first s(t) was passed through the multi-layer CNN module and the second input x(t) was passed through the unique powerful RNN layers, which are the Bi-LSTM (Hochreiter and Schmidhuber, 1997) layers. Then, the two inputs were joined together as the input of the following multi-layer Bi-LSTM module. Finally, the output ot at the t moment gave a probability distribution of called bases. Our module was designed with the core idea to convert the input s(t) and the input x(t) into a final transcription yt1 or yt2. Notably, we used center loss to help supervise the learning of our module.
Convolutional Neural Network Layers
Since our electrical signal input was a one-dimensional time-series sequence, we used a one-dimensional CNN layer with a filter size and stride set of 3 and 1, respectively. Then we applied the rectified linear unit (ReLU) function (Zhao et al., 2017) on the outputs of the CNN layer, which was the most common activation operation in the CNN models (Table 3). We also used the batch normalization to accelerate the training process.
TABLE 3.
Hyper-parameters of NanoReviser.
| Layer | Feature source | Hyper-parameters | Value |
| CNN | Signal input | Kernel size | 1 × 3 |
| Number of filters | 8 | ||
| Stride | 1 | ||
| Activation function | ReLUa | ||
| Bi-LSTM | Read input | State size | 16 |
| Activation function | tanhb | ||
| Bi-LSTM | Concatenated input | State size | 64 |
| Activation function | tanhb | ||
| Dropout | Concatenated input | Dropout rate | 0.2 |
| Center loss | Concatenated input | Proportion | 0.2 |
| Adam optimizer | Initial learning rate | 0.002 | |
| Decay rate | 0.05 | ||
| Beta_1 | 0.9 | ||
| Beta_2 | 0.999 |
aReLU is one of the commonly used activation functions in CNN layer. btanh is the default activation function in LSTM layer.
CNN is good at learning multi-level representations, but the traditional deep CNN faces a degradation problem (LeCun et al., 2015). Thus, in the current study, we used the residual CNN (He et al., 2015) to extract features from the raw signals. The residual network contained direct links between the higher layer outputs and the lower layer inputs, and the residual block in our module is illustrated in Figure 1C.
We used three residential blocks to build an identity block (Figure 1D) just like the ResNet model (He et al., 2015). Indeed, the first input s(t) went through the identity block and then was mixed with the second handled inputs.
Recurrent Neural Network Layers
Compared with the traditional recurrent layers, the LSTM model (Hochreiter and Schmidhuber, 1997) has been shown to be more effective, especially for the task of learning long-term dependencies (Yue and Wang, 2018). We applied the tanh function (Yue and Wang, 2018)on the outputs of the LSTM layer, which was the default activation operation in the LSTM models (Table 3). Since DNA polymers near the nanopores could contribute to changes in the electrical signal, a Bi-LSTM model (Schuster and Paliwal, 1997) allowed us to look at the bases both far ahead and far behind at will. Thus, we recruited the Bi-LSTM model in our module. Specifically, the second input, x(t), was fed into two Bi-LSTM layers, combined with batch normalization. Then, the latter Bi-LSTM layer received the combination of the handled signal data and read data. In order to avoid overfitting, we used dropout layers (Bouthillier et al., 2015). After formation of the two fully connected layers, model 1 finally output the likelihood of the input event as A, T, C, G, I, or D and model 2 output the likelihood of the input event as A, T, C, or G.
Loss Function
In order to make our module perform well with extremely unbalanced data, we recruited the center loss function (Wen et al., 2016) into the commonly used softmax cross-entropy loss function. According to Wen, the softmax cross-entropy loss function merely considers the inter-class distance, whereas the main idea of the center loss function is to reduce the intra-class distance during the training process.
The softmax cross-entropy loss function is normally defined as:
where xi, which belongs to the yith class, is the ith feature of the mini-batch inputs with m samples. Furthermore, Wand b are the weights and bias term in the last fully connected layer, and n is the number of classes. Obviously, the model could learn to distinguish the samples from different classes by minimalizing the softmax cross-entropy loss.
The center loss function used in our current work was expressed using the equation.
where m is the mini-batch number and cyi denotes as the center of features. The loss function we used in our module was just like the one Wen (Wen et al., 2016) used in his work and was represented as:
Here, λ is a hyper parameter used to control the proportion of the softmax cross-entropy loss and the center loss. We set λ to be 0.2.
Evaluation Criterion
Due to the sequencing protocols and default basecallers being quite different between the E. coli (K12 MG1655) and human (NA12878) datasets, we evaluated NanoReviser on both the E. coli and human datasets separately. We randomly used 100 reads that were not contained in training set from E. coli data apart from the training set to evaluate our module. As for the NA12878 datasets, 100 reads excluded from the training set from human NA12878 chromosome 20 and 2,100 reads from the other chromosomes (100 reads from each chromosome apart from chromosome 22, which is not provided in the rel3 data of the Nanopore WGS Consortium) were chosen to evaluate the performance of our module, and compared this performance to those of other modules.
The evaluation criteria we used were the deletion rate, the insertion rate, the mismatch rate and the error rate, where
and
According to methylation annotation of the E. coli genome on the MethSMRT database (Ye et al., 2017), there are three types of methylated areas: m6A, m4C and modified bases. Therefore, the deletion rate, the insertion rate, and the mismatch rate on the kth type of methylated areas could be denoted as:
Where X stands for deletion, insertion and mismatch. The error rate on the k type methylated areas could be denoted as:
Thus, the total deletion rate, the insertion rate, the mismatch rate and the error rate on the methylated areas could be denoted as:
Where X stands for deletion, insertion and mismatch.
We compared NanoReviser with DeepNano (Boža et al., 2017), Chiron Versions 0.6.1.1 (Teng et al., 2018) and Scrappie 1.4.01.
Results and Discussion
Implementation of NanoReviser
NanoReviser used the two outputs from two deep learning models to compute the final DNA sequences as shown in Figure 1A. Specifically, when model 1 or model 2 predicted the same base as the original read base, the original read base was preserved; whereas when model 1 and model 2 each predicted the same base as each other but different than the original read base, the read base was corrected. NanoReviser directly deleted the read base when model 1 considered the read base to be an insertion and model 2 predicted the same base as the original read base. When NanoReviser predicted a base as a deletion, the base inferred by model 2 was added to the DNA sequence. Except for these situations, NanoReviser tended to preserve the original read bases to avoid introducing new errors.
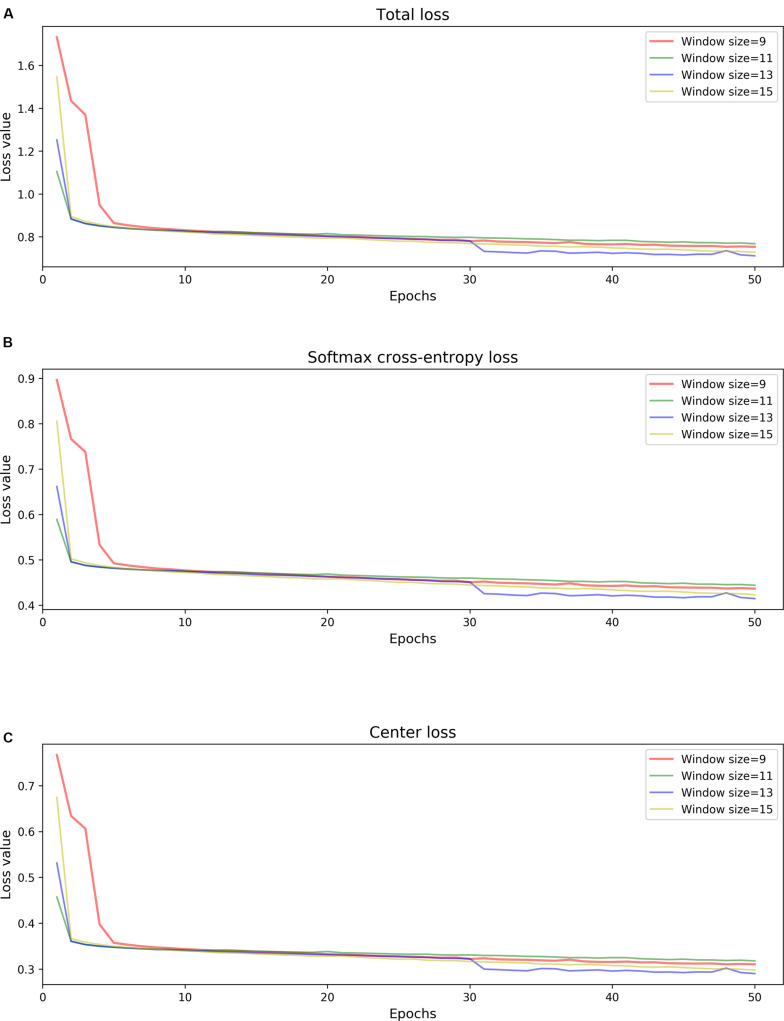
Since model 1 and model 2 share a similar structure, we have tested the parameters on the model 1 and used the same parameters for model 2. Table 3 summarizes the chosen hyper-parameters for NanoReviser. The batch size is set to 256 and the training sets were shuffled at the beginning of every training round. As described in the previous studies, the presence of multiple bases in the nanopore could contribute to the raw electrical signal produced and used to predict the identity of the central base (Rang et al., 2018). Although LSTM could relatively well memorize the long-term relationship between the surrounding bases and the central base in the nanopore, processing the whole nanopore sequencing read is still beyond the ability of LSTM. Therefore, the inputs must be divided into small pieces, and we tested window sizes ranging from 9 to 15. We observed that the model 1 performed the best when the window size was 13 and we inferred from our results that the model converged after 50 rounds of training (Figure 2). Since window size, training epochs and the data size of the training data are crucial for the final training result, these parameters could be set by users when training NanoReviser.
FIGURE 2.
NanoReviser fitting performances for various window sizes and over many iterations. (A) Total loss value. (B) Softmax cross-entropy loss value. (C) Center loss value.
Performance Comparison on the E. coli Dataset
To date, 560,000 nanopore sequencing reads have been collected in the E. coli dataset and the sequencing depth of the E. coli dataset used was near 650x. We randomly selected 100 reads from the 560,000 reads for evaluation. The details of the NanoReviser performance are presented in Table 4. Specifically, we separated 2x depth reads to train our module and the method was marked as NanoReviser (low coverage). Then, we trained our module including only the reads located in a particular area of the E. coli genome and the method was marked as NanoReviser (local). Moreover, we trained our module with a combination of both low-coverage reads and local reads, and this method was marked as NanoReviser. Both NanoReviser (local) and NanoReviser (low coverage) performed better than did the default basecaller, i.e., the Albacore program, as well as the Chiron program, which is currently considered to be the most accurate open-source basecaller (Wick et al., 2019). Unfortunately, although DeepNano could yield all the reads in test data, all the reads could not be aligned to the E. coli genome. Thus, DeepNano was excluded from the comparison on the E. coli data. When compared with the latest basecaller Scrappie, NanoReviser performed better than both the Scrappie events and Scrappie raw model. Notably, scrappie raw achieved a good performance at the expense of fewer reads production, which generated only 90 reads on the 100 reads test sets. After 50 rounds of training, NanoReviser reduced the error rate by over 5% compared with the default basecaller Albacore and achieved the best performance on the test set.
TABLE 4.
Performances of various tools on E. coli genome sequencing data.
| Dataset | Tool | Deletion rate (%) | Insertion rate (%) | Mismatch rate (%) | Error rate (%) |
| E. coli 100 reads | Albacore (default) | 6.66 | 5.14 | 7.74 | 19.55 |
| Chiron | 5.48 | 3.93 | 6.70 | 16.64 (−3.44) | |
| Scrappie events | 8.34 | 3.82 | 4.68 | 16.84 (−2.71) | |
| Scrappie rawa | 6.61 | 4.27 | 4.53 | 15.42 (−4.13) | |
| NanoReviser (low coverage) | 5.82 | 3.88 | 4.60 | 14.30 (−5.25) | |
| NanoReviser (local) | 5.37 | 4.74 | 4.94 | 15.05 (−4.50) | |
| NanoReviser | 5.62 | 3.91 | 4.69 | 14.22(−5.33) |
aScrappie raw only generate 90 reads. The number in bold denotes the lowest error rate in the corresponding columns.
Notably, we found that although NanoReviser improved the basecalling accuracy in general regardless of the data on which it was trained, the training data chosen nevertheless did slightly influence the type of error identified by the model. It seemed that NanoReviser best recognized the patterns of insertion errors from the low-coverage training sets whereas it was most sensitive to the deletion error type when trained on the local reads. Theoretically, the insertion error and the deletion error may be thought of as “two sides of a coin,” i.e., with an expectation that a model tending to be more sensitive to the deletion error type would introduce more insertion errors, and similarly a model tending to be more sensitive to the insertion error type would introduce more deletion errors. When trained with the combined datasets including both low-coverage data and local data, NanoReviser generally achieved a balanced sensitivity to the insertion and deletion error types.
Performance Comparison on the Human Dataset
Compared with the E. coli dataset, more advanced sequencing applications were involved in the sequencing of the NA12878 dataset. A total of over 13 million reads have been generated from several laboratories and the sequencing depth of the human dataset used was about 28x. We used the reads from chromosome 20 to train the NanoReviser. In order to test the generalization ability of the NanoReviser, we randomly selected 2,200 reads from chromosome 1–20 and chromosome X of the NA12878 dataset for evaluation. The results of these evaluations are reported in Table 5.
TABLE 5.
Performances of various tools on human NA12878 genome sequencing data.
| Dataset | Tool | Deletion rate (%) | Insertion rate (%) | Mismatch rate (%) | Error rate (%) |
| NA12878 Chr. 1-21 and Chr. X Total 2,200 reads | Albacore (default) | 12.32 | 4.53 | 5.88 | 22.74 |
| DeepNano | 11.67 | 5.15 | 5.53 | 22.36 (−0.38) | |
| Chiron | 9.46 | 4.21 | 4.47 | 18.13 (−4.61) | |
| Scrappie eventsa | 10.43 | 4.39 | 4.09 | 18.89 (−3.84) | |
| Scrappie rawb | 9.15 | 4.67 | 4.22 | 19.01 (−3.73) | |
| NanoReviser (low coverage) | 9.65 | 4.94 | 4.38 | 18.97 (−4.76) | |
| NanoReviser (local) | 8.64 | 4.81 | 4.35 | 17.80 (−4.93) | |
| NanoReviser | 8.84 | 4.40 | 3.83 | 17.07(−5.66) |
aScrappie events could only basecall 2,186 reads, we use the default reads basecalled by Albacore to instead the miss-basecalled 14 reads. bScrappie raw could only basecall 1,625 reads, we use the default reads basecalled by Albacore to instead the miss-basecalled 575 reads. The number in bold denotes the lowest error rate in the corresponding columns.
We used 1x depth reads to train the model and the method was marked as NanoReviser (low coverage) in Table 5. Then, we trained our module including only the reads located on a particular area and the method was marked as NanoReviser (local) in these tables. In both the low-coverage and local training methods, NanoReviser provided better performances than did the default basecaller Albacore. Since both Scrappie events and Scrapie raw could not produce all the reads contained in the test data, we used the reads basecalled by the default basecaller Albacore to make sure all the tools could yield the whole reads contained in the test datasets. As it is shown in Table 5, NanoReviser trained with the local and low-coverage data achieved the best performance, with an error rate of 17.07%, compared to the error rate of 22.74% for the default basecaller and error rate of 18.13% for the Chiron program.
When trained on the E. coli dataset, NanoReviser showed the same error recognition pattern as it did when trained on the human dataset, i.e., learning more about insertion errors from the low-coverage data and more about deletion errors from the local data. Moreover, unlike the Illumina sequencing technology whose sequencing errors have been shown to be random (Besser et al., 2018), Oxford Nanopore sequencing technology showed more deletion type errors both when trained on the E. coli data and human data. The deletion error rates were observed to be higher than the other types of error rates regardless of the kind of basecalling or revision tools used. Also, for all tools tested, we noticed that the deletion error rates for the human dataset were much higher than those for the E. coli dataset. Although the deletion error was indicated to be a serious issue for the Oxford Nanopore sequencing, sacrificing the insertion error rate in order to detect more deletion errors, from comparing the performance of NanoReviser (local) with the performance of NanoReviser (low coverage), was concluded to not be warranted. Fortunately, NanoReviser could learn the tradeoff between detecting deletion errors and insertion errors through a combination of using low-coverage data and local data, and in this way increase the overall performance.
Methylation and Error Revision on the E. coli Dataset
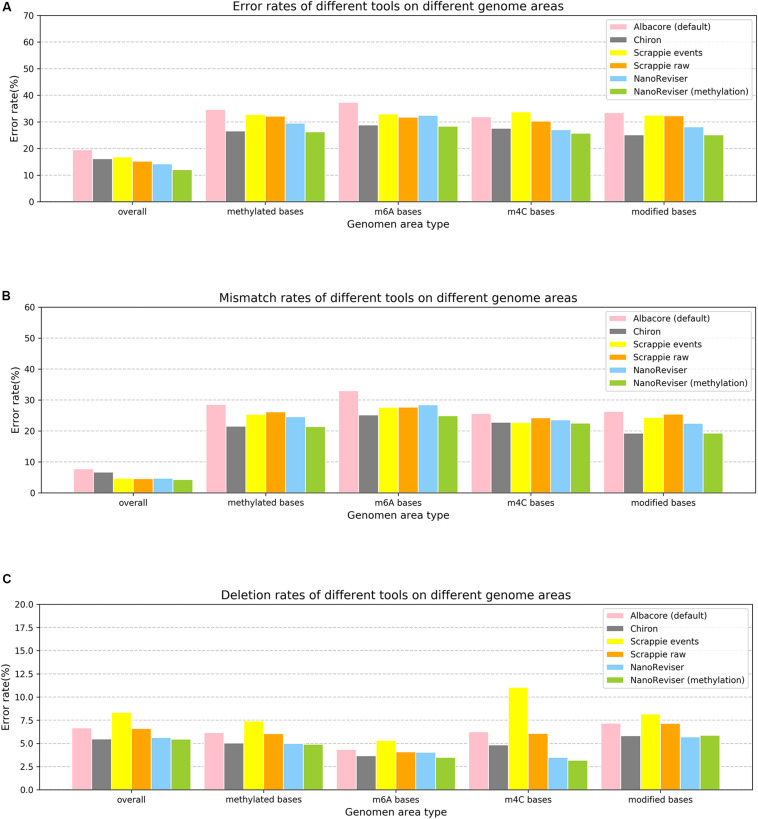
Studies have shown that methylation could severely affect the basecalling (Feng et al., 2015; Jain et al., 2016; Simpson et al., 2017; Rang et al., 2018). Therefore, we analyzed the error rates of the methylated bases of the E. coli genome. The Oxford Nanopore sequencing technology did encounter troubles in this regard. The overall error rate for the E. coli dataset was 19.55%, whereas the error rate for the methylated bases was over 34% and the mismatch error rate was as high as 28.53% (see Figure 3 and Table 5). The deletion error rate seemed to be similar to the overall genome deletion error rate whereas no insertion error happened for the methylated bases.
FIGURE 3.
Error rates of different error types on different genome areas. (A) Overall error rate. (B) Mismatch error rate. (C) Deletion error rate. NanoReviser (methylation) stands for NanoReviser trained with methylation information.
Since, promisingly, a tool was able to detect the modified bases at the same time as basecalling, we annotated the methylated areas of the reads based on the MethSMRT database (Ye et al., 2017) and took the methylation information as an additional input feature to train the model. We used the same 100-read E. coli test set here as described above, and the results are presented in Table 6. During the NanoReviser (methylation) training process, we used the combination of the low-coverage data and local data we had used in training NanoReviser without methylation. Interestingly, it seems that all tools reduced the error rate by revising the mismatch errors when basecalling the methylated bases. The analysis of insertion rates of different tools on the methylated bases was excluded from Figure 3 and Table 6 because all tools either translate methylated bases into other bases or skip methylated bases. In other words, insertion errors never happened on the methylated bases. As for the methylated area, the overall error rate of NanoReviser without the methylation information was observed to be higher than Chiron, yet was also observed to be lower than the overall error rates of Scrappie raw and Scrapie event (Figure 3). However, by taking the methylation positions into consideration, NanoReviser (methylation) reduced the error rate by about 7.5% compared with the default basecalling result and decreased the error rate by over 2% compared with the model without the methylation information, which achieved the best performance on all kinds of methylated bases including the m6A bases, m4C bases, and the modified bases.
TABLE 6.
Performances of various areas of the E. coli genome.
| Location |
Albacore (default) |
NanoReviser |
NanoReviser (methylation) |
|||
| Error rate | Mismatch rate | Error rate | Mismatch rate | Error rate | Mismatch rate | |
| Overall | 19.55 | 7.74 | 14.22 | 4.69 | 12.05 | 3.29 |
| Methylated bases | 34.69 | 18.54 | 29.53 | 24.55 | 26.27 | 21.38 |
| m6A bases | 37.32 | 32.97 | 32.45 | 28.41 | 28.38 | 24.89 |
| m4C bases | 31.89 | 25.66 | 27.08 | 23.60 | 25.72 | 22.53 |
| Modified bases | 33.46 | 26.27 | 28.13 | 22.43 | 25.12 | 19.25 |
The above results taken together indicated minimizing deletion errors to be at odds with minimizing insertion errors in training on low-coverage data or local data, and indicated methylation information to be crucial to the process of translating the electrical signal into a DNA sequence. Notably, decreasing the mismatch error was found to be the core improvement of NanoReviser (methylation), consistent with the high miscalling of the methylated bases.
Runtime Comparison
We have compared the computing speeds on a local PC (MacBook Air, 2017) with one CPU processor (2.2 GHz Intel Core i7) and DDR3 memory (8GB, 1600 MHz), as well as on a high-performance computer (Dell R930, 2019) with four CPU processors (2.1 GHz Intel Xeon E7-4809 v4), four GPU processors (Nvidia Tesla T4) and 18 DDR4 memories (18 × 32G, 2133 MHz). The results are listed in Table 7. An advantage of Chiron and Scrappie in the tests was its use of parallel computation to accelerate the basecalling process, which was also used in NanoReviser basecalling process. Notably, since Scrappie needs to run on a Linux Ubuntu system, we just compared Scrappie raw and Scrappie events on the high-performance computer (Dell R930, 2019). With the local PC, running the tests using NanoReviser was over 40 times faster than that using Chiron, and much faster than DeepNano. When running the tests on the high-performance computer, NanoReviser was twice faster than Chiron. Due to the efficient operation ability of C, Scrappie, which was code in C, run faster than tools written in Python, including DeepNano, Chiron as well as NanoReviser. However, tools, which written in Python, have an excellent cross platform capability. Notably, some researchers (Rang et al., 2018) believed that the high demand of computing resources primarily limited the wide use of Chiron despite its high accuracy on basecalling. However, NanoReviser, which takes advantage of more of the available sequencing information than does Chiron, is a better solution for obtaining high-quality nanopore sequencing reads, especially when the computing resources are limited.
TABLE 7.
Running speeds of various tools used on E. coli data and human data.
| Tool |
Local PC with only CPU (MacBook)> |
HPC with CPU and GPU (Dell R930) |
||
| E. coli | Human | E. coli | Human | |
| Chiron | 21 bp/s | 21 bp/s | 1,428 bp/s | 1,526 bp/s |
| DeepNano | 604 bp/s | 723 bp/s | 1,025 bp/s | 1,134 bp/s |
| Scrappie events | – | – | 6,240 bp/s | 7,628 bp/s |
| Scrappie Raw | – | – | 5,312 bp/s | 6,004 bp/s |
| NanoReviser | 875 bp/s | 1,122 bp/s | 3,050 bp/s | 3,042 bp/s |
| NanoReviser (local) | 887 bp/s | 1,089 bp/s | 3,036 bp/s | 3,094 bp/s |
| NanoReviser (low coverage) | 860 bp/s | 1,092 bp/s | 3,004 bp/s | 3,006 bp/s |
| NanoReviser (methylation) | 849 bp/s | – | 2,690 bp/s | – |
Conclusion
We have developed software based on a deep-learning algorithm and named NanoReviser, which was shown to efficiently correct the basecalling errors introduced by current basecallers provided by default. NanoReviser was validated using E. coli and H. sapiens data, and showed a higher accuracy than that of default basecaller, Albacore, the commonly regarded best basecaller, Chiron, as well as two separate models in Scrappie. Based on our elaborate training data construction, we found that NanoReviser learned more about insertion errors when trained on low-coverage data and more about deletion errors when trained on local reads. When trained on a combination of low-coverage data and local data, NanoReviser learned a compromise solution, which showed the importance of the training data selection. Moreover, due to our belief in the power of using genomics information when constructing a bioinformatics algorithm, we analyzed the methylated area of the E. coli genome. Here we found that the overall error rate for the modified area showed a different pattern than did the overall error rate for the whole genome. Specifically, the error rate was much higher for the modified areas, and mismatch error type contributed the most to the error rate. In order to handle the high mismatch error rate of the methylated bases, we used the methylation annotation to train NanoReviser. This training improved the performance of NanoReviser for both the methylated bases and on the whole genome scale. And when taking the methylation information into consideration, NanoReviser showed a reduced deletion error. Therefore, it may be concluded that there could be an enhanced relationship between modification detection, at least methylation detection, and basecalling, and we are looking forward to combining modification calling and basecalling. Furthermore, we believe that the error rate in the homopolymer region is a unique challenge in improving the basecalling accuracy, and according to the assessment on NA12878 chromosome 4, which contains the most homopolymer regions in all the NA12878 chromosomes, NanoReviser showed the ability to improve the basecalling accuracy on the homopolymer regions. Finally, we compared the running speeds of NanoReviser with DeepNano, Chiron and Scrappie on both our local PC and a high-performance computer. NanoReviser ran faster than did DeepNano and Chiron on the local PC and on the high-performance computer. On the high-performance computer, both Scrappie events and Scrappie raw are more efficient than NanoReviser. However, Scrappie can only supported by Linux Ubuntu system. Therefore, NanoReviser is expected to be a universal solution for the elimination of nanopore sequencing errors in the future.
Data Availability Statement
NanoReviser is an open source program and the source code is available at https://github.com/pkubioinformatics/NanoReviser.
Author Contributions
HZ, LW, and LQ conceived the study and wrote the manuscript. LW developed the code. LW, LY, and YW performed data analysis under the supervision of HZ. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Binbin Lai, Qi Wang, Xiaoqi Wang, Yongchu Liu, and Yang Li for their beneficial discussions and assistance with this manuscript.
Funding. This work was supported by the National Key Research and Development Program of China (2017YFC1200205), the National Natural Science Foundation of China (31671366), and the Special Research Project of “Clinical Medicine + X” of Peking University. Part of the analysis was performed on the High-Performance Computing Platform of the Center for Life Science of Peking University.
References
- Ameur A., Kloosterman W. P., Hestand M. S. (2018). Single-molecule sequencing: towards clinical applications. Trends Biotechnol. 37 72–85. 10.1016/j.tibtech.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Besser J., Carleton H. A., Gerner-Smidt P., Lindsey R. L., Trees E. (2018). Next-generation sequencing technologies and their application to the study and control of bacterial infections. Clin. Microbiol. Infect. 24 335–341. 10.1016/j.cmi.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthillier X., Konda K., Vincent P., Memisevic R. (2015). Dropout as data augmentation. arXiv [Preprint] Available online at: http://arxiv.org/abs/1506.08700 (accessed February 16, 2019). [Google Scholar]
- Boža V., Brejová B., Vinař T. (2017). DeepNano: deep recurrent neural networks for base calling in MinION nanopore reads. PLoS One 12:e0178751. 10.1371/journal.pone.0178751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. G., Clarke J. (2016). Nanopore development at Oxford Nanopore. Nat. Biotechnol. 34 810–811. 10.1038/nbt.3622 [DOI] [PubMed] [Google Scholar]
- David M., Dursi L. J., Yao D., Boutros P. C., Simpson J. T. (2017). Nanocall: an open source basecaller for Oxford Nanopore sequencing data. Bioinformatics 33 49–55. 10.1093/bioinformatics/btw569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Zhang Y., Ying C., Wang D., Du C. (2015). Nanopore-based fourth-generation DNA sequencing technology. Genom. Proteom. Bioinform. 13 4–16. 10.1016/j.gpb.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Zhang X., Ren S., Sun J. (2015). Deep residual learning for image recognition. arXiv [Prreprint], Available online at: http://arxiv.org/abs/1512.03385 (accessed February 16, 2019). [Google Scholar]
- Hochreiter S., Schmidhuber J. (1997). Long short-term memory. Neural Comput. 9 1735–1780. 10.1162/neco.1997.9.8.1735 [DOI] [PubMed] [Google Scholar]
- Jain M., Koren S., Miga K. H., Quick J., Rand A. C., Sasani T. A., et al. (2018). Nanopore sequencing and assembly of a human genome with ultra-long reads. Nat. Biotechnol. 36 338–345. 10.1038/nbt.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Olsen H. E., Paten B., Akeson M. (2016). The Oxford Nanopore MinION: delivery of nanopore sequencing to the genomics community. Genome Biol. 17:239. 10.1186/s13059-016-1103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Tyson J. R., Loose M., Ip C. L. C., Eccles D. A., O’Grady J., et al. (2017). MinION analysis and reference consortium: phase 2 data release and analysis of R9.0 chemistry. F1000Research 6:760. 10.12688/f1000research.11354.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G. (2016). Nanocorr:Error Correction For Oxford Nanopore Reads. Available online at: https://github.com/jgurtowski/nanocorr (accessed Februrary 28, 2016). [Google Scholar]
- Kalchbrenner N., Grefenstette E., Blunsom P. (2014). “A convolutional neural network for modelling sentences,” in Proceedings of the 52nd Annual Meeting of the Association for Computational Linguistics, Baltimore, MD. [Google Scholar]
- Kingma D. P., Ba J. (2014). Adam: a method for stochastic optimization. arXiv [Preprint] Available online at: http://arxiv.org/abs/1412.6980 (accessed December 21, 2019). [Google Scholar]
- LeCun Y., Bengio Y., Hinton G. (2015). Deep learning. Nature 521 436–444. 10.1038/nature14539 [DOI] [PubMed] [Google Scholar]
- Leggett R. M., Clark M. D. (2017). A world of opportunities with nanopore sequencing. J. Exper. Bot. 68 5419–5429. 10.1093/jxb/erx289 [DOI] [PubMed] [Google Scholar]
- Loman N. J., Quick J., Simpson J. T. (2015). A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat. Methods 12 733–735. 10.1038/nmeth.3444 [DOI] [PubMed] [Google Scholar]
- Lu H., Giordano F., Ning Z. (2016). Oxford nanopore MinION sequencing and genome assembly. Genom. Proteom. Bioinform. 14 265–279. 10.1016/j.gpb.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascanu R., Gulcehre C., Cho K., Bengio Y. (2013). How to construct deep recurrent neural networks. arXiv [Preprint] Available online at: http://arxiv.org/abs/1312.6026 (accessed December 20, 2018). [Google Scholar]
- Pollard M. O., Gurdasani D., Mentzer A. J., Porter T., Sandhu M. S. (2018). Long reads: their purpose and place. Hum. Mol. Genet. 27 R234–R241. 10.1093/hmg/ddy177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang F. J., Kloosterman W. P., de Ridder J. (2018). From squiggle to basepair: computational approaches for improving nanopore sequencing read accuracy. Genome Biol. 19:90. 10.1186/s13059-018-1462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Audhkhasi K., Sethy A., Ramabhadran B., Picheny M. (2017). “End-to-end speech recognition and keyword search on low-resource languages,” in Proceedings of the 2017 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA. [Google Scholar]
- Schuster M., Paliwal K. K. (1997). Bidirectional recurrent neural networks. IEEE Trans. Signal Process. 45 2673–2681. 10.1109/78.650093 [DOI] [Google Scholar]
- Simpson J. T., Workman R. E., Zuzarte P. C., David M., Dursi L. J., Timp W. (2017). Detecting DNA cytosine methylation using nanopore sequencing. Nat. Methods 14 407–410. 10.1038/nmeth.4184 [DOI] [PubMed] [Google Scholar]
- Sović I., Šikić M., Wilm A., Fenlon S. N., Chen S., Nagarajan N. (2016). Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nat. Commun. 7:11307. 10.1038/ncomms11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber M., James B. (2017). BasecRAWller: Streaming Nanopore Basecalling Directly from Raw Signal. Available online at: https://www.osti.gov/biblio/1358006 (accessed May 5, 2017). [Google Scholar]
- Stoiber M., Quick J., Egan R., Eun Lee J., Celniker S., Neely R. K., et al. (2017). Identification of DNA modifications enabled by genome-guided nanopore signal processing. bioRxiv [Preprint] 10.1101/094672 [DOI] [Google Scholar]
- Teng H., Cao M. D., Hall M. B., Duarte T., Wang S., Coin L. J. M. (2018). Chiron: translating nanopore raw signal directly into nucleotide sequence using deep learning. Gigascience 7:giy037. 10.1093/gigascience/giy037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Zhang K., Li Z., Qiao Y. (2016). “A discriminative feature learning approach for deep face recognition,” in Computer Vision - ECCV 2016, eds Leibe B., Matas J., Sebe N., Welling M. (Cham: Springer International Publishing; ), 499–515. 10.1007/978-3-319-46478-7_31 [DOI] [Google Scholar]
- Wick R. R., Judd L. M., Holt K. E. (2019). Performance of neural network basecalling tools for Oxford Nanopore sequencing. bioRxiv [Preprint] 10.1101/543439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Luan Y., Chen K., Liu Y., Xiao C., Xie Z. (2017). MethSMRT: an integrative database for DNA N6-methyladenine and N4-methylcytosine generated by single-molecular real-time sequencing. Nucleic Acids Res. 45 D85–D89. 10.1093/nar/gkw950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T., Wang H. (2018). Deep learning for genomics: a concise overview. arXiv [Preprint] Available online at: http://arxiv.org/abs/1802.00810 (accessed December 18, 2018). [Google Scholar]
- Zhao G., Zhang Z., Guan H., Tang P., Wang J. (2017). Rethink ReLU to training better CNNs. arXiv [Preprint] Available online at: http://arxiv.org/abs/1709.06247 (accessed February 14, 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NanoReviser is an open source program and the source code is available at https://github.com/pkubioinformatics/NanoReviser.