Abstract
Pancreatic cancer has one of the poorest prognoses of all malignancies, with little improvement in clinical outcome over the past 40 years. Pancreatic ductal adenocarcinoma is responsible for the vast majority of pancreatic cancer cases, and is characterised by the presence of a dense stroma that impacts therapeutic efficacy and drives pro-tumorigenic programs. More specifically, the inflammatory nature of the tumour microenvironment is thought to underlie the loss of anti-tumour immunity and development of resistance to current treatments. Inflammatory pathways are largely mediated by the expression of, and signalling through, cytokines, chemokines, and other cellular messengers. In recent years, there has been much attention focused on dual targeting of cancer cells and the tumour microenvironment. Here we review our current understanding of the role of IL-6, and the broader IL-6 cytokine family, in pancreatic cancer, including their contribution to pancreatic inflammation and various roles in pancreatic cancer pathogenesis. We also summarise potential opportunities for therapeutic targeting of these pathways as an avenue towards combating poor patient outcomes.
Keywords: Cytokine, Interleukin, Pancreatic
Introduction
Pancreatic cancer is a devastating malignancy with a 5-year relative survival rate of only 3–9%, dependent on the geographical location surveyed [1–4], with these statistics exhibiting only modest improvement over the last four decades [1–3]. The median survival post-diagnosis ranges from just 6–11 months for locally advanced disease, and 2–6 months for metastatic disease [4]. It was estimated by the World Health Organisation that pancreatic cancer is currently the 7th leading cause of cancer-related death, being responsible for over 430,000 deaths worldwide in 2018 [5]. With incidence increasing, pancreatic cancer has been predicted to be the third leading cause of cancer-related death in the European Union by 2025 [6], and the second leading cause of cancer-related death in the United States of America and Germany by 2030 [7,8].
Several factors contribute to the poor survival of pancreatic cancer patients. A current lack of reliable diagnostic markers that would enable early screening [9], coupled with largely non-specific symptoms of disease, results in over 50% of patients presenting with metastatic disease at diagnosis [1,3]. This subgroup of patients have limited therapeutic options, and are thus typically administered palliative chemotherapy aimed at prolonging survival and reducing symptoms during end-of-life care [10–12]. Moreover, whilst approximately 10–20% of patients present with localised disease that is eligible for potentially curative surgery [1,12,13], disease recurs in over 70% of patients post-resection [14,15]. Ultimately, these factors culminate in more than 90% of patients diagnosed with pancreatic cancer succumbing to disease [12].
These harrowing statistics highlight that despite research efforts, there remains a lack of understanding of the pathogenesis of disease, which in turn limits the development of new therapeutics.
Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma is the predominant pancreatic malignancy
Pancreatic cancer can arise from either the endocrine or the exocrine region of the pancreas. Tumours arising from the exocrine compartment are termed pancreatic ductal adenocarcinoma (PDAC) and account for over 85% of all pancreatic cancers [12,16].
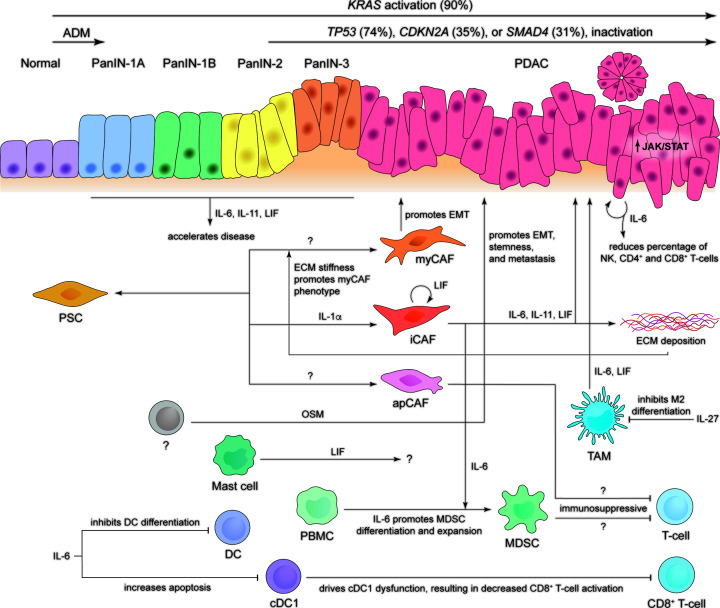
PDAC develops via a stepwise progression, from normal tissue through to invasive lesions, which is associated with distinct morphological characteristics [17–19]. It has been proposed that this process begins with a phenomenon termed acinar-to-ductal metaplasia (ADM), which is a normal homeostatic mechanism whereby acinar cells transdifferentiate into a ductal phenotype in response to particular stimuli [20]. However, if compounded by an oncogenic ‘hit’, cells are pushed towards a pathogenic phenotype that develops into pancreatic intraepithelial neoplasia (PanIN) [21–24]. Disease progresses through pre-invasive stages, termed PanIN-1A, PanIN-1B, PanIN-2, and PanIN-3, prior to invasive PDAC [25]. This progression is associated with increasing nuclear atypia, whereby the nuclei are no longer positioned basally, and loss of normal architecture as cells become more papillary in nature, with PanIN-3 lesions demonstrating increased mitoses [17]. As disease progresses to PDAC, cells become invasive and breach the basement membrane, growing through the extracellular matrix and metastasising to distant organs (Figure 1).
Figure 1. Our current understanding of the contribution of IL-6 family cytokines to PanIN and PDAC development.
Pre-invasive PanIN lesions develop from normal ductal epithelia, through PanIN stages 1A, 1B, 2, and 3, to stage 4 invasive and/or metastatic PDAC. This process is associated with acinar-to-ductal metaplasia (ADM) early in disease, combined with an accumulation of oncogenic mutations (most common mutations are indicated). A number of cells within the tumour microenvironment have been shown to secrete IL-6 family cytokines, which in turn results in the activation of a pro-tumorigenic signalling cascade. A better understanding of the relationship between each of these cells within the tumour microenvironment may reveal new therapeutic opportunities to manage cancer progression.
Less common precursor lesions include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) that also develop through multistep processes [25]. Whilst they share some common features, each lesion is morphologically and genetically distinct. In contrast with PanINs that form within small ducts, IPMNs develop within the primary or secondary branches of the main pancreatic duct, whilst MCNs lack ductal involvement [25].
An inflammatory tumour microenvironment contributes to PDAC pathogenesis
An archetypal feature of PDAC is the development of extensive stromal networks within the tumour microenvironment (TME) that can account for up to 90% of the total tumour volume [26–29]. This unique characteristic drives the inflammatory nature of PDAC that contributes to its aggressive phenotype [30]. Desmoplasia is driven by pancreatic stellate cells (PSCs) and cancer-associated fibroblasts (CAFs) that, upon activation, produce a range of extracellular matrix (ECM) components such as collagen, laminin, and fibronectin, which in turn form a physical barrier preventing the penetration of therapeutics [31–37]. Though PSCs and CAFs have been shown to support cancer metastasis and drug resistance, they interact with cancer cells in a bidirectional manner, with each promoting the survival, growth, and proliferation of the other [36–42]. Quiescent fibroblasts are able to differentiate into two unique subtypes, termed myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs) [36]. These two subtypes are distinct, whereby myCAFs express high levels of α-smooth muscle actin (α-SMA) and are located adjacent to cancer cells, while iCAFs express low levels of α-SMA and instead secrete high levels of inflammatory mediators, including IL-6, and are distributed distant from cancer cells within desmoplastic tumour regions [36]. Broadly, myCAFs appear to have roles in epithelial-to-mesenchymal transition (EMT) and ECM remodelling, whilst iCAFs appear to be involved in inflammation and ECM deposition [36,43]. A third less abundant subtype, termed antigen-presenting CAFs (apCAFs), has more recently been defined [43]. These cells express low levels of both α-SMA and IL-6, and instead express high levels of major histocompatibility complex class II (MHCII) and related genes [43]. As such, all three subtypes are transcriptionally and functionally distinct.
The wider TME contains a plethora of other cell types including endothelial cells, tumour-associated macrophages (TAMs) and neutrophils (TANs), mast cells, regulatory T-cells, myeloid-derived suppressor cells (MDSCs), dendritic cells, natural killer (NK) cells, and nerve cells [44]. Interactions between various cells within the TME can drive either pro-or anti-tumorigenic functions of others, for example, cancer cells can induce PSCs to secrete inflammatory cytokines that drive immune cells towards an immunosuppressive phenotype, and also form a positive feedback loop by increasing the tumorigenic potential of cancer cells [36,45,46]. The ECM itself has also been suggested to modify PSC behaviour, in particular, that ECM stiffness promotes the myCAF phenotype, indicated by increased α-SMA expression [47]. This results in substantial complexity that ultimately determines tumour phenotype [26,37].
The components of the microenvironment modify tumour behaviour through the production of cytokines, growth factors, and other signalling molecules that predominantly drive a pro-inflammatory and immunosuppressive program that enhances PDAC tumour growth and progression [28,40,44,48–52] (Figure 1). The ability of the TME to inhibit therapeutic efficacy through both molecular mechanisms and physical fibrotic barriers contributes to the intrinsic resistance of disease [34]. Thus, dual targeting of cancer cells and the TME may be required to induce a favourable therapeutic response, although this poses a signficant scientific and clinical challenge [28,51,53–56].
Molecular basis of pathogenesis
PDAC development is associated with accumulation of mutations
The progression of tumorigenesis through PanIN and PDAC stages is associated with the stepwise accumulation of specific genetic mutations that drive malignant transformation. The most frequent genetic alteration is an activating KRAS point mutation (codon 12) that occurs early on in tumour development [57], and is detected in over 90% of PDAC tumours [58–60]. Mutations in KRAS have been shown to drive development of precursor PanIN lesions, and when combined with an appropriate tumour suppressor mutation, these lesions progress to invasive or metastatic PDAC [61,62] (Figure 1). Patient tumours harbouring wild-type (WT) KRAS often carry activating mutations in downstream effector molecules, such as BRAF or PIK3CA [58].
Inactivation of a range of tumour suppressor proteins is also common, including mutations in TP53, CDKN2A, and SMAD4, in approximately 74%, 35%, and 31% of tumours, respectively [59,60]. Whilst each mutation has unique mechanistic outcomes, all three proteins are either directly or indirectly involved in the regulation of the G1/S cell cycle checkpoint. Analysis of patient tumours indicates that two or more of these mutations often occur together, with CDKN2A mutation being combined with either TP53, SMAD4, or both, usually in the background of KRAS mutation [63]. This suggests that by disrupting this checkpoint, cancer cells are able to overcome inhibitory mechanisms, allowing continued progression to invasive disease.
Unbiased sequencing efforts have also enabled identification of low prevalence PDAC mutations observed in less than 10% of cases [59]. These include mutations in genes involved in chromatin modification, KDM6A (10%); DNA damage repair, ATM (<2%); and other tumour-related processes such as growth (TGFBR1 or TGFBR2; <5%) [59]. Furthermore, it is important to note that technical advances are continuously uncovering epigenetic mechanisms that further modulate the PDAC transcriptional landscape and ultimately influence disease heterogeneity and tumour progression [64].
Molecular subtypes
The PDAC epithelial compartment is typically divided into two subtypes, including a classical subtype exhibiting an epithelial-like expression profile, and a squamous or mesenchymal-like subtype [65,66]. An additional third exocrine subtype is outlined in some analyses, and is characterised by a gene expression profile related to digestive enzyme production [67,68]. The classical or epithelial subtype has also been further divided into a pancreatic progenitor and an immunogenic subtype, whereby the immunogenic subtype is distinguished by significant immune infiltration and associated gene programmes [67]. Though there is no consensus on which classification system will allow the most valuable stratification of patients, the mesenchymal subtype is invariably associated with a poor prognosis [65,67,68]. The stromal compartment has also been classified into either normal or activated subtypes, reflecting the pro-and anti-tumorigenic capabilities of the TME, with the activated subtype associated with reduced survival [65]. This is particularly valuable as the extensive heterogeneity of PDAC complicates clinically relevant stratification of patients. Thus, the identification of molecularly unique subtypes may enable development of tailored therapeutic regimens that will provide improved clinical outcomes.
Current treatment options
Regardless of disease stage at time of diagnosis, patients have relatively limited treatment options. For the majority of patients, disease will be locally advanced or metastatic, disqualifying them from undergoing potentially curative surgery [1,3]. In these cases, patients are typically offered chemotherapy with palliative intent [10–12].
Surgery provides the only potentially curative treatment
Surgical resection remains the only potentially curative treatment option due to minimal efficacy of standard-of-care chemotherapy and radiotherapy. Due to its aggressive nature, the majority of patients present to clinic with locally advanced or metastatic disease, with only 10–20% of patients presenting with localised tumours that are eligible for surgical resection [1,12,69]. Even for those able to undergo surgical intervention, over 70% of patients relapse post-resection [14,15], with median survival improving to 18 months, and 5-year relative survival rate increasing modestly to 15–20% [70]. The use of neoadjuvant therapy is generally reserved for borderline resectable disease in an effort to enable patients to become eligible for surgery [71]. However, a range of recent trials have shown improved clinical outcomes, including overall survival, for neoadjuvant treatment of patients with resectable tumours [71]. Following surgical resection, patients are typically treated with adjuvant gemcitabine-based chemotherapy [72], although a recent study showed improved disease-free survival and overall survival with a modified FOLFIRINOX therapy (combination of oxaliplatin, irinotecan, leucovorin and fluorouracil) [73].
Radiotherapy provides variable clinical outcome
Whilst the use of radiotherapy and chemoradiotherapy (combination chemo- and radiotherapy) in the neoadjuvant and adjuvant settings have been investigated, there remains a lack of consensus regarding therapeutic benefit [74,75]. This is due to issues such as insufficient radiation dose and low participant numbers, as well as low uptake of modern techniques [76]. In the neoadjuvant setting, preliminary studies reported reduced lymph node positivity and rates of local recurrence for chemoradiotherapy compared to surgery with adjuvant chemotherapy [75,76]. However, the use of radiotherapy in the palliative setting was reported to modestly reduce overall survival [77]. More recent studies using ablative doses of radiation have shown a survival benefit, highlighting that technological advancements may provide an avenue for improved clinical outcomes following radiotherapy [78]. These contrasting results highlight the need to determine which subset of patients may benefit from the inclusion of radiotherapy approaches in standard treatment regimens.
Chemotherapy remains the cornerstone of treatment
Despite modest improvements in overall survival, palliative chemotherapy remains the standard treatment option for patients with locally advanced or metastatic disease. Gemcitabine monotherapy has been the mainstay treatment for pancreatic cancer since 1997 when it was demonstrated to improve median survival by just over 1 month, compared with fluorouracil [79]. Within the last decade there have been some further improvements in clinical outcome with combination chemotherapies gemcitabine/nab-paclitaxel and FOLFIRINOX providing median overall survival benefits of 1.8 and 4.3 months, respectively, compared with gemcitabine alone [80,81]. Although FOLFIRINOX treatment resulted in a lower percentage of patients experiencing reduced quality of life, it also had increased toxicity and adverse events, thus preventing its administration to patients with multiple comorbidities [73].
Therapeutic resistance remains a significant barrier to patient survival
Despite advances in chemotherapeutic options, treatment efficacy and patient prognosis remain poor due to the inherent therapeutic resistance of pancreatic cancer. It has been proposed that this drug resistance may be driven by the TME, including changes to cytokine signalling and metabolic pathways [82–84]. This intrinsic resistance is demonstrated by patients experiencing similar overall survival for chemotherapy treatment (3.0–8.6 months) compared with best supportive care (2.5–7.0 months), which encompasses the use of palliative surgery, psychological support, pain management and other methods of symptom control [85]. Whilst a range of targeted treatments, such as EGFR or checkpoint inhibitors, have been trialled with or without chemotherapy, they have shown limited success [86–88].
Emerging roles for the IL-6 family of cytokines in PDAC
Cytokines are soluble molecular messengers that enable efficient communication between a range of cell types, and have been recognised to be major contributors to the growth and metastasis of cancers [89–92]. In pancreatic cancer, cytokines mediate signalling between cancer cells, and the cells of the TME, including PSCs, CAFs, endothelial cells, and a range of immune cells including macrophages, mast cells, neutrophils, and regulatory T-cells [26,93–98]. It is the specific signalling pathways active within this community of cells that dictates the balance of pro- and anti-tumorigenic functions [99].
The IL-6 family of cytokines
The interleukin 6 (IL-6) family of cytokines includes IL-6, IL-11, leukaemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), neuropoietin (NP), IL-27, and IL-31 [100–104]. These cytokines are grouped as they share structural similarity, forming a four α-helical bundle (termed helices A-D) with an up-up-down-down topology [101,105,106].
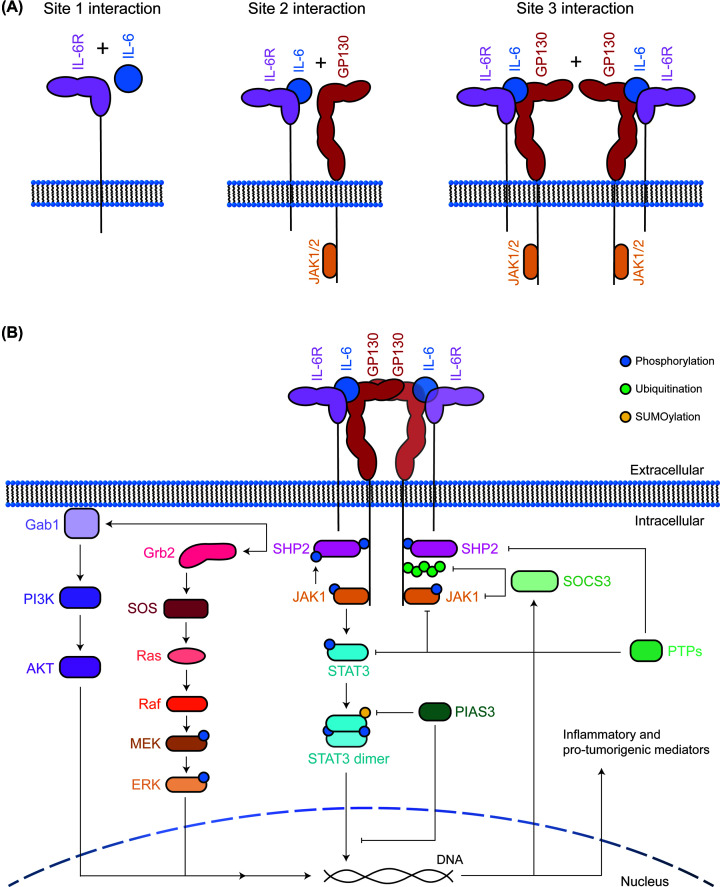
IL-6 and IL-11 utilise a hexameric signalling complex, consisting of two molecules each of the cytokine, α-receptor (either IL-6R or IL-11R, respectively), and β-receptor glycoprotein 130 (gp130) [107–109]. IL-6 and IL-11 are able to signal via two distinct mechanisms, termed classic and trans-signalling. Classic signalling involves the formation of a complex including membrane-bound IL-6R or IL-11R, with gp130 and the respective cytokine. Conversely, trans-signalling utilises soluble IL-6R or IL-11R molecules, which are able to form a signalling complex with gp130 and the respective cytokine [110–113]. In this way, classic signalling relies on the responding cell's intrinsic expression of IL-6R or IL-11R, whilst trans-signalling is able to activate any cell expressing gp130 [110,114].
LIF, OSM, IL-27, and IL-31 signal through trimeric complexes, with a single cytokine molecule engaging the respective receptor, LIFR, OSMR, IL-27R (WSX-1), or IL-31R, and either gp130 or OSMR (for IL-31) [100,101,105,115–117]. CNTF, CT1, CLC, and NP form tetrameric signalling complexes, composed of one cytokine molecule, one LIFR, one CNTFR, and one gp130 receptor [100,105]. In each case, the active signalling complex consists of two chains that are signalling competent, with a combination of either gp130, LIFR, OSMR, IL-27R, or IL-31R [105]. The requirement for multiple receptor subunits means that, although gp130 is ubiquitously expressed, the expression of other receptor subunits dictates the ability for any given cell to respond to cytokine, as signalling initiation requires the presence of cytokine and a compatible receptor complex [118] (Figure 2A).
Figure 2. IL-6 family cytokine signalling pathway.
(A) Schematic representation of the stepwise binding process for the IL-6 family members, with IL-6 as an example. The site 1 interaction involves cytokine binding to the respective receptor, with the site 2 interaction generally between the cytokine and the common gp130 receptor chain, finally site 3 interactions involve formation of the final active signalling complex, in this case, formation of the IL-6/IL-6R/gp130 hexameric complex. (B) General outline of the IL-6 family cytokine signalling pathway. Formation of an active hexameric complex leads to activation of JAKs with subsequent activation of the STAT3, MAPK, and PI3K pathways (left). This results in up-regulation of the negative regulator SOCS3, as well as a range of inflammatory and pro-tumorigenic molecules. The pathway is inhibited by SOCS3, PIAS3, and PTPs via dephosphorylation, ubiquitin-mediated proteasomal degradation, and SUMOylation (right).
Signalling complex assembly leads to trans-phosphorylation and activation of receptor-associated Janus tyrosine kinases (JAKs), largely JAK1, and to a lesser extent JAK2 and TYK2 [119,120]. In the case of gp130-mediated signalling this results in phosphorylation of the cytoplasmic domain of gp130 at tyrosine (Y) 683, 759, 767, 814, 905, and 915 [121]. Phosphotyrosine (pY) 767, 814, 905, and 915 of gp130 provide docking sites for signal transducer and activator of transcription (STAT) molecules, leading to their subsequent phosphorylation by JAK1 and formation of active STAT dimers [121–123]. Phosphorylated STAT (pSTAT) dimers then translocate to the nucleus and modulate target gene expression, including up-regulation of a range of genes involved in inflammatory and pro-tumorigenic pathways [122–124] (Figure 2B). Broadly, these STAT3-regulated genes can be categorised into pathways associated with inhibition of apoptosis, increased cell proliferation, modulation of immunity and inflammation, increased angiogenesis, and increased invasive and metastatic potential [125–132].
Although JAK/STAT signalling is the predominant pathway activated downstream of IL-6 family cytokines, the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways can also be activated [122]. The MAPK pathway has been suggested to be activated by a Src homology domain 2-containing phosphatase 2 (SHP2)-mediated mechanism, whereby SHP2 is recruited to pY759 on gp130, allowing JAK-mediated phosphorylation of SHP2 [106,133,134]. This promotes association with the adaptor protein, growth factor receptor bound protein 2 (Grb2), leading to activation of the G-protein, Ras, via son of sevenless (SOS), with a subsequent phosphorylation cascade including Raf, MEK, and ERK1/2 activity [106,135]. Following this, a MAPK-dependent phosphorylation event leads to the recruitment of Grb2-associated binding protein 1 (Gab1) to the plasma membrane, where Gab1 is suggested to act as a scaffold or adaptor protein to allow binding of PI3K and SHP2, leading to activation of the PI3K and MAPK pathways, respectively [133] (Figure 2B).
The suppressor of cytokine signalling 3 (SOCS3) is largely responsible for regulation of signalling, and is directly upregulated by STAT3 [100]. SOCS3 contains an SH2 domain, allowing it to bind to pY residues within the gp130 receptor [100], with preferential binding to Y759 [134,136,137]. Once bound, SOCS3 recruits an E3 ubiquitin ligase complex, containing Cullin5, Rbx2 and adaptors Elongin B and C, via its SOCS box domain [138]. This complex ubiquitinates the gp130 receptor, inducing its internalisation and targeting it for proteasomal degradation [138–143], and is also able to ubiquitinate JAK2 in vitro [144]. SOCS3 also mediates direct inhibition of the kinase activity of JAK1/2 via its kinase inhibitory region [145–148]. Thus, SOCS3 is able to down-regulate IL-6 family cytokine signalling pathways, through two distinct mechanisms.
The phosphotyrosine phosphatases (PTPs) and protein inhibitors of activated STATs (PIASs) also limit the strength and duration of cytokine signalling [122]. A range of PTPs, including SHP2, are responsible for de-phosphorylating tyrosine phosphorylated substrates, including JAKs, STATs and other SHP2 molecules [122,134,149]. PIAS3 preferentially binds pSTAT3 and inhibits activity either by preventing STAT3 interaction with DNA, by recruiting transcriptional repressors to STAT3 target genes, or by SUMOylating STAT3 to prevent its activity [150,151] (Figure 2B).
Interleukin 6 in PDAC
Elevation of serum IL-6 is a negative prognostic marker in human PDAC
Serum IL-6 levels were increased in PDAC patients compared with healthy patients [69,152–158] or those with chronic pancreatitis [152,154,157,158], and were also increased in patients with metastatic PDAC compared to those with locally advanced disease [157–161]. Moreover, elevated serum IL-6 positively correlated with increased disease burden, weight loss/cachexia, and metastasis [69,152–160,162–167], however, there are conflicting observations in the literature regarding IL-6 and cachexia [161]. Although, increased serum IL-6 levels correlate with increased disease stage, and in metastatic patients correlates with poor overall survival [161,168]. As such, it has been suggested that IL-6 may be a superior marker for diagnostic and prognostic purposes, compared with the standard C-reactive protein (CRP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) markers [157].
IL-6 is expressed within the TME
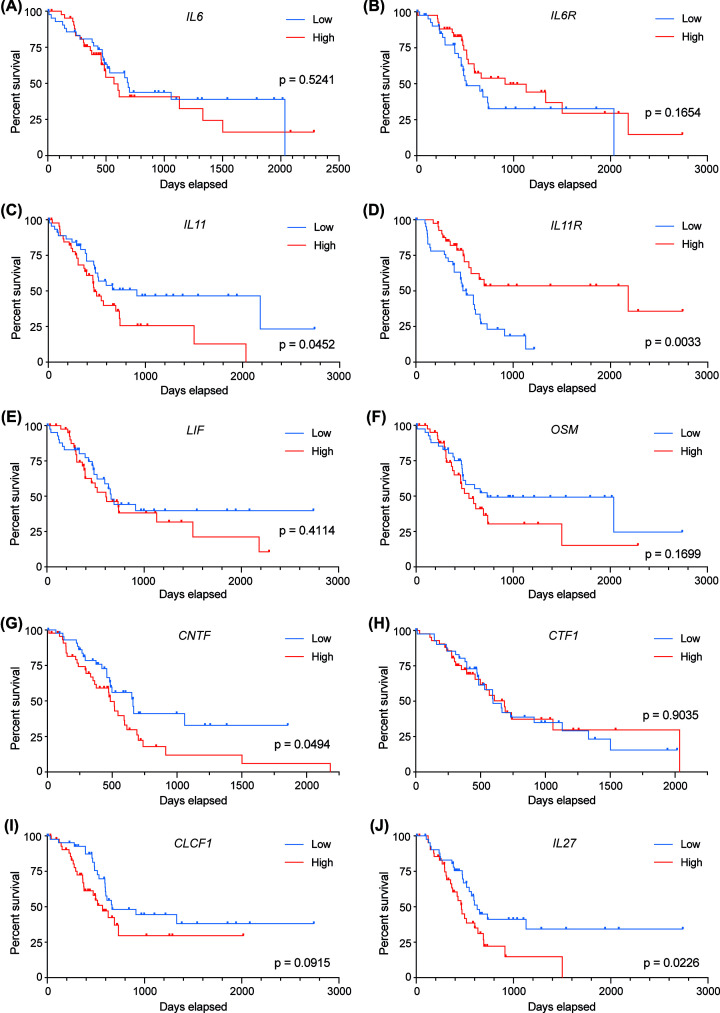
lL6/IL-6 was overexpressed in human PDAC tumours in comparison with adjacent normal tissue [165,169]. Whilst this tumour-specific elevation has been correlated with reduced survival in some studies [69,155,157,161,165], others showed no significant correlation with survival [160], similar to the data available in The Cancer Genome Atlas (TCGA) dataset for both IL-6 and IL-6R (Figure 3A,B). The TCGA comprise aggregate sequencing data, which does have limitations regarding interpretation of contributions of individual cell populations to disease outcome; however, it remains a widely used resource for exploratory investigations. However, overexpression of IL-6 has been observed at the mRNA and protein level in the pancreata of PDAC mice [46,170,171], with Il6 expression increasing with age, which is indicative of disease stage in these models [172].
Figure 3. IL-6 family cytokine expression in PDAC patients.
Overall survival for patients with high (top quartile) and low (bottom quartile) level expression of (A) IL6, (B) IL6R, (C) IL11, (D) IL11R, (E) LIF, (F) OSM, (G) CNTF, (H) CTF1 (CT-1), (I) CLCF1 (CLC), and (J) IL27 (n = 43 per group). Data and graphs obtained from OncoLnc [272] using data from The Cancer Genome Atlas (TCGA). Statistical significance determined by Mantel-Cox Log-rank test.
Despite the presence of IL-6 in tumours, primary human and commercial pancreatic cancer cell lines have been reported to exhibit variable expression levels of IL-6 and secreted cytokine, albeit consistently higher than normal pancreatic ductal epithelial cells [46,153,165,173,174]. In an organoid model, minimal IL-6 was expressed by pancreatic cancer cells (PCCs) or PSCs in monoculture, however in coculture, PCCs expressed only Il6ra, whilst iCAFs expressed high levels of IL-6, with this activating STAT3 within PCCs [36,171,175]. iCAFs also demonstrate an upregulation of the JAK/STAT pathway, with expression of IL-6 being dramatically increased in vitro when incubated with PCC conditioned media, indicating that soluble factor/s trigger IL-6 production [36,43,46]. More recently, PCC-derived IL-1α has been shown to induce autocrine LIF secretion, and thereby promote the iCAF phenotype, including activation of the JAK/STAT signalling pathway and IL-6 production [176].
In addition, TAMs have been identified as producers of IL-6 in pancreatic cancer by correlative immunohistochemistry and expression analysis of isolated cell populations [46,172,177]. Production of IL-6 by TAMs was shown to influence tumour development via bone-marrow chimeras, as mice reconstituted with IL-6 knockout (KO) (Il6-/-) myeloid cells developed low-grade PanINs, whilst those reconstituted with IL-6 WT cells developed PanIN-3 lesions [172].
IL-6 is a driver of PDAC pathogenesis
Both in vitro and in vivo studies suggest that the presence of IL-6 in the TME can drive activation of STAT3 [178], with IL-6 inhibition reducing STAT3 phosphorylation [172,179]. This IL-6/STAT3 program has been proposed to be a driver of PDAC pathogenesis, by enhancing tumour initiation and progression, angiogenesis, regulation of cytokine expression and immune cell behaviour, resistance to apoptosis, and promotion of metastasis [180–182]. In an inducible KRAS-driven mouse model, genetic deletion of Il6 resulted in a reduction of ADM and PanIN formation when KRAS mutation was initiated embryonically, compared with controls, suggesting a role for IL-6 in tumour initiation [46]. This was also observed in a constitutive KRAS mutant model, where genetic deletion of IL-6 prevented tumour initiation in vivo, with a reduction in the number of PanIN 1, 2, and 3 lesions [172]. Interestingly, oncogenic KRAS and hypoxic conditions, both features of PDAC tumours [12,183,184], were shown to induce IL-6 production [173,185,186], perhaps representing a feed-forward pathway enhancing tumorigenesis [126]. However, IL-6 is not absolutely required for PanIN formation, as induction of KRAS mutation at 4-6 weeks of age, in conjunction with an experimental pancreatitis model, drove formation of PanIN lesions that were not significantly different between IL-6 WT and KO mice [46].
Il6-/- mice exhibited reduced tumour progression with decreased proliferative capacity of both cancer and stromal cells, enabling regression of precursor lesions [46]. Furthermore, this inhibition of tumour progression by IL-6 deletion was due, at least in part, to the reversal of ADM, with ductal cells reverting to an acinar-like phenotype [46]. Increased apoptosis of cancer and stromal cells was also shown to contribute to this reduced tumour progression, as demonstrated by appropriate immunohistochemical analyses, with up-regulation of pro-apoptotic, and down-regulation of anti-apoptotic BCL-2 family members [46]. This is mirrored by in vitro data, whereby IL-6 stimulation increased the expression of anti-apoptotic BCL2/BCL-2 and BCL2L1/BCL-XL [187], with blockade of IL-6 signalling or STAT3 activation inducing apoptosis [169,179]. Collectively, these data suggest that whilst IL-6 contributes, it is not required for PDAC initiation and progression.
The process of angiogenesis supports tumour growth and progression by enabling adequate blood supply, which is enhanced by IL-6 signalling. Upon IL-6 stimulation, PDAC cell lines up-regulate key angiogenic factors such as vascular endothelial growth factor (VEGF/VEGF) and neurophilin-1 (NRP1/NRP1) [173,178,188,189], with significant correlation observed between the expression of IL-6R and VEGF on human PDAC sections [189]. IL-6-induced up-regulation of VEGF correlated with a growth advantage in PCCs, with both features inhibited by treatment with a JAK2 inhibitor [178].
Another facet of the pro-tumorigenic effects of IL-6 is the regulation of cytokine expression that enables modulation of the immune system [69]. In particular, it has been shown that IL-6 is able to up-regulate a type 2 cytokine profile in vitro that may inhibit anti-tumour immunity in disease [173]. IL-6 suppressed the differentiation of human CD14+ cells into dendritic cells (DCs) in vitro, whilst combination treatment with IL-6 and granulocyte colony-stimulating factor (G-CSF) inhibited the ability of DCs to respond to alloantigen, a process that is required for DC maturation and antigen presentation, where these effects were reversed by blockade of IL-6 and/or G-CSF [190]. IL-6 has also been implicated in driving increased apoptosis of type I conventional DCs (cDC1s), leading to cDC1 dysfunction early in tumorigenesis, and thereby decreased CD8+ T-cell activation [191]. This notion is further supported by in vivo studies whereby genetic deletion of IL-6 in a KRAS-driven PDAC mouse model exhibited a significant decrease in the percentage of intra-tumoral cancer-promoting macrophages and MDSCs [46]. Utilising primary human PSCs, MDSC differentiation was shown to be driven by PSC-derived IL-6, in a STAT3-dependent manner [45]. The resultant MDSCs were able to suppress T-cell proliferation [45], suggesting a role for IL-6 in promoting an immunosuppressive TME. Correlative analysis indicates that IL-6, through the generation of metabolic stress, indirectly causes a reduction in the percentage of intra-tumoral natural killer (NK) cells, CD4+, and CD8+ T-cells in pre-cachectic and cachectic mice [192]. This effect was coupled to a reduction in the expression of an array of genes involved in immune cell recruitment and T-cell effector function [192], indicating that IL-6 is able to, directly and indirectly, modulate immune regulation to enhance tumorigenesis.
EMT, migration, and invasion are prerequisite abilities that are required for tumour metastasis. Stimulation of PCCs with IL-6 modulated the expression of a range of proteins that drive EMT and migration, including up-regulation of SNAI1/SNAI1 (Snail), SNAI2 (Slug), CDH2 (N-cadherin), VIM (vimentin), FN1 (fibronectin), COL1A1 (collagen), and TWIST2, and down-regulation of CDH1/CDH1 (E-cadherin), with these effects mitigated by IL-6 or STAT3 inhibition [175,193,194]. IL-6-treated PCCs and pre-invasive PanINs exhibited morphological changes, with cells displaying up to three-fold increased invasive ability that was abrogated by IL-6, JAK2, or STAT3 inhibition [171,178,193–195]. These results suggest that IL-6 mediates its effects predominantly through the JAK/STAT3 signalling pathway. This IL-6 driven invasive ability was recapitulated in human xenograft-derived PCCs, with the mechanism reported to be due, at least in part, to activation of a small GTPase, CDC42, which promotes the development of migratory filopodia, but not lamellipodia [195]. In addition, IL-6 drives up-regulation of matrix metalloproteinase (MMP) 2 in vitro in a JAK2-dependent manner [178]. As such, it has been demonstrated that IL-6 enhances the migratory, invasive, and metastatic capability of PDAC cells.
Collectively, these data suggest that IL-6 is able to enhance tumorigenesis and metastatic potential through a variety of molecular pathways that are predominantly reliant on the JAK2/STAT3 signalling axis (Figure 1).
Anti-IL-6 treatment inhibits tumour growth in murine models
The vast majority of literature exploring the function of IL-6 in PDAC indicates a pathogenic role, providing a robust rationale for the targeted inhibition of this signalling pathway. Autocrine signalling of IL-6 by PCCs has also been demonstrated to prevent gemcitabine-induced apoptosis in vitro [42]. Knockdown of IL-6 by RNA interference (RNAi) resulted in increased sensitivity of PDAC cells to gemcitabine, leading to reduced proliferation, colony formation, and invasion, and increased apoptosis in vitro [169]. These results were mirrored in a PDAC cell line xenograft model, where IL-6 knockdown, in combination with gemcitabine, produced a substantial reduction in tumour burden [169]. Furthermore, pancreas-specific KrasG12D/+/p53fl/+ mutant mice with established disease were treated with an IL-6 neutralising antibody, resulting in a reduction in ADM and almost complete lack of PanIN lesions, compared to mice treated with isotype control that exhibited a range of ADM, PanIN-1, 2, and 3 lesions [46]. Combination treatment of anti-IL-6R with anti-programmed death-ligand 1 (PD-L1) immunotherapy was also demonstrated to be promising, with syngeneic xenograft models exhibiting reduced tumour growth, reduced abundance of α-SMA+ fibrobalsts (likely myCAFs), and increased infiltration of effector T-cells, and pancreas-specific KrasG12D/+/p53R270H//+/Brca2fl/fl mutant mice exhibiting decreased disease progression and increased overall survival [196].
Whilst these studies provide evidence that IL-6 inhibition may provide therapeutic benefit, the application of IL-6 targeted treatments will require assessment of the value of targeting classical and/or trans-signalling pathways. It has been proposed that trans-signalling is the predominant pathway by which IL-6 mediates pathogenic effects in pancreatic cancer [101]. The contribution of each signalling mechanism was explored in vivo by crossing KRAS-mutant mice with either Il6-/- or soluble gp130 (sgp130) transgenic mice (sgp130Tg) to prevent classic and trans-signalling [197], respectively [172]. In this model, sgp130Tg mice exhibited a more pronounced reduction in the number of PanIN lesions, than KrasG12D/Il6-/- mice, compared with KrasG12D mice [172]. As such, it may be beneficial to specifically target IL-6 trans-signalling using sgp130, preferentially to anti-IL-6 or anti-IL-6R therapies.
In order to investigate the contribution of classic and trans-signalling, PDAC cell line xenografts were treated with either anti-IL-6R or sgp130 [174]. Treatment during tumour growth (beginning 3 days post-injection) or following tumour resection (resected 10 days post-injection, treatment 3 days post-resection), resulted in reduced tumour burden compared with PBS or gemcitabine treatment, with sgp130 exhibiting enhanced results in each case; however, anti-IL-6R treatment exhibited fewer metastases post-resection [174]. In order to assess whether gemcitabine chemotherapy would improve responses, xenografts were treated with anti-IL-6R or sgp130 in combination with gemcitabine [174]. However, this strategy did not significantly improve inhibition of tumour growth compared to monotherapy, although gemcitabine alone or in combination reduced metastatic spread [174]. Whilst combination of all three treatments was not performed, it would be intriguing to explore whether bulk or staged treatment regimens would be capable of reducing both tumour growth and metastatic spread.
Interleukin 11 in PDAC
IL-11 expression is associated with poor prognosis in PDAC
IL-11 has been shown to be overexpressed in 64% of human PDAC tumours [165,198], with patients also displaying significantly elevated serum IL-11 levels compared to healthy controls [199]. Whilst two studies reported that reduced serum or tissue IL-11 levels correlated with poor survival [165,199], data available in the TCGA data demonstrated that high IL-11 expression, but low IL-11R expression correlates with poor overall survival (Figure 3C,D). This apparent dichotomy is not understood.
IL-11 may have a pro-tumorigenic role in PDAC
Both IL-11 and IL-11R are expressed by pancreatic organoids and PSCs, with higher expression in cells isolated from the primary tumour, compared with those from metastatic sites [36]. Moreover, it is the PSCs, especially those with an iCAF phenotype, rather than the tumour organoids, which are the prominent producers of IL-11 [36]. Recombinant IL-11 stimulation has been shown to induce the activation of STAT3 in pancreatic cancer cell lines, with neutralising antibodies able to block this effect [36]. Furthermore, it was shown that oncogenic KRAS was able to up-regulate IL11 expression, with inhibition of STAT3 preventing this effect [200], suggesting that STAT3 is involved in mutant KRAS-mediated expression of IL-11. Knockdown of IL-11 in pancreatic cancer cell lines resulted in reduced expression of MMP2 and MMP9 and a 50% reduction in invasive capacity [200]. These results suggest that IL-11 may have a pro-tumorigenic effect in pancreatic cancer, similar to its roles in colorectal [201], gastric [202], and breast [203] cancers.
LIF in PDAC
LIF may be associated with poor prognosis in human disease
Elevated LIF levels have been detected in the serum of PDAC patients, compared with healthy patients [170]. Increased serum levels of LIF have been shown to correlate with poor therapeutic response and reduced disease-free and overall survival [170], although this is not reflected in data available in the TCGA (Figure 3E). The correlation between serum LIF concentration was determined to be a superior indicator of response compared to the FDA-approved marker CA19-9 [170]. LIF overexpression is specifically observed in PDAC tumours, compared with adjacent normal tissue, healthy patients, or patients with chronic pancreatitis or benign pancreatic tumours, who exhibit normal low level expression [170,204,205]. This specific increase in LIF expression was correlated with tumour differentiation status and is thought to contribute to PDAC pathogenesis.
LIF overexpression promotes STAT3 activation, cell growth, and proliferation
LIF overexpression has been reported in a number of pancreatic cancer cell lines and organoids [36], with stimulation by a range of cytokines, such as TNF, IL-1β, IL-6, or LIF itself, leading to further increased expression of LIF [206]. The most potent producers of LIF are PSCs, particularly those with an iCAF phenotype, but macrophages and mast cells also secrete the cytokine [36,170,204,206]. It has been suggested that increased LIF expression may be driven by overactive KRAS, which is present in the vast majority of human PDAC tumours [12,207]. Furthermore, PCCs express high levels of LIFR [36], as well as gp130, with LIF stimulation resulting in further upregulation of LIFR, suggesting that these cells may be the target of TME-derived LIF [170,206]. LIF stimulation resulted in increased activation of STAT3, but not of other downstream molecules ERK or AKT, and knockdown of LIFR or treatment with LIF-neutralising antibodies reduced STAT3 activation in pancreatic cancer cell lines [36,170]. In vitro, LIF stimulation promotes cell growth and proliferation, potentially via up-regulation of CXCL-8 [206,208], among other pathways, with increased expression of CXCL-8 in the serum and tumours of PDAC patients correlating with increased tumour growth [209].
Anti-LIF treatment inhibits tumorigenesis and increases overall survival in murine models
Mouse models of PDAC mirror the overexpression of LIF observed in patients, with elevated levels detected in serum at 3 weeks of age, and in pancreas tissue at 5 weeks of age, concurrent with development of early-stage PanIN lesions, suggesting that increased expression of LIF correlates with increased disease progression [170,204]. Inhibition of LIF signalling, by genetic LIFR deletion, LIF knockdown, or treatment with LIF neutralising monoclonal antibodies, results in reduced tumour progression in PDAC mouse models and decreased tumour engraftment and growth in pancreatic cancer cell line xenograft models [170,207]. In combination with chemotherapy, LIF inhibition increased overall survival and prolonged response to therapy in PDAC mouse models, KRASG12D-driven syngeneic xenograft and patient-derived xenograft models [170,207]. Even in the context of chemotherapy pre-treated PDAC mice, combination treatment with gemcitabine plus anti-LIF significantly improved survival [170]. This survival advantage was proposed to be due to reduced EMT, increased cancer cell differentiation, and increased apoptosis, resulting in less aggressive disease that was more amenable to treatment [170]. Anti-LIF treatment of PDAC mice also demonstrated that LIF is involved in a process termed neural remodelling, which drives invasion into pancreatic nerves and enhances metastatic processes [204]. These data implicate LIF in PDAC pathogenesis, with particular roles in enhancing disease progression and therapeutic resistance.
Conversely, the down-regulation of LIFR was observed in approximately 70% of human tumours, with this decreased expression correlated with increased invasion and metastasis [210]. This finding was validated in pancreatic cancer cell lines and xenograft models, with the mechanism being linked to reduced expression of genes that drive EMT [210]. This disparity in the effects of increased LIF and LIFR expression highlight the need to gain a deeper understanding of the contribution of LIF signalling to PDAC pathogenesis.
OSM in PDAC
OSM is associated with poor prognosis in human disease
Elevated levels of OSM have been detected in the serum of untreated PDAC patients, as well as those treated with gemcitabine and erlotinib combination therapy, compared with healthy controls [211]. OSM and OSMR are overexpressed in human PDAC tumours compared with normal pancreas [212–215], with OSM expression correlating with increased expression of STAT3 target genes [216], but not with overall survival (Figure 3F).
Upon comparison of PDAC tumour and adjacent normal tissue, 22 pathways were found to be up-regulated, 4 of which involved OSM signalling via both the JAK/STAT and MAPK pathways [217]. While regulation of OSMR has not been thoroughly studied, 20% of human PDAC tumours exhibited OSMR methylation, a mechanism reported in melanoma cells to downregulate OSMR to evade OSM-mediated growth control [218].
OSM contributes to EMT of PDAC cells
A range of in vitro studies have highlighted that OSM is able to promote EMT, drive stem-like phenotypes, and inhibit apoptosis in PDAC [216,219–221]. For example, stimulation of human PDAC cell lines with OSM alone, or in combination with transforming growth factor β (TGFβ) or hepatocyte growth factor (HGF), promoted EMT, defined by up-regulation of vimentin and down-regulation of E-cadherin [216,221]. OSM and HGF combination treatment was found to modulate mRNA expression of 11 EMT-related genes, with three of these being solely OSM-driven [221]. In particular, expression of key EMT-related transcription factors, ZEB1 and SNAI1 (Snail), were increased following OSM stimulation, with associated JAK/STAT3 and STAT3/TGFβ/SMAD3 activation, respectively [216,220]. Furthermore, OSM stimulation promoted expression of OSMR, thus providing a positive feedback loop [216]. OSM-mediated modulation of EMT factors and associated morphological changes were partially blocked by MEK inhibition, and completely blocked by JAK and PI3K inhibition, with OSM/HGF-mediated changes being partially blocked by each inhibitor [221]. Similar observations have been made in 3D culture systems, indicating that multiple downstream pathways are involved in OSM-driven EMT [221].
OSM, but not IL-6, has been shown to drive conversion of human PDAC cell lines to a stem-like phenotype (defined as CD24low/CD44high) [222], and to suppress their re-differentiation [216,220]. This conversion to cancer stem cells (CSCs) was associated with EMT, whereby changes in ZEB1, Snail and E-cadherin expression correlated with the up-regulation of CD44 and down-regulation of CD24 [216]. This process was proposed to involve JAK activation and the EMT-promoting STAT3/TGFβ/SMAD3-mediated signalling pathway, as inhibition of JAK1/2 or SMAD3 was able to prevent CD44 expression and acquisition of CSC properties [216,220].
OSM stimulation of PCCs in vitro has also been shown to result in the emergence of a distinct cell population with high tumorigenic and metastatic potential [216]. Generation of OSM-overexpressing PDAC cell lines revealed that these cells had increased migratory potential and decreased sensitivity to gemcitabine, although proliferative capacity was not affected [216]. Another study demonstrated that radiotherapy-resistant PDAC cells overexpress OSM, which correlated with inhibition of apoptosis, enhanced DNA repair, and overexpression of ZEB1 [219]. These data suggest that OSM may contribute to increased tumorigenic potential and therapeutic resistance in PDAC.
OSM drives an aggressive PDAC phenotype in murine models
Orthotopic xenograft mouse models with WT or OSM-overexpressing PDAC cells have shown that OSM promotes a highly aggressive phenotype, with tumours 5–10 times larger, and with increased intraperitoneal metastases compared to their WT counterparts [216]. Interestingly, co-injection of WT PDAC cells with either human WT fibroblasts or OSM-overexpressing fibroblasts revealed that the latter was able to drive aggressive disease with increased tumour burden and metastasis, compared to WT [216]. These results suggest that an OSM-rich TME promotes growth, invasion, and metastasis, with TAMs colocalising with OSM in liver metastases, suggesting that they may be involved in endogenous OSM production [223]. In contrast, treatment with OSM-expressing viruses was explored using an orthotopic xenograft hamster model, in which localised expression of OSM within the TME significantly reduced tumour burden and increased survival [224]. These contradicting results emphasise the dual processes seemingly at play downstream of OSM and highlight the need to gain a refined understanding of its role during each stage of PDAC pathogenesis.
Other IL-6 family members in PDAC
CNTF
There is limited data surrounding the role of CNTF in PDAC, however publicly available RNA-seq data shows that it is expressed in the normal pancreas, with increased expression correlating with reduced overall survival in PDAC patients (Figure 3G).
CT-1
PDAC cell lines express relatively low levels of CTF1/CT-1 compared with colorectal cancer cells [225]. As such, whilst therapeutic neutralisation or genetic KO of CT-1 in mice prevented the hepatic engraftment of injected colorectal cancer cells, engraftment rates of PDAC cells were not affected [225]. Furthermore, expression levels of CT-1 in PDAC tumours do not correlate with overall survival in the TCGA dataset (Figure 3H).
CLC
Despite CLC having potential tumorigenic properties, particularly in KRAS mutant cancers [226], expression levels of CLC in PDAC tumours do not correlate with overall survival (Figure 3I), suggesting that it may not play a role in this malignancy.
IL-27
Both pro-and anti-tumorigenic roles have been suggested for IL-27, depending on the cancer type examined [227,228]. In PDAC, elevated levels of IL-27 correlate with poor overall survival (Figure 3J); however, it has been shown that IL-27 overexpression in PDAC cell line xenograft models exhibited reduced tumour growth, due to increased cell cycle arrest and apoptosis, and increased overall survival [229,230]. It was also shown by in vitro co-culture that IL-27 inhibited the polarisation of TAMs towards an M2 phenotype, thereby reducing cancer cell proliferation, migration, and invasion, inducing cancer cell apoptosis, and improving therapeutic response to gemcitabine [231].
IL-31
Human PCCs have been shown to express low levels of IL-31 and high levels of IL-31R, relative to a panel of cells originating from other cancer types [232]. Although no publications or publicly available datasets address the expression levels or role of IL-31 signalling in PDAC, administration of IL-31-IgG was shown to reduce tumour growth and angiogenesis in a colorectal cancer xenograft mouse model, suggesting that IL-31 may have anti-tumorigenic effects [232].
JAK1/2 and STAT3 in PDAC
JAK1/2 and STAT3 are activated in PDAC and promote tumorigenesis
Overactivation of JAK1/2 and STAT3 is implicated in human and mouse models of PDAC, with particular links to increased tumour growth and progression [126,129,130,172,182,233–235]. This overactivation of the JAK/STAT3 pathway provides a positive feedback loop through the production of cytokines, other growth factors, and ECM components, the latter of which is a classic hallmark of PDAC [233,236].
Activation of JAK was shown to correlate with reduced overall survival compared with patients with moderate or low activation levels [237]. JAK2 was shown to be crucial to the progression of cancer cachexia in PDAC mice [238], a condition that develops in 80% of patients with late-stage disease and is a common cause of death [239].
pSTAT3 is elevated in human PDAC tissue, compared to adjacent normal tissue [240]. Elevation of pSTAT3 is a negative prognostic marker in PDAC [241], and has been implicated in driving the up-regulation of STAT3 target genes that promote EMT, invasion, angiogenesis, and immunosuppression, and are required for tumour initiation and progression [126–128,131,132,193,242,243]. In line with these observations, pancreas-specific deletion of STAT3 in PDAC mouse models revealed reduced inflammation, tumour initiation, progression, and invasive potential [126,172,244], exhibited by significant reduction in ADM, and loss of PanIN-2 and PanIN-3 lesions [172,244]. Moreover, activation of JAK2 and STAT3 has been shown to drive chemotherapy resistance in mouse models [234,245]. Thus, the downstream activation of the JAK/STAT3 signalling axis is thought to be a major contributing factor driving PDAC pathogenesis, and has been proposed as an attractive therapeutic target [246,247].
Do IL-6 family cytokines represent a new therapeutic opportunity?
Based on pre-clinical data, a range of IL-6 family cytokines, as well as their downstream mediators, have been demonstrated to contribute to pancreatic cancer pathogenesis (Figure 1). Conflicting results suggest that we do not yet understand the precise temporal and mechanistic details of these pathways. However, there remains an opportunity to utilise current experimental evidence to develop novel therapeutics to improve clinical outcomes for pancreatic cancer patients.
Therapeutic benefit of JAK1/2 or STAT3 inhibition may be context dependent
Inhibition of JAK2 reduced pancreatic cancer cell proliferation, adhesion, and invasion, and induced apoptosis in vitro [178,248–250]. Similarly, treatment of human and murine PSCs with the JAK1/2 inhibitor ruxolitinib reduced STAT3 phosphorylation, proliferation, and expression of PSC activation markers [251]. Treatment of PDAC xenografts with WP1066, a dual inhibitor of JAK2/STAT3, reduced tumour growth in vivo [249]. Tofacitinib, an FDA approved JAK inhibitor, reduced expression of chemokines and immune stimulatory molecules, which correlated with a decrease in recruitment of TAMs and TANs to the TME, compared with vehicle [252]. However, tofacitinib yielded decreased tumour growth and delayed relapse following treatment only in combination with immunotoxins or antibody-drug conjugates targeting mesothelin [252], a protein up-regulated in PDAC [253,254].
Inhibition of STAT3 using small molecules or RNAi reduced cancer cell proliferation, migration and invasion, and induced apoptosis in vitro [179,255–257], whilst reducing tumour growth and angiogenesis in vivo [256,258]. Liposomal delivery of a small molecule STAT3 inhibitor reduced STAT3 activation and cell growth, and sensitised cells to radiotherapy and chemotherapy in vitro and in vivo [240]. This was supported by another study where a JAK/STAT3 inhibitor, in combination with gemcitabine, significantly reduced tumour growth in both an orthotopic xenograft and genetic mouse model of pancreatic cancer [235]. Conversely, other studies have shown that inhibition of STAT3 results in up-regulation of anti-apoptotic genes in PDAC cell lines [259]. Furthermore, whilst STAT3 inhibition alone reduced PDAC xenograft tumour growth, when used in combination with anti-PD-1, the beneficial effects of STAT3 inhibition were negated [260]. These conflicting results may be explained by the differential expression of STAT3 at various disease stages, or the ability of STAT3 to drive distinct responses in different genetic backgrounds and at different stages of disease [243].
Clinical trials targeting JAK1/2 or STAT3 are ongoing
Patients with metastatic pancreatic cancer who progressed following gemcitabine treatment were administered capecitabine with or without ruxolitinib, a JAK1/2 inhibitor [261]. In this trial, treatment was generally well tolerated and ruxolitinib extended median overall survival by 0.2 months, with patients who displayed systemic inflammation having a 0.9 month survival benefit [261]. This improved response in a subset of patients with a more inflammatory phenotype suggests that inhibition may be more valuable in certain physiological contexts. However, two follow-up studies focusing on patients that had failed one line of chemotherapy and had systemic inflammation were terminated early due to a lack of treatment efficacy [262]. Upon analysing the available data, no further safety issues were raised, however there was no observed improvement in quality of life, progression-free survival, or overall survival [262]. Based on these results, a concurrent study investigating the efficacy of gemcitabine and ruxolitinib, with or without nab-paclitaxel, for patients with metastatic pancreatic cancer was terminated early [263].
Application of another JAK1/2 inhibitor, momelotinib, in combination with gemcitabine and nab-paclitaxel for patients with previously untreated metastatic pancreatic cancer demonstrated that the combination was well tolerated but exhibited no clinical benefit compared with gemcitabine/nab-paclitaxel dual treatment [264]. Ongoing studies trialling the combination of STAT3 inhibitor napabucasin with gemcitabine/nab-paclitaxel in patients with metastatic pancreatic cancer demonstrated that the treatment is well tolerated, though conclusive results regarding efficacy are pending [265,266].
Whilst the limited success of these JAK1/2 and STAT3 inhibitors seem to indicate that targeting this pathway may not yield improved patient outcomes, only late stage patients were enrolled. Moreover, while well tolerated, it was not reported whether the concentrations administered were sufficient to inhibit STAT3 activation in patient PDAC tumour cells, or in cells within the TME. Increased dosing may not have been possible, as it is common for these small molecule kinase inhibitors to lack specificity. For JAK inhibitors, off-target effects arise as many drugs are designed to block the highly conserved ATP-binding pocket. Ruxolitinib exhibits considerable off-target effects, with a study of 368 kinases, representing approximately 60% of the human kinome, identifying 33 kinases that were more than 50% inhibited, and 11 that were more than 80% inhibited by the drug [267]. Whilst momelotinib was not included in that study, the two drugs show similar phenotypic response profiles, with momelotinib demonstrating off-target inhibition of kinases such as JNK1 that are involved in alternative signalling pathways. Similarly, though napabucasin has been shown to target STAT3 [268], small molecule inhibitors of STAT3 often have poor potency and pharmacokinetic profiles [269]. Hence, despite therapies targeting either JAK1/2 or STAT3 so far failing to provide consistent clinical benefit, the extent to which this may be due to inappropriate dosing, failure of drug to reach the tumour site, or off-target effects remains unclear. Thus, inhibiting this pathway by another approach may be an alternative avenue that provides therapeutic efficacy.
Therapeutic potential of targeting IL-6 family cytokines
There are advantages of targeting extracellular cytokine–receptor interactions, including the ease of drug accessibility to its target, and the ability to specifically inhibit the cytokine pathway/s identified to be responsible for disease. This reduces the potential for off-target effects that are implicated when inhibiting downstream signalling molecules that are highly conserved between multiple signalling pathways.
Of all the IL-6 family members, only the most studied, IL-6, has clinical trials currently being performed to investigate whether inhibition of this pathway will be efficacious in pancreatic cancer. One clinical trial has been conducted using the anti-IL-6 monoclonal antibody, siltuximab, as a monotherapy, however results are pending (NCT00841191). Siltuximab monotherapy has exhibited favourable results in clinical trials for metastatic renal carcinoma [270] and relapsed and refractory multiple myeloma [271]. Another Phase II trial is currently recruiting, to investigate whether addition of the anti-IL-6R monoclonal antibody, tocilizumab, to dual first-line gemcitabine/nab-paclitaxel treatment is safe and efficacious for patients with unresectable locally advanced or metastatic pancreatic cancer (NCT02767557). As these studies are completed, we will gain further insight into the potential of inhibiting IL-6 in pancreatic cancer, though much work is required to increase our understanding of the contribution of other IL-6 family members to disease and their potential to improve clinical outcomes.
Concluding remarks
The devastating prognosis of those diagnosed with pancreatic cancer, combined with the predicted increases in incidence worldwide, reinforces the requirement to address this unmet clinical need for new therapeutics. The tumour microenvironment, particularly in the inflammatory and fibrotic context of pancreatic cancer, has emerged as a major contributor to pathogenesis, but also to evasion of anti-tumour immune responses and drug resistance. Although these broad roles have been outlined, we lack the details regarding the intricacy of the molecular mechanisms governing these tumorigenic pathways, including the crucial temporal and contextual features that determine cellular fate. In particular, the IL-6 family of cytokines have been demonstrated to influence pancreatic cancer pathogenesis, and thus may represent a previously unleveraged therapeutic opportunity. As such, if we are able to understand more deeply the intricacies of these cytokine signalling pathways, we may identify the precise context in which inhibition, or activation, of these pathways will enable improved clinical benefit for pancreatic cancer patients.
Acknowledgements
We thank Riley Metcalfe for creation of display elements incorporated into Figure 2 of this manuscript.
Abbreviations
- CNTF
ciliary neurotrophic factor
- CLC
cardiotrophin-like cytokine
- CSC
cancer stem cell
- CT-1
cardiotrophin-1
- IL-6
interleukin 6
- LIF
leukaemia inhibitory factor
- NP
neuropoietin
- OSM
oncostatin M
- PTP
phosphotyrosine phosphatase
Contributor Information
Michael D.W. Griffin, Email: griffin.m@unimelb.edu.au.
Tracy L. Putoczki, Email: putoczki.t@wehi.edu.au.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Health & Medical Research Council of Australia [grant number APP1098643]. M.D.W.G was the recipient of an Australian Research Council Future Fellowship (project number FT140100544). T.L.P was the recipient of a Victorian Cancer Agency Fellowship [grant number MCRF16009]; and a WEHI Dyson Bequest centenary Fellowship. Funding from the Victorian Government Operational Infrastructure Support Scheme is acknowledged.
Open Access
Open access for this article was enabled by the participation of Walter and Eliza Hall Institute in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
References
- 1.Siegel R.L., Miller K.D. and Jemal A. (2019) Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Australian Institute of, H. and Welfare. (2019) Cancer in Australia 2019. CAN 123 Cancer series No 119 [Google Scholar]
- 3.Rawla P., Sunkara T. and Gaduputi V. (2019) Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 10, 10–27 10.14740/wjon1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G. and McCain R.S. (2018) Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 24, 4846–4861 10.3748/wjg.v24.i43.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health, O (2020) WHO report on cancer: setting priorities, investing wisely and providing care for all, World Health Organization, Geneva [Google Scholar]
- 6.Ferlay J., Partensky C. and Bray F. (2016) More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 55, 1158–1160 10.1080/0284186X.2016.1197419 [DOI] [PubMed] [Google Scholar]
- 7.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M. and Matrisian L.M. (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 8.Quante A.S., Ming C., Rottmann M., Engel J., Boeck S., Heinemann V. et al. (2016) Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 5, 2649–2656 10.1002/cam4.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou B., Xu J.W., Cheng Y.G., Gao J.Y., Hu S.Y., Wang L. et al. (2017) Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 141, 231–241 10.1002/ijc.30670 [DOI] [PubMed] [Google Scholar]
- 10.Dragovich T. and Kundranda M. (2016) Nab-paclitaxel plus gemcitabine in the treatment of metastatic pancreatic cancer: utility and experience from the clinic. Gastrointestinal Cancer: Targets and Thera. 10.2147/GICTT.S8210328190957 [DOI] [Google Scholar]
- 11.Gudjonsson B. (1987) Cancer of the pancreas. 50 years of surgery. Cancer 60, 2284–2303 [DOI] [PubMed] [Google Scholar]
- 12.Ryan D.P., Hong T.S. and Bardeesy N. (2014) Pancreatic adenocarcinoma. N. Engl. J. Med. 371, 1039–1049 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 13.Li D., Xie K., Wolff R. and Abbruzzese J.L. (2004) Pancreatic cancer. Lancet 363, 1049–1057 10.1016/S0140-6736(04)15841-8 [DOI] [PubMed] [Google Scholar]
- 14.Van den Broeck A., Sergeant G., Ectors N., Van Steenbergen W., Aerts R. and Topal B. (2009) Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 35, 600–604 10.1016/j.ejso.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Schnelldorfer T., Ware A.L., Sarr M.G., Smyrk T.C., Zhang L., Qin R. et al. (2008) Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 247, 456–462 10.1097/SLA.0b013e3181613142 [DOI] [PubMed] [Google Scholar]
- 16.Haeberle L. and Esposito I. (2019) Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 4, 50–50 10.21037/tgh.2019.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iovanna J., Mallmann M., Goncalves A., Turrini O. and Dagorn J.-C. (2012) Current Knowledge on Pancreatic Cancer. Front. Oncol. 2, 10.3389/fonc.2012.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubilla A.L. and Fitzgerald P.J. (1976) Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 36, 2690–2698 [PubMed] [Google Scholar]
- 19.Kozuka S., Sassa R., Taki T., Masamoto K., Nagasawa S., Saga S. et al. (1979) Relation of pancreatic duct hyperplasia to carcinoma. Cancer 43, 1418–1428 [DOI] [PubMed] [Google Scholar]
- 20.Stanger B.Z. and Hebrok M. (2013) Control of cell identity in pancreas development and regeneration. Gastroenterology 144, 1170–1179 10.1053/j.gastro.2013.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De La O.J., Emerson L.L., Goodman J.L., Froebe S.C., Illum B.E., Curtis A.B. et al. (2008) Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. U.S.A. 105, 18907–18912 10.1073/pnas.0810111105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedlander S.Y.G., Chu G.C., Snyder E.L., Girnius N., Dibelius G., Crowley D. et al. (2009) Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16, 379–389 10.1016/j.ccr.2009.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habbe N., Shi G., Meguid R.A., Fendrich V., Esni F., Chen H. et al. (2008) Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. U.S.A. 105, 18913–18918 10.1073/pnas.0810097105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp J.L., von Figura G., Mayes E., Liu F.F., Dubois C.L., Morris J.P.T. et al. (2012) Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, 737–750 10.1016/j.ccr.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hruban R.H., Takaori K., Klimstra D.S., Adsay N.V., Albores-Saavedra J., Biankin A.V. et al. (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 28, 977–987 10.1097/01.pas.0000126675.59108.80 [DOI] [PubMed] [Google Scholar]
- 26.Ansari D., Carvajo M., Bauden M. and Andersson R. (2017) Pancreatic cancer stroma: controversies and current insights. Scandinavian J. Gastroenterol. 52, 641–646 10.1080/00365521.2017.1293726 [DOI] [PubMed] [Google Scholar]
- 27.Neesse A., Michl P., Frese K.K., Feig C., Cook N., Jacobetz M.A. et al. (2011) Stromal biology and therapy in pancreatic cancer. Gut 60, 861 10.1136/gut.2010.226092 [DOI] [PubMed] [Google Scholar]
- 28.Erkan M., Hausmann S., Michalski C.W., Fingerle A.A., Dobritz M., Kleeff J. et al. (2012) The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. 9, 454–467 [DOI] [PubMed] [Google Scholar]
- 29.Pandol S., Edderkaoui M., Gukovsky I., Lugea A. and Gukovskaya A. (2009) Desmoplasia of pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 7, S44–S47 10.1016/j.cgh.2009.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padoan A., Plebani M. and Basso D. (2019) Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 20, 676–696 10.3390/ijms20030676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachem M.G., Schneider E., Gross H., Weidenbach H., Schmid R.M., Menke A. et al. (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115, 421–432 10.1016/S0016-5085(98)70209-4 [DOI] [PubMed] [Google Scholar]
- 32.Apte M.V., Haber P.S., Darby S.J., Rodgers S.C., McCaughan G.W., Korsten M.A. et al. (1999) Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44, 534–541 10.1136/gut.44.4.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masamune A., Watanabe T., Kikuta K. and Shimosegawa T. (2009) Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin. Gastroenterol. Hepatol. 7, S48–S54 10.1016/j.cgh.2009.07.038 [DOI] [PubMed] [Google Scholar]
- 34.Jacobetz M.A., Chan D.S., Neesse A., Bapiro T.E., Cook N., Frese K.K. et al. (2013) Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 10.1136/gutjnl-2012-302529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., Von Hoff D.D. and Hingorani S.R. (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M. et al. (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apte M., Pirola R.C. and Wilson J.S. (2015) Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr. Opin. Gastroenterol. 31, 416–423 [DOI] [PubMed] [Google Scholar]
- 38.Hwang R.F., Moore T., Arumugam T., Ramachandran V., Amos K.D., Rivera A. et al. (2008) Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vonlaufen A., Joshi S., Qu C., Phillips P.A., Xu Z., Parker N.R. et al. (2008) Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 68, 2085–2093 10.1158/0008-5472.CAN-07-2477 [DOI] [PubMed] [Google Scholar]
- 40.Xu Z., Vonlaufen A., Phillips P.A., Fiala-Beer E., Zhang X., Yang L. et al. (2010) Role of pancreatic stellate cells in pancreatic cancer metastasis. Am. J. Pathol. 177, 2585–2596 10.2353/ajpath.2010.090899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachem M.G., Schünemann M., Ramadani M., Siech M., Beger H., Buck A. et al. (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128, 907–921 10.1053/j.gastro.2004.12.036 [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., Wu H., Guan J., Wang L., Ren X., Shi X. et al. (2015) Paracrine SDF-1α signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget 6, 3085–3097 10.18632/oncotarget.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A. et al. (2019) Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 9, 1102–1123 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foucher E.D., Ghigo C., Chouaib S., Galon J., Iovanna J. and Olive D. (2018) Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front. Immunol, 10.3389/fimmu.2018.01044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mace T.A., Ameen Z., Collins A., Wojcik S., Mair M., Young G.S. et al. (2013) Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 73, 3007–3018 10.1158/0008-5472.CAN-12-4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Yan W., Collins M.A., Bednar F., Rakshit S., Zetter B.R. et al. (2013) Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 73, 6359–6374 10.1158/0008-5472.CAN-13-1558-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H.-Y., Greene T., Lin T.-Y., Dawes C.S., Korc M. and Lin C.-C. (2017) Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta Biomater. 48, 258–269 10.1016/j.actbio.2016.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moir J.A., Mann J. and White S.A. (2015) The role of pancreatic stellate cells in pancreatic cancer. Surg. Oncol. 24, 232–238 10.1016/j.suronc.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 49.Greer J.B. and Whitcomb D.C. (2009) Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol. 9, 411–418 10.1016/j.coph.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 50.Dougan S.K. (2017) The Pancreatic Cancer Microenvironment. Cancer J. 23, 321–325 10.1097/PPO.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 51.Liang C., Shi S., Meng Q., Liang D., Ji S., Zhang B. et al. (2017) Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp. Mol. Med. 49, e406–e406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerra C., Collado M., Navas C., Schuhmacher A.J., Hernandez-Porras I., Canamero M. et al. (2011) Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19, 728–739 10.1016/j.ccr.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neesse A., Algul H., Tuveson D.A. and Gress T.M. (2015) Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484 10.1136/gutjnl-2015-309304 [DOI] [PubMed] [Google Scholar]
- 54.Javle M., Golan T. and Maitra A. (2016) Changing the course of pancreatic cancer -Focus on recent translational advances. Cancer Treatment Rev. 44, 17–25 10.1016/j.ctrv.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 55.Erkan M. (2013) Understanding the stroma of pancreatic cancer: co-evolution of the microenvironment with epithelial carcinogenesis. J. Pathol. 231, 4–7 10.1002/path.4213 [DOI] [PubMed] [Google Scholar]
- 56.Thomas D. and Radhakrishnan P. (2019) Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 18, 14–14 10.1186/s12943-018-0927-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moskaluk C.A., Hruban R.H. and Kern S.E. (1997) p16 and K-ras Gene Mutations in the Intraductal Precursors of Human Pancreatic Adenocarcinoma. Cancer Res. 57, 2140. [PubMed] [Google Scholar]
- 58.Witkiewicz A.K., McMillan E.A., Balaji U., Baek G., Lin W.-C., Mansour J. et al. (2015) Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6, 6744–6744 10.1038/ncomms7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waddell N., Pajic M., Patch A.-M., Chang D.K., Kassahn K.S., Bailey P. et al. (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raphael B.J., Hruban R.H., Aguirre A.J., Moffitt R.A., Yeh J.J., Stewart C. et al. (2017) Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32, 185e113–203.e113 10.1016/j.ccell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A. et al. (2003) Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 10.1016/S1535-6108(03)00309-X [DOI] [PubMed] [Google Scholar]
- 62.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H. et al. (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 63.Yachida S., White C.M., Naito Y., Zhong Y., Brosnan J.A., Macgregor-Das A.M. et al. (2012) Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin. Cancer Res. 18, 6339–6347 10.1158/1078-0432.CCR-12-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lomberk G., Blum Y., Nicolle R., Nair A., Gaonkar K.S., Marisa L. et al. (2018) Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat. Commun. 9, 1978 10.1038/s41467-018-04383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffitt R.A., Marayati R., Flate E.L., Volmar K.E., Loeza S.G., Hoadley K.A. et al. (2015) Virtual microdissection identifies distinct tumor-and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 10.1038/ng.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyabayashi K., Baker L.A., Deschenes A., Traub B., Caligiuri G., Plenker D. et al. (2020) Intraductal transplantation models of human pancreatic ductal adenocarcinoma reveal progressive transition of molecular subtypes. Cancer Discov. 10.1158/2159-8290.CD-20-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.-M., Gingras M.-C. et al. (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47M–52M 10.1038/nature16965 [DOI] [PubMed] [Google Scholar]
- 68.Collisson E.A., Sadanandam A., Olson P., Gibb W.J., Truitt M., Gu S. et al. (2011) Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 10.1038/nm.2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebrahimi B., Tucker S.L., Li D., Abbruzzese J.L. and Kurzrock R. (2004) Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 101, 2727–2736 10.1002/cncr.20672 [DOI] [PubMed] [Google Scholar]
- 70.Latenstein A.E.J., van Roessel S., van der Geest L.G.M., Bonsing B.A., Dejong C.H.C., Groot Koerkamp B. et al. (2020) Conditional Survival After Resection for Pancreatic Cancer: A Population-Based Study and Prediction Model. Ann. Surg. Oncol. 27, 2516–2524 10.1245/s10434-020-08235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chawla A. and Ferrone C.R. (2019) Neoadjuvant Therapy for Resectable Pancreatic Cancer: An Evolving Paradigm Shift. Front. Oncol., 10.3389/fonc.2019.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yegya-Raman N., Shah M.M., Grandhi M.S., Poplin E., August D.A., Kennedy T.J. et al. (2018) Adjuvant therapeutic strategies for resectable pancreatic adenocarcinoma. Ann Pancreat Cancer 1, 20 10.21037/apc.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L. et al. (2018) FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 379, 2395–2406 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 74.Venkatesulu B.P., Hsieh C.-E., Sanders K.L. and Krishnan S. (2018) Recent advances in radiation therapy of pancreatic cancer. F1000Res. , F1000 Faculty Rev-1931 10.12688/f1000research.16272.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roeder F. (2016) Neoadjuvant radiotherapeutic strategies in pancreatic cancer. World J. Gastrointest. Oncol. 8, 186–197 10.4251/wjgo.v8.i2.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall W.A. and Goodman K.A. (2019) Radiation therapy for pancreatic adenocarcinoma, a treatment option that must be considered in the management of a devastating malignancy. Radiat. Oncol. 14, 114 10.1186/s13014-019-1277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vuong B., Dehal A., Graff-Baker A.N., Chang S.-C., Foshag L., Bilchik A. et al. (2017) Effect of palliative surgery, chemotherapy, and radiation in stage IV pancreatic cancer. J. Clin. Oncol. 35, e15707–e15707 10.1200/JCO.2017.35.15_suppl.e15707 [DOI] [Google Scholar]
- 78.Rudra S., Jiang N., Rosenberg S.A., Olsen J.R., Parikh P.J., Bassetti M.F. et al. (2017) High Dose Adaptive MRI Guided Radiation Therapy Improves Overall Survival of Inoperable Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. hysics 99, E184 10.1016/j.ijrobp.2017.06.1042 [DOI] [Google Scholar]
- 79.Burris H.A. III, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R. et al. (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 80.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y. et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 81.Von Hoff D.D., Ervin T.J., Arena F.P., Chiorean E.G., Infante J.R., Moore M.J. et al. (2013) Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT). J. Clin. Oncol. 31, LBA148–LBA148 10.1200/jco.2013.31.4_suppl.lba148 [DOI] [Google Scholar]
- 82.Dauer P., Nomura A., Saluja A. and Banerjee S. (2017) Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology 17, 7–12 10.1016/j.pan.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delitto D., Black B.S., Sorenson H.L., Knowlton A.E., Thomas R.M., Sarosi G.A. et al. (2015) The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. Bmc Cancer [Electronic Resource] 15, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swayden M., Iovanna J. and Soubeyran P. (2018) Pancreatic cancer chemo-resistance is driven by tumor phenotype rather than tumor genotype. Heliyon 4, e01055–e01055 10.1016/j.heliyon.2018.e01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chin V., Nagrial A., Sjoquist K., O'Connor C.A., Chantrill L., Biankin A.V. et al. (2018) Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 3, Cd011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S. et al. (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 87.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S. et al. (2010) Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33, 828–833 10.1097/CJI.0b013e3181eec14c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P. et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dunlop R.J. and Campbell C.W. (2000) Cytokines and Advanced Cancer. J. Pain Symptom Manag. 20, 214–232 10.1016/S0885-3924(00)00199-8 [DOI] [PubMed] [Google Scholar]
- 90.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C. and Hermoso M.A. (2014) Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 149185 10.1155/2014/149185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oppenheim J. and Fujiwara H. (1996) The role of cytokines in cancer. Cytokine Growth Factor Rev. 7, 279–288 [DOI] [PubMed] [Google Scholar]
- 92.David Roodman G. (2003) Role of stromal-derived cytokines and growth factors in bone metastasis. Cancer 97, 733–738 10.1002/cncr.11148 [DOI] [PubMed] [Google Scholar]
- 93.Farrow B., Albo D. and Berger D.H. (2008) The role of the tumor microenvironment in the progression of pancreatic cancer. J. Surg. Res. 149, 319–328 10.1016/j.jss.2007.12.757 [DOI] [PubMed] [Google Scholar]
- 94.Vonderheide R.H. and Bayne L.J. (2013) Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr. Opin. Immunol. 25, 200–205 10.1016/j.coi.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nielsen M.F., Mortensen M.B. and Detlefsen S. (2016) Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J. Gastroenterol. 22, 2678–2700 10.3748/wjg.v22.i9.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pietras K. and Ostman A. (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 316, 1324–1331 10.1016/j.yexcr.2010.02.045 [DOI] [PubMed] [Google Scholar]
- 97.West N.R. (2019) Coordination of Immune-Stroma Crosstalk by IL-6 Family Cytokines. Front. Immunol. 10, 1093 10.3389/fimmu.2019.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang D.Z., Ma Y., Ji B., Wang H., Deng D., Liu Y. et al. (2011) Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 17, 7015–7023 10.1158/1078-0432.CCR-11-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Setrerrahmane S. and Xu H. (2017) Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol. Cancer 16, 153 10.1186/s12943-017-0721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morris R., Kershaw N.J. and Babon J.J. (2018) The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 27, 1984–2009 10.1002/pro.3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rose-John S. (2018) Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol., 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huising M.O., Kruiswijk C.P. and Flik G. (2006) Phylogeny and evolution of class-I helical cytokines. J. Endocrinol. 189, 1 10.1677/joe.1.06591 [DOI] [PubMed] [Google Scholar]
- 103.Murakami M., Kamimura D. and Hirano T. (2004) New IL-6 (gp130) family cytokine members, CLC/NNT1/BSF3 and IL-27. Growth Factors (Chur, Switzerland). 22, 75–77 10.1080/08977190410001715181 [DOI] [PubMed] [Google Scholar]
- 104.Derouet D., Rousseau F., Alfonsi F., Froger J., Hermann J., Barbier F. et al. (2004) Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proceed. Nat. Acad. Sci. United States Am. 101, 4827 10.1073/pnas.0306178101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Lupardus P., Laporte S.L. and Garcia K.C. (2009) Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60 10.1146/annurev.immunol.24.021605.090616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heinrich P.C., Behrmann I., Müller-Newen G., Schaper F. and Graeve L. (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–314 10.1042/bj3340297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dahmen H., Horsten U., Küster A., Jacques Y., Minvielle S., Kerr I.M. et al. (1998) Activation of the signal transducer gp130 by interleukin-11 and interleukin-6 is mediated by similar molecular interactions. Biochemical J. 331, 695–702 10.1042/bj3310695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matadeen R., Hon W.-C., Heath J.K., Jones E.Y. and Fuller S. (2007) The dynamics of signal triggering in a gp130-receptor complex. Structure (London, England) 15, 441–448 10.1016/j.str.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barton V.A., Hall M.A., Hudson K.R. and Heath J.K. (2000) Interleukin-11 signals through the formation of a hexameric receptor complex. J. Biol. Chem. 275, 36197–36203 10.1074/jbc.M004648200 [DOI] [PubMed] [Google Scholar]
- 110.Rose-John S. and Heinrich P.C. (1994) Soluble receptors for cytokines and growth factors: generation and biological function. Biochemical J. 300, 281–290 10.1042/bj3000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pflanz S., Tacken I., Grötzinger J., Jacques Y., Dahmen H., Heinrich P.C. et al. (1999) A fusion protein of interleukin-11 and soluble interleukin-11 receptor acts as a superagonist on cells expressing gp130. FEBS Lett. 450, 117–122 10.1016/S0014-5793(99)00477-9 [DOI] [PubMed] [Google Scholar]
- 112.Frieling J.T., Sauerwein R.W., Wijdenes J., Hendriks T. and van der Linden C.J. (1994) Soluble interleukin 6 receptor in biological fluids from human origin. Cytokine 6, 376–381 10.1016/1043-4666(94)90061-2 [DOI] [PubMed] [Google Scholar]
- 113.Lokau J., Nitz R., Agthe M., Monhasery N., Aparicio-Siegmund S., Schumacher N. et al. (2016) Proteolytic cleavage governs interleukin-11 trans-signaling. Cell Rep. 14, 1761–1773 10.1016/j.celrep.2016.01.053 [DOI] [PubMed] [Google Scholar]
- 114.Montero-Julian F.A. (2001) The soluble IL-6 receptors: serum levels and biological function. Cell. Mol. Biol. (Noisy-le-grand) 47, 583–597 [PubMed] [Google Scholar]
- 115.Cornelissen C., Luscher-Firzlaff J., Baron J.M. and Luscher B. (2012) Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 91, 552–566 10.1016/j.ejcb.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q., Putheti P., Zhou Q., Liu Q. and Gao W. (2008) Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 19, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J.F. et al. (2004) WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 10.4049/jimmunol.172.4.2225 [DOI] [PubMed] [Google Scholar]
- 118.Jones S.A., Scheller J. and Rose-John S. (2011) Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J. Clin. Invest. 121, 3375–3383 10.1172/JCI57158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng J., Witthuhn B.A., Matsuda T., Kohlhuber F., Kerr I.M. and Ihle J.N. (1997) Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17, 2497–2501 10.1128/MCB.17.5.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodig S.J., Meraz M.A., White J.M., Lampe P.A., Riley J.K., Arthur C.D. et al. (1998) Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93, 373–383 10.1016/S0092-8674(00)81166-6 [DOI] [PubMed] [Google Scholar]
- 121.Schmitz J., Dahmen H., Grimm C., Gendo C., Muller-Newen G., Heinrich P.C. et al. (2000) The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J. Immunol. 164, 848–854 10.4049/jimmunol.164.2.848 [DOI] [PubMed] [Google Scholar]
- 122.Heinrich P.C., Behrmann I., Haan S., Hermanns H.M., Muller-Newen G. and Schaper F. (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 10.1042/bj20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Putoczki T.L. and Ernst M. (2015) IL-11 signaling as a therapeutic target for cancer. Immunotherapy 7, 441–453 10.2217/imt.15.17 [DOI] [PubMed] [Google Scholar]
- 124.Ernst M. and Putoczki T.L. (2014) Molecular pathways: IL11 as a tumor-promoting cytokine-translational implications for cancers. Clin. Cancer Res. 20, 5579–5588 10.1158/1078-0432.CCR-13-2492 [DOI] [PubMed] [Google Scholar]
- 125.Carpenter R.L. and Lo H.-W. (2014) STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel) 6, 897–925 10.3390/cancers6020897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fukuda A., Wang S.C., Morris J.P.T., Folias A.E., Liou A., Kim G.E. et al. (2011) Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 19, 441–455 10.1016/j.ccr.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li B. and Huang C. (2017) Regulation of EMT by STAT3 in gastrointestinal cancer (Review). Int. J. Oncol. 50, 753–767 10.3892/ijo.2017.3846 [DOI] [PubMed] [Google Scholar]
- 128.Saitoh M., Endo K., Furuya S., Minami M., Fukasawa A., Imamura T. et al. (2016) STAT3 integrates cooperative Ras and TGF-beta signals that induce Snail expression. Oncogene 35, 1049–1057 10.1038/onc.2015.161 [DOI] [PubMed] [Google Scholar]
- 129.Scholz A., Heinze S., Detjen K.M., Peters M., Welzel M., Hauff P. et al. (2003) Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 125, 891–905 10.1016/S0016-5085(03)01064-3 [DOI] [PubMed] [Google Scholar]
- 130.Sharma N.K., Shankar S. and Srivastava R.K. (2014) STAT3 as an emerging molecular target in pancreatic cancer. Gastrointest. Cancer: Targets Ther. 2014, 115–122 [Google Scholar]
- 131.Wei D., Le X., Zheng L., Wang L., Frey J.A., Gao A.C. et al. (2003) Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22, 319–329 10.1038/sj.onc.1206122 [DOI] [PubMed] [Google Scholar]
- 132.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W. et al. (2016) FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3–CCL2 Signaling. Cancer Res. 76, 4124 10.1158/0008-5472.CAN-15-2973 [DOI] [PubMed] [Google Scholar]
- 133.Eulenfeld R., Dittrich A., Khouri C., Müller P.J., Mütze B., Wolf A. et al. (2012) Interleukin-6 signalling: More than Jaks and STATs. European J. Cell Biol. 91, 486–495 10.1016/j.ejcb.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 134.Lehmann U., Schmitz J., Weissenbach M., Sobota R.M., Hortner M., Friederichs K. et al. (2003) SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. J. Biol. Chem. 278, 661–671 10.1074/jbc.M210552200 [DOI] [PubMed] [Google Scholar]
- 135.Fukada T., Hibi M., Yamanaka Y., Takahashi-Tezuka M., Fujitani Y., Yamaguchi T. et al. (1996) Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5, 449–460 10.1016/S1074-7613(00)80501-4 [DOI] [PubMed] [Google Scholar]
- 136.Nicholson S.E., De Souza D., Fabri L.J., Corbin J., Willson T.A., Zhang J.-G. et al. (2000) Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proceed. Nat. Acad. Sci. 97, 6493 10.1073/pnas.100135197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmitz J., Weissenbach M., Haan S., Heinrich P.C. and Schaper F. (2000) SOCS3 exerts its inhibitory function on interleukin-6 signal transduction through the SHP2 recruitment site of gp130. J. Biol. Chem. 275, 12848–12856 10.1074/jbc.275.17.12848 [DOI] [PubMed] [Google Scholar]
- 138.Babon J.J., Sabo J.K., Soetopo A., Yao S., Bailey M.F., Zhang J.G. et al. (2008) The SOCS box domain of SOCS3: structure and interaction with the elonginBC-cullin5 ubiquitin ligase. J. Mol. Biol. 381, 928–940 10.1016/j.jmb.2008.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Babon J.J., Sabo J.K., Zhang J.G., Nicola N.A. and Norton R.S. (2009) The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. J. Mol. Biol. 387, 162–174 10.1016/j.jmb.2009.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamizono S., Hanada T., Yasukawa H., Minoguchi S., Kato R., Minoguchi M. et al. (2001) The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J. Biol. Chem. 276, 12530–12538 10.1074/jbc.M010074200 [DOI] [PubMed] [Google Scholar]
- 141.Kershaw N.J., Murphy J.M., Liau N.P., Varghese L.N., Laktyushin A., Whitlock E.L. et al. (2013) SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat. Struct. Mol. Biol. 20, 469–476 10.1038/nsmb.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang J.G., Farley A., Nicholson S.E., Willson T.A., Zugaro L.M., Simpson R.J. et al. (1999) The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. U.S.A. 96, 2071–2076 10.1073/pnas.96.5.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang J.G., Metcalf D., Rakar S., Asimakis M., Greenhalgh C.J., Willson T.A. et al. (2001) The SOCS box of suppressor of cytokine signaling-1 is important for inhibition of cytokine action in vivo. Proc. Natl. Acad. Sci. U.S.A. 98, 13261–13265 10.1073/pnas.231486498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kershaw N.J., Laktyushin A., Nicola N.A. and Babon J.J. (2014) Reconstruction of an active SOCS3-based E3 ubiquitin ligase complex in vitro: identification of the active components and JAK2 and gp130 as substrates. Growth Factors (Chur, Switzerland). 32, 1–10 10.3109/08977194.2013.877005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Babon J.J., Kershaw N.J., Murphy J.M., Varghese L.N., Laktyushin A., Young S.N. et al. (2012) Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity 36, 239–250 10.1016/j.immuni.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sasaki A., Yasukawa H., Suzuki A., Kamizono S., Syoda T., Kinjyo I. et al. (1999) Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4, 339–351 10.1046/j.1365-2443.1999.00263.x [DOI] [PubMed] [Google Scholar]
- 147.White C.A. and Nicola N.A. (2013) SOCS3: An essential physiological inhibitor of signaling by interleukin-6 and G-CSF family cytokines. JAKSTAT 2, e25045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yasukawa H., Misawa H., Sakamoto H., Masuhara M., Sasaki A., Wakioka T. et al. (1999) The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18, 1309–1320 10.1093/emboj/18.5.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anhuf D., Weissenbach M., Schmitz J., Sobota R., Hermanns H.M., Radtke S. et al. (2000) Signal transduction of IL-6, leukemia-inhibitory factor, and oncostatin M: structural receptor requirements for signal attenuation. J. Immunol. 165, 2535–2543 10.4049/jimmunol.165.5.2535 [DOI] [PubMed] [Google Scholar]
- 150.Heppler L.N. and Frank D.A. (2017) Targeting Oncogenic Transcription Factors: Therapeutic Implications of Endogenous STAT Inhibitors. Trends Cancer 3, 816–827 10.1016/j.trecan.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu M., Song D., Li H., Yang Y., Ma X., Deng S. et al. (2019) Negative regulators of STAT3 signaling pathway in cancers. Cancer Manag. Res. 11, 4957–4969 10.2147/CMAR.S206175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Okada S., Okusaka T., Ishii H., Kyogoku A., Yoshimori M., Kajimura N. et al. (1998) Elevated Serum Interleukin-6 Levels in Patients with Pancreatic Cancer. Japanese J. Clin. Oncol. 28, 12–15 10.1093/jjco/28.1.12 [DOI] [PubMed] [Google Scholar]
- 153.Wigmore S.J., Fearon K.C., Sangster K., Maingay J.P., Garden O.J. and Ross J.A. (2002) Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int. J. Oncol. 21, 881–886 [DOI] [PubMed] [Google Scholar]
- 154.Błogowski W., Deskur A., Budkowska M., Sałata D., Madej-Michniewicz A., Dąbkowski K. et al. (2014) Selected cytokines in patients with pancreatic cancer: a preliminary report. PLoS ONE 9, e97613–e97613 10.1371/journal.pone.0097613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miron N., Miron M.M., Milea V.G. and Cristea V. (2010) Proinflammatory cytokines: an insight into pancreatic oncogenesis. Roum. Arch. Microbiol. Immunol. 69, 183–189 [PubMed] [Google Scholar]
- 156.Barber M.D., Fearon K.C. and Ross J.A. (1999) Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin. Sci. (Lond.) 96, 83–87 10.1042/cs0960083 [DOI] [PubMed] [Google Scholar]
- 157.Mroczko B., Groblewska M., Gryko M., Kedra B. and Szmitkowski M. (2010) Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J. Clin. Lab. Anal. 24, 256–261 10.1002/jcla.20395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Talar-Wojnarowska R., Gasiorowska A., Smolarz B., Romanowicz-Makowska H., Kulig A. and Malecka-Panas E. (2009) Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig. Dis. Sci. 54, 683–689 10.1007/s10620-008-0390-z [DOI] [PubMed] [Google Scholar]
- 159.Miura T., Mitsunaga S., Ikeda M., Shimizu S., Ohno I., Takahashi H. et al. (2015) Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas 44, 756–763 10.1097/MPA.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 160.Kim H.W., Lee J.-C., Paik K.-H., Kang J., Kim J. and Hwang J.-H. (2017) Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine 96, e5926 10.1097/MD.0000000000005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ramsey M.L., Talbert E., Ahn D., Bekaii-Saab T., Badi N., Bloomston P.M. et al. (2019) Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology 19, 80–87 10.1016/j.pan.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lukaszewicz M., Mroczko B. and Szmitkowski M. (2007) Clinical significance of interleukin-6 (IL-6) as a prognostic factor of cancer disease. Pol. Arch. Med. Wewn. 117, 247–251 [PubMed] [Google Scholar]
- 163.Chen I., Dehlendorff C., Jensen B.V., Pfeiffer P., Nielsen S.E., Holländer N.H. et al. (2016) Serum interleukin-6 as a prognostic biomarker for survival in patients with unresectable pancreatic cancer. Annals Oncol. 27, 10.1093/annonc/mdw363.34 [DOI] [Google Scholar]
- 164.Feng L., Qi Q., Wang P., Chen H., Chen Z., Meng Z. et al. (2018) Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J. Int. Med. Res. 46, 5228–5236 10.1177/0300060518800588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bellone G., Smirne C., Mauri F.A., Tonel E., Carbone A., Buffolino A. et al. (2006) Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol. Immunother. 55, 684–698 10.1007/s00262-005-0047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wenger F.A., Jacobi C.A., Zieren J., Docke W., Volk H.D. and Muller J.M. (1999) Tumor size and lymph-node status in pancreatic carcinoma -is there a correlation to the preoperative immune function? Langenbecks Arch. Surg. 384, 473–478 10.1007/s004230050233 [DOI] [PubMed] [Google Scholar]
- 167.Martignoni M.E., Kunze P., Hildebrandt W., Kunzli B., Berberat P., Giese T. et al. (2005) Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin. Cancer Res. 11, 5802–5808 10.1158/1078-0432.CCR-05-0185 [DOI] [PubMed] [Google Scholar]
- 168.Farren M.R., Mace T.A., Geyer S., Mikhail S., Wu C., Ciombor K. et al. (2016) Systemic Immune Activity Predicts Overall Survival in Treatment-Naïve Patients with Metastatic Pancreatic Cancer. Clin. Cancer Res.: An Official J. Am. Assoc. for Cancer Res. 22, 2565–2574 10.1158/1078-0432.CCR-15-1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xing H.B., Tong M.T., Wang J., Hu H., Zhai C.Y., Huang C.X. et al. (2018) Suppression of IL-6 Gene by shRNA Augments Gemcitabine Chemosensitization in Pancreatic Adenocarcinoma Cells. Biomed. Res. Int. 2018, 3195025 10.1155/2018/3195025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shi Y., Gao W., Lytle N.K., Huang P., Yuan X., Dann A.M. et al. (2019) Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135 10.1038/s41586-019-1130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagathihalli N.S., Castellanos J.A., VanSaun M.N., Dai X., Ambrose M., Guo Q. et al. (2016) Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget 7, 65982–65992 10.18632/oncotarget.11786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lesina M., Kurkowski M.U., Ludes K., Rose-John S., Treiber M., Kloppel G. et al. (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 10.1016/j.ccr.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 173.Feurino L.W., Zhang Y., Bharadwaj U., Zhang R., Li F., Fisher W.E. et al. (2007) IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer Biol. Ther. 6, 1096–1100 10.4161/cbt.6.7.4328 [DOI] [PubMed] [Google Scholar]
- 174.Goumas F.A., Holmer R., Egberts J.H., Gontarewicz A., Heneweer C., Geisen U. et al. (2015) Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer 137, 1035–1046 10.1002/ijc.29445 [DOI] [PubMed] [Google Scholar]
- 175.Hamada S., Masamune A., Yoshida N., Takikawa T. and Shimosegawa T. (2016) IL-6/STAT3 Plays a Regulatory Role in the Interaction Between Pancreatic Stellate Cells and Cancer Cells. Dig. Dis. Sci. 61, 1561–1571 10.1007/s10620-015-4001-5 [DOI] [PubMed] [Google Scholar]
- 176.Biffi G., Oni T.E., Spielman B., Hao Y., Elyada E., Park Y. et al. (2019) IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discover. 9, 282–301 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gregory L.B., Elena G.C., Matthew P.F., Babak S., Ursina R.T., Weijing S. et al. (2011) CD40 Agonists Alter Tumor Stroma and Show Efficacy Against Pancreatic Carcinoma in Mice and Humans. Science 331, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang C., Yang G., Jiang T., Huang K., Cao J. and Qiu Z. (2010) Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J. Exp. Clin. Cancer Res. 29, 51 10.1186/1756-9966-29-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu A., Liu Y., Li P.K., Li C. and Lin J. (2011) LLL12 inhibits endogenous and exogenous interleukin-6-induced STAT3 phosphorylation in human pancreatic cancer cells. Anticancer Res. 31, 2029–2035 [PMC free article] [PubMed] [Google Scholar]
- 180.Holmer R., Goumas F.A., Waetzig G.H., Rose-John S. and Kalthoff H. (2014) Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat. Dis. Int. 13, 371–380 10.1016/S1499-3872(14)60259-9 [DOI] [PubMed] [Google Scholar]
- 181.Lesina M., Wormann S.M., Neuhofer P., Song L. and Algul H. (2014) Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin. Immunol. 26, 80–87 10.1016/j.smim.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 182.Pop V.V., Seicean A., Lupan I., Samasca G. and Burz C.C. (2017) IL-6 roles -Molecular pathway and clinical implication in pancreatic cancer -A systemic review. Immunol. Lett. 181, 45–50 10.1016/j.imlet.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 183.Tang D., Wang D., Yuan Z., Xue X., Zhang Y., An Y. et al. (2013) Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int. J. Cancer 132, 993–1003 10.1002/ijc.27715 [DOI] [PubMed] [Google Scholar]
- 184.Liu H., Ma Q., Xu Q., Lei J., Li X., Wang Z. et al. (2012) Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Curr. Pharm. Des. 18, 2395–2403 10.2174/13816128112092395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ancrile B., Lim K.H. and Counter C.M. (2007) Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 21, 1714–1719 10.1101/gad.1549407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bao B., Ali S., Ahmad A., Azmi A.S., Li Y., Banerjee S. et al. (2012) Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE 7, e50165 10.1371/journal.pone.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miyamoto Y., Hosotani R., Doi R., Wada M., Ida J., Tsuji S. et al. (2001) Interleukin-6 inhibits radiation induced apoptosis in pancreatic cancer cells. Anticancer Res. 21, 2449–2456 [PubMed] [Google Scholar]
- 188.Tang R.F., Wang S.X., Zhang F.R., Peng L., Wang S.X., Xiao Y. et al. (2005) Interleukin-1alpha, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 4, 460–463 [PubMed] [Google Scholar]
- 189.Masui T., Hosotani R., Doi R., Miyamoto Y., Tsuji S., Nakajima S. et al. (2002) Expression of IL-6 receptor in pancreatic cancer: involvement in VEGF induction. Anticancer Res. 22, 4093–4100 [PubMed] [Google Scholar]
- 190.Bharadwaj U., Li M., Zhang R., Chen C. and Yao Q. (2007) Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 67, 5479–5488 10.1158/0008-5472.CAN-06-3963 [DOI] [PubMed] [Google Scholar]
- 191.Lin J.H., Huffman A.P., Wattenberg M.M., Walter D.M., Carpenter E.L., Feldser D.M. et al. (2020) Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 217, 10.1084/jem.20190673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Flint T.R., Janowitz T., Connell C.M., Roberts E.W., Denton A.E., Coll A.P. et al. (2016) Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 24, 672–684 10.1016/j.cmet.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huang C., Yang G., Jiang T., Zhu G., Li H. and Qiu Z. (2011) The effects and mechanisms of blockage of STAT3 signaling pathway on IL-6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma 58, 396–405 10.4149/neo_2011_05_396 [DOI] [PubMed] [Google Scholar]
- 194.Wu Y.S., Chung I., Wong W.F., Masamune A., Sim M.S. and Looi C.Y. (2017) Paracrine IL-6 signaling mediates the effects of pancreatic stellate cells on epithelial-mesenchymal transition via Stat3/Nrf2 pathway in pancreatic cancer cells. Biochimica et Biophysica Acta (BBA) -General Subjects 1861, 296–306 10.1016/j.bbagen.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 195.Razidlo G.L., Burton K.M. and McNiven M.A. (2018) Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J. Biol. Chem. 293, 11143–11153 10.1074/jbc.RA118.003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mace T.A., Shakya R., Pitarresi J.R., Swanson B., McQuinn C.W., Loftus S. et al. (2018) IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 67, 320–332 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jostock T., Mullberg J., Ozbek S., Atreya R., Blinn G., Voltz N. et al. (2001) Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 268, 160–167 [DOI] [PubMed] [Google Scholar]
- 198. Oncomine Buchholz Dataset. ed.)∧eds.)
- 199.Ren C., Chen Y., Han C., Fu D. and Chen H. (2014) Plasma interleukin-11 (IL-11) levels have diagnostic and prognostic roles in patients with pancreatic cancer. Tumor Biol. 35, 11467–11472 10.1007/s13277-014-2459-y [DOI] [PubMed] [Google Scholar]
- 200.Shin S.Y., Choi C., Lee H.G., Lim Y. and Lee Y.H. (2012) Transcriptional regulation of the interleukin-11 gene by oncogenic Ras. Carcinogenesis 33, 2467–2476 10.1093/carcin/bgs297 [DOI] [PubMed] [Google Scholar]
- 201.Putoczki T.L., Thiem S., Loving A., Busuttil R.A., Wilson N.J. and Ziegler P.K. (2013) Interleukin-11 Is the Dominant IL-6 Family Cytokine during Gastrointestinal Tumorigenesis and Can Be Targeted Therapeutically. Cancer Cell 24, 257–271 10.1016/j.ccr.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 202.Ma J., Song X., Xu X. and Mou Y. (2019) Cancer-Associated Fibroblasts Promote the Chemo-resistance in Gastric Cancer through Secreting IL-11 Targeting JAK/STAT3/Bcl2 Pathway. Cancer Res. Treat. 51, 194–210 10.4143/crt.2018.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Johnstone C.N., Chand A., Putoczki T.L. and Ernst M. (2015) Emerging roles for IL-11 signaling in cancer development and progression: Focus on breast cancer. Cytokine & Growth Factor Rev. 26, 489–498 [DOI] [PubMed] [Google Scholar]
- 204.Bressy C., Lac S., Nigri J., Leca J., Roques J., Lavaut M.-N. et al. (2018) LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res. 78, 909. [DOI] [PubMed] [Google Scholar]
- 205.Peng F., Zhou J., Sheng W., Zhang D. and Dong M. (2014) Expression and significance of leukemia inhibitory factor in human pancreatic cancer. Zhonghua Yi Xue Za Zhi 94, 90–95 [PubMed] [Google Scholar]
- 206.Kamohara H., Ogawa M., Ishiko T., Sakamoto K. and Baba H. (2007) Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: Involvement of regulation of LIF and its receptor expression. Int. J. Oncol. 30, 977–983 [PubMed] [Google Scholar]
- 207.Wang M.T., Fer N., Galeas J., Collisson E.A., Kim S.E., Sharib J. et al. (2019) Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat. Commun. 10, 3055 10.1038/s41467-019-11044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kamohara H., Takahashi M., Ishiko T., Ogawa M. and Baba H. (2007) Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int. J. Oncol. 31, 627–632 [PubMed] [Google Scholar]
- 209.Chen Y., Shi M., Yu G.-Z., Qin X.-R., Jin G., Chen P. et al. (2012) Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J. Gastroenterol. 18, 1123–1129 10.3748/wjg.v18.i10.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ma D., Jing X., Shen B., Liu X., Cheng X., Wang B. et al. (2016) Leukemia inhibitory factor receptor negatively regulates the metastasis of pancreatic cancer cells in vitro and in vivo. Oncol. Rep. 36, 827–836 10.3892/or.2016.4865 [DOI] [PubMed] [Google Scholar]
- 211.Torres C., Perales S., Alejandre M.J., Iglesias J., Palomino R.J., Martin M. et al. (2014) Serum cytokine profile in patients with pancreatic cancer. Pancreas 43, 1042–1049 10.1097/MPA.0000000000000155 [DOI] [PubMed] [Google Scholar]
- 212.Ishikawa M., Yoshida K., Yamashita Y., Ota J., Takada S., Kisanuki H. et al. (2005) Experimental trial for diagnosis of pancreatic ductal carcinoma based on gene expression profiles of pancreatic ductal cells. Cancer Sci. 96, 387–393 10.1111/j.1349-7006.2005.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Badea L., Herlea V., Dima S.O., Dumitrascu T. and Popescu I. (2008) Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepato-Gastroenterol. 55, 2016–2027 [PubMed] [Google Scholar]
- 214.Logsdon C.D., Simeone D.M., Binkley C., Arumugam T., Greenson J.K., Giordano T.J. et al. (2003) Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 63, 2649–2657 [PubMed] [Google Scholar]
- 215.Segara D., Biankin A.V., Kench J.G., Langusch C.C., Dawson A.C., Skalicky D.A. et al. (2005) Expression of HOXB2, a retinoic acid signaling target in pancreatic cancer and pancreatic intraepithelial neoplasia. Clin. Cancer Res. 11, 3587–3596 10.1158/1078-0432.CCR-04-1813 [DOI] [PubMed] [Google Scholar]
- 216.Smigiel J.M., Parameswaran N. and Jackson M.W. (2017) Potent EMT and CSC Phenotypes Are Induced By Oncostatin-M in Pancreatic Cancer. Mol. Cancer Res. 15, 478–488 10.1158/1541-7786.MCR-16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lili L.N., Matyunina L.V., Walker L.D., Daneker G.W. and McDonald J.F. (2014) Evidence for the importance of personalized molecular profiling in pancreatic cancer. Pancreas 43, 198–211 10.1097/MPA.0000000000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deng G., Kakar S., Okudiara K., Choi E., Sleisenger M.H. and Kim Y.S. (2009) Unique Methylation Pattern of Oncostatin M Receptor Gene in Cancers of Colorectum and Other Digestive Organs. Clin. Cancer Res. 15, 1519 10.1158/1078-0432.CCR-08-1778 [DOI] [PubMed] [Google Scholar]
- 219.Nguyen A.M., Zhou J., Sicairos B., Sonney S. and Du Y. (2020) Upregulation of CD73 Confers Acquired Radioresistance and is Required for Maintaining Irradiation-selected Pancreatic Cancer Cells in a Mesenchymal State. Mol. Cell. Proteomics 19, 375–389 10.1074/mcp.RA119.001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Junk D.J., Bryson B.L., Smigiel J.M., Parameswaran N., Bartel C.A. and Jackson M.W. (2017) Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene 36, 4001–4013 10.1038/onc.2017.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Argast G.M., Mercado P., Mulford I.J., O'Connor M., Keane D.M., Shaaban S. et al. (2011) Cooperative Signaling between Oncostatin M, Hepatocyte Growth Factor and Transforming Growth Factor-β Enhances Epithelial to Mesenchymal Transition in Lung and Pancreatic Tumor Models. Cells Tissues Organs 193, 114–132 10.1159/000320179 [DOI] [PubMed] [Google Scholar]
- 222.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V. et al. (2007) Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 10.1158/0008-5472.CAN-06-2030 [DOI] [PubMed] [Google Scholar]
- 223.Benson D.D., Meng X., Fullerton D.A., Moore E.E., Lee J.H., Ao L. et al. (2012) Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1067–R1075 10.1152/ajpregu.00320.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nistal-Villan E., Bunuales M., Poutou J., Gonzalez-Aparicio M., Bravo-Perez C., Quetglas J.I. et al. (2015) Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol. Cancer 14, 210 10.1186/s12943-015-0479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bustos M., Dubrot J., Martinez-Anso E., Larequi E., Castaño D., Palazon A. et al. (2012) Cardiotrophin-1 determines liver engraftment of syngenic colon carcinoma cells through an immune system-mediated mechanism. Oncoimmunology 1, 1527–1536 10.4161/onci.22504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim J.W., Marquez C.P., Kostyrko K., Koehne A.L., Marini K., Simpson D.R. et al. (2019) Antitumor activity of an engineered decoy receptor targeting CLCF1–CNTFR signaling in lung adenocarcinoma. Nat. Med. 25, 1783–1795 10.1038/s41591-019-0612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kourko O., Seaver K., Odoardi N., Basta S. and Gee K. (2019) IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer. Front. Oncol. 9, 10.3389/fonc.2019.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fabbi M., Carbotti G. and Ferrini S. (2017) Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediators Inflamm. 2017, 3958069–3958069 10.1155/2017/3958069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Liu L.H., Hao G.Z., Zhang C., Ai J., Shao L.L. and Shan B.E. (2010) IL-27 promotes apoptosis of human pancreatic carcinoma Aspc1 cells. Chinese J. Cancer Biother. 17, 634–638 [Google Scholar]
- 230.Liu L., Meng J., Zhang C., Duan Y., Zhao L., Wang S. et al. (2012) Effects on apoptosis and cell cycle arrest contribute to the antitumor responses of interleukin-27 mediated by retrovirus in human pancreatic carcinoma cells. Oncol. Rep. 27, 1497–1503 [DOI] [PubMed] [Google Scholar]
- 231.Yao L., Wang M., Niu Z., Liu Q., Gao X., Zhou L. et al. (2017) Interleukin-27 inhibits malignant behaviors of pancreatic cancer cells by targeting M2 polarized tumor associated macrophages. Cytokine 89, 194–200 10.1016/j.cyto.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 232.Davidi S., Fremder E., Kan T., Raviv Z., Timaner M., Karin N. et al. (2017) The antiangiogenic role of the pro-inflammatory cytokine interleukin-31. Oncotarget 8, 16430–16444 10.18632/oncotarget.14857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Laklai H., Miroshnikova Y.A., Pickup M.W., Collisson E.A., Kim G.E., Barrett A.S. et al. (2016) Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505 10.1038/nm.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wormann S.M., Song L., Ai J., Diakopoulos K.N., Kurkowski M.U., Gorgulu K. et al. (2016) Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology. 151, 180e112–193.e112 10.1053/j.gastro.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 235.Nagathihalli N.S., Castellanos J.A., Shi C., Beesetty Y., Reyzer M.L., Caprioli R. et al. (2015) Signal Transducer and Activator of Transcription 3, Mediated Remodeling of the Tumor Microenvironment Results in Enhanced Tumor Drug Delivery in a Mouse Model of Pancreatic Cancer. Gastroenterology 149, 1932e1939–1943.e1939 10.1053/j.gastro.2015.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rath N. and Olson M.F. (2016) Regulation of pancreatic cancer aggressiveness by stromal stiffening. Nat. Med. 22, 462–463 10.1038/nm.4099 [DOI] [PubMed] [Google Scholar]
- 237.Denley S.M., Jamieson N.B., McCall P., Oien K.A., Morton J.P., Carter C.R. et al. (2013) Activation of the IL-6R/Jak/Stat Pathway is Associated with a Poor Outcome in Resected Pancreatic Ductal Adenocarcinoma. J Gastrointestinal Sur. 17, 887–898 10.1007/s11605-013-2168-7 [DOI] [PubMed] [Google Scholar]
- 238.Gilabert M., Calvo E., Airoldi A., Hamidi T., Moutardier V., Turrini O. et al. (2014) Pancreatic Cancer-Induced Cachexia Is Jak2-Dependent in Mice. J. Cellular Physiol. 229, 1437–1443 10.1002/jcp.24580 [DOI] [PubMed] [Google Scholar]
- 239.Muliawati Y., Haroen H. and Rotty L.W. (2012) Cancer anorexia -cachexia syndrome. Acta Med. Indones 44, 154–162 [PubMed] [Google Scholar]
- 240.Wu X., Tang W., Marquez R.T., Li K., Highfill C.A., He F. et al. (2016) Overcoming chemo/radio-resistance of pancreatic cancer by inhibiting STAT3 signaling. Oncotarget 7, 11708–11723 10.18632/oncotarget.7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu P., Wu D., Zhao L., Huang L., Shen G., Huang J. et al. (2016) Prognostic role of STAT3 in solid tumors: a systematic review and meta-analysis. Oncotarget 7, 19863–19883 10.18632/oncotarget.7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li H., Huang C., Huang K., Wu W., Jiang T., Cao J. et al. (2011) STAT3 knockdown reduces pancreatic cancer cell invasiveness and matrix metalloproteinase-7 expression in nude mice. PLoS ONE 6, e25941 10.1371/journal.pone.0025941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.D'Amico S., Shi J., Martin B.L., Crawford H.C., Petrenko O. and Reich N.C. (2018) STAT3 is a master regulator of epithelial identity and KRAS-driven tumorigenesis. Genes Dev. 32, 1175–1187 10.1101/gad.311852.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Corcoran R.B., Contino G., Deshpande V., Tzatsos A., Conrad C., Benes C.H. et al. (2011) STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 71, 5020–5029 10.1158/0008-5472.CAN-11-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang X., Ren D., Wu X., Lin X., Ye L., Lin C. et al. (2018) miR-1266 Contributes to Pancreatic Cancer Progression and Chemoresistance by the STAT3 and NF-κB Signaling Pathways. Mol. Ther. Nucleic Acids 11, 142–158 10.1016/j.omtn.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yu H., Lee H., Herrmann A., Buettner R. and Jove R. (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer 14, 736–746 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- 247.Yu H., Pardoll D. and Jove R. (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang C., Cao J., Huang K.J., Zhang F., Jiang T., Zhu L. et al. (2006) Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 97, 1417–1423 10.1111/j.1349-7006.2006.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Guha S., Chakraborty A., Szymanski S., Fokt I., Abbruzzese J., Kazerooni R. et al. (2007) WP1066, a potent inhibitor of Jak2/STAT3 pathway inhibits pancreatic tumor growth both in vitro and in vivo. Cancer Res. 67, 2393 [Google Scholar]
- 250.Palagani V., Bozko P., El Khatib M., Belahmer H., Giese N., Sipos B. et al. (2014) Combined inhibition of Notch and JAK/STAT is superior to monotherapies and impairs pancreatic cancer progression. Carcinogenesis 35, 859–866 10.1093/carcin/bgt394 [DOI] [PubMed] [Google Scholar]
- 251.Komar H.M., Serpa G., Kerscher C., Schwoegl E., Mace T.A., Jin M. et al. (2017) Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci. Rep. 7, 1787–1787 10.1038/s41598-017-01973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Simon N., Antignani A., Hewitt S.M., Gadina M., Alewine C. and FitzGerald D. (2019) Tofacitinib enhances delivery of antibody-based therapeutics to tumor cells through modulation of inflammatory cells. JCI Insight. 4, 10.1172/jci.insight.123281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hassan R. and Ho M. (2008) Mesothelin targeted cancer immunotherapy. European J. Cancer (Oxford, England: 1990). 44, 46–53 10.1016/j.ejca.2007.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Argani P., Iacobuzio-Donahue C., Ryu B., Rosty C., Goggins M., Wilentz R.E. et al. (2001) Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 7, 3862–3868 [PubMed] [Google Scholar]
- 255.Wang J., Guo X.J., Ding Y.M. and Jiang J.X. (2017) miR-1181 inhibits invasion and proliferation via STAT3 in pancreatic cancer. World J. Gastroenterol. 23, 1594–1601 10.3748/wjg.v23.i9.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bai E., Yang L., Xiang Y., Hu W., Li C., Lin J. et al. (2018) L61H46 shows potent efficacy against human pancreatic cancer through inhibiting STAT3 pathway. Cancer Manag. Res. 10, 565–581 10.2147/CMAR.S159090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lin L., Hutzen B., Zuo M., Ball S., Deangelis S., Foust E. et al. (2010) Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 70, 2445–2454 10.1158/0008-5472.CAN-09-2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Huang C., Jiang T., Zhu L., Liu J., Cao J., Huang K.J. et al. (2011) STAT3-targeting RNA interference inhibits pancreatic cancer angiogenesis in vitro and in vivo. Int. J. Oncol. 38, 1637–1644 [DOI] [PubMed] [Google Scholar]
- 259.Glienke W., Hausmann E. and Bergmann L. (2011) Targeting STAT3 signaling in pancreatic cancer promotes antiapoptotic gene expression. Pancreas 40, 323–324 10.1097/MPA.0b013e318204ea7b [DOI] [PubMed] [Google Scholar]
- 260.Ashizawa T., Iizuka A., Maeda C., Tanaka E., Kondou R., Miyata H. et al. (2019) Impact of combination therapy with anti-PD-1 blockade and a STAT3 inhibitor on the tumor-infiltrating lymphocyte status. Immunol. Letters 216, 43–50 10.1016/j.imlet.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 261.Hurwitz H.I., Uppal N., Wagner S.A., Bendell J.C., Beck J.T., Wade S.M. III et al. (2015) Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J. Clin. Oncol. 33, 4039–4047 10.1200/JCO.2015.61.4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hurwitz H., Van Cutsem E., Bendell J., Hidalgo M., Li C.-P., Salvo M.G. et al. (2018) Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest. New Drugs 36, 683–695 10.1007/s10637-018-0580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bauer T.M., Patel M.R., Forero-Torres A., George T.J. Jr, Assad A., Du Y. et al. (2018) A Phase Ib study of ruxolitinib + gemcitabine +/-nab-paclitaxel in patients with advanced solid tumors. Onco. Targets Ther 11, 2399–2407 10.2147/OTT.S157331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ng K., Hendifar A., Starodub A., Chaves J., Yang Y., Koh B. et al. (2019) Phase 1 dose-escalation study of momelotinib, a Janus kinase 1/2 inhibitor, combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. Invest. New Drugs 37, 159–165 10.1007/s10637-018-0650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bekaii-Saab T.S., Starodub A., El-Rayes B.F., Shahda S., O'Neil B.H., Noonan A.M. et al. (2018) Phase 1b/2 trial of cancer stemness inhibitor napabucasin (NAPA) + nab-paclitaxel (nPTX) and gemcitabine (Gem) in metastatic pancreatic adenocarcinoma (mPDAC). J. Clin. Oncol. 36, 4110–4110 10.1200/JCO.2018.36.15_suppl.4110 [DOI] [Google Scholar]
- 266.Sonbol M.B., Ahn D.H., Goldstein D., Okusaka T., Tabernero J., Macarulla T. et al. (2019) CanStem111P trial: a Phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Future Oncol. 15, 1295–1302 10.2217/fon-2018-0903 [DOI] [PubMed] [Google Scholar]
- 267.Zhou T., Georgeon S., Moser R., Moore D.J., Caflisch A. and Hantschel O. (2014) Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348). Leukemia 28, 404–407 10.1038/leu.2013.205 [DOI] [PubMed] [Google Scholar]
- 268.Han D., Yu T., Dong N., Wang B., Sun F. and Jiang D. (2019) Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J. Exp. Clin. Cancer Res. 38, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wong A.L.A., Hirpara J.L., Pervaiz S., Eu J.Q., Sethi G. and Goh B.C. (2017) Do STAT3 inhibitors have potential in the future for cancer therapy? Exp. Opin. Investig. Drugs 26, 883–887 10.1080/13543784.2017.1351941 [DOI] [PubMed] [Google Scholar]
- 270.Rossi J.F., Négrier S., James N.D., Kocak I., Hawkins R., Davis H. et al. (2010) A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. British J. Cancer 103, 1154–1162 10.1038/sj.bjc.6605872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chari A., Pri-Chen H. and Jagannath S. (2013) Complete remission achieved with single agent CNTO 328, an anti-IL-6 monoclonal antibody, in relapsed and refractory myeloma. Clin. Lymphoma Myeloma Leuk. 13, 333–337 10.1016/j.clml.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 272.Anaya J. (2016) OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Sci. 2, 10.7717/peerj-cs.6728286867 [DOI] [Google Scholar]