Abstract
Background and aims
Physical activity is beneficial in several chronic disorders including Crohn′s disease, but the preferred type of exercise is unknown. Our study aimed to examine and compare the safety, feasibility and potential beneficial effects of individual moderate endurance and moderate muscle training in patients with Crohn’s disease.
Methods
Quiescent or mildly active (Crohn’s disease activity index <220) patients with Crohn’s disease were randomly allocated to either a control, endurance, or muscle training group. Participants exercised individually for 3 months three times per week. Endpoints included dropout rate, disease activity, inflammatory parameters including faecal calprotectin, anthropometric data, quality of life, physical activity and strength.
Results
A total of 45 patients with Crohn’s disease were randomly allocated. In the endurance group (n = 17), the dropout rate was significantly higher (47% vs. 13%) compared with the muscle group (n = 15). In both groups the maximal and average strength in the upper and lower extremities increased significantly (all P < 0.04). In the endurance group emotional function was significantly improved (P = 0.03). Statistically significant changes of disease activity and other outcome parameters were not observed in this pilot cohort.
Conclusion
Both individual moderate endurance and muscle training can be safely performed in patients with mild or quiescent Crohn’s disease. Muscle training appears more feasible and may be favoured. Both forms of exercise have beneficial effects on strength. Quality of life increased in both intervention groups, although statistical significance was only reached in one subgroup.
Keywords: Crohn’s disease, exercise, physical activity, endurance, sport, fatigue, strength
Key summary
Summarise the established knowledge on this subject. • Physical activity has been demonstrated to exert beneficial effects in a variety of diseases, e.g. cancer and depressive disorders, and has been associated with a decreased risk of developing Crohn’s disease. • Crohn’s disease patients are reluctant to undertake physical activity and are less active than the average population. • The preferred training programme for Crohn’s disease patients is unknown.
What are the significant and/or new findings of this study? • Individual physical activity and especially muscle training is safe to perform in patients with quiescent or mildly active Crohn’s disease. • Muscle training appears to be more feasible in younger Crohn’s disease patients than endurance training. • Muscle and endurance training both increase the strength in the upper and lower extremities.
Introduction
Physical activity has a positive effect on disease activity, comorbidities and general wellbeing in many diseases. A positive effect has been consistently observed in various oncological diseases including breast or colon cancer,1,2 psychiatric diseases such as depression3 or cardiovascular diseases.4
There is also some promising but only limited evidence on the role of exercise both in the protection from inflammatory bowel disease (IBD) development and disease management.5 However, a comparison of different exercise methods in Crohn’s disease (CD) has not been evaluated thus far. This may be especially of interest as frequent CD symptoms such as diarrhoea or abdominal pain may cause reluctance to exercise regularly. CD is an intermittent chronic IBD that usually affects the entire gastrointestinal tract from the anus to the mouth. The exact aetiopathogenesis of CD still remains unclear despite enormous progress in the pathophysiological understanding of this disease. A dysregulation of the immune system is supposed to play a key role in the pathogenesis of CD. A general healthy lifestyle has been reported to decrease the morbidity and mortality in IBD patients,6 while the majority of patients with CD fail the World Health Organization (WHO) recommendations for physical activity.7 Interestingly, physically active patients tend to have a lower relapse rate indicating that physical activity may confer beneficial effects in CD patients.8 In addition, CD is significantly less frequently observed in active people.9 CD patients often experience fatigue and also reduced muscle strength and bone mineral density.10,11
A few prospective studies have investigated the effects of endurance training in CD patients.12–14 It has recently been shown in a small number of cases that high-intensity endurance training can be performed safely in CD patients.15 In addition to endurance training, the WHO recommends muscle training at least 2 days per week.16 So far, there have been only three studies investigating the effects of muscle training in CD patients.17–19 Only one investigation included a control group, however, not assessing effects on disease activity, wellbeing and strength.18 The majority of these previous studies focused on supervised exercise programmes which took place in research facilities or fitness centres.
Although the number of published investigations in the field of IBD and physical activity is growing, it remains unknown which type of training is more feasible for CD patients. Standardised exercise recommendations still do not exist.
Our primary aim was to evaluate whether individual non-supervised moderate endurance and muscle training can be carried out safely in patients with quiescent or mildly active CD without causing disease flares and which exercise programme would be more feasible.
Furthermore, we wanted to assess the effects of specific training programmes on disease activity, strength, psychological parameters and the patients’ wellbeing.
Materials and methods
Study design and setting
This study was designed as a pilot trial as it is the first trial to compare directly moderate endurance and independent moderate muscle training in CD patients. Primary endpoints were the assessment of safety and feasibility of the interventions in quiescent or mildly active CD patients. As a pilot study, multiple secondary endpoints were set to investigate potential beneficial effects. Due to the limitations of a pilot study a primary calculation of power could not be performed. The study was performed as a single centre, three arm parallel group randomised controlled trial. The patients were randomly allocated to either a control group without any exercise intervention or a moderate endurance training or a moderate muscle workout group. The study assessments were performed at inclusion into the study and after 3 months. After 6 months the patients were re-assessed using various questionnaires. Patients were recruited in the outpatient clinic of the Agaplesion Markus Hospital in Frankfurt/Main, Germany, with support by study calls of the German Crohn and Colitis Foundation (DCCV). The study was performed according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Hesse Medical Association (FF101/201) on 19 September 2017. Written, informed consent was obtained from each patient included in the study. As this trial was a pilot study with non-invasive interventions a trial registry was not performed. Recruitment occurred from January 2018 to August 2019 with the last follow-up in January 2020.
Patients
We included male and female CD patients aged between 18 and 65 years and diagnosed with CD for at least 6 months. Additional inclusion criteria were quiescent or mildly active disease as assessed by a Crohn’s disease activity index (CDAI) less than 220 and stable medication for at least 4 weeks.
Patients were excluded if they had contraindications to physical activity or if they were already exercising more than two times per week for more than 60 minutes. Patients with severe extraintestinal manifestation such as arthralgia or a concomitant autoimmune disease such as rheumatoid arthritis were also excluded. Pregnancy or a planned pregnancy were also an exclusion criterion, as well as a difficult vein access, a planned modification in the treatment or scheduled surgery within the next 3 months, if they had undergone surgery in the past 6 months or if they were participating in another interventional clinical trial.
Randomisation
The study participants were aleatorily randomly assigned in either a control or one of two intervention groups. The participants were informed about their random assignment after the inclusion test.
Intervention
Participants randomly allocated to the muscle exercise group received detailed instructions how to exercise after the assessment. The muscle training took about 30–40 minutes and patients were asked to exercise at least three times per week for 12 weeks. The exercise programme consisted of 12 exercises which were performed with the patient’s own bodyweight only. Thus, special exercise machines, training facilities or supervision were not required. The plan included well-known exercises like push-ups or sit-ups. Patients had to do two to three sets of each exercise, with an increasing number of repetitions. The plan was designed by an accredited sports therapist from our department. Patients received instructions either through an online program and smartphone application or in written paper form.
Moderate endurance exercise training was performed for 30 minutes three times per week for 12 weeks. Participants could perform their preferred form of exercise; for example, jogging, cycling or walking on their own. They were instructed to exercise at a heart rate of 60–80% of their maximum capacity heart rate (220 – age in years). Patients were instructed to strive according to the Borg scale, a validated tool that is based on the correlation between received perception of exertion and pulse rate.20
Patients with questions concerning the workout programme received support by email and telephone or an arranged visit in our outpatient clinic. Patients had to keep record of their training success and adherence. Every 4 weeks the patients’ records were reviewed to ensure correct and sufficient execution of the exercise programme.
Assessment of patients
The CDAI as well as the patient reported outcome score 2 (PRO-2) were used to determine disease activity.21 PRO-2 is a modification of the CDAI and consists only of the first two questions (bowel movement and abdominal pain) multiplied by two and five, respectively. Safety as a primary endpoint was measured as the absence of serious adverse events and no significant worsening of disease symptoms, defined as an increase of CDAI above 150 or an increase of 100 points from baseline.22 Feasibility was evaluated as a significant difference in dropout rates.
The German version of the short inflammatory bowel disease questionnaire (sIBDQ) with its four subgroups, bowel symptoms, systemic symptoms, emotional function and social function was used to determine current wellbeing23 and the short international physical activity questionnaire (sIPAQ) was used to evaluate average physical activity.24 Subjects had to indicate in the sIPAQ how often they have performed strong, moderate and low physical activities on average within the previous 7 days. Depending on the number of metabolic equivalents (METs), patients are grouped into high, moderate or low activity.
Strength in the lower extremity was determined by isometric measurement of the strength in the quadriceps muscle using the force transducer Bosche FH 1K at a 45° angle.25 For hand grip strength, the digital hand dynamometer DHD-1 (SH1001) from Saehan was used. Handgrip strength has been demonstrated to be an effective parameter to predict the functional nutritional status and muscular health in CD patients.26
At inclusion and after 3 months of intervention anthropological data (blood pressure, pulse, height, weight, body mass index, respiratory rate) were collected and complete blood count, measurements of kidney, liver, pancreatic, iron status, cholesterol, triglycerides, faecal calprotectin and inflammatory laboratory parameters were performed. Strength measurements in the upper and lower extremity as well as the analysis of the sIBDQ, sIPAQ, PRO-2 and CDAI were conducted at the same time. SIBDQ, sIPAQ and PRO-2 and changes in medication or surgical interventions during the study period were evaluated again by mail after 6 months of follow-up.
Sample size
This study was designed as a pilot and proof-of-concept study. We aimed at a sample size of 12 participants in each group to complete the study.27
Statistical analysis
Statistical analysis was performed by SPSS Statistics version 26 Python 3. A P value less than 0.05 was considered significant. For standard distributed values the paired t-test was used and for not normally distributed values we used the Wilcoxon signed rank test.
Results
Inclusion
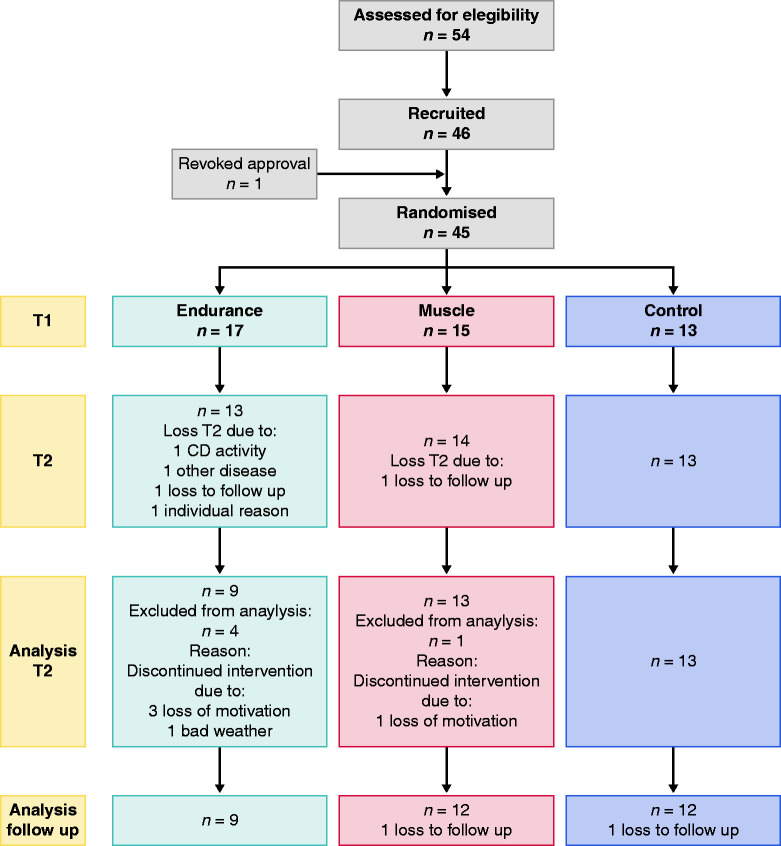
A flow chart of the study is depicted in Figure 1.
Figure 1.
Flowchart of study.
Fifty-four patients responded to our study call, of which 46 patients met the inclusion criteria. Of these patients, 45 were randomly assigned. One patient withdrew shortly before inclusion. The most frequent reasons for exclusion from the study were excessive disease activity (four) followed by high levels of exercise (three) and age (one). Two patients could only be randomly assigned after 6 months due to a change in medication or surgery. The patient disposition is summarised in Table 1.
Table 1.
Patient disposition.
| Patient disposition | Endurance training |
Muscle training |
Control |
|||
|---|---|---|---|---|---|---|
| Included (n = 17) | Analysed (n = 9) | Included (n = 15) | Analysed (n = 13) | Included (n = 13) | Analysed (n = 13) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Sex, female | 11 (64.7) | 6 (66.7) | 8 (53.3) | 7 (53.8) | 9 (69.2) | 9 (69.2) |
| Smoking | 1 (5.9) | 1 (11.1) | 2 (13.3) | 2 (15.4) | 4 (30.7) | 4 (30.7) |
| Working | 15 (88.2) | 7 (77.8) | 13 (86.7) | 12 (92.3) | 8 (61.5) | 8 (61.5) |
| Student | 1 (5.9) | 1 (11.1) | 2 (13.3) | 1 (7.7) | 2 (15.4) | 2 (15.4) |
| Retired | 1 (5.9) | 1 (11.1) | 0 (0) | 0 (0) | 2 (15.4) | 2 (15.4) |
| On sick leave | 1 (5.9) | 1 (11.1) | 2 (13.3) | 2 (15.4) | 0 (0) | 0 (0) |
| Azathioprine | 1 (5.9) | 0 (0) | 1 (6.7) | 1 (7.7) | 2 (15.4) | 2 (15.4) |
| Budenoside | 2 (11.8) | 2 (22.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TNF alpha inhibitor | 3 (17.6) | 1 (11.1) | 3 (20) | 3 (23.1) | 7 (53.8) | 7 (53.8) |
| Ustekinumab/ Vedolizumab | 4 (23.5) | 2 (22.2) | 4 (26.7) | 3 (23.1) | 0 (0) | 0 (0) |
| Mesalazine | 1 (5.9) | 1 (11.1) | 0 (0) | 0 (0) | 2 (15.4) | 2 (15.4) |
| No therapy | 5 (29.4) | 2 (22.2) | 7 (46.7) | 6 (46.2) | 2 (15.4) | 2 (15.4) |
| Ileocoecal resection | 9 (52.9) | 3 (33.3) | 12 (80) | 10 (76.9) | 6 (46.2) | 6 (46.2) |
| Colon or small bowel resection | 3 (17.6) | 1 (11.1) | 1 (6.7) | 1 (7.7) | 1 (7.7) | 1 (7.7) |
| Fistula surgery | 3 (17.6) | 1 (11.1) | 4 (26.7) | 4 (30.8) | 4 (30.8) | 4 (30.8) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age in years | 39.6 (12.0) | 45.3 (12.4) | 42 (13.1) | 45.0 (11.3) | 43.7 (12.0) | 43.7 (12.0) |
| Age at initial diagnosis | 23.2 (8.8) | 24.2 (11.1) | 23.4 (8.8) | 25.0 (8.3) | 26.3 (13.5) | 26.3 (13.5) |
| BMI | 24.0 (5.6) | 23.1 (6.2) | 23.6 (4.2) | 24 (4.2) | 23.5 (3.9) | 23.5 (3.9) |
| CDAI | 68.5 (61.4) | 84.4 (67.1) | 88.9 (60.5) | 95.5 (62.2) | 64 (70.5) | 64 (70.5) |
| Pro-2 | 35.9 (37.3) | 40.4 (28.1) | 32.7 (25.2) | 35.7 (25.7) | 22.8 (29.7) | 22.8 (29.7) |
| Leukocytes per nl | 8.5 (3.4) | 7.7 (1.1) | 7.4 (2.2) | 7.5 (2.3) | 8.8 (2.9) | 8.8 (2.9) |
| Haemoglobin (g/dl) | 14.4 (1.5) | 14.6 (1.7) | 13.1 (2.7) | 13.1 (2.9) | 14.5 (0.8) | 14.5 (0.8) |
| CRP (mg/dl) | 3.6 (4.6) | 3.1 (5.6) | 3.5 (4.8) | 2.4 (3.2) | 1.4 (1.6) | 1.4 (1.6) |
| Pulse in BPM | 76.1 (10.6) | 77 (9) | 74.9 (12.3) | 73 (6.9) | 73.2 (10.9) | 73.2 (10.9) |
| Respiratory frequency/minute | 12.3 (2.3) | 12.4 (2.6) | 10.5 (1.6) | 10.5 (1.7) | 13.1 (3.2) | 13.1 (3.2) |
| Systolic blood pressure (mmHg) | 120.2 (11.8) | 122 (12.2) | 117.7 (10.2) | 116 (9.5) | 125.3 (11.1) | 125.3 (11.1) |
TNF: tumour necrosis factor; BMI: body mass index; BPM: beats per minute; CDAI: Crohn’s disease activity index; CRP: C-reactive protein.
Withdrawal rate
A total of 17 patients were randomly allocated to the endurance group and nine out of 17 subjects (52.9%) completed the study.
Three patients (37.5%) indicated a lack of time and motivation as reasons for the discontinuation, one patient did not exercise regularly due to bad weather. Two patients had to discontinue the study due to illness, of which only one patient experienced CD symptoms directly within one week of the start of the exercise programme. One patient discontinued the study due to a personal situation not related to the study and disease. Another patient did not respond to our multiple contact attempts. Randomisation into the endurance group was ended before reaching the initial target of 12 patients completing the study. The reason for this was the high rate of dropouts.
In total, 15 patients were randomly allocated to the muscle group. Of these 15 subjects, 13 subjects (86.7%) completed the study. One patient stopped the study after a few weeks due to lack of time and motivation (50%). One patient did not respond to our contact attempts. In general, the dropout rate in the muscle group was significantly lower compared with the endurance group (P = 0.04). No subject discontinued the study due to CD-related symptoms,
A total of 13 patients were randomly allocated to the control group. All patients were included in the final analysis and there were no dropouts.
Disease activity
Clinical activity as assessed by the CDAI did not change significantly in all groups (see Figure 2). The CDAI decreased in the muscle group from 95 to 88, while in the control group the CDAI increased from 64 to 73. In the endurance group, the CDAI remained approximately the same at 84 and 85, respectively. No analysed patient experienced a relapse or significant worsening of disease activity.
Figure 2.
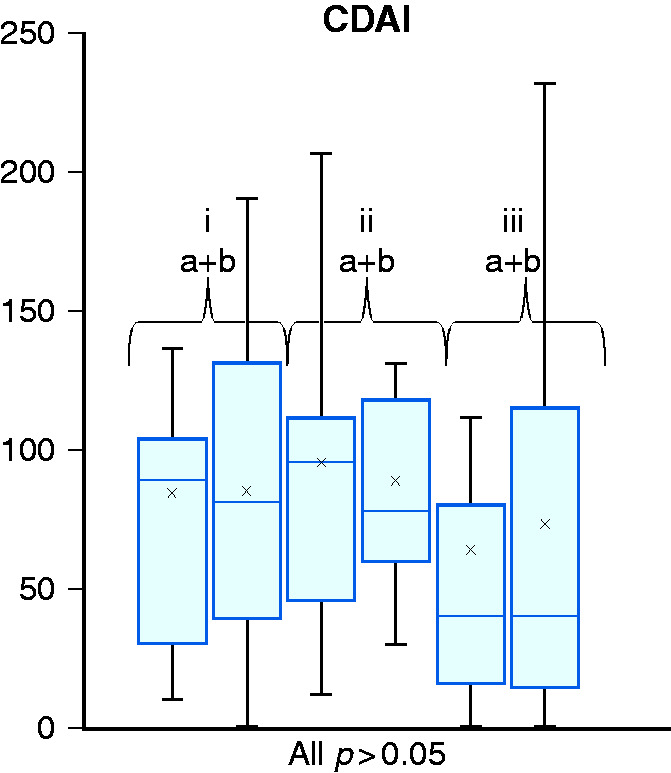
Crohn’s disease activity index (CDAI) score at inclusion (a) and at final test (b) after 12 weeks of intervention for endurance (i), muscle (ii) and control group (iii).
General wellbeing
In the endurance and muscle groups, the sIBDQ improved on average in all subgroups (see Table 2). However, statistical significance after 3 months of training was only achieved in the endurance group for the subgroup emotional function (13.78 to 15.67; P = 0.03).
Table 2.
Effects on CDAI, calprotectin, sIBDQ and sIPAQ pre, and post-intervention and at follow-up after 6 months.
| Endurance (n = 9)(follow-up 9) | Muscle (n = 13) (follow-up 12) | Control (n = 13) (follow-up 12) | |
|---|---|---|---|
| CDAI | Mean (SD) | Mean (SD) | Mean (SD) |
| Pre | 84.4 (67.1) | 95.5 (62.2) | 64 (70.5) |
| Post | 85.1 (61.6) | 88.8 (49.8) | 73.4 (76.6) |
| Calprotectin µg/g | Median (SD) | Median (SD) | Median (SD) |
| Pre | 69 (248) | 163 (377) | 33 (603) |
| Post | 74 ( 67) | 128 (198) | 49 (598) |
| sIBDQ | Mean (SD) | Mean (SD) | Mean (SD) |
| BS pre | 15.2 (3) | 15.5 (4.2) | 17.2 (3.1 |
| Post | 16.6 (3.2) | 16.4 (3.2) | 17.1 (3.2) |
| Follow-up | 16.9 (1.2) | 16.5 (2.2) | 17.7 (2.9) |
| SS pre | 9.6 (2.7) | 8.5 (2.7) | 10.7 (1.6) |
| Post | 9.8 (2.9) | 8.9 (3) | 11.6 (1.7) |
| Follow-up | 10.2 (2.2) | 8.8 (2.5) | 11.2 (2.4) |
| EF pre | 13.8 (3.5) | 14.2 (4.9) | 15.4 (3.2) |
| Post | 15.7 (2.9) | 14.4 (4.3) | 15.4 (3.2) |
| Follow-up | 15.4 (4) | 14.7 (3.8) | 17.7 (2.6) |
| SF pre | 12.6 (2.9) | 11.9 (3.1) | 12 (2.4) |
| Post | 12.6 (1.9) | 12.3 (1.9) | 12.8 (2) |
| Follow-up | 12.3 (2.1) | 12.3 (2.8) | 13.1 (2.1) |
| Sum pre | 51 (9.8) | 50.1 (12.7) | 55.3 (8.1) |
| Post | 54.7 (8.1) | 52 (10) | 56.9 (8.2) |
| Follow-up | 54.9 (10.4) | 52.3 (9.6) | 59.7 (7.7) |
| sIPAQ pre | 1.1 (0.3) | 1.7 (0.5) | 1.8 (0.7) |
| Post | 2.3 (0.7) | 2.3 (0.6) | 1.7 (0.8) |
| Follow-up | 2.3 (0.5) | 2.2 (0.6) | 2.1 (0.7) |
CDAI: Crohn’s disease activity index; sIBDQ: short inflammatory bowel disease questionnaire; sIPAQ: short international physical activity questionnaire; BS: bowel symptoms; SS: systemic symptoms; EF: emotional function; SF: social function.
Physical activity
Patients completing the study exercised on average 31.15 times in the muscle group in 12 weeks compared with 31 times in the endurance group. In the control group no subject increased physical activity to more than two activities per week. The average number of physical exercises per week at the final test was 0.42 compared with 0.38 at inclusion. In addition to the diaries, the sIPAQ score was used as a parameter to determine the daily exercise activity. In the muscle and endurance group, there was a significant increase in daily exercise from inclusion to the final test from 547 METs (1.1) to 1758 METs (2.3) and from 1346 METs (1.7) to 2120 METs (2.3) (P = 0.015 and 0.002), respectively (see Figure 3). In the control group, average daily activity decreased from 1652 METs (1.8) to 1258 METs (1.7) (P = 0.23).
Figure 3.
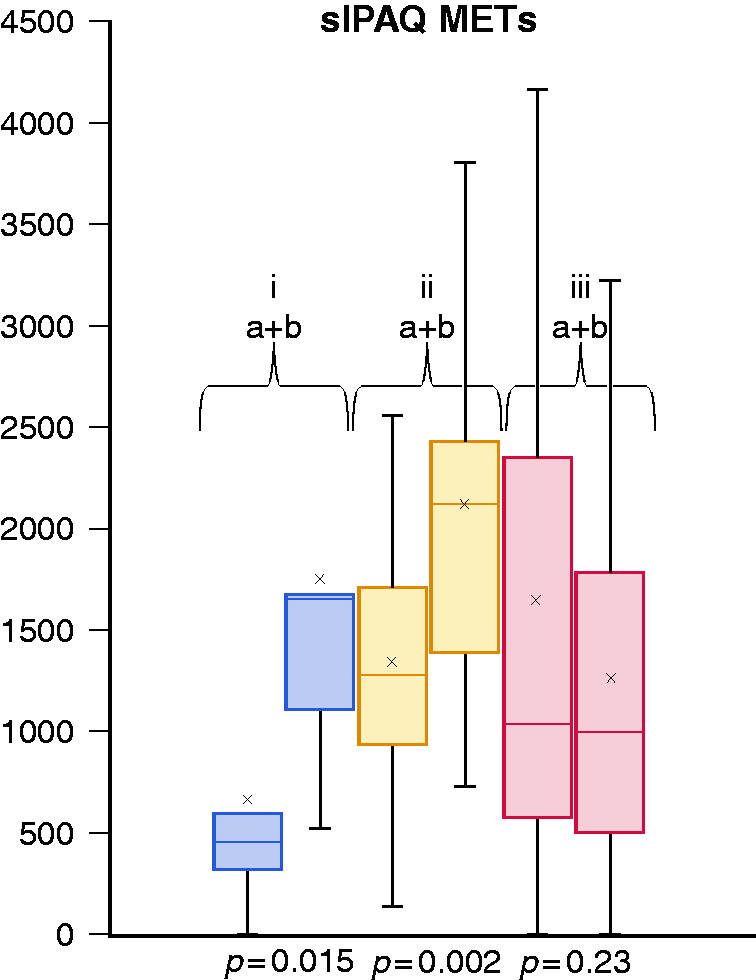
Short international physical activity questionnaire (sIPAQ) score in metabolic equivalents (METs) at inclusion (a) and at final test (b) after 12 weeks of intervention for endurance (i), muscle (ii) and control group (iii).
Patients in both intervention groups also continued to have an increased daily physical activity at follow-up after 6 months (endurance group 1.1 to 2.3; P = 0.01 and muscle group 1.7 to 2.2; P = 0.01).
Increase in strength
In the dominant hand, the maximum and average strength increased both in the endurance group and in the muscle group (all P < 0.02), respectively. In the control group strength decreased significantly (P = 0.01 and 0.04) (see Figure 4).
Figure 4.
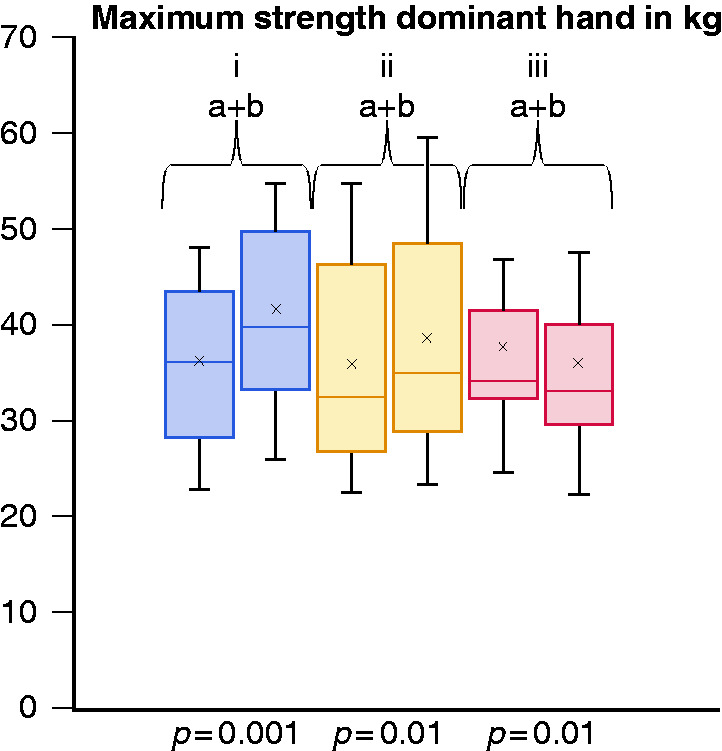
Maximum strength in the dominant hand in kilograms at inclusion (a) and at final test (b) after 12 weeks of intervention for endurance (i), muscle (ii) and control group (iii).
The maximum and average strength in the quadriceps of the non-dominant leg increased in the endurance and muscle group (all P < 0.04). In the control group there was no significant change (see Figure 5).
Figure 5.
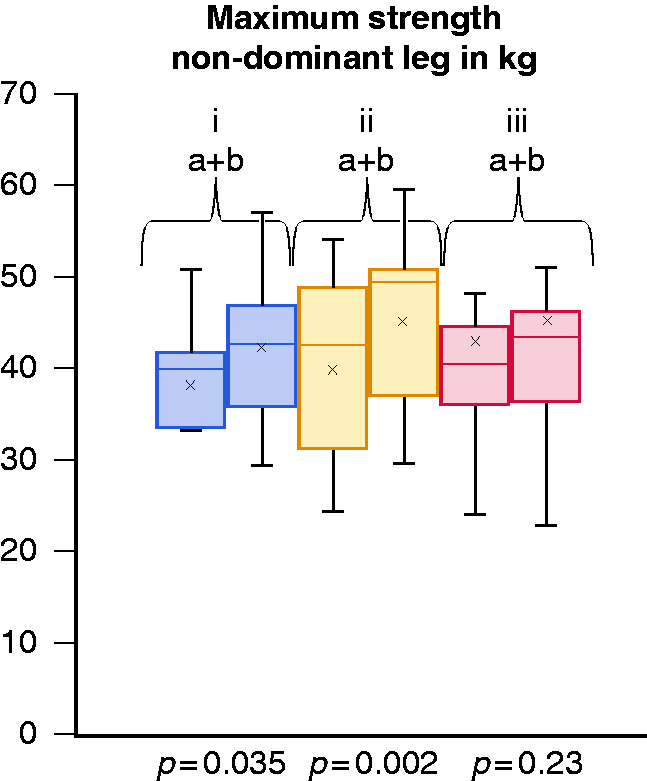
Maximum strength in the non-dominant leg in kilograms at inclusion (a) and at final test (b) after 12 weeks of intervention for endurance (i), muscle (ii) and control group (iii).
Laboratory parameters and anthropometric data
In all three groups there were no significant changes in the inflammatory parameters and anthropometric data.
Discussion
Our study demonstrates that both moderate endurance and moderate muscle training is safe to perform and beneficial for muscular strength and wellbeing in patients with mildly active or quiescent CD. Previously, there were controversies about the safety of physical activity in CD patients, as a previous study had suggested that strenuous exercise may cause acute gastrointestinal symptoms such as heartburn and diarrhoea.28 Furthermore, myositis has rarely been observed in patients treated with tumour necrosis factor (TNF) alpha inhibitors29 and infliximab therapy is rarely associated with increased creatinine kinase levels in the blood.30 None of our participants terminated the study due to muscle cramps or other signs of muscle alterations and only one participant in the endurance group discontinued the study due to CD-specific symptoms. These findings are consistent with previous investigations with other types of exercise programmes.12–15,17–19 Both moderate endurance training and moderate muscle training can therefore be categorised as safe modalities and recommended to patients according to their personal preferences and needs.
To our knowledge, our study is the first to compare the feasibility of muscle and endurance training, showing superiority to resistance training. While physical activity is inversely associated in CD patients with disease activity and depression,31 and decreases the risk of relapse and future active disease,8 CD patients are for various reasons reluctant to undertake physical activity32 and even patients with low disease activity complain that IBD symptoms such as fatigue and joint pain limit their ability to exercise.33 As the dropout rate in the muscle group was significantly lower than in the endurance group, we believe that muscle training may be more feasible for our – to a large extent – working cohort. We assume muscle training can be more easily integrated into the everyday lives of mostly young and active patients. Regardless of weather conditions, short days in the wintertime and other distracting problems, muscle training can easily be performed and integrated into the daily lives of patients. In addition, even if it was not directly pointed out by the patients, the permanent proximity to sanitary facilities could also be an advantage of the muscle programme.
In both intervention groups, patients who completed the training programme up to the final test were still significantly more active in their everyday lives at follow-up. It seems to be crucial to motivate the patients to carry out the programme intensively for the first 3 months.
We were surprised by the high and unexpected dropout rate in the endurance group, which has not been observed in previous studies.12,14,15 In contrast to previous investigations, our patients exercised individually without supervision and without fixed exercise appointments. We speculate that a supervised training programme may increase the motivation and may cause a reduced dropout rate.
Patients with CD have a reduced strength, even being in remission, compared with the general population.34 Our study showed an increase in strength in the upper and lower extremities for both training programmes after a short exercise time of only 3 months. We therefore assume that both types of exercise could cause beneficial effects on other comorbidities of CD patients such as osteopenia and osteoporosis, because muscle strength serves as a predictor of bone mineral density in CD.35 In addition, an increase in strength ameliorates fatigue, as recently shown by van Erp et al.19 Positive effects on other comorbidities such as fatigue, depression and the immune system have also been reported in other diseases such as cancer, mood disorders or cardiovascular diseases.1–4
Earlier studies have shown an increase in general wellbeing through physical activity.12,13,19 We could observe a significant difference only in the subclass emotional function in the endurance group. As stated before, in previous studies patients were supervised while exercising.12,13,19 Social support and increased exchange with other affected people and medical staff could be a cause for the significant increase in scores for general wellbeing not observed by us.36
Our study has several strengths. It is the first study to compare the effects of muscle training and endurance training on disease activity, strength and general wellbeing and includes a control group. From the beginning, the training programme was designed in a way that no supervision and contact with other people, investigators or sports therapists was necessary during the study. Patients could exercise individually on their own, at their preferred times and without dependence on training facilities. This may especially provide in the current Covid-19 situation a big advantage, as decreasing physical contacts for immunocompromised patients seems to be highly important.
A weakness of our study is the small study population. Because of the high dropout rate in the endurance group no significant statistical analysis could be performed and statements about the potential beneficial effects in this group are limited. Due to the small number of patients, the individual groups are not completely homogeneous and observation periods could have been extended also to determine a long-term effect on disease activity and other parameters. An improvement of disease activity would have been unlikely to be observed, as only patients with low CDAI were included. The implementation of the training programme was independently documented by the patients and could not be objectively verified by us. For future investigations a wearable tracking of the heart rate may be useful to monitor adherence. However, our study serves as a very good starting point for further studies in this field with a larger number of patients and also including patients with more active disease activity.
Conclusion
In conclusion, this study shows that both individual endurance and muscle training can be safely performed in patients with quiescent or mildly active CD. Muscle training appears to be more feasible for this patient group and should therefore be favoured. Both forms of exercise have beneficial effects on strength. Quality of life increased in both intervention groups, although statistical significance was only achieved for the subgroup emotional function in the endurance group. Further research in a larger population, also including patients with higher disease activity is needed to examine the effects of moderate exercise on disease activity, immunological changes and also comorbidities such as osteopenia in CD patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The study was performed according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Hesse Medical Association (FF101/201) on 19 September 2017.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a research award of the DCCV e.V. and the IBD DACH group.
Informed consent
Written, informed consent was obtained from each patient included in the study.
ORCID iDs
Jürgen Stein https://orcid.org/0000-0003-3558-3341
Axel Dignass https://orcid.org/0000-0002-9724-054X
References
- 1.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012; 104: 815–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuecher K, Bolling C, Vogt L, et al. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: a single-blind RCT. Support Care Cancer 2019; 27: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 3.Wegner M, Helmich I, Machado S, et al. Effects of exercise on anxiety and depression disorders: review of meta-analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets 2014; 13: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 4.Alves AJ, Viana JL, Cavalcante SL, et al. Physical activity in primary and secondary prevention of cardiovascular disease: overview updated. World J Cardiol 2016; 8: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres J, Ellul P, Langhorst J, et al. European Crohn’s and Colitis Organisation topical review on complementary medicine and psychotherapy in inflammatory bowel disease. J Crohns Colitis 2019; 13: 673–685e. [DOI] [PubMed] [Google Scholar]
- 6.Lo CH, Khalili H, Song M, et al. Healthy lifestyle is associated with reduced mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020; Epub ahead of print DOI: 10.1016/j.cgh.2020.02.047. [DOI] [PMC free article] [PubMed]
- 7.Mack DE, Wilson PM, Gilmore JC, et al. Leisure-time physical activity in Canadians living with Crohn disease and ulcerative colitis: population-based estimates. Gastroenterol Nurs 2011; 34: 288–294. [DOI] [PubMed] [Google Scholar]
- 8.Jones PD, Kappelman MD, Martin CF, et al. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis 2015; 21: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ 2013; 347: f6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Langenberg DR, Della Gatta P, Warmington SA, et al. Objectively measured muscle fatigue in Crohn’s disease: correlation with self-reported fatigue and associated factors for clinical application. J Crohns Colitis 2014; 8: 137–146. [DOI] [PubMed] [Google Scholar]
- 11.Jahnsen J, Falch JA, Mowinckel P, et al. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol 2003; 98: 1556–1562. [DOI] [PubMed] [Google Scholar]
- 12.Klare P, Nigg J, Nold J, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: a prospective randomized controlled trial. Digestion 2015; 91: 239–247. [DOI] [PubMed] [Google Scholar]
- 13.Loudon CP, Corroll V, Butcher J, et al. The effects of physical exercise on patients with Crohn’s disease. Am J Gastroenterol 1999; 94: 697–703. [DOI] [PubMed] [Google Scholar]
- 14.Ng V, Millard W, Lebrun C, et al. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin J Sport Med 2007; 17: 384–388. [DOI] [PubMed] [Google Scholar]
- 15.Tew GA, Leighton D, Carpenter R, et al. High-intensity interval training and moderate-intensity continuous training in adults with Crohn’s disease: a pilot randomised controlled trial. BMC Gastroenterol 2019; 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Global recommendations on physical activity for health Geneva: WHO, 2010. [PubMed]
- 17.de Souza Tajiri GJ, de Castro CL, Zaltman C. Progressive resistance training improves muscle strength in women with inflammatory bowel disease and quadriceps weakness. J Crohns Colitis 2014; 8: 1749–1750. [DOI] [PubMed] [Google Scholar]
- 18.Robinson RJ, Krzywicki T, Almond L, et al. Effect of a low-impact exercise program on bone mineral density in Crohn’s disease: a randomized controlled trial. Gastroenterology 1998; 115: 36–41. [DOI] [PubMed] [Google Scholar]
- 19.van Erp LW, Roosenboom B, Komdeur P, et al. Improvement of fatigue and quality of life in patients with quiescent inflammatory bowel disease following a personalized exercise program. Dig Dis Sci 2020; Epub ahead of print DOI: 10.1007/s10620-020-06222-5. [DOI] [PubMed] [Google Scholar]
- 20.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 21.Best WR, Becktel JM, Singleton JW, Jr, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 22.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- 23.Rose M, Fliege H, Hildebrandt M, et al. [ Validation of the new German translation version of the “Short Inflammatory Bowel Disease Questionnaire” (SIBDQ)]. Z Gastroenterol 2000; 38: 277–286. [DOI] [PubMed] [Google Scholar]
- 24.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 25.Kim WK, Kim DK, Seo KM, et al. Reliability and validity of isometric knee extensor strength test with hand-held dynamometer depending on its fixation: a pilot study. Ann Rehabil Med 2014; 38: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu ZL, Wang TR, Qiao YQ, et al. Handgrip strength index predicts nutritional status as a complement to body mass index in Crohn’s disease. J Crohns Colitis 2016; 10: 1395–1400. [DOI] [PubMed] [Google Scholar]
- 27.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stats 2005; 4: 287–291 [Google Scholar]
- 28.Peters HP, De Vries WR, Vanberge-Henegouwen GP, et al. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001; 48: 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riolo G, Towheed TE. Anti-tumor necrosis factor inhibitor therapy-induced dermatomyositis and fasciitis. J Rheumatol 2012; 39: 192–194. [PubMed] [Google Scholar]
- 30.Theodoraki E, Orfanoudaki E, Foteinogiannopoulou K, et al. Asymptomatic hyperCKemia during infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 1266–1271. [DOI] [PubMed] [Google Scholar]
- 31.Tew GA, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis 2016; 22: 2933–2942. [DOI] [PubMed] [Google Scholar]
- 32.Gatt K, Schembri J, Katsanos KH, et al. Inflammatory bowel disease [IBD] and physical activity: a study on the impact of diagnosis on the level of exercise amongst patients with IBD. J Crohns Colitis 2019; 13: 686–692. [DOI] [PubMed] [Google Scholar]
- 33.DeFilippis EM, Tabani S, Warren RU, et al. Exercise and self-reported limitations in patients with inflammatory bowel disease. Dig Dis Sci 2016; 61: 215–220. [DOI] [PubMed] [Google Scholar]
- 34.Wiroth JB, Filippi J, Schneider SM, et al. Muscle performance in patients with Crohn’s disease in clinical remission. Inflamm Bowel Dis 2005; 11: 296–303. [DOI] [PubMed] [Google Scholar]
- 35.Lee N, Radford-Smith GL, Forwood M, et al. Body composition and muscle strength as predictors of bone mineral density in Crohn’s disease. J Bone Miner Metab 2009; 27: 456–463. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira S, Zaltman C, Elia C, et al. Quality-of-life measurement in patients with inflammatory bowel disease receiving social support. Inflamm Bowel Dis 2007; 13: 470–474. [DOI] [PubMed] [Google Scholar]