Abstract
Background
Guidelines suggest computed tomography colonography (CTC) following incomplete optical colonoscopy (OC). Colon capsule endoscopies (CCE) have been suggested as an alternative, although completion rates have been unsatisfactory. Introduction of artificial intelligence (AI)-based localization algorithms of the camera capsules may enable identification of incomplete CCE investigations overlapping with incomplete OCs.
Objective
The study aims to investigate relative sensitivity of CCE compared with CTC following incomplete OC, investigate the completion rate when combining results from the incomplete OC and CCE, and develop a forward-tracking algorithm ensuring a safe completeness of combined investigations.
Methods
In this prospective paired study, patients with indication for CTC following incomplete OC were included for CCE and CTC. Location of CCE abortion and OC abortion were registered to identify complete combined investigations. AI-based algorithm for localization of capsules were developed reconstructing the passage of the colon.
Results
In 237 individuals with CTC indication; 105 were included, of which 97 underwent both a CCE and CTC. CCE was complete in 66 (68%). Including CCEs which reached most oral point of incomplete OC, 73 (75%) had complete colonic investigations; 78 (80%) had conclusive investigations. Relative sensitivity of CCE compared with CTC was 2.67 (95% confidence interval (CI) 1.76;4.04) for polyps >5 mm and 1.91 (95% CI 1.18;3.09) for polyps >9 mm. An AI-based algorithm was developed.
Conclusion
Sensitivity of CCE following incomplete OC was superior to CTC. Introducing and improving algorithm-based localization of capsule abortion may increase identification of overall complete investigation rates following incomplete OC.
ClinicalTrials.gov identifier: NCT02826993
Keywords: Colon capsule endoscopy, camera capsule endoscopy, computed tomography colonography, CT colonography, artificial intelligence, optical colonoscopy, colonoscopy, colon cancer, colorectal cancer, endoscopy
Introduction
Large bowel screening and the increased focus on bowel cancer symptoms has increased optical colonoscopy (OC) demands.1 Colon capsule endoscopy (CCE) is one way to reduce demands. Recent publications have indicated CCE investigations as feasible after incomplete OC,2–7 with completion rates as good as in unselected patients.8 The dissemination of CCE in clinical practice is slow, probably in part due to the high rate of incomplete investigations.9,10 An obstacle is the time used to investigate images from CCE, performed by specialized doctors. Studies indicate that CCE is preferred by patients compared with OC,11,12 and complication rates from CCE has been very low in intention-to-treat designed studies.13 Better bowel cleanliness and faster transit times should be aimed for, but a combination of OC and CCE results might also contribute in cases of CCE after incomplete OC.
Pathological findings have been shown to be frequent in patients with incomplete OCs,14 and CCE has been suggested instead of computed tomography colonography (CTC). Several publications have confirmed the feasibility and the advantages of this.2–8 CCE can be administered immediately after the OC attempt, and is more sensitive than CTC.4 Incomplete OC resulting from poor bowel cleanliness can be followed by a continued bowel preparation before CCE administration. European recommendations do suggest CCE on this indication.15
The fraction of incomplete CCEs is mostly a result of incomplete transit rather than poor bowel preparation.9 The bowel section missed in incomplete CCE is most often the anal part of the colon, whereas missed sections in incomplete OCs are the most oral part. It should be possible to reduce the fraction of incompletely investigated individuals by combining those two incomplete investigations, but it is necessary to ensure complete visualization with overlapping sections.
Development in image processing has propelled artificial intelligence (AI) solutions to estimate the location of capsules,16–18 which can provide the endoscopist with the position of the capsule and of any pathological process to be removed or biopsied.
We re-designed an AI-based algorithm for the localization of camera capsules and estimating its location by the means of statistical inference. The algorithm tracks the capsule backwards from the anus to the coecum. Every location estimation has some margin of error/uncertainties attached. These margins will accumulate as the point of interest gets further away from the starting point, meaning that the locations estimated will have the highest accuracy at the starting point and lowest at the coecum. By backwards tracking of the capsule’s movement we achieved an accuracy of ± 6 cm.16 The backwards tracking was preferred because the starting point is easy to identify. As the scope of this work is concerned with incomplete investigation, it is necessary to deploy the localization algorithm in a forward-tracking manner, as the anus might not have been observed/reached by the capsule.
We aimed to investigate the sensitivity of CCE compared with CTC in the case of incomplete OC, to investigate the completion rate for CCE in this population, the completion rate when combining results from the incomplete OC with an incomplete CCE, and to develop a forward-tracking algorithm to ensure safe completeness of the combined investigations.
Materials and methods
This prospective paired study assesses the relative sensitivity and diagnostic yield of CCE and CTC after incomplete OC, for the detection of all colorectal neoplasia sized >5 mm and >9 mm. After incomplete index colonoscopy, patients were informed of this study by a research nurse. Patients who did not meet the exclusion criteria and signed informed consent were included and underwent CCE and CTC. Patients with any neoplasia >9 mm or more than two polyps of any size identified by CCE, CTC and/or incomplete OC were referred to a therapeutic second OC in propofol sedation or general anaesthesia after an intensified bowel preparation. This risk stratification did not differ between indicating investigation type. The sample size was calculated with the assumption of non-inferiority between CCE and CTC. Prevalence of patients with at least one polyp/mass equal to or larger than 6 mm after an incomplete colonoscopy was assumed to be 10%. Non-inferiority was declared if the estimated difference between the diagnostic yield of CCE and CTC was ≤11%. To maintain that hypothesis as well as the type I error (α) of 5% and power (=1-β) of 80%, the required sample size was estimated to be 92 patients. Adding a dropout rate of 5% resulted in a total study size of 97 patients.
Population and flow of investigations
All adult patients that underwent an incomplete OC at our endoscopy unit in Nyborg, Denmark, from May 2016 until December 2018 with indication for CTC were possible candidates for this study. Exclusion criteria were previous gastrointestinal surgery (except for appendectomy), known inflammatory bowel disease, ostomy, diabetes, symptoms of bowel obstruction, pacemaker and/or severe kidney disease.
CCE was performed after a repeated bowel preparation, no earlier than 5 days past incomplete OC and no later than 66 days past incomplete OC (mean was 14 days). CTC was performed the day after CCE. CCE pictures were evaluated by Corporate Health International Hamburg, Germany.19 Patients were classified as no, low, medium or high risk based upon findings. Therapy and follow-up was organized accordingly.20 Reclassification of findings occurred on 12 occasions by the project surgeon, downgrading their risk from medium to low, usually in cases of more than two polyps which were smaller than 9 mm and located in the rectum or sigmoid.
Optical colonoscopy procedure
OCs were performed by the department’s doctors including formally trained trainees. In case of a difficult colonoscopy, an experienced doctor would assist. The standard colonoscope was Olympus Evis Exera III 190® (Olympus, Tokyo, Japan). Unit of Position Detection (UPD) was available. OCs were defined as complete when the coecum was reached.
CCE procedure
CCE investigations were performed with the second-generation CCE (PillCam2®, Medtronic, Minneapolis, MN, US). Bowel preparation procedures are included in Table 1. Images were uploaded and analysed by gastroenterologists, blinded to OC findings, with at least 2 years of experience, performed by Corporate Health International, Hamburg, Germany.19 They evaluated completeness of the investigation, quality of bowel cleansing, and number, size and location of neoplasia. CCE procedures were defined as complete when capsules were excreted within recording time and bowel cleanliness was graded as 2–4 by the Leighton–Rex scale. Relying on excretion rather than visualization of the hemorrhoidal plexus for complete investigations was chosen as a precautionary principle to ensure that capsules reached the lowest part of the rectum, as visualization could occur from the higher part.
Table 1.
Procedure bowel preparation for computed tomography (CT) colonography and colon capsule endoscopies (CCE), including booster regimen for CCE.
| Day | Procedure |
|---|---|
| −2 | 1000 mg oral magnesium-oxide 2 l of water 1000 mg oral magnesium-oxide |
| −1 | Clear fluids diet 1 l Moviprep + 2½ l of water |
| 0 | 1 l Moviprep + 1½ l of water 30 min fasting Capsule ingestion with 20 mg oral domperidon Booster: ¼ l Eziclen + 1 l of water Booster: ¼ l Eziclen + 1 l of water 10 mg rectal bisacodyl Clear fluids diet (incl. fruitless yoghurt, soup with no fillings, coffee/tea with milk and fizzy drinks) 50 ml Omnipaque mixed with non-carbonated fruit juice |
| 1 | CT colonography |
CTC procedure
CTC investigations were performed with a Siemens Somatom Definition Edge 64-slice CT scanner. Slice thickness was 1 mm, and reconstructions were made with slice thickness of 0.6, 1.0 and 2.0 mm. CTC was performed the day after CCE on the same bowel preparation (Table 1). Initially the colon was inflated with 3–4 l of CO2. Additional CO2 was inflated during the CTC to ensure sufficient distention. Before the CTC investigation, buscopan i.v. was administered. Scans were evaluated by certified abdominal radiologists (Department of Radiology of Odense University Hospital), blinded to OC and CCE findings. They evaluated the quality of the bowel cleanliness as well as number, size and location of neoplasia. CTCs were defined as complete when accepted by the radiologists, who would clearly mark the patient journal in the case that bowel preparation had been insufficient or parts of the colon had not been properly visualized.
Statistical analysis
Data were collected prospectively during investigations and entered into a secure database using RedCap® (REDCap consortium, Vanderbilt, the Netherlands). All data management and descriptive statistics were conducted using SAS 9.4 (SAS, Gary, North Carolina). Relative sensitivity and diagnostic yield were calculated using principles of Cheng and Maculuso.21 Diagnostic yields were defined as the proportions of patients with at least one polyp >5 mm and >9 mm respectively. Analyses of polyp sensitivity in CCE compared with CTC were conducted per patient. As follow-up second colonoscopy was not performed in all patients, calculations of relative sensitivity compared the number of patients with a polyp >5 mm and >9 mm, respectively, identified from CCE with the number of patients with a polyp >5 mm and >9 mm, respectively, identified from CTC. Thereby the CTC investigations were the reference. Subgroup analysis of patients undergoing a second follow-up colonoscopy was omitted, as such calculations would be very sensitive to selection bias.
Developing the algorithm
For the reconstruction of the large intestine we follow the model proposed by Herp et al.16 This model assumes a cylinder shape of the bowel. The movement and orientation of the capsule can be estimated by sampling the radius of the cylinder from a prior distribution and matching features points between consecutive frames. The algorithm was composed by finding feature points between consecutive frames (between any two frames, the same ‘objects’ were found and mapped). Next these features were translated to movement, while still assuming a cylindrical shape of the bowel. Last, this procedure was repeated for the length of the investigation and prior distributions, a point-wise classifier was then used to predict the end of the investigation. Whereas the framework of the original model intents to begin the reconstruction from the anus,16 we adapted it to start the reconstruction from the entrance to the large intestine, which can be applied for capsule investigations not reaching the rectum before battery depletion. As this approach is based on randomly sampling from prior distributions on the radius of the cylinder and sampling frequency of the capsule, the outcome of the reconstruction model is given in terms of the probability of the reconstruction ending in specific sections of the colon. We divided the colon into the following sections: right colon, transverse colon, left colon (descending and sigmoid) and rectum, and we identified right and left flexure as points of reference. The original work was evaluated on the ground truth established by an expert panel, in which two internationally recognized experts, two medical doctors from Odense University Hospital and an additional expert evaluation from Corporate Health International formed the panel.19
Ethics
Participants were informed orally and in writing by a research nurse, and signed an informed consent form before inclusion. They could withdraw their consent at any time without consequences for further diagnostics or treatment. There was no financial gain for the participants. The study was approved by the regional ethics committee (S-20150140) and the Danish Data Protection Agency (16/16125). The study was registered with ClinicalTrials.gov (identifier: NCT02826993).
Results
Out of 237 patients with incomplete index colonoscopy, 105 individuals were included. Eight withdrew their consent to participate. Ninety-seven underwent both CCE and CTC. CCE was complete in 66 patients and CTC in 93. Thirty-two individuals were referred for a second colonoscopy. Twenty-eight were performed and all were complete (Figure 1). Three of these individuals had stenosis as reason for incomplete index colonoscopy. Four OCs were not initiated due to patient preference (n = 3) or downgrading of risk by surgeon (n = 1). Indication for index colonoscopy was mostly positive faecal immunochemical test (FIT) from screening or symptoms. The majority had an incomplete index colonoscopy because of pain or no progression. In 53% the scope did not reach beyond the sigmoid colon (Table 2). CCE bowel cleanliness was reported acceptable, good or perfect in 76%, and poor or unacceptable in 22%.
Figure 1.
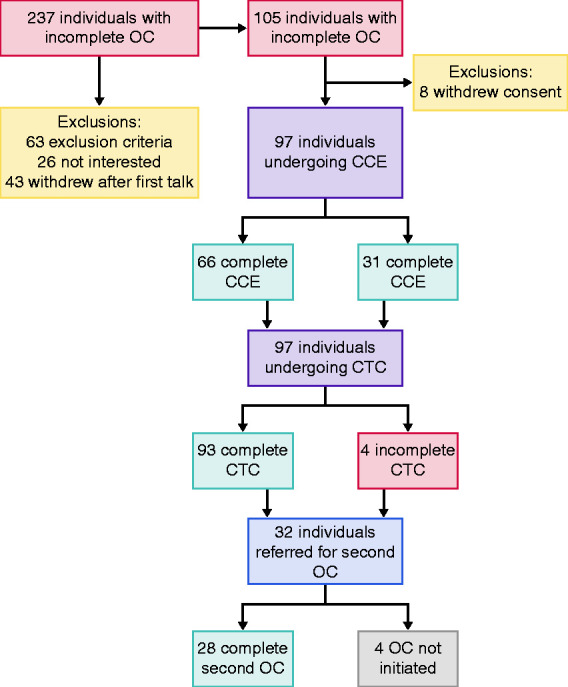
Flow chart.
CCE: colon capsule endoscopies; CTC: computed tomography colonography; OC: optical colonoscopy.
Table 2.
Baseline characteristics.
| Frequency (%) | |
|---|---|
| Gender | |
| Female | 72 (74) |
| Male | 25 (26) |
| Age | |
| <50 | 17 (18) |
| 50–59 | 24 (25) |
| 60–69 | 27 (28) |
| >69 | 29 (30) |
| Indication for index colonoscopy | |
| Follow-up after previous surgery | 6 (6) |
| Screening | 41 (42) |
| Symptoms | 50 (52) |
| Reason incomplete index colonoscopy | |
| Pain | 39 (40) |
| Severe angulations | 22 (23) |
| Looping | 22 (23) |
| Stenosis | 4 (4) |
| Redundant colon | 5 (5) |
| Suspected adhesions | 3 (3) |
| Not specified/Other technical challenges | 2 (2) |
| Location of abortion of index colonoscopy | |
| Rectum | 10 (10) |
| Sigmoideum | 41 (42) |
| Descendens | 19 (20) |
| Splenic flexure | 5 (5) |
| Transversum | 11 (11) |
| Hepatic flexure | 6 (6) |
| Ascendens | 5 (5) |
Complete investigations
CCE was complete in 66 (68%) individuals; 73 (75%) including CCE investigations that overlapped with the most oral site reached by the incomplete index colonoscopy. In the remaining 24 individuals with incomplete CCE investigations, five had conclusive investigations and patients were referred to therapeutic colonoscopy, and the last 19 patients had no findings at the following complete CTC. In total, 78 (80%) individuals had a conclusive investigation after incomplete index colonoscopy and complete or incomplete CCE. Overview of abort locations from index colonoscopy and CCE for participants with incomplete CCE is provided in Table 3.
Table 3.
Locations of abortion per patient in OC and CCE in 31 individuals with incomplete CCE.
|
|
|
CCE abort location |
||||
|---|---|---|---|---|---|---|
| Rectum | Left colon | Transverse colon | Right colon | Small bowel | ||
| OC abort location | Rectum | a1 | 4 | 0 | 0 | 0 |
| Sigmoideum | 0 | b3 | 1 | 5 | 1 | |
| Descending colon | 0 | 3 | 0 | 5 | 0 | |
| Splenic flexure | 0 | 0 | 0 | 0 | 0 | |
| Transverse colon | 1 | 2 | 0 | 0 | 0 | |
| Hepatic flexure | 0 | 3 | 0 | 0 | 0 | |
| Ascending colon | 0 | 0 | 0 | 1 | 1 | |
CCE: colon capsule endoscopies; OC: optical colonoscopy.
a:  Green coloured fields mark overlapping colon investigations
Green coloured fields mark overlapping colon investigations
b:  Yellow coloured fields mark possible overlapping colon
investigation
Yellow coloured fields mark possible overlapping colon
investigation
Diagnostic yield
Overall, 19 polyps >5 mm were identified by CTC (0.2 per participant) and 71 polyps >5 mm by CCE (0.7 per participant). CTC found polyps in two individuals who had no findings from CCE. CCE found polyps in 49 individuals who had no findings from CTC. CTC found polyps over 5 mm in 15 participants, of whom 14 underwent a second colonoscopy confirming 10 of them. CTC found polyps over 9 mm in 11 participants, of whom 10 underwent a second colonoscopy confirming four of them. CCE found polyps over 5 mm in 40 participants, of whom 25 underwent a second colonoscopy confirming 15 of them. CCE found polyps over 9 mm in 21 participants, of whom 18 underwent a second colonoscopy confirming five of them. A total of 19 polyps >9 mm from 13 participants were not confirmed by second colonoscopy (Figure 2). The diagnostic yield of CTC was 15.5% (95% confidence interval (CI) 8.9; 24.2) for polyps >5 mm and 11.3% (95% CI 5.8; 19.4) for polyps >9 mm. The diagnostic yield of CCE was 41.2% (95% CI 31.34; 51.7) for polyps >5 mm and 21.6% (95% CI 13.9; 31.2) for polyps >9 mm. The relative sensitivity of CCE compared with CTC was 2.67 (95% CI 1.8; 4.0) for polyps >5 mm and 1.91 (95% CI 1.2; 3.1) for polyps >9 mm (Table 4).
Figure 2.
False positive polyps estimated >9 mm from colon capsule endoscopy investigations.
The figure includes all findings from colon capsule endoscopy identified as polyps >9 mm, from patients who had no polyps >9 mm found in therapeutic optical colonoscopy.
Table 4.
Diagnostic yield of CCE and CTC for polyps >5 mm and for polyps >9 mm.
| Diagnostic yield % (95% CI) | Relative Sensitivity (95% CI) | |
|---|---|---|
| >5 mm | ||
| CCE | 41.24 % | 2.67 |
| 31.34 %;51.69 % | 1.76;4.04 | |
| CTC | 15.46 % | |
| 8.92 %;24.22 % | ||
| >9 mm | ||
| CCE | 21.65 % | 1.91 |
| 13.93 %;31.17 % | 1.18;3.09 | |
| CTC | 11.34 % | |
| 5.80 %;19.39 % |
CCE: colon capsule endoscopies; CI: confidence interval; CTC: computed tomography colonography.
In 31 patients with incomplete CCE, 10 individuals were referred to second colonoscopy. Two had polyps detected by index colonoscopy whereas eight of them were detected by CCE. Two had findings in CTC as well, but none of the 31 patients were referred to second colonoscopy because of CTC findings alone. In the study population of 97 individuals, one patient was referred to second colonoscopy exclusively based on findings from CTC. This individual had a 7 mm polyp indicated in the sigmoid colon by CTC, but the polyp was not seen in second colonoscopy. No adverse events occurred during the trial.
Colon capsule located by algorithm
To benchmark the automated localization of CCE, we compared the model output with a ground truth established by physicians. Possible outputs were right colon, transverse colon, left colon (descending and sigmoid) and rectum. We distinguished, for each patient, between the capsule’s two camera heads (CH1 and CH2). The physician’s assessment was directly compared with the model output. Figure 3 shows the probability of ending an investigation in the sections. Each line represents a camera head’s relative position at the end of the investigation. The misclassification is never larger than one section. For the one case of rectum, the algorithm is not able to identify this section and therefore places the end of the investigation in the left colon. Overall, the agreement between ground truth and model assessment has a mean value of 77% and a median of 85%.
Figure 3.
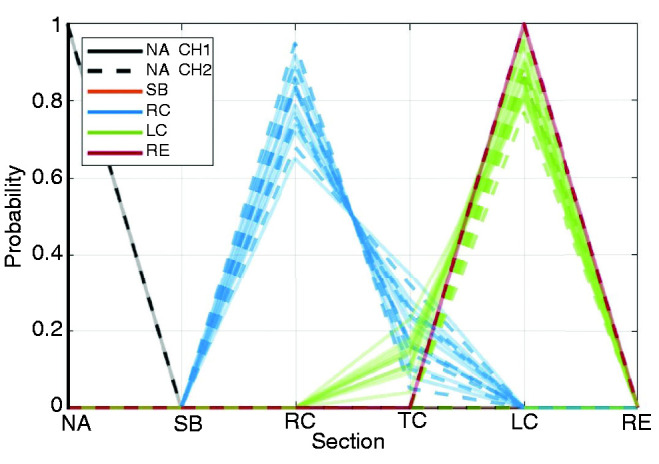
Probability of reconstructions ending in a given section.
Coloured according to doctor’s assessment and line style by camera head. Not available (NA), Small Bowel (SB), Right Colon (RC), Transverse Colon (TC), Left Colon (LC), Rectum (RE).
Discussion
CCE was complete in 66 patients, but overlapping investigations enabled complete visualization of the colon mucosa in 73. This may be low compared with other study populations, but all patients meeting inclusion criteria were included if no exclusion criteria were present. No patients were excluded because of non-compliance to bowel preparation, constipation or history of poor bowel preparation. The population age was high compared with previous studies, and risk of incomplete CCE may be increased as much as fivefold in those over 50 after incomplete OC.5 Some 83% of the study population were 50 years of age or older. The definition of complete CCE was capsule excretion within recording time. A definition accepting CCE as complete when the hemorrhoidal plexus has been visualized could potentially increase completion rate, although at the time of study initiation a precautionary principle seemed logical as information on CCE in this population was limited.
Seven individuals were categorized as potentially having a complete visualization of colonic mucosa; six of them as their CCE was reported to abort in the left colon, while their OC reached splenic flexure, descending colon or sigmoideum. One individual’s investigations were potentially overlapping as the CCE and the OC both aborted in the right/ascending colon (table 3). These individuals may well have had a complete visualization, but the limited localization accuracy of CCE abortion does not assure this. Therefore, further improvement of the algorithm, enabling recognition of landmarks (e.g. splenic flexure), will increase the rate of identified overlapping investigations. When the algorithm can safely identify the flexures as landmarks, the accumulated margins of error could be reset, thereby increasing the accuracy of following landmarks. Individuals with incomplete CCE, but with a definite investigation of right and transverse colon, may only need OC to advance as far as the splenic flexure. The location of OC abortion was in this study indicated as rectum, sigmoideum, descending colon, splenic flexure, transverse colon, hepatic flexure or ascending colon by the endoscopists. The accuracy of this location could also be improved by either dye marking or clipping at the OC abort location.5
Patients underwent a new bowel preparation before undergoing CCE in our study. Bowel preparation is associated with discomfort,22,23 and it may be preferable to conduct the CCE shortly after the incomplete OC with no additional bowel preparation. This has been tested, and completion rates were not significantly lower compared with group undergoing a new bowel preparation.8
A limitation to our study was that not all individuals have undergone a following complete OC to confirm findings from CCE and CTC, enabling us to calculate specificity, but this would increase the risk of unnecessary adverse events in patients and was therefore omitted. The number of polyps identified by CCE may be overestimated if the CCE identifies the same polyp more than once because of backwards progression of the capsule. This has not affected relative sensitivities and diagnostic yields reported, as these are calculated per patient and not per polyp. An AI algorithm to estimate the risk of two seen polyps being the same could help out in this matter.24
Estimated polyp sizes may differ between CCE, CTC, OC and pathology.24,25 In this study, CCE often identified larger polyp sizes than reported in pathology reports following polypectomy. If CCE identifies polyps <6 mm, but estimates their diameter to be greater than 5 mm, the relative sensitivity would be affected. CTC are less reliable for polyps <6 mm,26 and may not report those polyps. CTC should be more sensitive to larger polyps, but recent results show a higher sensitivity even to large polyps by CCE compared with CTC.27
Our study shows that CCE can safely be performed, and is a more sensitive investigation compared with CTC, with a relative sensitivity of 1.91 for polyps >9 mm and 2.67 for polyps >5 mm. The results confirm previous findings from Spada and colleagues, who report relative sensitivities of 1.67 for polyps ≥10 mm and 2.0 for polyps ≥6 mm,4 and a high rate of patients could be spared radiation from CTC. Our study showed a higher diagnostic yield than did the findings of the Italian study,4 which is possibly because of differences in methods. Spada and colleagues performed a second follow-up colonoscopy in all individuals with at least one polyp ≥6 mm, enabling them to identify false positives, which was not possible in the current study. Relative sensitivity was significantly increased for CCE, especially for polyps >5 mm. Sensitivity in CTC versus CCE has previously also been reported to be equal,28 but in a population with initial incomplete OC, CCE seems superior. Optimizing the bowel preparation regimen could potentially increase CCE completion rate, leading to an even higher superiority of CCE compared with CTC.
Conclusion
Sensitivity of CCE following incomplete OC was superior to CTC. The rate of complete CCE must be improved. The introduction and improvement of AI-based localization of capsule abortion may increase identification of complete colonic mucosa visualization rates in individuals with incomplete OC followed by incomplete CCE. To develop a more accurate forward-tracking algorithm, more investigations have to be included in the training of the algorithm, but we have demonstrated the feasibility of producing such an algorithm.
Supplementary Material
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Ethics approval
The study was approved by the regional ethics committee (S-20150140) and the Danish Data Protection Agency (16/16125). The study was registered with ClinicalTrials.gov (identifier: NCT02826993).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Medtronic® (Minneapolis, MN, US) provided the capsules, but had no influence on study protocol, analysis or interpretation of the results.
Informed consent
Participants were informed orally and in writing by a research nurse, and signed an informed consent form before inclusion. They could withdraw their consent at any time without consequences for further diagnostics or treatment. There was no financial gain for the participants.
ORCID iDs
Supplemental material
Supplemental material for this article is available online.
References
- 1.Greuter M, De Klerk CM, Meijer GA. Screening for colorectal cancer with fecal immunochemical testing with and without postpolypectomy surveillance colonoscopy: A cost-effectiveness analysis. Ann Intern Med 2017; 167: 11. [DOI] [PubMed] [Google Scholar]
- 2.Baltes P, Bota M, Albert J, et al. PillCamColon2 after incomplete colonoscopy - A prospective multicenter study. World J Gastroenterol 2018; 24: 3556–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pioche M, Ganne C, Gincul R, et al. Colon capsule versus computed tomography colonography for colorectal cancer screening in patients with positive fecal occult blood test who refuse colonoscopy: A randomized trial. Endoscopy 2018; 50: 761–769. [DOI] [PubMed] [Google Scholar]
- 4.Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: A prospective, comparative trial. Gut 2015; 64: 272–281. [DOI] [PubMed] [Google Scholar]
- 5.Hussey M, Holleran G, Stack R, et al. Same-day colon capsule endoscopy is a viable means to assess unexplored colonic segments after incomplete colonoscopy in selected patients. United European Gastroenterol J 2018; 6: 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alarcon-Fernandez O, Ramos L, Adrian-de-Ganzo Z, et al. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol 2013; 11: 534–540. [DOI] [PubMed] [Google Scholar]
- 7.Nogales O, Garcia-Lledo J, Lujan M, et al. Therapeutic impact of colon capsule endoscopy with PillCam COLON 2 after incomplete standard colonoscopy: A Spanish multicenter study. Rev Esp Enferm Dig 2017; 109: 322–327. [DOI] [PubMed] [Google Scholar]
- 8.Triantafyllou K, Viazis N, Tsibouris P, et al. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc 2014; 79: 307–316. [DOI] [PubMed] [Google Scholar]
- 9.Kroijer R, Dyrvig AK, Kobaek-Larsen M, et al. Booster medication to achieve capsule excretion in colon capsule endoscopy: A randomized controlled trial of three regimens. Endosc Int Open 2018; 6: E1363–E1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobaek-Larsen M, Kroijer R, Dyrvig AK, et al. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Colorectal Dis 2018; 20: 479–485. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen MK, Baatrup G, Petersen C, et al. Screening individuals’ experiences of colonoscopy and colon capsule endoscopy; a mixed methods study. Acta Oncol 2019: 1–6. [DOI] [PubMed]
- 12.Negreanu L, Babiuc R, Bengus A, et al. PillCam Colon 2 capsule in patients unable or unwilling to undergo colonoscopy. W J Gastrointest Endosc 2013; 5: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spada C, Hassan C, Marmo R, et al. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol 2010; 8: 7. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: A population-based case-control study. Ann Intern Med 2012; 157: 8. [DOI] [PubMed] [Google Scholar]
- 15.Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2012; 44: 527–536. [DOI] [PubMed] [Google Scholar]
- 16.Herp J, Majtner T, Buijs MM, et al. Movement estimation for capsule endoscopy videos based on feature points tracking. Submitted to Artificial Intelligence in Medicine 2020.
- 17.Wahid K, Kabir SML, Khan HA, et al. A localization algorithm for capsule endoscopy based on feature point tracking. In: 2016 International Conference on Medical Engineering, Health Informatics and Technology (MediTec). IEEE, 2016, pp. 1–5.
- 18.Bao G, Pahlavan K, Mi L. Hybrid localization of microrobotic endoscopic capsule inside small intestine by data fusion of vision and RF sensors. IEEE Sensors J 2015; 15: 10. [Google Scholar]
- 19.Buijs MM, Kroijer R, Kobaek-Larsen M, et al. Intra and inter-observer agreement on polyp detection in colon capsule endoscopy evaluations. United European Gastroenterol J 2018; 6: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan C, Quintero E, Dumonceau J, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013; 45: 10. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Macaluso M. Comparison of the accuracy of two tests with a confirmatory procedure limited to positive results. Epidemiology 1997; 8: 3. [DOI] [PubMed] [Google Scholar]
- 22.Restall G, Michaud V, Walker JR, et al. Patient experiences with colonoscopy: A qualitative study. J Can Assoc Gastroenterol 2019; Epub: 8. [DOI] [PMC free article] [PubMed]
- 23.Sharara AI, El Reda ZD, Harb AH, et al. The burden of bowel preparations in patients undergoing elective colonoscopy. United European Gastroenterol J 2016; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanes-Vidal V, Nadimi ES, Buijs MM, et al. Capsule endoscopy vs. colonoscopy vs. histopathology in colorectal cancer screening: Matched analyses of polyp size, morphology, and location estimates. Int J Colorectal Dis 2018; 33: 1309–1312. [DOI] [PubMed] [Google Scholar]
- 25.de Vries AH, Bipat S, Dekker E, et al. Polyp measurement based on CT colonography and colonoscopy: Variability and systematic differences. Eur Radiol 2010; 20: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickhardt PJ. Missed lesions at CT colonography: Lessons learned. Abdom Imaging 2013; 38: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utano K, Katsuki S, Matsuda T, et al. Colon capsule endoscopy versus CT colonography in patients with large non-polypoid tumours: A multicentre prospective comparative study (4CN Study). Digestion 2019; Epub: 9. [DOI] [PubMed]
- 28.Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenter Hepatol 2014; 12: 1303–1310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.