Abstract
The increasing knowledge on ulcerative colitis’ pathophysiology has contributed to the expansion of the therapeutic arsenal for this condition. However, to date, 25–40% of patients with ulcerative colitis remain primary or secondary non-responders to therapy, and up to 10% need to eventually undergo a colectomy. Janus kinase inhibitors block cytokine signalling involved in the pathogenesis of several inflammatory conditions. Tofacitinib is the first drug of this class approved for moderate-to-severely active ulcerative colitis in patients for whom disease worsened and those who did not improve with conventional therapy (aminosalicylates, corticosteroids and immunosuppressants) or monoclonal antibodies. We aimed to review the main aspects and concerns related to the current use of tofacitinib and to explore its future application.
Keywords: Case report, inflammatory bowel disease, Janus kinase inhibitors, tofacitinib, ulcerative colitis
Case report
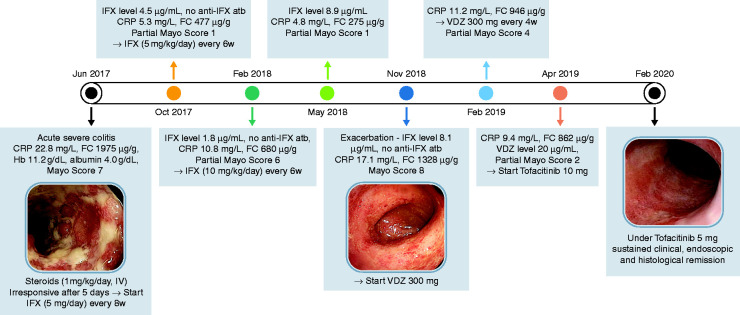
A 29-year-old Caucasian woman, without previous medical records of interest, was admitted to the emergency service, in June 2017, with a 7-week history of mild colicky abdominal pain (relived by defaecation) and bloody stools (6–8 times per day). In the last 4 weeks, she gradually developed rectal tenesmus and lost 2 kg; at hospital admission, she had fever (38.1°C). Faecal calprotectin (FC) was 1975 µg/g (considered significantly elevated for values >250 µg/g); stool culture, cytomegalovirus (CMV) and the Clostridium difficile toxin test were negative. The endoscopic assessment showed confluent deep ulcers until splenic flexure and the histological analysis revealed the presence of ulcer, corresponding to a Geboes score 5.4 (Figure 1). Therefore, the patient was diagnosed with acute severe ulcerative colitis (UC) and started intravenous steroids (1 mg/kg/day). By the fifth day of hospitalization, the patient was apyretic and painless, but maintained seven stools per day, with blood in less than half of them – severe according to the Truelove and Witts index. Therefore, infliximab (IFX) 5 mg/kg/day was added (at 0, 2 and 6 weeks). After 15 days, the patient was free of rectal tenesmus and achieved a partial Mayo score = 2; C-reactive protein (CRP) was 4.7 mg/l (normal value <3 mg/l) and FC was 1013 µg/g.Fourteen weeks after starting IFX, she was asymptomatic and FC decreased to 477 µg/g; the IFX level was 4.5 µg/ml (normal trough levels 3–7 µg/ml), antibodies targeting IFX could not be detected and the endoscopic Mayo score = 1. In this context, it was decided to shorten the time between infusions to 6 weeks. Eight months after starting IFX, the patient lost response. Thus, the IFX dose was maximized to 10 mg/kg/day, every 6 weeks. Four months later, the IFX levels were 8.9 µg/ml, and the patient was in clinical and endoscopic remission. After 6 months, the patient asked for an earlier medical appointment and reported abdominal cramping and 5–6 blood-streaked loose stools per day, in the previous week. The blood analysis revealed an increase in CRP (17.1 mg/l); FC was 1328 µg/g, and IFX levels were 8.1 µg/ml. Endoscopy revealed continuous colonic inflammation with marked erythema, absent vascular pattern and friability, and erosions without ulceration; corresponding to Mayo score = 8. In this context, the patient started vedolizumab (VDZ) 300 mg. Eight weeks later, she was free from abdominal pain but maintained 4–5 stools per day, of which 50% with visible blood (partial Mayo score = 3). The dosing frequency was adjusted to every 4 weeks and trough level was 20 µg/ml, 4 weeks later. In this context, it was decided to stop VDZ and start tofacitinib 10 mg, twice daily. Six days after treatment start, the patient was free from pain and had 3 daily bowel movements without blood; FC decreased to 195 µg/g. The induction treatment with tofacitinib went uneventfully; slight and transitory changes in the lipid profile were observed (maximum levels of total cholesterol and triglycerides of 219 and 183 mg/dl, 4 weeks after treatment start), no other relevant analytical changes were observed. After 8 weeks the patient was in clinical, endoscopic and histological (Geboes Score [GS] = 2.1) remission.Since July 2019, the patient has been receiving tofacitinib 5 mg, twice daily, for maintenance, and remains free of symptoms and endoscopic activity.
Figure 1.
Clinical case summary. FC: faecal calprotectin; IFX: infliximab; VDZ: vedolizumab.
Janus kinase (JAK) inhibitors, a new therapeutic class
The therapeutic arsenal for inflammatory bowel disease (IBD) was much increased in recent decades. Currently, several alternatives are available among which are immunosuppressors and monoclonal antibodies targeting tumour necrosis factor (TNF)-α, α4β7integrin or anti-interleukins 12/23. This target diversity suggests that the pathogenesis underlying IBD is complex and depends on several molecular pathways.1 The JAK family comprises JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). This family of cytoplasmic protein tyrosine kinases has an important role in the transduction pathway of several pro-inflammatory cytokines known to play a role in IBD (interleukin (IL) 2, 4, 7, 9, 13, 15, 17, 21 and 23, but also interferons and growth factors)2 and binding is mediated through JAK1 and JAK3.3 When cytokines bind to their cell-surface receptors the ligand receptors dimerize, resulting in the phosphorylation of JAK molecules, which then trigger intracellular signal transduction and transcription. JAK inhibitors prevent the phosphorylation of the signal transducer and activator of transcription (STAT) protein, thereby decreasing the synthesis of pro-inflammatory proteins.4 Besides this ‘broad-spectrum’ anti-inflammatory activity, JAK inhibitors present several advantages in comparison with monoclonal antibodies: (a) oral administration; (b) predictable pharmacokinetics with a reduced plasma half-life, rapid onset of action and quick clearance (beneficial in cases of severe infections and need for surgery); (c) intracellular target; and (d) lack of immunogenicity.5,6
Notwithstanding, it must be taken into account that the inhibition of each type of JAK blocks the signalling of a particular subset of cytokines, influencing both efficacy and safety7 and that broad cytokine inhibition may lead to unwanted off-target activity and adverse effects. It has been suggested, for example, that the inhibition of JAK2 may be related to unfavourable haematological side effects due to the inhibition of erythropoietin, IL-3, IL-5, prolactin and growth hormone – prompting the development of more selective JAK inhibitors.8
To date, tofacitinib is the unique JAK inhibitor approved for UC. Even though this small molecule was developed to be a JAK3-specific inhibitor, it also has affinity for JAK1 and JAK2, being a pan-JAK inhibitor.8 Several other molecules are currently in phase 3 trials for UC: the selective JAK1 inhibitors filgotinib (SELECTION study, NCT02914522) and upadacitinib (U-ACCOMPLISH study, NCT03653026; U-ACHIEVE study, NCT02819635) and the pan-JAK inhibitor TD-1473 (RHEA Study, NCT03758443).9
Besides UC, tofacitinib is also approved for rheumatological and dermatological conditions and very relevant safety information comes from these settings. In 2012 this drug was initially approved for moderate-to-severe active rheumatoid arthritis (RA); in 2017 it was also approved for the treatment of active psoriatic arthritis.
Efficacy - which UC patients are candidates for JAK inhibitors?
The OCTAVE trials, the results of which were published in 2017, aimed to assess the efficacy and safety of tofacitinib, an oral reversible pan-JAK inhibitor, in two settings of moderate-to-severe active UC: (a) patients refractory to previous conventional therapy; and (b) patients previously treated with a TNF-α antagonist. In these trials conventional immunosuppressors were suspended at baseline, allowing the exploration of the usefulness of tofacitinib as single treatment. In the OCTAVE Induction 1 trial, tofacitinib 10 mg given twice daily induced remission in 18.5% of UC patients (versus 8.2% for placebo; p = 0.007), while In OCTAVE Induction 2 remission occurred in 16.6% (versus 3.6%; p<0.001) at week 8. In both trials the therapeutic effect of tofacitinib 10 mg twice daily was similar between patients who had previously received a TNF antagonist and those who had not.10 In the OCTAVE Sustain, remission at 52 weeks occurred in 34.3% of the patients in the 5 mg tofacitinib group and 40.6% in the 10 mg tofacitinib group (versus 11.1% of placebo; p<0.001).10 In June 2018, tofacitinib was approved for the treatment of adult patients with moderate-to-severe active UC with an inadequate response, loss of response or intolerance to corticosteroids, immunosuppressive agents and/or biological therapies.6 The benefits of this drug as induction therapy may be observed as soon as after 3 days of treatment, with improvement in the frequency of bloody stools.11 In addition, tofacitinib improved health-related quality of life from week 4 and this effect was sustained for at least 52 weeks.12
In the face of the current evidence the clinical guidelines of the American College of Gastroenterology,13 published in March 2019, went further and recommended the use of tofacitinib in three different settings: as first-line therapy in TNF-α naïve patients; as a second line in those who had already received TNF-α; and as a third line in those refractory to VDZ. The recommendations of the European Crohn’s and Colitis Organization are awaited.
The real-world effectiveness of tofacitinib was confirmed in three retrospective studies14–16 and a recent prospective trial.17
Positioning tofacitinib in current treatment strategies
Tofacitinib is the first IBD therapy with rapid onset of action, reducing symptoms as early as 3 days, and thus may have a role in patients who have failed first-line therapy (intravenous corticosteroids and immunosuppressors) for acute moderate-to-severe UC.11 In addition, due to the rapid clearance, in case of emergent colectomy, adverse surgical outcomes may be less likely.18 Also, tofacitinib has robust efficacy, including in patients who have already failed corticosteroids, thiopurines or anti-TNF.10 Indeed, in the OCTAVE induction studies the difference in clinical remission between tofacitinib 10 mg and placebo was 12.3% (95% confidence interval (CI), 5.0–19.5), for UC patients without previous anti-TNF failure, and 10.6% (95% CI, 7.3–13.9) for anti-TNF failures.19 Regarding maintenance with 5 mg, the difference from placebo of sustained steroid-free remission was 35.2% (95% CI, 19.4–50.9) in previous non-failures, and 17.5% (95% CI, –3.8–38.7) in patients who failed anti-TNF therapy.19
JAK/STAT pathway inhibitors (differently from TNF, integrins and interleukin inhibitors) are capable of simultaneously blocking multiple cytokines, allowing a more extensive resolution of intestinal inflammatory response. However, to date, no UC head-to-head clinical trials comparing biologics with tofacitinib exist. However, a network meta-analysis compared the efficacy and safety of several first-line (biologic-naïve) and second-line (previous exposure to anti-TNF) therapies for moderate-severe UC.20 In this study, IFX and VDZ were ranked highest as first-line agents, while, among patients with prior anti-TNF exposure, tofacitinib was ranked highest both for induction of clinical remission (odds ratio (OR) 11.88, 95% CI 2.32–60.89) and mucosal healing (OR 4.71, 95% CI 2.23–9.92). Regarding maintenance of remission, tofacitinib and VDZ were comparable.20
Potential risks associated with tofacitinib therapy
Infections
An open-label extension study was performed to assess the safety of tofacitinib 5 mg or 10 mg, twice a day, in the treatment of RA for up to 9.5 years. In this study, stable background therapy was continued and adjustments were allowed at the investigators’ discretion.21 The severity of the adverse effects was mostly mild (59%) or moderate (36%). The most common events were infections (9.0%), of which 11.3% were caused by herpes zoster (HZ). All infection-related events had incidence ratios (IRs) below 0.5; the exceptions were combined serious infections (IR of 2.4) and HZ (IR of 3.4).
In the IBD setting, the pooled analysis of the UC trials with tofacitinib (completing 4.4 years of follow-up) revealed that the occurrence of opportunistic infections, excluding HZ, was small and comparable with placebo. The trials reported one case each of CMV colitis, CMV hepatitis, histoplasmosis and pulmonary cryptococcosis.22 The IR of serious non-HZ infections was similar to that found in the placebo group, and included appendicitis (n = 4), anal abscess (n = 2) and Clostridium difficile infection (n = 2).22 On the other hand, a higher dose-related incidence of HZ infection was reported. Indeed, in comparison with placebo, a 5 mg twice-daily maintenance dose of tofacitinib had an IR of HZ of 2.1 (95% CI, 0.4–6.0), while a 10 mg twice-daily dose had an IR of 6.6 (95% CI, 3.2–12.2).23
The incidence of HZ infection may be decreased with vaccination, yet the use of attenuated-live vaccines is contraindicated in patients on immunosuppressants. Recombinant vaccines have appeared as a sound alternative, preventing the risk of disease due to the replication of the attenuated virus.24 A recent recombinant HZ vaccine was tested in phase 1 and 2 clinical trials enrolling immunosuppressed patients.25,26 Its efficacy was also confirmed in a phase 3 trial enrolling patients above 50 years old in whom the risk of HZ infection was reduced by 97.2% (95% CI 93.7–99.0).24
The IR of tuberculosis (0.02–0.75)27 and hepatitis B (no reactivations)28 appears to be low in tofacitinib trials enrolling RA patients. Notwithstanding, testing for those infections and, when needed, implementing prophylactic therapy is recommended before tofacitinib administration, as occurs with biologics.23
Malignancies
In the extension study of tofacitinib safety on RA, the overall risk of malignancies was low (IR ≤ 0.9%),21 a significant value even more after taking into account that these patients have an inherent predisposition to cancer due to their underlying disease.
In the IBD setting, non-melanoma skin cancer (NMSC) was the most frequent malignancy, occurring in six UC patients in the OCTAVE trials.29 This finding is consistent with the observations reported for the use of tofacitinib in other indications like RA.21 However, it is important to consider that all UC patients with NMSC had prior exposure to thiopurines and some had prior history of NMSC. More recently, in the global analysis of UC induction and maintenance trials, including the study A3921063 (NCT00787202)30 and the four OCTAVE trials (NCT01465763, NCT01458951, NCT01458574, NCT01470612),10 the IR of malignancies (NMSC and other neoplasms) was similar in patients under tofacitinib and placebo: 1.0 (95% CI, 0.0–5.4) and 0.7 (95% CI, 0.3–1.2), respectively.22
Cardiovascular events
Based on the preliminary results of a post-marketing study (NCT02092467) enrolling RA patients above 50 years old with cardiovascular risk factors, both the Food and Drug Administration31 and the European Medicines Agency32 highlighted the risk of pulmonary embolism. In that study, tofacitinib 10 mg was associated with increased all-cause mortality and pulmonary embolism compared with anti-TNF, while tofacitinib 5 mg and anti-TNF had comparable safety profiles. Notwithstanding, the pooled analysis of 1157 tofacitinib-treated UC patients enrolled in three induction studies, one maintenance trial and one ongoing long-term extension study provided an IR for major adverse cardiovascular events (MACEs) of 0.24 (95% CI, 0.07–0.62).33 Importantly, in this study only four MACEs were registered, and three of these patients had at least four cardiovascular risk factors.
Along the same lines, a recent meta-analysis including more than 13,000 patients with different inflammatory disorders advocated that, comparing with placebo, tofacitinib did not increase the risk of cardiovascular events (OR 1.07, 95% CI 0.49–2.34) or MACEs (OR 1.54, 95% CI 0.42–5.59).34 This study reported that tofacitinib 10 mg was associated with significantly lower incidence of cardiovascular events (OR 0.56, 95% CI 0.33–0.96), in what may be due to the higher dose-related anti-inflammatory effect. In addition, tofacitinib has been associated with a dose-dependent reversible increase in total cholesterol (TC), low-density lipoprotein (LDL) and high-density lipoprotein (HDL), with maximum levels at the eighth week of treatment.33 Notwithstanding, no meaningful changes were observed on LDL/HDL and TC/HDL ratios, or in the 10-year cardiovascular risk.33 Therefore, tofacitinib appears not to systematically increase the risk of atherosclerotic cardiovascular diseases, although larger studies with longer follow-ups must be developed.
Therefore, in the face of current knowledge, the introduction and maintenance of tofacitinib must be careful, weighing the expected benefits against the potential adverse events. Close monitoring throughout the treatment and the awareness of the special cases and the high-risk populations is essential to maximize the benefits. Further trials are warranted to assess whether selectivity in JAK inhibition may have safety advantages.
Precautions and contraindications
Warnings and precautions
Tofacitinib is metabolized by cytochrome CYP3A4 and, thus, interactions with other medications may occur. Like most drugs, adjustment for hepatic or renal impairments is required, as well as in the case of anaemia, neutropenia or lymphocytopaenia.35 Caution must be taken in patients with risk factors for venous thromboembolism including older age, obesity, smoking, long-term bedridden status, recent major surgery, major trauma, heart failure, cancer, use of combined hormonal contraceptives and congenital abnormality of coagulant factors.36,37
Contraindications
Tofacitinib is contraindicated in cases of hypersensitivity and serious infection. Studies enrolling patients with UC, RA or psoriasis suggest that prenatal exposure to tofacitinib did not change the pregnancy and newborn outcomes, in comparison with those of general population,38,39 nevertheless tofacitinib is contraindicated in these settings. The preliminary analysis of safety endpoints in RA (NCT02092467) revealed a significantly higher risk of serious infections in patients aged above 65 years. Therefore, even though those events may be related to the clinical condition and comorbidities, tofacitinib is currently not recommended for patients above that age. No data are available on the use of tofacitinib in children; future studies are warranted to define the therapeutic approaches for this population.5
Tofacitinib work-up
Baseline
Before the introduction of tofacitinib, TC and HDL levels must be checked,33 as well as patients’ cardiovascular risk factors (obesity/overweight, physical inactivity, hypertension, hyperlipidaemia, smoking, diabetes mellitus and family history of premature coronary disease) (Table 1). Independent of history and vaccination status, serological analysis should be performed to check whether the patient is protected against varicella, measles, mumps and rubella.
Table 1.
Tofacitinib treatment monitoring.
|
Baseline | ||
| Complete anamnesis, including cardiovascular risk factors, history of venous thromboembolism, lung disease, gastrointestinal perforation, herpes zoster, pregnancy; complete blood count, serum creatinine, liver function tests, fasting lipid panel, serum creatinine phosphokinase, serology for HIV, hepatitis B and hepatitis C, Mantoux test or IFN-γ release assay | ||
|
Not recommended to start if: | ||
| - Hypersensitive to any component | ||
| - Pregnant or breastfeeding | ||
| - Lymphocyte count <500/mm3, neutrophil count <1000/mm3 and/or haemoglobin <9 g/dl | ||
| - Positive serology for hepatitis B and/or C, HIVa | ||
| - Active tuberculosis, serious localized or opportunistic infections | ||
|
Throughout the treatment, after 4–8 weeks of treatment start and then every 3 months | ||
| Lymphocyte count | <500 cells/mm3 | Not recommended to maintain |
| Neutrophil count | <1000 cells/mm3 | Avoid maintaining |
| <500 cells/mm3 | Not recommended to maintain | |
| Haemoglobin | <8 g/dl or drop ≥2 g/dl | Interrupt |
| Liver enzymes and serum creatinine | Moderate renal impairment (creatinine clearance <49 ml/min) Moderate hepatic impairment (Child-Pugh score ≥Grade B, 7 points) | Dose reduction to 5 mg |
| Lipids | Generally, maximum levels in 6 weeks. If persistent elevation, statin therapy may be started; manage according to clinical hyperlipidaemia guidelinesb | |
IFN: interferon.
aThese patients were excluded from tofacitinib trials.
bFor example, the guidelines for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS).
It is currently recommended that, IBD patients aged 50 or more years should receive a zoster vaccination;40 however, given the increased risk of HZ, it remains to be seen whether it may be reasonable to administer the vaccine to all IBD patients treated with tofacitinib, regardless of their age.41 Live attenuated vaccines are contraindicated in patients on immunosuppressors and, thus, patients already in tofacitinib should receive a recombinant zoster vaccine.23,42 In addition, all adult patients should receive non-live vaccines including pneumococcal (PCV13 and PPSV23), hepatitis A, hepatitis B (in seronegative patients), Haemophilus influenza B, human papilloma virus, tetanus and pertussis.40
The induction dose of tofacitinib is 10 mg twice daily, for 8 weeks. If clinical remission is not achieved after this period, treatment with 10 mg twice daily may be maintained for 8 more weeks, according to the specific clinical situation. In the OCTAVE Open study, this extension strategy resulted in clinical response and remission in 59.7% and 16.2%, respectively, of the subjects that failed to achieve remission in 8 weeks.43 If after 16 weeks no therapeutic benefit is observed, tofacitinib must be suspended.10 The maintenance dose is 5 mg, twice daily;33 however, it may be kept at 10 mg in previous anti-TNFα failures, after weighing risks and benefits. Indeed, in this group of patients, sustained corticosteroid-free remission is significantly higher with a maintenance dose of 10 mg than with 5 mg (38.9% vs 22.2%).44 In addition, patients who lose response to 5 mg (as maintenance therapy) may benefit from dose escalation to 10 mg. In fact, in the OCTAVE Open study, clinical response and remission were recovered in 59% and 34%, 8 weeks after dose escalation.45
Throughout the treatment
During treatment with tofacitinib potential changes in haemoglobin, lymphocytes, neutrophils, serum creatinine, liver enzymes and the lipid panel must be monitored. A summary of the analytical monitoring and work-up recommended by the retailer,36 the Food and Drug Administration46 and the European Medicines Agency44 is summarized in Table 1.
The annual seasonal administration of inactivated influenza vaccine is recommended. In addition, it is important to check the vaccination status of the immunocompetent individuals who share the household with the IBD patient.40 Regarding cancer prevention and screening, all patients should follow a programme of sun protection and dermatological surveillance and women should undergo an annual cervical examination.40 Surveillance colonoscopies for colorectal carcinoma should be started 8 years after the onset of pancolitis or 12–15 years after onset of left-sided colitis.47
Tofacitinib – guessing the future
According to the American College of Gastroenterology13 and in the face of current evidence, tofacitinib may be used at four points of the moderate-to-severe active UC therapeutic algorithm: (a) as first-line therapy even before thiopurines; (b) after failure of conventional therapy; (c) as a second-line option, before VDZ; or (d) as a third-line therapy (after anti-TNF and VDZ) (Figure 2). Indeed, tofacitinib was reported to be the most effective therapy in anti-TNF failures, even though VDZ may be a reasonable alternative.13,20
Figure 2.

The place of Janus kinase (JAK) inhibitors in the therapeutic algorithm of ulcerative colitis (UC), four hypotheses. TNF: tumour necrosis factor.
In order to optimize the use of this promising therapeutic weapon, head‐to‐head comparisons, in both biologic‐naïve and biologic‐exposed patients are warranted, in the context of moderate-to-severe UC but also in the management of acute severe flares. In the opposite spectrum of the disease, the usefulness of tofacitinib in mild-to-moderate active UC, is yet to be explored. In the future, the potential combination of tofacitinib, or other JAK inhibitors, with other immunosuppressors (either ‘classical’ or monoclonal antibodies) should also be explored. Indeed, some case reports have described the use of combination strategies in the setting of acute severe colitis, as rescue therapy to avoid colectomy (tofacitinib plus IFX)48 and also in the management of patients with UC plus rheumatic diseases (tofacitinib plus VDZ)49 Further studies on combination therapy are warranted in order to explore its efficacy and safety profile50 and to extend the range of applications of JAK inhibitors. In addition, combination therapy may be a rescue alternative in cases of loss of response to tofacitinib through, for example, the addition of VDZ. Plus, even though tofacitinib allows the reduction of costs associated with intravenous administration and therapeutic drug monitoring,5 cost-effectiveness analyses are warranted to aid therapeutic decision-making. Finally, the development of biomarkers identifying the subset of patients most likely to respond to JAK inhibitors would be of utmost importance.
Acknowledgements
The authors thank Paula Pinto (Pharmaceutical Medicine Academy (PMA)) for providing editorial assistance.
Declaration of conflicting interests
F Magro served as speaker and received honoraria from Merck Sharp and Dohme, Abbvie, Vifor, Falk, Laboratórios Vitória, Ferring, Hospira and Biogen. MM Estevinho has no conflict of interests to disclose.
Ethics approval
There was no requirement for taking ethical clearance, thereby, there is nothing to declare.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iDs
Fernando Magro https://orcid.org/0000-0003-2634-9668
Maria Manuela Estevinho https://orcid.org/0000-0001-7171-0139
References
- 1.Hazel K, O’Connor A. . Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis 2020; 35: 1746–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soendergaard C, Bergenheim FH, Bjerrum T, et al. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther 2018; 192: 100–111. [DOI] [PubMed] [Google Scholar]
- 3.Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020; 17: 323--337. [DOI] [PubMed] [Google Scholar]
- 4.Varyani F, Argyriou K, Phillips F, et al. Profile of tofacitinib in the treatment of ulcerative colitis: An evidence-based review of recent data. Drug Des Devel Ther 2019; 13: 4091–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese S, D’Amico F, Bonovas S, et al. Positioning tofacitinib in the treatment algorithm of moderate to severe ulcerative colitis. Inflamm Bowel Dis 2018; 24: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Clotet A, Castro-Poceiro J, Panes J, et al. JAK inhibition: The most promising agents in the IBD pipeline? Curr Pharm Des 2019; 25: 32–40. [DOI] [PubMed] [Google Scholar]
- 7.Olivera P, Danese S, Peyrin-biroulet L. Expert review of clinical immunology JAK inhibition in inflammatory bowel disease. Expert Rev Clin Immunol 2017; 13: 693–703. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Argollo M, Le Berre C, et al. JAK selectivity for inflammatory bowel disease treatment: Does it clinically matter? Gut 2019; 68: 1893–1899. [DOI] [PubMed] [Google Scholar]
- 9.Shivaji UN, Nardone OM, Cannatelli R, et al. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol Hepatol 2020; 1253: 30414–30415. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 11.Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 139–147. [DOI] [PubMed] [Google Scholar]
- 12.Panes J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: Health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis 2018; 12: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 14.Lair-Mehiri L, Stefanescu C, Vaysse T, et al. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis 2020; 52: 268–273. [DOI] [PubMed] [Google Scholar]
- 15.Ungaro R, Fenster M, Dimopoulos C, et al. P344 real-world effectiveness of tofacitinib in ulcerative colitis: A multi-centre study. J Crohn’s Colitis 2019; 13: S274–S275. [Google Scholar]
- 16.Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci 2019; 64: 1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biemans VBC, Sleutjes JAM, De Vries AC, et al. Tofacitinib for ulcerative colitis: Results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther 2020; 51: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative Colitis: A real-world experience. J Crohn’s Colitis 2020 Feb 5; jjaa018. Epub ahead of print. doi: 10.1093/ecco-jcc/jjaa018. [DOI] [PubMed] [Google Scholar]
- 19.Dubinsky MC, Peyrin-Biroulet L, Melmed GY, et al. Efficacy of Tofacitinib in Patients With Ulcerative Colitis by Prior Tumor Necrosis Factor Inhibitor Treatment Status: Results From OCTAVE Induction and Maintenance Studies. Am J Gastroenterol 2017; 112: S354 https://journals.lww.com/ajg/Fulltext/2017/10001/Efficacy_of_Tofacitinib_in_Patients_With.640.aspx [Google Scholar]
- 20.Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: First- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 162–175. [DOI] [PubMed] [Google Scholar]
- 21.Wollenhaupt J, Lee E, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019; 21: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019; 17: 1541–1550. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal M, Kim E, Colombel J. JAK inhibitors safety in ulcerative colitis: Practical implications. J Crohn’s Colitis 2020 Feb 1; jjaa017. Epub ahead of print. doi: 10.1093/ecco-jcc/jjaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
- 25.Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood 2014; 124: 2921–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: A phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winthrop KL, Park SH, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YM, Huang WN, Wu YD, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: A real-world study. Ann Rheum Dis 2018; 77: 780–782. [DOI] [PubMed] [Google Scholar]
- 29.Tran V, Shammas RM, Sauk JS, et al. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: Design, development and positioning of therapy. Clin Exp Gastroenterol 2019; 12: 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandborn W, Ghosh S, Panes J, et al. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012; 367: 616–624. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. FDA Drug Safety Communication - safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients, https://www.fda.gov/drugs/drug-safety-and-availability/safety-trial-finds-risk-blood-clots-lungs-and-death-higher-dose-tofacitinib-xeljanz-xeljanz-xr (2019, accessed 15 May 2020).
- 32.European Medicines Agency. Increased risk of blood clots in lungs and death with higher dose of Xeljanz (tofacitinib) for rheumatoid arthritis - EMA/180287/2019, https://www.ema.europa.eu/en/documents/press-release/increased-risk-blood-clots-lungs-death-higher-dose-xeljanz-tofacitinib-rheumatoid-arthritis_en.pdf (2020, accessed 24 May 2020).
- 33.Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020; 18: 123–132.e3. [DOI] [PubMed] [Google Scholar]
- 34.Xie W, Xiao S, Huang Y, et al. Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis 2019; 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amico FD, Parigi TL, Fiorino G, et al. Tofacitinib in the treatment of ulcerative colitis: Efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol 2019; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfizer Laboratories. Xeljanz/Xeljanz XR (tofacitinib) - highlights of prescribing information, https://www.pfizermedicalinformation.com/en-us/xeljanz#S5.7 (2019, accessed 21 May 2020)
- 37.Crous-Bou M, Harrington LB, Kabrhel C. Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost 2016; 42: 808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016; 39: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahadevan U, Dubinsky MC, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018; 24: 2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: Preventive care in inflammatory bowel disease. Am J Gastroenterol 2017; 112: 241–258. [DOI] [PubMed] [Google Scholar]
- 41.Greenfest A, Szvarca D, Clarke L, et al. New vaccine, new guidelines, same problem: The rates of herpes zoster infection and vaccination in IBD patients. Inflamm Bowel Dis 2019; 25: e60. [DOI] [PubMed] [Google Scholar]
- 42.Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: Mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018; 24: 2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin DT, Dubinsky MC, Danese S, et al. P653 efficacy and safety of an additional 8 weeks of tofacitinib induction therapy: Updated results of the OCTAVE Open study for tofacitinib 8-week induction non-responders. J Crohn’s Colitis 2020; 14: S537–S539. [Google Scholar]
- 44.European Medicines Agency. Assessment report Xeljanz - EMA/414633/2018, https://www.ema.europa.eu/en/documents/variation-report/xeljanz-h-c-4214-x-0005-epar-assessment-report-variation_en.pdf (2018, accessed 24 May 2020).
- 45.Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: Results from OCTAVE Open. Aliment Pharmacol Ther 2020; 51: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Food and Drug Administration. Xeljanz/Xeljanz XR - highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf (2018, accessed 15 May 2020).
- 47.Keller D, Windsor A, Cohen R, et al. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech Coloproctol 2019; 23: 3–13. [DOI] [PubMed] [Google Scholar]
- 48.Griller N, Cohen L. Rapid onset of tofacitinib induction therapy for the treatment of ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 1213. [DOI] [PubMed] [Google Scholar]
- 49.Le Berre C, Loeuille D, Peyrin-Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol 2019; 17: 794–796. [DOI] [PubMed] [Google Scholar]
- 50.Paschos P, Katsoula A, Salanti G, et al. Systematic review with network meta-analysis: The impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment Pharmacol Ther 2018; 48: 1174–1185. [DOI] [PubMed] [Google Scholar]