Abstract
Background
Diagnosis of early chronic pancreatitis is a clinical challenge and hindered by the lack of a gold standard. Endoscopic ultrasound (EUS) and the endoscopic pancreatic function test (ePFT) are the most sensitive morphological and functional methods in this setting. EUS-elastography allows for the quantification (strain ratio) of pancreatic fibrosis, and the dynamic evaluation of the main pancreatic duct compliance provides additional information. We developed a multimodal EUS-based approach for the evaluation of the pancreas by integrating these four methods in a single procedure.
Objective
We aim to describe morphological and functional pancreatic abnormalities in patients with clinical suspicion of chronic pancreatitis and inconclusive EUS findings by using the multimodal EUS-based approach.
Methods
This was a prospective, cross-sectional, observational study of patients with clinically suspected chronic pancreatitis and indeterminate EUS criteria of the disease. EUS criteria of chronic pancreatitis, quantitative pancreatic elastography, ePFT and compliance of the main pancreatic duct were evaluated in a single procedure.
Results
In total, 53 patients with 3–4 EUS criteria of chronic pancreatitis were included (mean age 39.7 years, 29 male). Strain ratio was abnormally high in all patients. Peak bicarbonate concentration was decreased in 43 patients (81.1%) and the main pancreatic duct compliance was reduced in 41 patients (77.3%). Some 34 patients (64.1%) had abnormal results at EUS, elastography, ePFT and compliance of the main pancreatic duct.
Conclusions
A multimodal EUS-based test for the morphological and functional evaluation of the pancreas is presented, which allows detecting mild pancreatic abnormalities in patients with suspected early chronic pancreatitis. The presence of abnormal morphological and functional evaluation of the pancreas could support the clinical suspicion of early chronic pancreatitis in the appropriate clinical setting.
Keywords: Pancreatic secretion, ductal compliance, pancreatic fibrosis, secretin, chronic pancreatitis
Introduction
Chronic pancreatitis (CP) is characterized by chronic inflammation of the pancreas, fibrosis and loss of functioning cells.1 Diagnosis of CP is usually easy at advanced stages of the disease, when severe morphological changes (e.g. pancreatic calcifications, atrophy and irregular dilatation of the pancreatic duct) can be demonstrated by imaging techniques. Diagnosis of early CP is, however, challenging due to the limited sensitivity and specificity of usual diagnostic methods and the lack of a widely accepted definition of the disease at that stage.2 A recent consensus report suggested that early CP can be theoretically diagnosed based on a combination of the presence of risk factors, low risk for other disorders with overlapping features, appropriate clinical context and supportive biomarkers.2 Biochemical analytes, pain scales, imaging features, pancreatic function tests and histology are included among biomarkers supporting the diagnosis of early CP.
The topic is highly controversial. Risk factors for CP are absent in patients with idiopathic disease, the clinical context can be variable, and biomarkers are not accurate for early CP. Demonstration of significant pancreatic infiltration of inflammatory cells would be needed for the definite diagnosis of early CP, as pancreatic fibrosis may develop in pancreatopathies different from CP (e.g. pancreatic abnormalities associated with diabetes mellitus, aging or smoking). Histological diagnosis of early CP is nowadays unfeasible, and accurate biomarkers are still lacking.3 Demonstration of combined mild morphological and functional abnormalities in the appropriate clinical context by using sensitive methods could nowadays be used to support the clinical suspicion of early CP. Progression of those abnormalities over time will further support the diagnosis of the disease.
Together with magnetic resonance imaging (MRI) and secretin-stimulated magnetic resonance pancreatography (sMRP), endoscopic ultrasound (EUS) is the most sensitive method for the detection of mild morphological changes of CP.1,2,4,5 EUS findings such as hyperechoic foci and strands, parenchymal lobularity and hyperechoic ductal wall are signs of pancreatic fibrosis.6,7 These findings are sensitive but not specific of CP.4,8,9
Pancreatic elastography has been shown to be highly accurate for the diagnostic evaluation of pancreatic solid masses as well as CP.10,11 Fibrosis of pancreatic parenchyma in the context of CP is associated with an increased stiffness that can be measured by EUS-elastography.10
The endoscopic pancreatic function test (ePFT) is currently considered the most sensitive test for the functional diagnosis of CP.1,12,13 The test consists of the quantification of bicarbonate concentration in duodenal samples obtained endoscopically after stimulation of pancreatic secretion with intravenous secretin.13 In addition, the dynamic behaviour of the main pancreatic duct after secretin stimulation can be evaluated if an echoendoscope is used for the ePFT. This is of interest as lack or reduced main pancreatic duct compliance after intravenous secretin is considered a feature of periductal fibrosis, which is seen early in CP.14–17
We have developed a multimodal EUS-based approach for the evaluation of early pancreatic changes suggesting CP by integrating the four methods described above in one single procedure: (a) EUS for the sensitive evaluation of pancreatic morphology, (b) EUS-elastography to quantify the pancreatic stiffness and degree of pancreatic fibrosis, (c) ePFT for accurate evaluation of pancreatic secretion, and (d) dynamic EUS evaluation of the pancreatic duct compliance after intravenous secretin. The aim of the present study was to describe the morphological and functional changes of the pancreas by using the multimodal EUS-based approach in patients with clinical suspicion of CP and inconclusive EUS findings.
Material and methods
A prospective, cross-sectional, observational study of patients with clinically suspected CP and inconclusive EUS findings was designed and carried out at the Pancreas and Endoscopy Unit of the University Hospital of Santiago de Compostela, Spain.
Patients aged ≥18 years presenting with epigastric pain and normal findings at upper gastrointestinal endoscopy and abdominal ultrasound, who were referred for EUS from January 2013 to December 2019, were considered for the study. From this cohort of patients, those with clinical suspicion of CP and indeterminate findings of the disease at EUS18 as the only abnormality potentially explaining pain were invited to participate in the study and included after signing the corresponding informed consent. For the purposes of this study, history of acute pancreatitis or acute relapsing pancreatitis were considered as exclusion criteria. Patients with history of pancreatic or upper gastrointestinal surgery, significant coronary heart disease or heart failure, severe chronic obstructive pulmonary disease, chronic liver disease with portal hypertension, any malignant tumour, active infection or fever during the previous 7 days, pregnancy, breast-feeding or inability to give informed consent were also excluded.
Multimodal EUS-based dynamic morphological and functional evaluation of the pancreas
Included patients underwent the multimodal EUS-based evaluation of the pancreas under anaesthesiologist-guided propofol sedation. The following protocol was carried out:
EUS was performed using a slim linear echoendoscope (EG-3270-UK; Pentax Europe GmbH, Germany) and the platform HITACHI-Ascendus (Hitachi Medical Systems Europe, Switzerland). Standard EUS criteria of CP according to the Rosemont classification18 were evaluated and recorded.
After EUS examination, an elastographic evaluation of the head, body and tail of the pancreas was performed as previously described.10 Two different areas (A and B) were selected: area A was the largest possible area of the pancreatic parenchyma. Area B referred to a soft (red) reference area, corresponding to normal surrounding gut wall. The result of the elastographic evaluation was defined as the quotient B/A (strain ratio, SR). The mean SR at the head, body and tail of the pancreas was considered as the result of the elastographic evaluation (normal SR <2.25).10
After aspirating residual gastric and duodenal fluid content, secretin (Secrelux, Sanochemia Diagnostic GmbH, Germany) was administered as an intravenous bolus of 0.2 μg/kg body weight for the ePFT. Samples of duodenal fluid (3–5 ml each) were collected at 15-min intervals for 45 min using a standard ERCP catheter (Tandem™ XL Triple-Lumen ERCP Cannula, Boston Scientific, USA) (Figure 1). Samples of duodenal fluid were immediately placed into 7 ml glass tubes, closed, kept on ice during the test and immediately analysed or frozen at −80°C until analysis. Bicarbonate concentration was quantified in all samples by using the autoanalyser Rapidpoint® 500 system (Siemens, Germany) and the maximal concentration (peak) was considered as the result of the test (normal ≥80 mEq/l).19
The diameter of the main pancreatic duct was measured at the body of the pancreas before and 5, 10, 25 and 40 min after secretin stimulation. There is no universally accepted normal pancreatic duct compliance after intravenous secretin. Based on previous studies, a dilatation of at least 50% from baseline (100*[maximal diameter-baseline]/baseline) was defined as normal.17
Figure 1.
Pancreatic fluid collection after the intravenous injection of secretin during the endoscopic pancreatic function test. Fluid (3–5 ml) is aspirated using a standard ERCP catheter.
In total, the multimodal EUS-based dynamic morphological and functional evaluation of the pancreas takes 55–60 min. Complications related to the procedure were recorded.
To avoid inter-individual variability and bias, all examinations were carried out by the same two expert endosonographers together, who agreed with EUS criteria of CP, elastographic evaluation and measurement of the pancreatic duct compliance.
Statistical analysis
A descriptive data analysis was performed. Quantitative variables are shown as mean ± standard deviation and box plot. Categorical variables are shown as percentages. Sample size was defined by the duration of the study; all patients fulfilling inclusion criteria and none of the exclusion criteria over the study period from January 2013 to December 2019 were included.
Ethical aspects
The study was approved by the Clinical Research Ethics Committee of the Galician Ministry of Health (Comité Ético de Investigación Clínica de Galicia, Consellería de Sanidad, www.ceic.sergas.es) on 10 October 2011, with the approval number 2011/281. All patients provided written informed consent to the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study was conducted in accordance with the Declaration of Helsinki and its amendments, and Good Clinical Practice guidelines.
Results
In total, 131 patients had clinically suspected CP and indeterminate EUS findings of the disease over the study period (Figure 2). Out of them 54 patients were finally included in the study (mean age 39.7 years, range 18–66, 29 male) (Table 1). Fifteen patients were moderate drinkers and smokers (1.5 ± 0.6 drinks/day and 10.2 ± 8.6 cigarettes/day), 11 were smokers (10.1 ± 8.1 cigarettes/day) but not drinkers, eight patients were drinkers (1.6 ± 0.5 drinks/day) but not smokers, whereas the remaining 20 patients had no toxic risk factor for CP. Frequency of additional risk factors of CP according to the TIGAR-O classification are shown in Table 1.20
Figure 2.
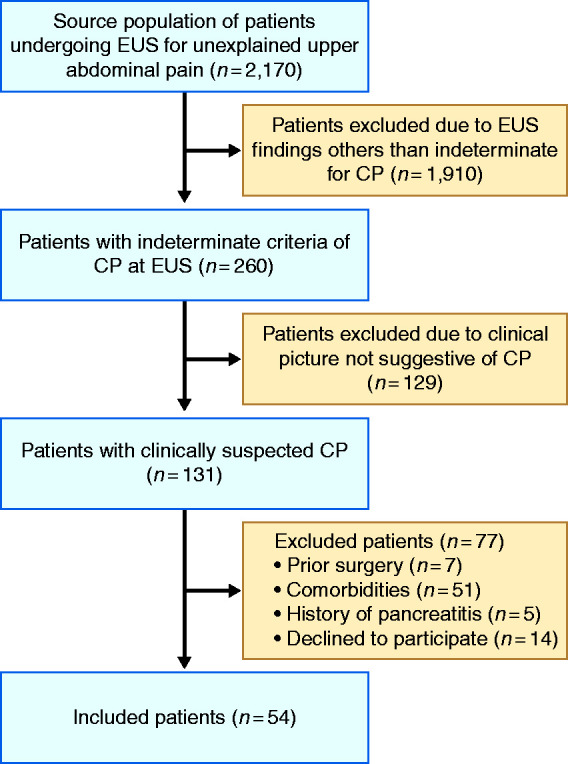
Flow chart of patients.
Table 1.
Risk factors of chronic pancreatitis in the study population according to the TIGAR-O version 2.20
| Risk factor | n (%) |
|---|---|
| Toxic-metabolic | |
| Alcohol use | |
| • Non-drinkers | 31 (57.4%) |
| • 1–2 drinks/day | 23 (42.6%) |
| • ≥3 drinks/day | 0 |
| Smoking | |
| • Non-smoker (<100 cigarettes in lifetime) | 28 (51.8%) |
| • Past smoker | 6 (11.1%) |
| • Current smoker | 20 (37.1%) |
| ο Cigarettes/day (mean ± SD) | 10.2 ± 8.2 |
| Hypercalcaemia (>12.0 mg/dl) | 0 |
| Hypertriglyceridemia (>300 mg/dL) | 0 |
| Medications* | |
| - No medications | 31 (57.4%) |
| - Proton pump inhibitors | 13 (24.1%) |
| - Doxazosin | 2 (3.7%) |
| - Statins | 3 (5.5%) |
| - Calcium antagonist | 1 (1.8%) |
| - Prokinetics | 2 (3.7%) |
| - Benzodiazepines | 3 (5.5%) |
| - Oral contraceptives | 2 (3.7%) |
| - Paracetamol | 2 (3.7%) |
| - Insulin therapy | 1 (1.8%) |
| - Corticoids | 1 (1.8%) |
| - Tiroxin | 2 (3.7%) |
| End-stage chronic kidney disease | 0 |
| Diabetes mellitus | 2 (3.7%) |
| No identifiable risk factor | |
| • ≤35 years of age | 22 (40.74%) |
| • >35 years of age | 32 (59.25%) |
| Genetic | |
| • Not evaluated | 30 (55.5%) |
| • Autosomal dominant (PRSS1 mutations) | 0/54 |
| • Autosomal recessive (CFTR, SPINK1, CTCR) | 2/54 (3.7%) |
| Autoimmune disorders | |
| • IgG4-related diseases | 0 |
| • Type 2 or previous diagnosis of inflammatory bowel disease |
1 (1.8%) |
| Previous AP or ARP** | 0 |
| Obstructive** | |
| • Pancreas divisum | 0 |
| • Ampullary stenosis | 0 |
| • Main pancreatic duct strictures | 0 |
| • Localized mass causing duct obstruction | 0 |
*Name of medications and number of patients. **Acute pancreatitis (AP), acute relapsing pancreatitis (ARP) and pancreatic calcifications were considered as exclusion criteria.
Multimodal EUS evaluation was feasible in 53 cases. The procedure was interrupted in the remaining patient due to a significant sedation-related respiratory depression.
Out of the 53 cases finally included in the study, 36 patients had three EUS criteria of CP (67.9%), and the remaining 17 cases presented four EUS criteria of the disease (32.1%) (Figure 2). Among parenchymal criteria, all 53 cases presented hyperechoic foci and strands, whereas none of them presented lobularity, cystic lesions or calcifications. Thirty-six cases (67.9%) presented hyperechoic ductal wall as the only EUS ductal criteria, and 17 cases (32.1%) presented hyperechoic wall and ductal irregularity. None of the cases presented ductal dilatation or calcifications.
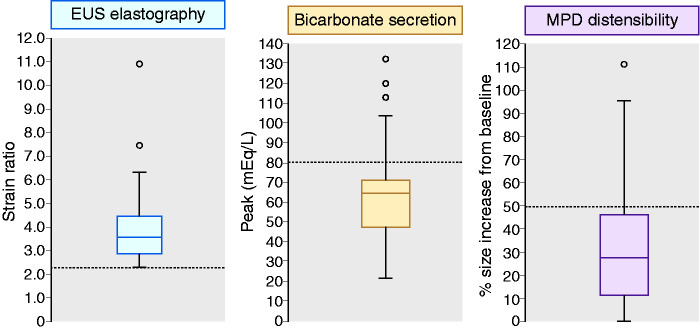
All 53 patients had an abnormally high SR at EUS-elastography (3.94 ± 1.57) (Figures 3 and 4). Bicarbonate concentration peak in duodenal fluid during the ePFT was 64.7 ± 23.9 mEq/l (Figure 4). An abnormally low bicarbonate peak was observed in 43 patients (81.1%). Finally, compliance of the pancreatic duct was reduced in 41 patients (77.3%) (Figure 4). Basal diameter of the main pancreatic duct at the pancreas body in these patients before intravenous secretin injection was 1.7 ± 0.4 mm, whereas maximal diameter after intravenous secretin was 2.2 ± 0.5 mm.
Figure 3.
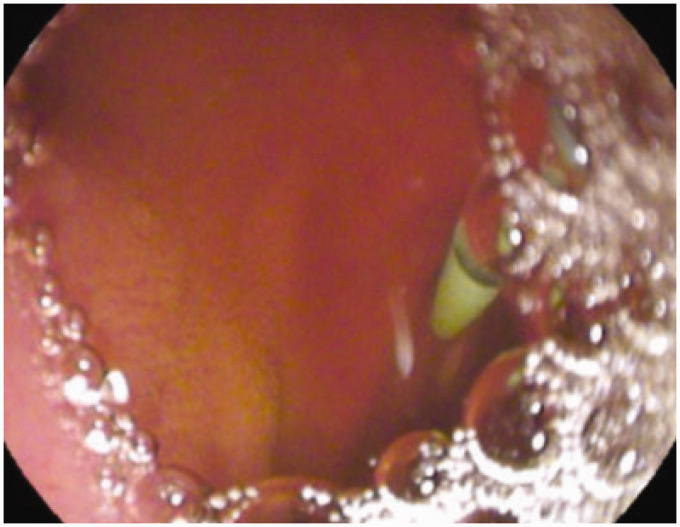
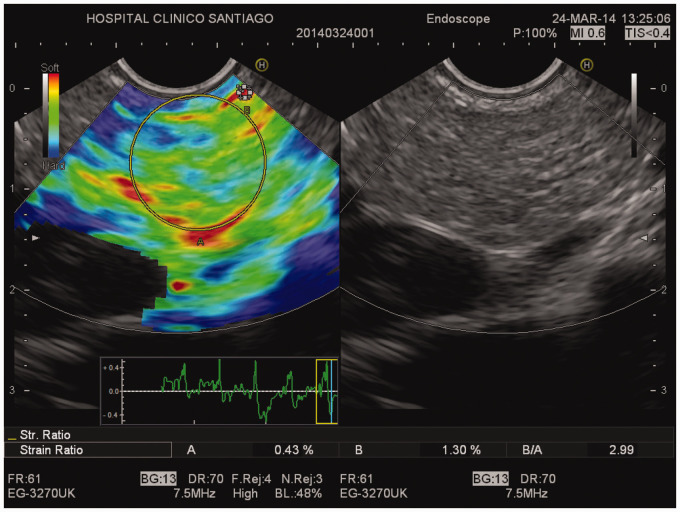
Endoscopic ultrasound (EUS) and EUS-guided elastographic evaluation of the pancreas. Right picture: B-mode EUS showing mild pancreatic abnormalities (hyperechoic foci, strands, hyperechoic pancreatic duct wall with duct irregularity). Left picture: EUS-guided elastography showing pancreatic fibrosis (strain ratio 2.99).
Figure 4.
Box plot representing the median, quartiles, interquartile range, superior and inferior whiskers and outliers of the elastographic evaluation of the pancreas (left), stimulated pancreatic secretion of bicarbonate (centre) and compliance of the main pancreatic duct (right). Limits of normality are shown as dotted lines.
Overall, and in addition to the presence of 3–4 EUS criteria of CP, 34 patients (64.1%) had abnormal results at elastography, ePFT and ductal compliance; nine patients (17.0%) had abnormal results at elastography and ePFT, but normal compliance of the pancreatic duct; seven patients (13.2%) had abnormal results at elastography and ductal compliance, but normal result at ePFT; finally, three patients (5.7%) had only abnormal result at elastography. In summary, 64.1%, 30.2% and 5.7% of the patients had abnormal results in all four, three or two pancreatic tests, respectively, out of the four tests included in the multimodal method.
Two complications (3.7%) related to the procedure were documented: a case of mild acute pancreatitis that required hospital admission for 48 h, and a second case of respiratory depression related to deep sedation with the patient being discharged without further complications 2 h after interrupting the procedure.
Discussion
A new dynamic morphological and functional multimodal EUS-based evaluation of the pancreas for the detection of mild pancreatic abnormalities is presented. These combined abnormalities could allow supporting the diagnosis of early CP in the appropriate clinical context. Together with the standard B-mode EUS evaluation of the pancreas, this method includes the quantification of pancreatic fibrosis by EUS-elastography, the quantification of secretin-stimulated pancreatic bicarbonate secretion, and the dynamic evaluation of the pancreatic ductal compliance after intravenous secretin. In addition to changes in B-mode EUS, all these parameters are altered in 64% of our cohort of patients with clinically suspected CP, thus probably supporting the diagnosis of early CP in them. The progression of these pancreatic abnormalities over time could further support the diagnosis of CP.
Diagnosis of early noncalcific CP is a clinical challenge due to the lack of sensitive and specific methods.2 Pain is not a consistent feature, and steatorrhoea or pancreatogenic diabetes may be the first clinical manifestation of CP in patients with otherwise painless disease.21,22 In the present study, patients with chronic epigastric pain and no relevant findings at standard blood tests, upper gastrointestinal endoscopy and abdominal ultrasound, in whom the diagnosis of early CP was clinically suspected as a potential cause of pain, were evaluated for inclusion. Patients with history of acute pancreatitis or acute relapsing pancreatitis were excluded because consistent morphological pancreatic abnormalities in them easily supports the diagnosis of CP.
A recent international consensus on early CP failed to agree on a definition of diagnostic criteria of early CP.2 They reached consensus on defining ‘early’ as disease state, not disease duration. Genetic variants and environmental risk factors can add specificity or provide evidence to support the diagnosis of early CP, but they are neither necessary nor sufficient to make a diagnosis. According to them, the differential diagnosis for early CP includes other disorders with morphological and functional overlapping features.2
Morphological changes of CP as evaluated by imaging procedures such as MRI, CT scan and EUS are the consequence of pancreatic fibrosis. Pancreatic fibrosis may however develop in clinical conditions others than CP, such as diabetes mellitus, smoking and aging.23–25 A relevant pancreatic infiltration of inflammatory cells characterizes CP and, together with fibrosis, allows the histological diagnosis of the disease.26 EUS-guided pancreatic biopsy has been attempted to confirm the diagnosis of early CP in patients with mild EUS changes, but the authors failed at obtaining a core tissue sample adequate for histological evaluation.3
According to the international consensus on early CP mentioned above, early CP cannot be diagnosed based on currently available imaging techniques alone.2 Theoretically early CP can be diagnosed based on a combination of the presence of high-risk factors for CP, low risk for other disorders with features that overlap CP, appropriate clinical context and the presence of supportive biomarkers including biochemical analytes, pain scales, imaging features and pancreatic function tests.2 Risk factors for CP are, however, absent in patients with idiopathic disease, and the clinical context can be variable and not always typical as stated above. Imaging findings and function tests are by themselves not accurate enough, and their combination is highly recommended. Demonstration of mild morphological and functional pancreatic abnormalities could allow supporting the diagnosis of the disease in the appropriate clinical context.
Several studies on the accuracy of EUS criteria for the diagnosis of CP have been reported.27–30 The higher the number of detected criteria is, the higher is the specificity and positive predictive value of the EUS finding. The presence of ≥4 out of nine EUS criteria in one study had a sensitivity of 91% and a specificity of 86% for the diagnosis of CP as compared with histology from surgical specimens.29 In another study, the presence of three or more EUS criteria had a sensitivity of 83% with a specificity of 80% for the diagnosis of noncalcific CP.30 In addition, a significant correlation has been reported between the number of EUS criteria and the histological fibrosis score.29,30 The inclusion of patients with three or four EUS criteria of CP in our study was concordant with these data, as these are mainly the patients in whom the diagnosis of CP requires confirmation by additional methods.
The secretin-CCK test is considered the most sensitive function test for the diagnosis of early CP.31 Previous studies have shown that the accuracy of the ePFT is equivalent to that of the classical secretin test using a drilling tube.32 The sensitivity and specificity of this test for diagnosing CP both exceed 90%.13 A previous study reported on a moderate correlation and concordance of EUS and ePFT results in patients with suspected minimal change CP.15 EUS and ePFT therefore provide complementary functional and structural information for the diagnosis of CP.7,15 Some 43 patients (81.1%) of our cohort of patients with clinically suspected CP and 3–4 EUS criteria of the disease had an abnormally low bicarbonate secretion.
The efficacy of EUS-elastography for the quantification of pancreatic fibrosis and the diagnosis of early CP was previously evaluated by our group.10 A diagnostic accuracy of 91.1% for CP was obtained by using a SR cut-off of 2.25. Itoh et al. supported our findings by showing an agreement of 0.9 between EUS-guided elastography and the histological fibrosis score from surgically resected specimens.33 In the present study, every patient with clinically suspected CP and morphological abnormalities at EUS had an abnormally high SR supporting the presence of pancreatic fibrosis. Despite the potential bias associated with the selection of the reference area, SR was preferred in this study over the hue histogram34 because SR is widely validated and accepted for quantitative elastography. In addition, SR has been previously evaluated in healthy pancreas and CP.10
Although the inadequate distension of the main pancreatic duct after secretin stimulation is one of the criteria used for the diagnosis of CP during sMRP,35,36 there is no universally accepted normal stimulated pancreatic duct compliance. In patients with suspected CP with normal sMRP but abnormal duodenal filling, a mean pancreatic duct calibre change of 1.1 to 1.2 mm (<50% from basal size) after secretin stimulation was described.35 Gardner et al. firstly documented data on EUS-based evaluation of the dynamic behaviour of the main pancreatic duct after secretin stimulation.17 These authors reported on a different degree of ductal distensibility in patients suffering from CP and controls (44% vs. 140% size increase in the tail in CP and controls, respectively). In the present study, 41 patients (77.3%) with clinically suspected CP presented a pancreatic ductal compliance of less than 50% from basal size. Based on these previous studies together with our results, a pancreatic duct dilatation after secretin of less than 50% from basal can be considered as abnormal.
Taking all together, about two out of three patients with clinically suspected CP and mild features of CP at EUS present with abnormal dynamic morphological and functional pancreatic findings. Although these findings are probably not specific of early CP, combined demonstration of pancreatic fibrosis by EUS-elastography, together with a low bicarbonate secretion and reduced ductal compliance after secretin stimulation in patients with 3–4 EUS criteria of the disease could allow supporting the diagnosis of early CP if used in the appropriate clinical context. All patients included in the study are now on long-term follow-up to evaluate the natural history of the pancreatic morphological and functional changes. The demonstration of progression of these abnormalities over time would further support the diagnosis of the disease.
The prospective design and the high number of patients included with unexplained epigastric pain, clinical suspicion of CP and mild EUS changes of CP are strengths of the study. On the contrary, the lack of a gold standard for the diagnosis of early CP is a limitation that cannot be solved today. Because of that, a descriptive analysis of the frequency of highly sensitive static and dynamic morphological and functional pancreatic abnormalities is presented instead of analysis of diagnostic accuracy in this setting.
In conclusion, a multimodal EUS-based test for the morphological and functional evaluation of the pancreas in patients with clinically suspected CP is presented. This test provides valuable morphological and functional information that could help to support the diagnosis of early CP in the appropriate clinical context.
Supplemental Material
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620936810 for Endoscopic ultrasound-based multimodal evaluation of the pancreas in patients with suspected early chronic pancreatitis by J Enrique Domínguez-Muñoz, Jose Lariño-Noia, Ana Alvarez-Castro, Laura Nieto, Santiago Lojo, Saul Leal, Daniel de la Iglesia-Garcia and Julio Iglesias-Garcia in United European Gastroenterology Journal
Conflicts of interest
Authors have no conflict of interest relevant to the manuscript to disclose.
Ethics approval
The study was approved by the Clinical Research Ethics Committee of the Galician Ministry of Health (Comité Ético de Investigación Clínica de Galicia, Consellería de Sanidad, www.ceic.sergas.es) on 10 October 2011, with the approval number 2011/281.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Instituto de Salud Carlos III and European Regional Development Fund (ISCIII and Fondos FEDER; MICINN; Spain; PI15/00802, PI18/00340).
Informed consent
All patients provided written informed consent to the study.
ORCID iDs
J Enrique Domínguez-Muñoz https://orcid.org/0000-0001-8283-3185
Daniel de la Iglesia-Garcia https://orcid.org/0000-0002-1826-2282
References
- 1.Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur Gastroenterol J 2017; 5: 153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcomb DC, Shimosegawa T, Chari ST, et al. International consensus statements on early chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with The International Association of Pancreatology, American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology Epub ahead of print 21 May 2018. DOI: 10.1016/j.pan.2018.05.008. [DOI] [PMC free article] [PubMed]
- 3.Iglesias García J, Lariño-Noia J, Abdulkader Nallib I, et al. Endoscopic ultrasound (EUS) guided fine needle biopsy (FNB) with the Procore™ needle provides inadequate material for the histological diagnosis of early chronic pancreatitis. Rev Esp Enferm Dig 2018; 110: 510–514. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias-García J, Lariño-Noia J, Lindkvist B, et al. Endoscopic ultrasound in the diagnosis of chronic pancreatitis. Rev Esp Enferm Dig 2015; 107: 221–228. [PubMed] [Google Scholar]
- 5.Issa Y, Kempeneers MA, van Santvoort HC, et al. Diagnostic performance of imaging modalities in chronic pancreatitis: A systematic review and meta-analysis. Eur Radiol 2017; 27: 3820–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutani MS, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38: 820–824. [DOI] [PubMed] [Google Scholar]
- 7.Albashir S, Bronner MP, Parsi MA, et al. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: Correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105: 2498–2503. [DOI] [PubMed] [Google Scholar]
- 8.Anaizi A, Hart PA, Conwell DL. Diagnosing chronic pancreatitis. Dig Dis Sci 2017; 62: 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza SL, Anderson MA, Korsnes SJ, et al. EUS diagnostic criteria for chronic pancreatitis: A comparison of conventional versus Rosemont criteria. Dig Dis Sci 2015; 60: 3782–3787. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias-Garcia J, Domínguez-Muñoz JE, Castiñeira-Alvariño M, et al. Quantitative elastography associated with endoscopic ultrasound for the diagnosis of chronic pancreatitis. Endoscopy 2013; 45: 781–788. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. New imaging techniques: Endoscopic ultrasound-guided elastography. Gastrointest Endosc Clin N Am 2017; 27: 551–567. [DOI] [PubMed] [Google Scholar]
- 12.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas 2014; 43: 1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens T, Parsi MA. Update on endoscopic pancreatic function testing. World J Gastroenterol 2011; 17: 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirkes T, Shah ZK, Takahashi N, et al. Reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography: The Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Radiology 2019; 290: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens T, Dumot JA, Parsi MA, et al. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55: 2681–2687. [DOI] [PubMed] [Google Scholar]
- 16.Sainani NI, Kadiyala V, Mortele K, et al. Evaluation of qualitative magnetic resonance imaging features for diagnosis of chronic pancreatitis. Pancreas 2015; 44: 1280–1289. [DOI] [PubMed] [Google Scholar]
- 17.Gardner TB, Purich ED, Gordon SR. Pancreatic duct compliance after secretin stimulation: A novel endoscopic ultrasound diagnostic tool for chronic pancreatitis. Pancreas 2012; 41: 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc 2009; 69: 1251–1261. [DOI] [PubMed] [Google Scholar]
- 19.Conwell DL, Zuccaro G, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc 2003; 57: 37–40. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb DC, North American Pancreatitis Study Group. Pancreatitis: TIGAR-O Version 2 Risk/Etiology Checklist with topic reviews, updates, and use primers. Clin Transl Gastroenterol 2019; 10: e00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammann RW, Buehler H, Muench R, et al. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas 1987; 2: 368–377. [DOI] [PubMed] [Google Scholar]
- 22.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994; 107: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 23.Mohapatra S, Majumder S, Smyrk TC, et al. Diabetes mellitus is associated with an exocrine pancreatopathy: Conclusions from a review of literature. Pancreas 2016; 45: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Geenen EJM, Smits MM, Schreuder TCMA, et al. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol 2011; 106: 1161–1166. [DOI] [PubMed] [Google Scholar]
- 25.Detlefsen S, Sipos B, Feyerabend B, et al. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch Int J Pathol 2005; 447: 800–805. [DOI] [PubMed] [Google Scholar]
- 26.Shrikhande SV, Martignoni ME, Shrikhande M, et al. Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg 2003; 90: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 27.Trikudanathan G, Munigala S, Barlass U, et al. Evaluation of Rosemont criteria for non-calcific chronic pancreatitis (NCCP) based on histopathology - A retrospective study. Pancreatology 2017; 17: 63–69. [DOI] [PubMed] [Google Scholar]
- 28.Jimeno-Ayllón C, Pérez-García JI, Gómez-Ruiz CJ, et al. Standard criteria versus Rosemont classification for EUS-diagnosis of chronic pancreatitis. Rev Esp Enferm Dig 2011; 103: 626–631. [DOI] [PubMed] [Google Scholar]
- 29.Varadarajulu S, Eltoum I, Tamhane A, et al. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: A prospective tissue characterization study. Gastrointest Endosc 2007; 66: 501–509. [DOI] [PubMed] [Google Scholar]
- 30.Chong AKH, Hawes RH, Hoffman BJ, et al. Diagnostic performance of EUS for chronic pancreatitis: A comparison with histopathology. Gastrointest Endosc 2007; 65: 808–814. [DOI] [PubMed] [Google Scholar]
- 31.Domínguez Muñoz JE. Diagnosis of chronic pancreatitis: Functional testing. Best Pract Res Clin Gastroenterol 2010; 24: 233–241. [DOI] [PubMed] [Google Scholar]
- 32.Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67: 458–466. [DOI] [PubMed] [Google Scholar]
- 33.Itoh Y, Itoh A, Kawashima H, et al. Quantitative analysis of diagnosing pancreatic fibrosis using EUS-elastography (comparison with surgical specimens). J Gastroenterol 2014; 49: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 34.Săftoiu A, Vilmann P, Gorunescu F, et al. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol 2012; 10: 84–90. [DOI] [PubMed] [Google Scholar]
- 35.Balci NC, Alkaade S, Magas L, et al. Suspected chronic pancreatitis with normal MRCP: Findings on MRI in correlation with secretin MRCP. J Magn Reson Imaging 2008; 27: 125–131. [DOI] [PubMed] [Google Scholar]
- 36.Balcı C. MRI assessment of chronic pancreatitis. Diagn Interv Radiol Ank Turk 2011; 17: 249–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ueg-10.1177_2050640620936810 for Endoscopic ultrasound-based multimodal evaluation of the pancreas in patients with suspected early chronic pancreatitis by J Enrique Domínguez-Muñoz, Jose Lariño-Noia, Ana Alvarez-Castro, Laura Nieto, Santiago Lojo, Saul Leal, Daniel de la Iglesia-Garcia and Julio Iglesias-Garcia in United European Gastroenterology Journal