Abstract
Background
Reports of liver injury in patients with novel coronavirus disease 2019 (COVID-19) are emerging from China and the USA. A wide variety of liver function test abnormalities and few cases of severe liver failure have been reported. No data on the hepatic phenotype from Europe are available at current.
Methods
We report a case series of 44 consecutive patients hospitalized for COVID-19 in Germany.
Results
At the time of admission, aspartate aminotransferase greater than the upper limit of normal was present in 70%, while alanine aminotransferase was elevated in 15.8%. Markers of cholestatic liver injury were altered only in a minority of patients. During hospitalization, 31% and 22% experienced increasing aspartate aminotransferase and alanine aminotransferase, respectively, when transaminases were normal at admission. Severe liver injury defined by 3×> upper limit of normal was observed in 9.1% over a mean time of 10.5 days. Importantly, patients exhibited cytotoxicity including lactate dehydrogenase and creatinine kinase elevations, but no signs of relevant liver function impairment.
Conclusion
In summary, in a case series of hospitalized patients in Germany, cytotoxicity in the absence of severe liver dysfunction at admission and only few cases suggestive of severe liver injury during hospital were observed.
Keywords: Liver, hepatology, COVID-19, inflammation, cohort study
Key summary
Abnormal aspartate aminotransferase levels are frequent in patients with coronavirus disease 2019 (COVID-19) at the time of hospital admission.
During hospitalization, the potential causes of liver injury are multifactorial and heterogeneous, partly influenced by disease severity.
We observed two patterns of liver injury in patients with COVID-19. First, a predominant aspartate aminotransferase elevation at admission – potentially liver-unrelated cytotoxicity.
Second, increasing alanine aminotransferase and aspartate aminotransferase during hospitalization in 20–30% of patients without significant liver function impairment.
Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV2 infection is a serious global health threat. A number of case series have suggested that liver injury is a relevant complication in the context of COVID-19.1,2 First reports showed mostly mild elevation of liver function tests (LFTs);3 however, these could be of concern when initiating potentially hepatotoxic drug regimens. In an early report from Shanghai 50.7% of patients exhibited abnormal liver function and increasing LFTs were observed, predominantly with HIV protease inhibitor (lopinavir/ritonavir) treatment.4 In another case series 21 patients with a mean age of 70 years and a high rate of comorbidities (86%) exhibited 38% of abnormal LFTs. The rate of elevated LFTs appears to be different between mild and severe cases. Expectedly, cases with multi-organ affection have a higher rate of liver function impairment. Importantly, the role of pre-existing liver disease, and in particular end-stage liver disease, has not been adequately explored.3 Infection with chronic hepatitis B virus, which was prevalent in 2.7% of the initial Chinese cohorts, did not lead to more severe cases of liver injury.5
Currently, few data from Germany are available and the mortality rates appear to be lower compared with other regions. A study on the first 50 hospitalized patients in the area that experienced the earliest and largest outbreak in Germany reported a lethal course in seven out of 50 patients and included four patients with not further specified ‘hepatic insufficiency’.6 This study reported prominent signs of inflammation and cell injury, including a lactate dehydrogenase (LDH), creatinine kinase (CK) and interleukin-6 increase that was more pronounced in patients with acute respiratory distress syndrome. Also, obesity and diabetes type 2 have been replicated as factors predisposing for a complicated course of COVID-19.
The factors that contribute to liver injury in the context of COVID-19 are threefold. First, SARS CoV2 cellular entry is mediated through angiotensin-converting enzyme 2 and thus hepatocytes and cholangiocytes are likely targets of this infection.7 Viral mediated injury to cholangiocytes can give rise to hepatocyte cell injury,8 as cholangiocytes are crucial for hepatocyte homeostasis.9 Disruption of the hepatic microenvironment through inflammatory factors is well established as contributing to progressive liver injury.10 A direct effect of COVID-19 in the liver parenchyma is supported by autopsy findings of patients that died from other coronavirus infections and demonstrated viral genome to be present in the liver tissue.11 Second, activation of the innate immune system at the level of the liver or systemically is an important factor that will progress liver injury. A maximum has been observed in patients with strong immune activation, a condition that has been termed cytokine storm.12 Third, drug-induced liver injury is of concern in patients that receive non-steroidal anti-inflammatory medications for the control of fever, antibiotics for superinfection or antiviral treatment. Importantly, all these factors can affect patients with advanced liver disease more severely and exacerbation with declining liver function leading to acute-on-chronic liver failure (ACLF) with increased mortality is of concern. This has previously been reported for patients with cirrhosis and influenza infection.13 The aim of this case series report is to specify the hepatic involvement in relation to the clinical presentation and outcomes of incident COVID-19 cases in Germany and consider these findings in relation to the case cohorts that have been reported in Asia.
Methods
Study population and clinical assessment
The study population was enrolled at the University Medical Centre Mainz (UMCM). UMCM is a tertiary medical centre and includes a certified infectious disease centre and a liver transplant centre. Patients were treated and assessed by standard operating procedures that have been established in the context of the COVID-19 pandemic as part of the standard of care. This involves hospitalization when requiring oxygen therapy (seen in 42/44 patients). The criterion for intensive care unit (ICU) admission was respiratory distress requiring mechanical ventilation related to acute respiratory distress syndrome. Patients with confirmed COVID-19 infection (PCR positive for SARS-CoV-2 by throat swab) admitted to the UMCM between 3 March and 30 April 2020 were included. Patient data were extracted retrospectively from the electronic health records and deidentified. Laboratory and clinical parameters were assessed at the day of hospital admission. Descriptive statistics were performed (SPSS Statistics V23; GraphPad Prism). Because of the small sample size no statistical measures were calculated.
Results
A total of 44 patients (mean age 68 years (range 23–86 years)), 68% male, were included. Patient characteristics are summarized in Table 1. Initial symptoms included dyspnoea, fever and cough. The mean oxygen saturation was 92.3% (range 74–99%) and respiratory rate 17/min (range 12–25/min). The mean time of the hospital stay was 10.5 days with two patients being discharged within 24 h and 13 (29.5%) requiring ICU therapy. In this cohort, pre-existing liver disease was present in 23% of patients and the spectrum included non-alcoholic fatty liver disease, alcoholic liver disease and previously treated hepatitis B virus/hepatitis C virus co-infection. One patient was HIV positive. Comorbidities included diabetes (25%), arterial hypertension (48%) and dyslipidaemia (32%). Only 33% were not on concomitant medication at the time of admission. Treatment was predominantly symptomatic and included antipyretics. Only two patients received hydroxychloroquine or protease inhibitors for the treatment and remdesivir was not available to the patients.
Table 1.
Clinical characteristics and outcomes of patients hospitalized for COVID-19.
| Variables | ||
|---|---|---|
| Total cohort, n | 44 | |
| Age mean in years (range) | 68 (23; 86) | |
|
Male gender (%) |
30/44 (68.2%) |
|
| Laboratory at admission a | Median (min; max) | % abnormal (reference range) |
| Alanine aminotransaminase, U/l | 28 (7; 142) | 15.8% (6/38)(male <50; female <35) |
| Aspartate aminotransaminase, U/I | 46 (20; 197) | 70% (26/37) (male <35; female <31) |
| Alkaline phosphatase, U/l | 76 (29; 836) | 25% (9/36) (male <138; female <111) |
| Gamma-GT, U/l | 34 (15; 504) | 19.4% (7/36) (male <64; female <36) |
| Total bilirubin, mg/dl | 0.7 (0.3; 2.9) | 13.6% (<1.2) |
| Albumin, g/l | 27 (15; 50) | 45.8%(<34) |
| CK, U/l | 128 (7; 3033) | 40.4% (17/42) (male <200; female <170) |
| LDH, U/l | 390 (188; 1445) | 95.2% (40/42)(ULN <245) |
| Leukocytes, /nl | 6.8 (1.24; 16) | |
| Cholinesterase, kU/l | 6.4 (2.9; 9.5) | |
| INRb | 1.1 (0.9; 1.5) | |
| Haemoglobin, g/dl | 13.4 (6.8; 16.3) | |
| aPTT, s | 28.3 (23; 42) | |
| Thrombocytes, /nl | 206 (6; 440) | |
| D-dimer, mg/FEU | 1.2 (0.3; 13.5) | |
| Fibrinogen, mg/dl | 585 (295; 933) | |
| Surrogates of advanced fibrosis | ||
| APRI >1c | 11/36 (31%) | |
| FIB-4 >2.67c | 19/35 (54%) | |
| Comorbidities | ||
| Diabetes type 2 | 11/44 (25%) | |
| Art. HTN | 21/44 (48%) | |
| Dyslipidaemia | 14/44 (32 %) | |
| Pre-existing liver diseased | 10/44 (23%) | |
| Treatment | ||
| Hydroxychloroquinee | 2/44 (5%) | |
| Protease inhibitore | 2/44 (5%) | |
| Antibiotics | 12/44 (27%) | |
| Symptomatic only | 32/44 (73%) | |
| Course/outcome | ||
| Acute liver injury, >3× ULN ALT or ASTf | 4/44 (9.1%) | |
| Dischargedf | 24/44 (54.5%) | |
| Treated on ID ward | 31/44 (70.5%) | |
| Treated on ICU | 13/44 (29.5%) | |
| Death | 2/44 (4.5%) |
aLabs at the time of admission available in 30 patients.
bExcluded two patients on phenprocoumon.
cAPRI: four patients excluded and FIB-4: five patients excluded for missing data.
dPre-existing liver disease included 2× alcoholic liver disease, 7× non-alcoholic fatty liver disease, 1× history of hepatitis B virus/hepatitis C virus.
eOne patient receiving both protease inhibitor and hydroxycholoroquine.
fNo patient exhibited bilirubin >2× ULN.
gAs of 4 April.
hAll on co-medication potentially leading to gGT increase.
Gamma GT: Gamma-Glutamyl Transferase; CK: creatinine kinase; LDH: lactate dehydrogenase; ULN: upper limit of normal; INR: International normalized ratio; aPTT: activated partial thromboplastin time; APRI: AST to Platelet Ratio Index; FIB-4: Fibrosis-4; Art. HTN: arterial hypertension; ALT: alanine aminotransaminase; AST: aspartate aminotransaminase; AP: Alkaline phosphatase; ID: infectious disease; ICU: intensive care unit
LFTs at the time of admission were available in 38 patients, while two patients were discharged without additional laboratory tests being performed and four patients receiving delayed laboratory diagnostic. At admission, the pattern of LFT elevation was predominantly aspartate aminotransferase (AST) elevations in 70% while only 15.8% of patients exhibited alanine aminotransferase (ALT) above the upper limit of normal (ULN) (see Table 1). Bilirubin was elevated in 13.6% and International normalized ratio (INR) in 15% of patients (>1.2 mg/dl), excluding two patients receiving anticoagulation. Cholestatic markers were elevated in 19.4% for Gamma-Glutamyl Transferase (gGT) and 25% Alkaline phosphatase (AP). No patient had evidence or a history of advanced liver diseases. Surrogate scores of advanced fibrosis were calculated and are provided in Table 1. Thirty-one per cent of patients exhibited an AST to Platelet Ratio Index (APRI) score above 1 and 55% a Fibrosis-4 (FIB-4) index of >2.67.
During the observation time of a mean of 10.5 days 57.1% of patients exhibited increasing LFTs with a maximum at day 5. The dynamics of ALT and AST changes are displayed in Figure 1. An increase above 3× ULN indicative of severe liver injury was observed for AST in 4/42 patients and ALT in 2/42 patients at any time of the treatment (see Figure 1). Importantly, these patients displayed extensive laboratory abnormalities indicative of cellular injury, including elevated CK (50%) and LDH (87.5%). No haemolysis was detectable.
Figure 1.
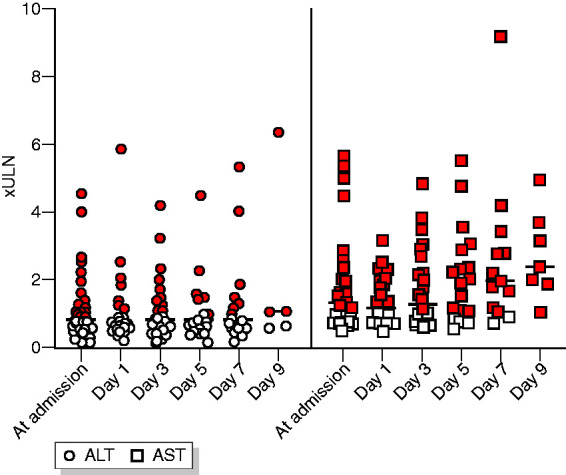
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) over the time of hospitalization. Red colour denotes >upper limit of normal (ULN).
Discussion
This is the first report of hospitalized patients with COVID-19 from Germany specifically exploring the hepatic phenotype. While other case series have indicated that liver injury related to SARS-CoV2 infection could be a prominent feature, this has not been systematically studied in Europe. In the explored cohort of consecutively admitted patients, we observed a dominant elevation of AST, while ALT and cholestatic markers were not as frequently elevated and, importantly, liver function was not markedly abnormal. This laboratory pattern – in the absence of relevant cholestasis or impaired liver function – argues against relevant liver injury at the time of admission. Additionally, only few cases exhibited an increase of gGT levels – a marker with pure hepatic origin – and concomitant medications that are known to cause liver function test abnormalities were identified in a majority of these patients. Importantly a majority of patients exhibited markers of cytolysis, including LDH and CK, with no evidence of haemolysis or clinical obvious myositis. This is in line with previous reports and highlights the degree of cellular injury in patients with COVID-19 that is responsible for the release of intracellular markers even before hospitalization.
Second, we observed increasing AST in hospitalized patients with no clear association with treatment or the clinical severity in this cohort. Other reports have identified prolonged hospitalization related to a severe course of COVID-19 as one risk factor of ALT elevations.14 Importantly, these patients were also more likely to exhibit metabolic comorbidities. The role of pre-existing liver disease in relation to abnormal LFTs is less clear. Despite that few data related to the role of surrogate scores of hepatic steatosis in identifying patients at risk for prolonged hospitalization and more severe disease course,14 this has not been convincingly shown. Other studies have highlighted that acute liver injury occurs later compared with pulmonary or renal complications starting not before day 10 of treatment.15 The use of non-invasive surrogate scores to assess the prevalence of advanced liver disease appears to be inappropriate in COVID-19 as the parameters that define the scores are not in a steady state.16 In the explored cohort an implausibly high rate of advanced fibrosis was detected using the FIB-4 and APRI and the relevant number of patients above the upper cut-off in the current case series is more likely related to the cellular injury rather than the presence of advanced fibrosis. Importantly these scores have been developed in a different context of use and therefore their value in a COVID-19 positive population remains undetermined at this time.
This cases series included patients with pre-existing liver disease; however, these were at an early stage and none exhibited an advanced stage consistent with cirrhosis. While liver injury should be monitored in patients with COVID-19 infection, only a minority of patients developed severe elevations of more than 3× ULN of ALT and, importantly, we did not observe any cases of impaired liver function.
The limitations of this study are related to the small number of patients from a single centre. However, we provide important early experience arguing against relevant liver injury at the time of admission or hospital treatment in patients in the absence of end-stage liver disease.
In summary, the current study highlights a number of relevant findings for physicians treating COVID-19 infected patients. First, while the majority of patients exhibited elevated AST, this seems to reflect not severe liver injury but, rather, a cytotoxic response, potentially involving the musculature. Second, in patients with pre-existing, early stage liver disease no relevant decline of liver function was observed. This could be very different in patients with underlying cirrhosis, as these patents have previously been reported to be susceptible to developing ACLF during the course of viral infection.13 Despite the lack of relevant liver injury in these early cases reported from Germany, monitoring of liver function, in particular after antiviral therapy is initiated and in vulnerable patient populations, is warranted.
Acknowledgements
We want to acknowledge the patients and their families. In addition, our thanks extend to all physicians that provide care to patients at these times to overcome the health threat of COVID-19.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Consultancy: BMS, Echosens, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Madrigal, Novartis, Pfizer, Roche.
Ethics approval
The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local ethics committee (Landesärztekammer Rheinland Pfalz Nr. 2020-14931-NIS in March 2020).
Funding
The current work was funded by intramural funds of the University Medical Center Mainz.
Informed consent
The study was approved by the Ethical Committee of the Landesärztekammer Rheinland-Pfalz (ID 2020-14931-NIS). Retrospective analysis was performed on anonymized data and informed consent was not applicable.
ORCID iD
Jörn M Schattenberg https://orcid.org/0000-0002-4224-4703
References
- 1.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol 2020. Epub ahead of print 8 March 2020. DOI: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed]
- 2.Cai Q, Huang D, Yu H, et al. Characteristics of liver tests in COVID-19 patients. J Hepatol 2020. Epub ahead of print 13 April 2020. DOI: 10.1016/j.jhep.2020.04.006.
- 3.Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020. Epub ahead of print 15 March 2020. DOI: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed]
- 4.Fan Z, Chen L, Li J, et al. Clinical features of COVID-19 related liver damage. medRxiv 2020. Epub ahead of print 27 February 2020. DOI: 10.1101/2020.02.26.20026971.
- 5.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med Epub ahead of print 29 February 2020. DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 6.Dreher M, Kersten A, Bickenbach J, et al. Charakteristik von 50 hospitalisierten COVID-19-Patienten mit und ohne ARDS. Dtsch Arztebl Int 2020; 117: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell Epub ahead of print 7 March 2020. DOI: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 8.Barnes BH, Tucker RM, Wehrmann F, et al. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int 2009; 29: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raven A, Lu WY, Man TY, et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017; 547: 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson I. Liver: Cholangiocytes regenerate hepatocytes during severe liver injury. Nat Rev Gastroenterol Hepatol 2017; 14: 503. [DOI] [PubMed] [Google Scholar]
- 11.Farcas GA, Poutanen SM, Mazzulli T, et al. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis 2005; 191: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schutte A, Ciesek S, Wedemeyer H, et al. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol 2019; 70: 797–799. [DOI] [PubMed] [Google Scholar]
- 14.Ji D, Qin E, Xu J, et al. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: A preliminary analysis. J Hepatol 2020. Epub ahead of print 08 April 2020. DOI: 10.1016/j.jhep.2020.03.044.
- 15.Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020. Epub ahead of print 3 May 2020. DOI: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed]
- 16.Schattenberg JM. Reading the stars for hepatic fibrosis or how to predict the severity of liver disease in patients with NASH. Liver Int 2019; 39: 812–814. [DOI] [PubMed] [Google Scholar]


