Abstract
The cardiopulmonary system is a physiological cornerstone in the adaptive response to hypobaric hypoxia. Portable devices make it feasible nowadays to precisely assess the response to high altitude (HA) expeditions. In this study, we investigated breathing and arterial blood pressure responses during a Himalayan trek from 665 m to 4,780 m altitude in a white European (Italian) sojourner and a native Nepali (Tamang) guide, both healthy males. Resting diurnal and nocturnal data were acquired by means of ambulatory blood pressure monitoring (ABPM) and sleep apnea monitoring. We found an increase in the mean diurnal arterial blood pressure. Nocturnal blood pressure dipping was confirmed at all altitudes. Oxygen saturation decreased at altitude, with its additional nocturnal fall. Sleep apneic episodes, present in the Italian only, increased with altitude. We conclude that the nocturnal, more than diurnal, cardiorespiratory function is affected by HA hypoxia. Further studies should address the role of ethnicity, medications, and sociodemographic factors in the cardiorespiratory responses to hypobaric hypoxia.
Keywords: blood pressure, breathing, Himalayas, hypobaric hypoxia, physiological monitoring, sleep
In this study, we investigated cardiopulmonary nocturnal and diurnal responses during a Himalayan trek in a white European (Italian) sojourner and a native Nepali (Tamang) guide, by means of ambulatory blood pressure and sleep‐apnea monitoring. We found an increase in the mean BP. Oxygen saturation decreased at altitude, with its additional nocturnal fall. Sleep apneic episodes increased with altitude.

1. INTRODUCTION
High altitude (HA) has a multitude of pathophysiological effects (Young & Reeves, 2002). The condition is characterized by hypobaric hypoxia, decreased air density, and cold stress. Inspired oxygen pressure (PO2) and, consequently, arterial and tissue PO2 decrease. Nevertheless, there are native populations living at HA, such as Sherpas in the Himalayas, Tibetans on the Tibetan Plateau, or Andeans in the Andes. These peoples are chronically adapted to chronic HA in all body systems (Horscroft et al., 2017; Julian & Moore, 2019). Concerning cardiorespiratory function, adaptive responses to HA consist of increases in cardiac output and ventilation, and increases in the number of capillaries and mitochondria (Di Giulio et al., 2003; Mohsenin, 2015). In non‐acclimatized lowlanders, HA hypoxia causes an increase in cardiac output in a few days, primarily explained by increased heart rate (HR). In contradistinction, in HA‐acclimated lowlanders as well as in natives, cardiac output stays similar to that at sea level (Naeije, 2010). With acute ascent, HR at rest and at a standardized workload is higher than that at sea level, resulting in a systolic volume reduction (Princi, Zupet, Finderle, & Accardo, 2008).
Acute exposure to HA also increases blood pressure (BP), especially in the first days of exposure in lowlander (Calbet & Lundby, 2009) which persists after acclimatization (Parati et al., 2015) and is accompanied by reduced nocturnal BP dipping, due likely to a nighttime reduction in PO2 (Parati et al., 2014). The chronic response is an increase in BP, despite improved systemic O2 delivery with acclimatization (Calbet, 2003). The association of hypertension with altitude is population specific, for example Andean residents have a low prevalence of hypertension, whereas Tibetan highlanders have a high prevalence (Norboo et al., 2015). HA also evokes bradyarrhythmia during apneic periods in lowlanders, but not in Sherpas (Busch et al., 2018). However, population specificity of BP response to middle‐term hypobaric hypoxia remains to be elucidated.
HA critically affects respiration. A hypobaric reduction in alveolar PO2 limits the perimetric oxygen supply leading to a drop in arterial oxygen saturation (SaO2) (Calbet & Lundby, 2009). A hallmark of HA is periodic breathing, especially during non‐REM sleep (Ainslie, Lucas, & Burgess, 2013). Periodic breathing is characterized by periods of apnea lasting for 10–15 s, followed by hyperpnea caused by increased central chemoreceptor sensitivity to PaCO2 accumulation (Mohsenin, 2015). The hypoxic ventilatory response (HVR) to acute hypoxia is increased at HA, facilitating and extending nighttime periodic breathing (Lahiri, Maret, Sherpa, & Peters, 1984). Periodic breathing, duration and efficiency of sleep, and nighttime desaturations are life‐threatening at HA. Of AMS based on the assessment of oxygen saturation and sleep abnormalities is a matter of controversy (Naeije, 2010; Tannheimer et al., 2017).
A recent massive increase in tourism and sports activities at HA raises a need for the evaluation of adaptive cardiorespiratory variables, with adequate and portable devices (Ridolfi, Vetter, Solà, & Sartori, 2010). Photoplethysmography (PPG) associated with sleep apnea monitors and probes assessing respiration and PO2 have been widely used in this regard (Allen, 2007). Additionally, ambulatory blood pressure monitoring (ABPM) system has been used to investigate arterial BP at HA (Bilo et al., 2011). Indeed, altitude‐induced sleep disturbances have been widely reported and may negatively affect daytime performances, despite some uncertainty in specific adaptations due to age, gender, altitude working habit, living altitude, and altitude sickness (Bloch, Buenzli, Latshang, & Ulrich, 2015).
The purpose of this study was to investigate the cardiovascular and respiratory responses to HA hypobaric hypoxia, with attention to nocturnal adaptive response, in two mountaineers trekking high Himalayas: a white European (Italian) sojourner and a native Nepali Tamang. We also aimed to search for the plausible differences depending on ethnicity and chronic living conditions.
2. MATERIALS AND METHODS
The study was part of the medical research “Kanchenjunga Exploration & Physiology”, a subset of the broad project approved by the Ethics Review Board of the Nepal Health Research Council (NHRC) and performed under the ethical standards of the 1964 Helsinki declaration and all its amendments. All participants provided written informed consent to participate in the study.
The two participants completed a 19‐day trekking covering a circuit of 300 km over 16,000 m difference in altitude, with a daily average of 6 hr walking in the Nepali Himalayas. They underwent the assessment of nocturnal cardiovascular and breathing function, which was conducted in Dobhan (665 m), Ghunsa (3,427 m altitude), and Lhonak (4,780 m of altitude) (Figure 1). Both participants were considered well‐acclimated to altitude and neither suffered from altitude sickness. The Italian participant only took one acetazolamide pill of 250 mg daily, at 6 p.m. 2 days before reaching the highest altitude point.
Figure 1.
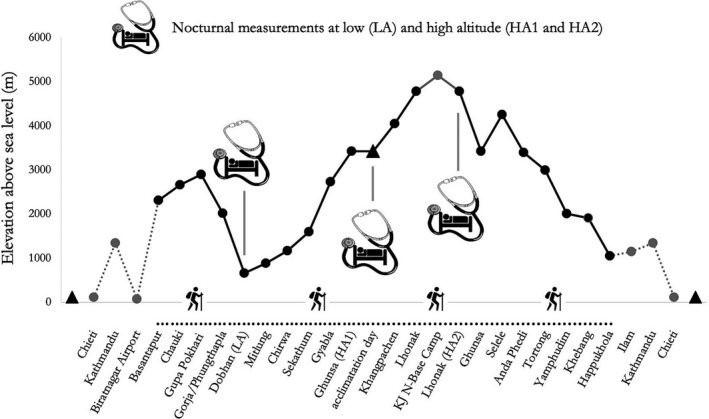
Altimetric scheme of “Kanchenjunga Exploration and Physiology”. Vertical lines denote three time points of assessment of nocturnal arterial blood pressure and oxygen saturation
Characteristics of the two participants were as follows: ethnic white European (Italian trekker), nonsmoker, aged 48, body mass index (BMI) of 30.5 kg/m2; and ethnic Tamang (Nepali guide), light smoker, aged 40, BMI of 28.8 kg/m2. Neither participant reported any cardiovascular or respiratory disease, particularly heart arrhythmias or upper airway obstruction. Both participants wore two portable devices during sleep (Figure 2). The Italian trekker's abode normally was at sea level. However, he reported past HA experience more than 3 years before the current expedition. The Tamang guide also resided at low altitude, but reported frequent work‐related exposure to HA; on average, 3–5 expeditions per year.
Figure 2.

Tamang and Italian participants equipped with ABPM and sleep apnea monitor devices before sleeping
Breathing and SpO2 were measured with two identical sleep apnea screening devices with 1 s intervals through a finger (WHO, 2011) and nasal probe (APN‐100; Contec Medical Systems Co. China). Mean arterial (MAP), systolic (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) were measured automatically every 30 min with two identical oscillometric recorders (ABPM50; Contec Medical Systems Co. China). Nocturnal assessment took place between 22:00 and 6:00 o'clock. The average sleep duration was approximately 6–7 hr for both participants. We noted the time of going to sleep and waking time, along with changes in sleep behavior, urinary frequency (0–2 per night), and sleeping difficulty, such as delayed sleep onset or middle‐of‐the‐night insomnia.
Additionally, we assessed diurnal BP and SpO2 at daily basis in Kathmandu (1,450 m), before the expedition, and in Ghunsa and Lhonak, during the expedition. SBP and DBP data were taken in a sitting position at rest, with the cuff at heart level (Whelton et al., 2018), along with SpO2 and HR, in the morning before breakfast. Additionally, body weight and waist circumference were measured (World Health Organization, 2011). All the measurements were performed in duplicate.
Apnea was interpreted as a 80% decline in airflow or more of the baseline level lasting for 10 to 40 s (Thiriet, 2015). Breathing rate (BR) was normalized for the non‐apnea time, subtracting apnea time from the total time, and dividing the result by the number of breaths. From diurnal BP data, PP was calculated as a diastolic‐systolic difference, and MAP was calculated as SBP + 2DBP ÷ 3. Owing to the individual diaries of sleeping time, we controlled for any missing or disordered data caused by untoward changes in sleeping time or behavior. Any software‐related automatic removal of artifacts was manually rechecked.
3. RESULTS
Nighttime SBP and DBP increased during the ascent from 665 m to 3,427 m in both trekkers. During continuing ascent to 4,780 m, SBP remained at the increased level, while DBP further increased in the Tamang but not in the Italian. There was an increase in MAP during the ascent, which was also greater in the Tamang. PP remained about stable in the Tamang, while it decreased in the Italian at mid‐altitude, to increase even more at the highest altitude. HR continually increased from the lowest to highest altitude (Table 1), but the increase started from a lower value, being therefore relatively greater, in the Tamang. The percent of apneic episodes at sleep increased during the ascent in the Italian, who primarily showed such episodes also at low altitude, while it remained relatively low in Tamang. BR increased at the highest altitude in the Tamang, while it decreased in the Italian (Table 1).
Table 1.
Nocturnal blood pressure (BP), heart rate (HR), breathing function, and oxygen saturation (SpO2) changes in high altitude trekkers
| Trekker | Altitude | SBP1 | DBP2 | PP3 | MAP4 | HR5 | SpO2 6 | Apnea time7 | BR8 | nBR9 | HR10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Italian | 665 m | 115.7 | 70.1 | 45.7 | 81.6 | 57.2 | 95 | 13.0 | 13 | 15.0 | 56 |
| 3,427 m | 118.6 | 79.9 | 38.8 | 90.4 | 72.7 | 80 | 38.2 | 10 | 16.2 | 75 | |
| 4,780 m | 128.7 | 77.8 | 50.9 | 96.1 | 65.7 | 84 | 44.7 | 7 | 12.4 | 62 | |
| Tamang | 665 m | 90.9 | 53.8 | 37.1 | 64.5 | 73.1 | 95 | 1.3 | 13 | 12.7 | 66 |
| 3,427 m | 103.8 | 67.8 | 36.0 | 78.0 | 72.9 | 89 | 0.7 | 17 | 17.3 | 77 | |
| 4,780 m | 114.4 | 76.4 | 38.0 | 86.2 | 92.8 | 79 | 2.6 | 18 | 18.4 | 92 |
Accuracy of the BP oscillometric recorder was as follows: HR 2 bpm, and BR 2 rpm. Interval between measurement was 30 min. Accuracy of the sleep apnea monitor was as follows: SpO2 2%, BR 2 rpm, and HR 2 bpm. Interval between measurements was 1 s.
1Systolic blood pressure (mmHg); 2Diastolic blood pressure (mmHg); 3Pulse pressure (mmHg); 4Mean arterial pressure (mmHg); 5Heart rate per min, measured by the BP oscillometric recorder; 6Peripheral oxygen saturation (%); 7(% of sleep time); 8Breaths per min; 9normalized breaths per min; 10Heart rate per min, measured by the sleep apnea monitor.
Diurnal SpO2 reflected a rebound after nocturnal reductions at all altitude levels. BR tended to be lower than that at night, and remained overall higher in the Tamang (Table 2). HR fluctuated at about the same level as that at nighttime. The increment in BP during diurnal ascent was primarily expressed by DBP and MAP values, while PP, differently from nocturnal data, was reduced at altitude in both trekkers. They both lost weight progressively with altitude. This reduction was more pronounced in the Italian, as he also had a clear reduction in waist circumference when compared with the Tamang.
Table 2.
Diurnal blood pressure (BP) and oxygen saturation (SpO2) in high altitude trekkers. Accuracy of the devices was as follows: SpO2 2%, HR 2 bpm, BR 2 rpm, and BP 3 mmHg
| Trekker |
Altitude (m) |
BMI1 (kg/m2) |
WC2 (cm) |
SpO2 3* (%) |
HR4* (bpm) |
BR5 (bpm) |
SBP6^ (mmHg) |
DBP7^ (mmHg) |
PP8 (mmHg) |
MAP9 (mmHg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Italian | 1,450 | 30.6 | 110 | 97 | 64 | 14 | 136 | 82 | 54 | 100 |
| 3,427 | 29.1 | 106 | 94 | 88 | 13 | 139 | 92 | 47 | 108 | |
| 4,780 | 28.7 | 103 | 92 | 86 | 10 | 137 | 94 | 43 | 108 | |
| Tamang | 1,450 | 28.8 | 97 | 96 | 72 | 18 | 131 | 89 | 42 | 103 |
| 3,427 | 28.3 | 96 | 93 | 76 | 10 | 124 | 94 | 30 | 104 | |
| 4,780 | 27.7 | 96 | 85 | 89 | 13 | 129 | 94 | 30 | 106 |
Data are means of two measurements. 1Body mass index; 2Waist circumference; 3Peripheral oxygen saturation; 4Heart rate per min; 5Breaths per min; 6Systolic blood pressure; 7Diastolic blood pressure; 8Pulse pressure; 9Mean arterial pressure.
4. DISCUSSION
Sleep disturbances at altitude are common: in the systematic review of Bloch and colleagues (Bloch et al., 2015), the authors argued that subjective insomnia occurs at HA (i.e., 4,559 m) rather than at low (1,630 m) and moderate (2,590 m) altitudes, periodic breathing and arousals occur in lowlanders sojourning at altitude, and the effects of acclimatization on sleep are altitude dependent. However, sleep‐disordered breathing occur in a large proportion of highlanders, and a greater prevalence of sleep central apneas in highlanders compared with lowlanders has been reported (Pham et al., 2017), with a pathophysiological link to pulmonary hypertension (Latshang et al., 2017). So far, there is still uncertainty about the role of ethnicity and that of altitude working habit on cardiorespiratory nocturnal response depending on ethnicity and altitude working habit. In this scenario, this study is a comparative report of the nocturnal responses of the cardiorespiratory system to environmental hypobaric hypoxia in two ethnically different male Himalayas trekkers, a sojourner Italian and a native Nepali Tamang.
We found that oxygen saturation decreased in a way characteristic for HA sojourn in both participants (Baertschi, Dayhaw‐Barker, & Flammer, 2016). Arterial blood pressure showed a characteristic increase at HA (Narvaez‐Guerra, Herrera‐Enriquez, Medina‐Lezama, & Chirinos, 2018). The mean arterial pressure was the variable that showed the most expressive upward trends. We supplement current findings by showing day/night profile of BP changes and distinctly stronger increases at nighttime, which point to the potential peril of brain episodes developing during sleep in mountaineers. PP changes followed a diverse trend, ranging from 39 to 51 mmHg at nighttime in the Italian, while remained stable in the Tamang. In contrast, diurnal data rather showed a reduction in PP with altitude. If we consider the nocturnal data at the lowest and the highest point, HR and PP changes were unrelated. A larger increase in PP in Italian versus Tamang (11.4% vs. 2.4%, respectively) was in contrast to a larger increase in HR in Tamang versus Italian (26.9% vs. 14.9%, respectively). We can speculate that a larger fall in nocturnal SpO2 in Tamang versus Italian (−16.8% vs. −11.6%, respectively) might provoke a more intense compensatory chronotropic response. A reason may lie in changes of arterial stiffness (Said, Eppinga, Lipsic, Verweij, & van der Harst, 2018) caused by higher sympathetic activation with altitude.
The present study showed a nocturnal dipping of BP, with diurnal hypertension at altitude. Referring to nocturnal BP, although neither trekker had remarkable sleep disorders, the Italian took acetazolamide pills. Considering the time course of acetazolamide clearance (Van Berkel & Elefritz, 2018), it is rather doubtful that differential trends in BP changes between the Italian and Tamang trekkers could be due to the acetazolamide's diuretic effect.
We found that diurnal SpO2 was higher than nocturnal. However, the Italian had a lower SpO2 at moderate rather than HA. Considering the percentage of sleep‐related apneic episodes, which was increasing with altitude, there is a biological plausibility that an increase in periodic breathing, mostly consisting of central apneas, a characteristic feature of HA particularly in mountaineers aged over 40 (Said et al., 2018), could actually increase SpO2 during sleep, as an adaptive response. Even if we could not differentiate between peripheral obstructive and central apneic episodes, it is a reasonable assumption that we dealt central apneas. In this context, acetazolamide fails to prevent the increase in sleep apneas (Ainslie et al., 2013).
Breathing rate appeared not to be subject to adaptive responses at HA as the diurnal resting rate was unrelated to nocturnal rate. Of note, breathing rate of our trekkers was in a range characteristic for healthy adults, that is below 20 breaths/min (Cretikos et al., 2008). The Tamang trekker's breathing followed a typical response to hypoxia consisting of increased rate (San et al., 2013). In contrast, the Italian trekker's breathing rate, normalized for non‐apneic sleep, decreased at HA; a trend that would have been masked by a lack of data normalization. The pattern of sleep in the Italian, with the presence of apneic episodes at HA, a likely adaptive feature of sleep‐breathing function, deserves further exploratory research, particularly in the face of a report by Heinzer et al. showing the lack of breathing rate adaptation in response to hypobaric hypoxia (Heinzer et al., 2016). We suggest that a decrease in body weight that we noticed in the trekkers mass could affect the respiratory system, which entails an intertwined function (Homma & Masaoka, 2008; Littleton, 2012; Salome, King, & Berend, 2010; Thomas, Cowen, Hulands, & Milledge, 1989). We noticed an appreciable reduction in BMI and waist circumference in the Italian trekker as opposed to the Tamang. Therefore, in the context of typical respiratory adaptation, even in the absence of a specific mechanistic analysis, we suggest that a decrease in metabolism could reverse the hypoxia‐related adaptive augmentation of breathing rate.
The study has typical limitations of a field research in extreme conditions. We conclude that the cardiorespiratory function at extreme HA seems more affected during nighttime sleep, than during daytime physical activities related to ascent. That implies the plausibility of potentially life‐threatening brain episodes, such as HA cerebral edema, that could happen during nighttime rest and sleep at HA. Further studies should address the role of ethnicity, medications, and sociodemographic factors in the cardiorespiratory responses to hypobaric hypoxia.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The funders had no role in the design of the study; data collection, analyses, or interpretation of the data; writing of the manuscript; or in the decision to publish the results.
AUTHORS CONTRIBUTIONS
Conceptualization, D.B. and V.V.; methodology, D.B. and V.V.; formal analysis, D.B. and V.V.; investigation, D.B. and V.V.; resources, V.V.; data curation, D.B. and V.V.; writing—original draft preparation, D.B., S.B., and V.V.; writing—review and editing, D.B., S.B., and V.V.; visualization, D.B., S.B., and V.V.; supervision, V.V.; project administration, V.V.; funding acquisition, V.V.
ACKNOWLEDGMENTS
Authors thank Mission Nepal Holidays Pvt. Ltd (Kathmandu, Nepal) for logistical support, and all the Italian trekkers and Nepalese porters involved in this project.
Bondi D, Bhandari S, Verratti V. Case studies in physiology: Nocturnal cardiorespiratory adaptive differences between an Italian trekker and a Nepali guide. Physiol Rep. 2020;8:e14537 10.14814/phy2.14537
Funding information
Funded by the Department of Psychological, Health and Territorial Sciences, “G. d’Annunzio” University of Chieti‐Pescara, Italy.
REFERENCES
- Ainslie, P. N. , Lucas, S. J. E. , & Burgess, K. R. (2013). Breathing and sleep at high altitude. Respiratory Physiology and Neurobiology, 188(3), 233–256. 10.1016/j.resp.2013.05.020 [DOI] [PubMed] [Google Scholar]
- Allen, J. (2007). Photoplethysmography and its application in clinical physiological measurement. Physiological Measurement, 28(3), R1–R39. 10.1088/0967-3334/28/3/R01 [DOI] [PubMed] [Google Scholar]
- Baertschi, M. , Dayhaw‐Barker, P. , & Flammer, J. (2016). The effect of hypoxia on intra‐ocular, mean arterial, retinal venous and ocular perfusion pressures. Clinical Hemorheology and Microcirculation, 63(3), 293–303. 10.3233/CH-152025 [DOI] [PubMed] [Google Scholar]
- Bilo, G. , Caldara, G. , Styczkiewicz, K. , Revera, M. , Lombardi, C. , Giglio, A. , … Parati, G. (2011). Effects of selective and nonselective beta‐blockade on 24‐h ambulatory blood pressure under hypobaric hypoxia at altitude. Journal of Hypertension, 29(2), 380–387. 10.1097/HJH.0b013e3283409014 [DOI] [PubMed] [Google Scholar]
- Bloch, K. E. , Buenzli, J. C. , Latshang, T. D. , & Ulrich, S. (2015). Sleep at high altitude: Guesses and facts. Journal of Applied Physiology, 119(12), 1466–1480. 10.1152/japplphysiol.00448.2015 [DOI] [PubMed] [Google Scholar]
- Busch, S. A. , Davies, H. , van Diepen, S. , Simpson, L. L. , Sobierajski, F. , Riske, L. , … Steinback, C. D. (2018). Chemoreflex mediated arrhythmia during apnea at 5,050 m in low‐ but not high‐altitude natives. Journal of Applied Physiology, 124(4), 930–937. 10.1152/japplphysiol.00774.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet, J. A. L. (2003). Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. Journal of Physiology, 551(Pt 1), 379–386. 10.1113/jphysiol.2003.045112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet, J. A. L. , & Lundby, C. (2009). Air to muscle O2 delivery during exercise at altitude. High Altitude Medicine and Biology, 10(2), 123–134. [DOI] [PubMed] [Google Scholar]
- Cretikos, M. A. , Bellomo, R. , Hillman, K. , Chen, J. , Finfer, S. , & Flabouris, A. (2008). Respiratory rate: The neglected vital sign. Medical Journal of Australia, 188(11), 657–659. 10.5694/j.1326-5377.2008.tb01825.x [DOI] [PubMed] [Google Scholar]
- Di Giulio, C. , Bianchi, G. , Cacchio, M. , Macrì, M. A. , Ferrero, G. , Rapino, C. , … Artese, L. (2003). Carotid body HIF‐1alpha, VEGF and NOS expression during aging and hypoxia. Advances in Experimental Medicine and Biology, 536, 603–610. [DOI] [PubMed] [Google Scholar]
- Heinzer, R. , Saugy, J. J. , Rupp, T. , Tobback, N. , Faiss, R. , Bourdillon, N. , … Millet, G. P. (2016). Comparison of sleep disorders between real and simulated 3,450‐m altitude. Sleep, 39(8), 1517–1523. 10.5665/sleep.6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma, I. , & Masaoka, Y. (2008). Breathing rhythms and emotions. Experimental Physiology, 93(9), 1011–1021. 10.1113/expphysiol.2008.042424 [DOI] [PubMed] [Google Scholar]
- Horscroft, J. A. , Kotwica, A. O. , Laner, V. , West, J. A. , Hennis, P. J. , Levett, D. Z. H. , … Murray, A. J. (2017). Metabolic basis to Sherpa altitude adaptation. Proceedings of the National Academy of Sciences of the United States of America, 114(24), 6382–6387. 10.1073/pnas.1700527114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian, C. G. , & Moore, L. G. (2019). Human genetic adaptation to high altitude: Evidence from the andes. Genes, 10(2), 150 10.3390/genes10020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri, S. , Maret, K. H. , Sherpa, M. G. , & Peters, R. M. Sleep and periodic breathing at high altitude: Sherpa natives versus sojourners In: West J. B., & Lahiri S. editors. High Altitude and Man [Internet]. New York, NY: Springer; 1984. [cited 2020 Mar 12]. p. 73–90. (Clinical Physiology). Doi: 10.1007/978-1-4614-7525-5_8. [DOI] [Google Scholar]
- Latshang, T. D. , Furian, M. , Aeschbacher, S. S. , Ulrich, S. , Osmonov, B. , Mirrakhimov, E. M. , … Bloch, K. E. (2017). Association between sleep apnoea and pulmonary hypertension in Kyrgyz highlanders. European Respiratory Journal, 49(2), 1601530 10.1183/13993003.01530-2016 [DOI] [PubMed] [Google Scholar]
- Littleton, S. W. (2012). Impact of obesity on respiratory function. Respirology, 17(1), 43–49. 10.1111/j.1440-1843.2011.02096.x [DOI] [PubMed] [Google Scholar]
- Mohsenin, V. (2015). Common high altitudes illnesses a primer for healthcare provider. British Journal of Medicine and Medical Research, 7(12), 1017–1025. 10.9734/BJMMR/2015/17501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeije, R. (2010). Physiological adaptation of the cardiovascular system to high altitude. Progress in Cardiovascular Diseases, 52(6), 456–466. 10.1016/j.pcad.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Narvaez‐Guerra, O. , Herrera‐Enriquez, K. , Medina‐Lezama, J. , & Chirinos, J. A. (2018). Systemic hypertension at high altitude. Hypertension, 72(3), 567–578. 10.1161/HYPERTENSIONAHA.118.11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norboo, T. , Stobdan, T. , Tsering, N. , Angchuk, N. , Tsering, P. , Ahmed, I. , … Okumiya, K. (2015). Prevalence of hypertension at high altitude: Cross‐sectional survey in Ladakh, Northern India 2007–2011. British Medical Journal Open, 5(4), e007026 10.1136/bmjopen-2014-007026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati, G. , Bilo, G. , Faini, A. , Bilo, B. , Revera, M. , Giuliano, A. , … Mancia, G. (2014). Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high‐altitude exposure: A randomized clinical trial. European Heart Journal, 35(44), 3113–3122. 10.1093/eurheartj/ehu275 [DOI] [PubMed] [Google Scholar]
- Parati, G. , Ochoa, J. E. , Torlasco, C. , Salvi, P. , Lombardi, C. , & Bilo, G. (2015). Aging, high altitude, and blood pressure: A complex relationship. High Altitude Medicine and Biology, 16(2), 97–109. 10.1089/ham.2015.0010 [DOI] [PubMed] [Google Scholar]
- Pham, L. V. , Meinzen, C. , Arias, R. S. , Schwartz, N. G. , Rattner, A. , Miele, C. H. , … Schwartz, A. R. (2017). Cross‐sectional comparison of sleep‐disordered breathing in native peruvian highlanders and lowlanders. High Altitude Medicine and Biology, 18(1), 11–19. 10.1089/ham.2016.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princi, T. , Zupet, P. , Finderle, Z. , & Accardo, A. (2008). Linear and nonlinear assessment of heart rate variability in nonacclimatized middleaged subjects at different hypoxic levels. Biomedical Sciences Instrumentation, 44, 380–385. [PubMed] [Google Scholar]
- Ridolfi, A. , Vetter, R. , Solà, J. , & Sartori, C. (2010). Physiological monitoring system for high altitude sports. Procedia Engineering, 2(2), 2889–2894. 10.1016/j.proeng.2010.04.083 [DOI] [Google Scholar]
- Said, M. A. , Eppinga, R. N. , Lipsic, E. , Verweij, N. , & van der Harst, P. (2018). Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. Journal of the American Heart Association, 7(2), 10.1161/JAHA.117.007621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome, C. M. , King, G. G. , & Berend, N. (2010). Physiology of obesity and effects on lung function. Journal of Applied Physiology, 108(1), 206–211. 10.1152/japplphysiol.00694.2009 [DOI] [PubMed] [Google Scholar]
- San, T. , Polat, S. , Cingi, C. , Eskiizmir, G. , Oghan, F. , & Cakir, B. (2013). Effects of high altitude on sleep and respiratory system and theirs adaptations. The Scientific World Journal, 2013, 1–7. 10.1155/2013/241569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannheimer, M. , van der Spek, R. , Brenner, F. , Lechner, R. , Steinacker, J. M. , & Treff, G. (2017). Oxygen saturation increases over the course of the night in mountaineers at high altitude (3050–6354 m). Journal of Travel Medicine, 24(5), 3050–6354. 10.1093/jtm/tax041. [DOI] [PubMed] [Google Scholar]
- Thiriet, M. (2015). Diseases of the cardiac pump. Cham, Switzerland: Springer, 125 p. [Google Scholar]
- Thomas, P. S. , Cowen, E. R. , Hulands, G. , & Milledge, J. S. (1989). Respiratory function in the morbidly obese before and after weight loss. Thorax, 44(5), 382–386. 10.1136/thx.44.5.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel, M. A. , & Elefritz, J. L. (2018). Evaluating off‐label uses of acetazolamide. American Journal of Health‐System Pharmacy, 75(8), 524–531. 10.2146/ajhp170279. [DOI] [PubMed] [Google Scholar]
- Whelton, P. K. , Carey, R. M. , Aronow, W. S. , Casey, D. E. , Collins, K. J. , Dennison Himmelfarb, C. , … Wright, J. T. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology, 71(19), e127–e248. [DOI] [PubMed] [Google Scholar]
- WHO . Pulse Oximetry Training Manual. Geneva: WHO Press, World Health Organization; 2011. [Google Scholar]
- World Health Organization . (2011). Waist circumference and waist‐hip ratio: Report of a WHO expert consultation, Geneva, 8–11 December 2008. Geneva: World Health Organization. [Google Scholar]
- Young, A. J. , & Reeves, J. T. (2002). Human adaptation to high terrestrial altitude In Burr R. E. (Ed.), Pandoff KB (pp. 644–688). Medical aspects of harsh environments: Ist. USA: Walter Reed Army Medical Center Borden Institute. [Google Scholar]


