Abstract
Sensorimotor transformations require spatiotemporal coordination of signals, that is, through both time and space. For example, the gaze control system employs signals that are time‐locked to various sensorimotor events, but the spatial content of these signals is difficult to assess during ordinary gaze shifts. In this review, we describe the various models and methods that have been devised to test this question, and their limitations. We then describe a new method that can (a) simultaneously test between all of these models during natural, head‐unrestrained conditions, and (b) track the evolving spatial continuum from target (T) to future gaze coding (G, including errors) through time. We then summarize some applications of this technique, comparing spatiotemporal coding in the primate frontal eye field (FEF) and superior colliculus (SC). The results confirm that these areas preferentially encode eye‐centered, effector‐independent parameters, and show—for the first time in ordinary gaze shifts—a spatial transformation between visual and motor responses from T to G coding. We introduce a new set of spatial models (T‐G continuum) that revealed task‐dependent timing of this transformation: progressive during a memory delay between vision and action, and almost immediate without such a delay. We synthesize the results from our studies and supplement it with previous knowledge of anatomy and physiology to propose a conceptual model where cumulative transformation noise is realized as inaccuracies in gaze behavior. We conclude that the spatiotemporal transformation for gaze is both local (observed within and across neurons in a given area) and distributed (with common signals shared across remote but interconnected structures).
In this Review we describe challenges the literature has faced to study spatial transformations in the visuomotor pathway required for orienting to visual stimuli during natural head‐unrestrained gaze behavior. We summarize our relatively new method developed to address this question and review our recent studies that apply it to neural activity in two key structures in gaze control, the frontal eye field and superior colliculus. We synthesize these results and supplement it with the previous literature to propose a new conceptual model where cumulative transformation noise, within local and distributed circuits, is realized as inaccuracies in gaze behavior.
![]()
1. INTRODUCTION
A central question in sensorimotor neuroscience concerns what sequence of events takes place in order to transform vision into voluntary action (Bremner & Andersen, 2014; Bruce & Goldberg, 1985; Crawford, Henriques, & Medendorp, 2011; DeCharms & Zador, 2000; Flanders, Tillery, & Soechting, 1992; Gallivan & Culham, 2015; Gnadt, Bracewell, & Andersen, 1991; Goodale, 2011; Helmbrecht, Dal Maschio, Donovan, Koutsouli, & Baier, 2018; Optican, 2005; Pouget & Snyder, 2000; Robinson, 1973; Schall, 2019; Schall & Thompson, 1999; Sparks, 1986, 2002; Westendorff, Klaes, & Gail, 2010). One of the best studied experimental models in sensorimotor neuroscience is the gaze control system, which serves to orient the fovea toward visual stimuli. A gaze shift to a visual stimulus requires the appropriate movements of the eyes (and often the head) in space and time. Therefore, sensorimotor transformation is as much of a spatial problem as it is a temporal problem (Andersen, Snyder, Li, & Stricanne, 1993; Crawford et al., 2011; Franklin, Reichenbach, Franklin, & Diedrichsen, 2016; Heitz, 2014; Optican, 2005; Snyder, 2000). Surprisingly, the spatiotemporal transformations for ordinary gaze shifts (made directly or after a short delay toward a visual stimulus) have only recently been demonstrated.
Macaques have proven to be useful experimental models for studying gaze control circuitry due to the anatomical and functional similarities with the human system (Kaas, 2004; Passingham, 2009). Many neurons in the primate gaze system exhibit elevated discharge rate in response to a visual stimulus (visual response) and/or around the time of movement (motor/movement response; Bruce & Goldberg, 1985; Goldberg, Colby, & Duhamel, 1990; Hikosaka & Wurtz, 1983; Mays & Sparks, 1980; Mohler & Wurtz, 1976; Schall, 2015; Schlag‐Rey & Schlag, 1984). However, the spatial mapping between these temporal codes is not trivial. Numerous modeling and experimental studies have attempted to address this question (e.g. Basso & May, 2017; Cohen & Andersen, 2002; Crawford et al., 2011; Funahashi, Takeda, & Watanabe, 2004; Fuster, 2001; Gandhi & Katnani, 2011; Sato & Schall, 2003; Snyder, 2000; Sparks, 2002; Sparks & Mays, 1990). As we shall see, each of these approaches have provided important advances in understanding spatial coding for gaze control, and each has its limitations. Most importantly, traditional methodologies did not allow one to simultaneously test all spatial models, or track their progress through time. So much of what we believe about ordinary gaze transformations relies on inferences integrated from more complex laboratory paradigms.
The goals of this review are to (a) summarize a relatively new method to identify spatiotemporal codes in the brain, (b) describe the application of this method in two important oculomotor structures—the frontal eye field (FEF) and superior colliculus (SC)—during fairly ordinary head‐unrestrained gaze shifts, (c) use this as an opportunity to directly compare the neurophysiology of these two structures, and (d) contextualize these new results with respect to the classic oculomotor literature. The novelty of our approach is the use of a sophisticated computational analysis method that is able to simultaneously test between all of the known, as well as novel, spatial models in these structures through different task events (Keith, DeSouza, Yan, Wang, & Crawford, 2009; Sajad et al., 2015; Sajad, Sadeh, Yan, Wang, & Crawford, 2016). As we shall see, similar spatiotemporal transformations occur in both structures at the level of within and between neurons, suggesting that they occur at the level of shared, distributed signals rather than specific brain structures. First, we will provide some general background and review of the SC and FEF, of the spatial models that have been proposed, and the ways these have been tested.
2. OVERVIEW OF SC AND FEF ANATOMY AND ROLES IN GAZE CONTROL
In macaques, the FEF is a cortical structure located at the bank of the arcuate sulcus, with large pyramidal neurons in layer 5, characteristic of cortical motor structures (Stanton, Deng, Goldberg, & McMullen, 1989; reviewed by Schall et al., 2017), whereas the SC is a multilayered subcortical structure located on the roof of midbrain (Mohler & Wurtz, 1976; reviewed by May, 2006). These two structures are intimately connected (Figure 1a): the FEF sends projections to the SC directly (Künzle, Akert, & Wurtz, 1976; Stanton, Goldberg, & Bruce, 1988a), and via the basal ganglia (Astruc, 1971; Hikosaka & Wurtz, 1983; Stanton, Goldberg, & Bruce, 1988b). The SC sends projections back to the FEF via the dorsomedial thalamus (Benevento & Fallon, 1975; Barbas & Mesulam, 1981; Goldman‐Rakic & Porrino, 1985; Lynch, Hoover, & Strick, 1994; Figure 1a). The SC and (to a lesser extent) the FEF project directly to the brainstem and spinal cord burst generators that innervate motoneurons for eye and head motion (Castiglioi, Gallaway, & Coulter, 1978; Harting, 1977; Huerta, Krubitzer, & Kaas, 1986; Isa & Sasaki, 2002; Kawamura, Brodal, & Hoddevik, 1974; Segraves, 1992; Stanton et al., 1988a). The causal role of the SC and FEF in gaze shift production is well established through various microstimulation (Bruce, Goldberg, Bushnell, & Stanton, 1985; Klier, Wang, & Crawford, 2001; Monteon, Constantin, Wang, Martinez‐Trujillo, & Crawford, 2010; Paré, Crommelinck, & Guitton, 1994), lesion (Schiller, Sandell, & Maunsell, 1987), and inactivation studies (Bollimunta, Bogadhi, & Krauzlis, 2018; Dias, Kiesau, & Segraves, 1995; Hanes & Wurtz, 2001; Hikosaka & Wurtz, 1985; McPeek & Keller, 2004). Both SC and FEF also receive direct visual input from the thalamus and visual cortex (Kaas & Huerta, 1988; Lynch et al., 1994; Schall, Morel, King, & Bullier, 1995). The superficial layer of the SC also receives direct visual input from the retina (Perry & Cowey, 1984).
FIGURE 1.
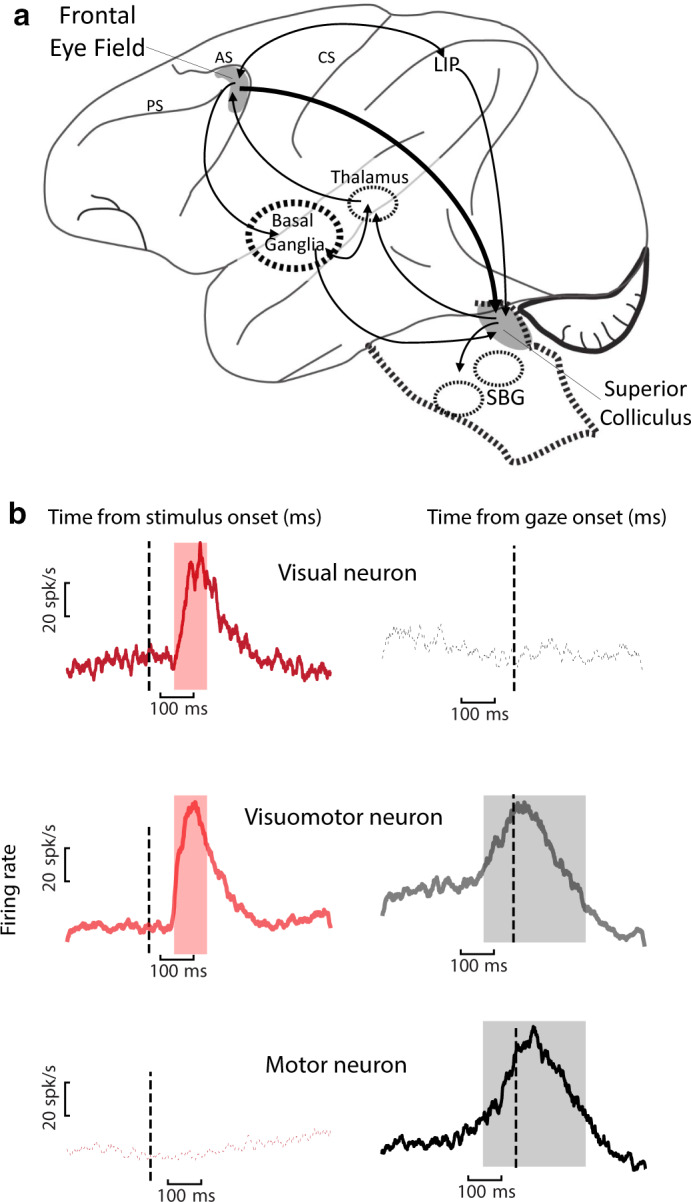
Schematic of key areas in dorsal visual pathway and representative visuomotor signals. (a) FEF and SC (shaded in gray) are shown in relation to interconnected structures. AC: arcuate sulcus, PC: Principal sulcus, CS: Central sulcus, SBG: Saccade Burst Generator. (b) Target‐ and gaze movement‐ aligned population responses of three general classes of neurons: Visual, Visuomotor, and Motor neurons in FEF (data from Sajad et al., 2015). Similar response profiles observed in SC (Sadeh et al., 2015). For the results reviewed in this manuscript, FEF and SC visual responses were sampled from 80 to 180 ms (pink shade) and 60 to 160 ms (not shown) following target presentation, respectively. FEF and SC motor responses included the bulk of the motor burst for each neuron (gray shade indicates mean FEF motor response). For SC, this window was fixed from −50 to +50 ms from gaze onset (not shown)
Both the FEF and SC can exhibit visual and motor responses (Bruce & Goldberg, 1985; Hanes & Schall, 1996; Mohler & Wurtz, 1976; Munoz & Wurtz, 1995; Paré & Hanes, 2003; Schiller, 1984). Neurons in these structures are often classified according to their temporal responses (Figure 1b): Visual neurons exhibit visual response, Motor (or movement) neurons exhibit motor response, and Visuomotor (or Visuomovement) neurons exhibit both response types (Bruce & Goldberg, 1985; Wurtz & Albano, 1980; but see Lowe & Schall, 2018). Visual and motor responses in the FEF and SC are often spatially selective for a restricted patch of space called a “response field” (or “receptive field” for the visual response; Bruce & Goldberg, 1985; Mohler, Goldberg, & Wurtz, 1973; Mohler & Wurtz, 1976; Sparks, 1988). FEF and SC response fields are often tuned for the contralateral visual field, and SC receptive fields show anatomic topographic organization.
3. SPATIAL MODELS FOR GAZE
As noted in the introduction, just because a neural event coincides temporally with an externally observable event (i.e. visual stimulus or saccade onset), it does not mean that one can assume which spatial variable is encoded. This is particularly true of motor‐locked signals, which may (or may not) have undergone considerable processing after the initial sensory input. At the input level for gaze saccades, light from visual stimuli hit the photoreceptors on the retina. Because the retina is fixed on the eye, we can say that the retina encodes visual stimuli in an eye‐centered frame where the fovea is the origin and positions can be defined by vectors projecting outwards along the spherical retina (Demb & Singer, 2015). Ultimately, the gaze system uses this to evoke patterns of muscle contractions to move the eye (rotation in head) and head (rotation on body) toward the stimulus. What remains unclear is how eye‐centered stimulus representations are transformed into muscle coordinates. Despite decades of work, there is still no consensus on the sequence of spatial transformations in the gaze system. Here, we briefly review some of the alternatives that have been proposed, and ways they have been experimentally tested.
3.1. Canonical models in gaze control
To characterize spatial processing in the brain, it is important to ask two questions: (a) what spatial parameter is encoded? and (b) what is the reference frame used to encode that parameter? (e.g. Soechting & Flanders, 1992). In the head‐unrestrained gaze control system, one might expect to encode spatial parameters, such as the visual target (T; e.g. Optican, 2005; Steenrod, Phillips, & Goldberg, 2012), eye motion (E), head motion (H), or their combination: gaze motion (G; e.g. Chen, 2006; Cowie & Robinson, 1994; Freedman & Sparks, 1997; Gandhi & Katnani, 2011). Spatial parameters related to these motions might be encoded either as displacement vectors relative to initial position (dE, dH, and dG) or final positions irrespective of initial position (Crawford & Guitton, 1997; Daemi & Crawford, 2015; Kardamakis & Moschovakis, 2009). Finally, each of these parameters might be encoded relative to various egocentric frames of references, including the eye (Te, Ge, Ee, and He), the head (Th, Gh, Eh, and Hh), or the body/space (body and space frames are indissociable when body does not move) (Ts, Gs, Es, and Hs; see Figure 2a; Boussaoud & Bremmer, 1999; Colby, 1998; Crawford et al., 2011; Lappi, 2016; Soechting & Flanders, 1992). Noteworthy that in experiments conducted in complete darkness, where the surrounding objects are not visible, egocentric frames are the focus because the possibility for object‐centered (i.e. allocentric) spatial representations is eliminated (but see Bharmauria, Sajad, Li, et al., 2020; Li et al., 2017). Many early conceptual models assumed that low‐level representations, such as Te, must be transformed into higher level frames, such as Th, to control movement (Andersen & Zipser, 1988; Soechting, Tillery, & Flanders, 1990), but more recent neural network studies have shown that this is not necessarily the case (Blohm, Keith, & Crawford, 2009; Pouget & Snyder, 2000; Smith & Crawford, 2005). Instead, the brain might make use of partial or intermediate reference frames, as discussed next.
FIGURE 2.
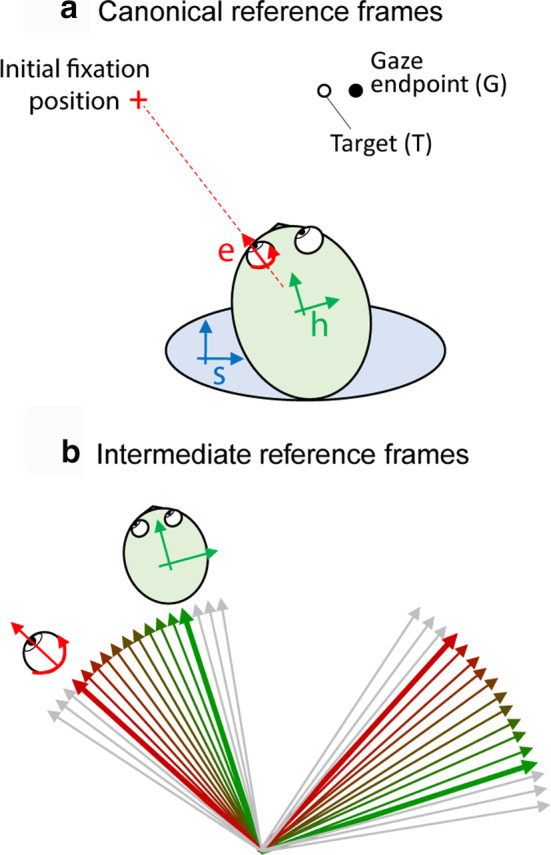
Spatial models in gaze control. (a) The location of the peripheral visual target (T) and the eventual location of the gaze shift (G) relative to egocentric reference eye (e), head (h), and body/space (s) reference frames at the time of fixation on the red cross. The spatial difference between T and G reflects inaccuracy in gaze behavior. (b) Intermediate eye‐head reference frames obtained by the linear combination of eye‐reference frame (red) and head‐reference frame (green) ranging from a more eye‐centered frame to a more head‐centered frame (color shade). Adapted from Sajad et al., (2015)
3.2. Intermediate reference frames
While the canonical reference frames (above) describe the egocentric representations in gaze control, empirical data as well as computational studies have suggested evidence for reference frames that are intermediate (or hybrid) between these frames (Avillac, Deneve, Olivier, Pouget, & Duhamel, 2005; Jay & Sparks, 1984; Martinez‐Trujillo, Medendorp, Wang, & Crawford, 2004; Pouget & Snyder, 2000; Stricanne, Andersen, & Mazzoni, 1996). Quantitatively, intermediate reference frames are obtained from the linear combination of two canonical frames (Figure 2b). In Figure 2b, the eye frame (red) and head frame (green) and nine intermediate frames with different degrees of eye‐ and head‐centeredness are shown. Constructing these intermediate frames of reference allows one to test nuances that are missed when one forces the data into predefined categories (e.g. Caruso, Pages, Sommer, & Groh, 2018). Recently, we have extended the concept of intermediate spatial coding to the coding of spatial parameters within the same reference frame, a key topic which we will return to below. Other coding mechanisms—such as gaze‐dependent “gain fields” are likely important for implementing reference frame transformations (Andersen & Zipser, 1988; Blohm & Crawford, 2009; Salinas & Abbott, 2001; Smith & Crawford, 2005), but will not be the focus of the current review.
4. TRADITIONAL APPROACHES TO STUDYING SPATIAL ENCODING
While a spatially tuned stimulus‐locked response is most likely related to stimulus location, at least in the absence of recurrent feedback, further processing means that a movement‐locked response may either be related to the stimulus or the metrics of the imminent movement (Marino, Rodgers, Levy, & Munoz, 2008; Omrani, Kaufman, Hatsopoulos, & Cheney, 2017; Stanford & Sparks, 1994). Most behavioral paradigms that dissociate these locations suggest the latter: imminent movement (Everling, Dorris, Klein, & Munoz, 1999; Everling & Munoz, 2000; Funahashi, 2013; Zhang & Barash, 2000; but see Edelman & Goldberg, 2002; Frens & Van Opstal, 1997; Quessy, Quinet, & Freedman, 2010). Further, whereas most studies involve head‐restrained eye motion, in natural head‐unrestrained conditions, the same signal might encode eye motion, head motion, or the combination: gaze (Chen, 2006; Cullen, Galiana, & Sylvestre, 2000; Guitton, Munoz, & Galiana, 1990; Knight, 2012; Paré & Guitton, 1990; Sparks, Freedman, Chen, & Gandhi, 2001; Walton, Bechara, & Gandhi, 2007). Under such conditions, many of the models described in the previous section become impossible to disentangle. Below we will review the traditional approach to investigating spatial parameters and their respective reference frames in FEF and SC.
4.1. Differentiating spatial parameters
The simple geometry of the oculomotor system actually imposes a challenge for testing spatial parameters. Unlike the reach system (where the visual vector and hand movement vector do not align unless the hand starts moving from the location of the eyeball; e.g. Blohm & Crawford, 2007), in the saccadic system, sensory and motor parameters are highly correlated (Freedman & Sparks, 1997; Marino et al., 2008; Smith & Crawford, 2005; Snyder, 2000). One way to overcome this challenge is to study random variations between these parameters (Bremmer, Kaminiarz, Klingenhoefer, & Churan, 2016; Keith et al., 2009; Platt & Glimcher, 1998; Wimmer, Nykamp, Constantinidis, & Compte, 2014), but neurophysiology techniques that rely on averaging often wash these out. For example, the variable scatter of gaze endpoint around target in many cases averages to zero, making it impossible to know if the activity of neurons is best described by target location or the gaze endpoint position. To overcome this limitation, experimenters have used clever paradigms that spatially dissociate the location of visual stimulus from the gaze target. Some have used motor adaptation paradigms in which after a training period, the motor system generates a movement that is spatially distinct from that of the visual stimulus (Edelman & Goldberg, 2002; Frens & Van Opstal, 1997; Quessy et al., 2010; Takeichi, Kaneko, & Fuchs, 2007). Others have used experimental tasks that require a deliberate (rule‐based) calculation of the gaze target to another location defined by (but different from) the visual stimulus (Everling et al., 1999; Everling & Munoz, 2000; Sato & Schall, 2003; Watanabe & Funahashi, 2007; Zhang & Barash, 2000). The most popular example of such tasks is the antisaccade task in which the subject is required to elicit an eye movement opposite to the direction of the target (Munoz & Everling, 2004). However, such transformations appear to be driven by top‐down feedback, propagating “backwards” from frontal to parietal to occipital cortex (Blohm et al., 2019; Paneri & Gregoriou, 2017). These techniques are thus valuable for understanding how the brain implements rule‐based, top‐down transformations, but they do not trivially map onto the standard bottom‐up sensorimotor transformations (Hawkins, Sayegh, Yan, Crawford, & Sergio, 2013; Jamadar, Johnson, Clough, Egan, & Fielding, 2015; Johnston, DeSouza, & Everling, 2009).
4.2. Differentiating reference frames
Classically, reference frames under head‐immobilized conditions are investigated by systematically switching the initial eye orientation between several discrete positions (Figure 3a). Because the head is stationary relative to the body, the head and body/space frames remain in register. If the neural response shows systematic changes as a function of position relative to one effector but not the other, then the neuron's response is in the reference frame fixed to that effector (Avillac et al., 2005; Caruso et al., 2018; Cohen & Andersen, 2002; Jay & Sparks, 1984; Russo & Bruce, 1994). To investigate intermediate frames of reference, some studies use the quantitative definition to explicitly test for these frames (e.g. Avillac et al., 2005; Jay & Sparks, 1984; Figure 2b). However, these techniques do not separate the head and body frames, and require repetition and averaging that are difficult to replicate under natural head‐unrestrained conditions (Keith et al., 2009).
FIGURE 3.
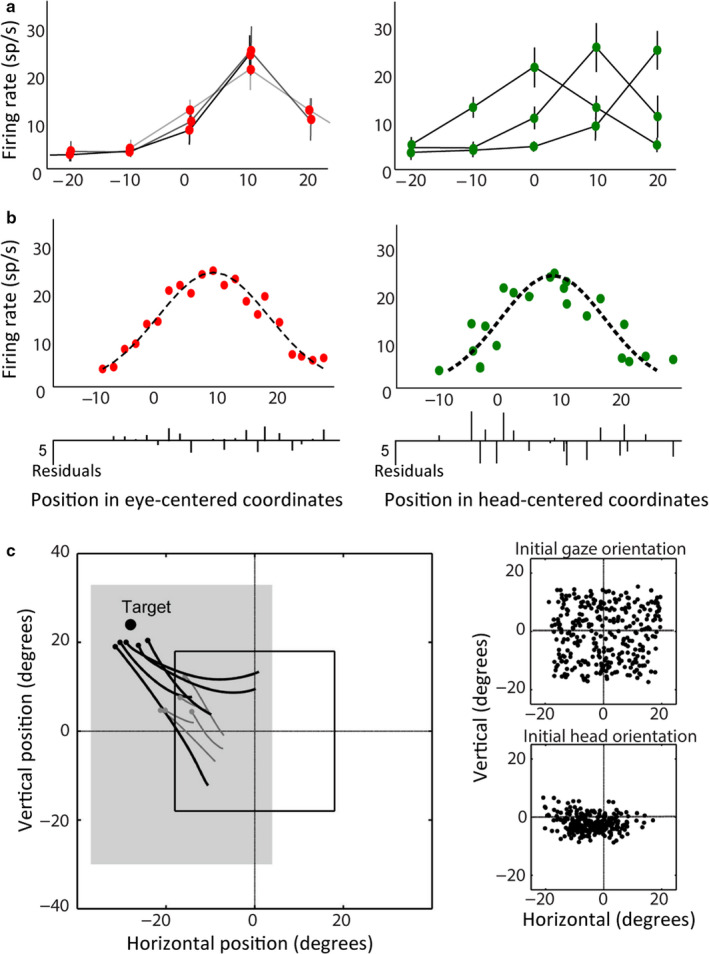
Classic and new methods of neural reference frame analysis for gaze control. (a) Response field plots obtained using the traditional approach to identify reference frames for an example neuron (e.g. Cohen & Andersen, 2002). Many trials are sampled while the head faces the front and the eye initial orientation varies between discrete positions. The shift in response field based on varying initial positions is assessed. The profile of the response field is conserved when plotted relative to the initial position of the eye (left), but shifts when plotted based on the initial position of the head (right), hence eye‐centered. (b) Response field plots reduced to one‐dimension, illustrating the logic for the statistical modeling method developed by Keith et al., (2009). Response fields were plotted by placing firing rate data over positions in space as defined by the tested model and the quality of the fit was assessed by measuring PRESS residuals obtained from a “remove one–fit–replace” approach (bottom panel shows residuals for all data points to a single fit). The response field is more spatially organized when plotted relative to initial eye orientation (left) compared to initial head orientation (right) as the data points (dots) fall closer to the nonparametric fit (dashed line, here looks Gaussian), hence eye‐centered. (c) In head‐unrestrained conditions, the dissociation of spatial parameters in gaze behavior were achieved by variability in eye‐head behavior. Gaze (black) and head (gray) movement trajectories to a single target (large circle) for five trials in the memory‐guided gaze task are shown (left panel). Gaze and head endpoint positions (small circles) fall at variable positions for the same target. Initial gaze position was randomly varied within a central square (black square) to increase variability in starting gaze orientation (upper‐right panel) and head orientation (lower‐right panel). This variability allowed for a differentiation between eye‐, head‐, and space‐ (or body) frames of reference. Adapted from Sajad et al. (2015)
An overarching theme is that, while various ingenious methods have been used to test spatial models for vision and gaze control, they each have their own limitations, testing only parts of the question. In the following section, we describe a method that allows one to test all such models simultaneously during natural, head‐unrestrained conditions.
5. A MODEL‐FITTING APPROACH DEVELOPED FOR IDENTIFYING NEURONAL SPATIAL CODES
The following section describes an analytic approach that was developed to test between multiple models of spatial coding in neural activity during head‐unrestrained gaze behavior. The method can be viewed as complementary to decoding approaches, where machine learning algorithms are used to derive specified information from neural data (Bremmer et al., 2016; Leavitt, Pieper, Sachs, & Martinez‐Trujillo, 2017; Glaser et al., 2017; Pruszynski & Zylberberg, 2019). The latter approach tests for implicit population codes, whereas the current method tests for explicit coding, at the level of both single units and neural populations. To do this in the presence of complex and “sloppy” head‐unrestrained behavior, several technical challenges had to be overcome.
5.1. Challenges and benefits of head‐unrestrained gaze recordings
Head‐unrestrained experiments provide the potential benefits of allowing more natural gaze behavior, testing effector coding specificity (gaze vs. eye vs. head), and separating more frames of reference (eye vs. head vs. space/body). However, they also produce major analytic challenges. One is that correlative techniques are insufficient because gaze, eye, and head motion always correlate with each other. Another is that in the range of head‐unrestrained gaze motion, three‐dimensional (3‐D; horizontal, vertical, and torsional) measurements become important because torsional rotation of the eyes and head becomes more prominent, and linear operations on 2‐D gaze/eye/head signals (only horizontal and vertical) yield large errors related to noncommutativity (Tweed, Haslwanter, Happe, & Fetter, 1999). Likewise, this requires a 3‐D analysis to accurately compute positions, such as Te and Ge, which are positions in true retinal (i.e. eye‐centered) coordinates (Crawford et al., 2011). A third challenge is that even for the same gaze orientation, the relative orientations of eye and head can be highly variable (DeSouza et al., 2011; Freedman & Sparks, 1997). Consequently, the traditional approach for identifying the reference frames (Figure 3a) is difficult to replicate. On the other hand, as we shall see, these same problems can be turned into advantages (Figure 3b,c).
Figure 3c illustrates the aspects of gaze behavior that we have utilized to map SC and FEF response fields in several of our recent studies (Sadeh, Sajad, Wang, Yan, & Crawford, 2015, 2018, 2020; Sajad et al., 2015, 2016). Important for addressing the spatial code is the pattern of various spatial parameters during this task. The (largely self‐generated) variability in the behavior tends to separate spatial parameters. The animal's gaze end‐points form a scatter around a given target, separating T and G (Figure 3c, left panel). The animal itself uses different combinations of eye and head rotation (Figure 3c. top‐right panel; including torsion, not shown) to achieve a given gaze shift, separating different effectors. Likewise, the animal uses different combinations of initial eye and head position (Figure 3c, bottom‐right panel; including torsion, not shown), which separate out different frames of reference. To increase the separation between the frames of reference, we introduced an additional variability in the initial gaze positions. Now, all that is needed is some statistical method able to account for these variations and utilize them to fit various spatial models against neural activity.
5.2. New approach to studying spatial encoding using PRESS statistics
To overcome the above challenges, Keith et al., (2009) introduced a method, which takes advantage of the property that neurons have spatially organized response fields. To identify the spatial parameter and reference frame that best describe variations in the neuron response, they exploited the natural variability in behavior described above. Figure 3b depicts the logic for this approach. Neural activity is plotted against each set of spatial parameters derived from the behavioral data. Spatial models were constructed by nonparametric fits through the distribution of data. Then, the quality of the fit for each model is quantified using Predicted Residual Error Sum of Squares (PRESS) statistics which is a form of cross‐validation used in regression analysis (Keith et al., 2009). In other words, for each data point, the residual is calculated relative to a fit to all the other data points, excluding the point in question. The spatial model that yields the lowest PRESS residuals (i.e. the best‐fit) is assumed to characterize the spatial parameter the neuron encodes, and models that yield significantly larger residuals (at the single neuron or population level) can be eliminated from consideration. This method can also be adapted to fitting intermediate models. For example, one can construct models based on points between and beyond the Te and Th (Figure 2b) and determine which weighting yields the lowest overall residuals (e.g. Figures 4b,c and 5b,c). As shown in these figures, this method is easiest to visualize with 2‐D response fields, but in principle, it can be applied to neurons that encode any spatially variable behavior in any multidimensional coordinate system. In the following sections, we review the use of these methods to describe response fields and spatial coding, for the first time directly comparing our results from the FEF and SC.
FIGURE 4.
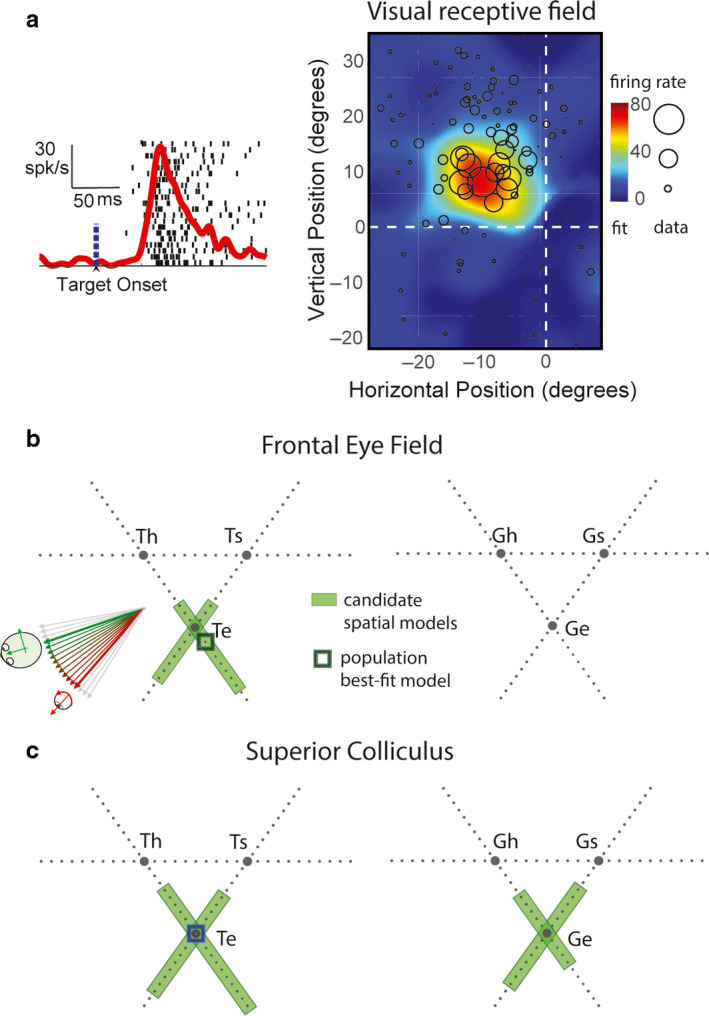
Spatial analysis of visual receptive fields in FEF and SC. (a) Raster and spike density function aligned on target onset (left) and the visual receptive field plot (right) of a representative visual response in FEF. Circles (radius: firing rate) represent data points for response field mapping. Activity was sampled from the 80–180 ms after target presentation (Figure 1b). Color‐map represents the nonparametric fit to the data. (b) Triangular plots represent intermediate models constructed from three pairs of canonical models: eye (e), head (h), and body/space (s) frames based on target location (left) and gaze endpoint (right). The continua between eye and head intermediate frames (Te‐Th, and Ge‐Gh) are also shown in Figure 2b. Green shade indicates intermediate spatial models that are not significantly eliminated. Black square indicates the population best‐fit model. (c) Similar conventions as (b) for superior colliculus. Green shades in (b) and (c) cluster around eye‐centered T (Te) and G (Ge) models. The population best‐fit (dark green square) was at intermediate spatial model at or close to Te for both FEF and SC. Adapted from Sajad et al., (2015) and Sadeh et al., (2015)
FIGURE 5.
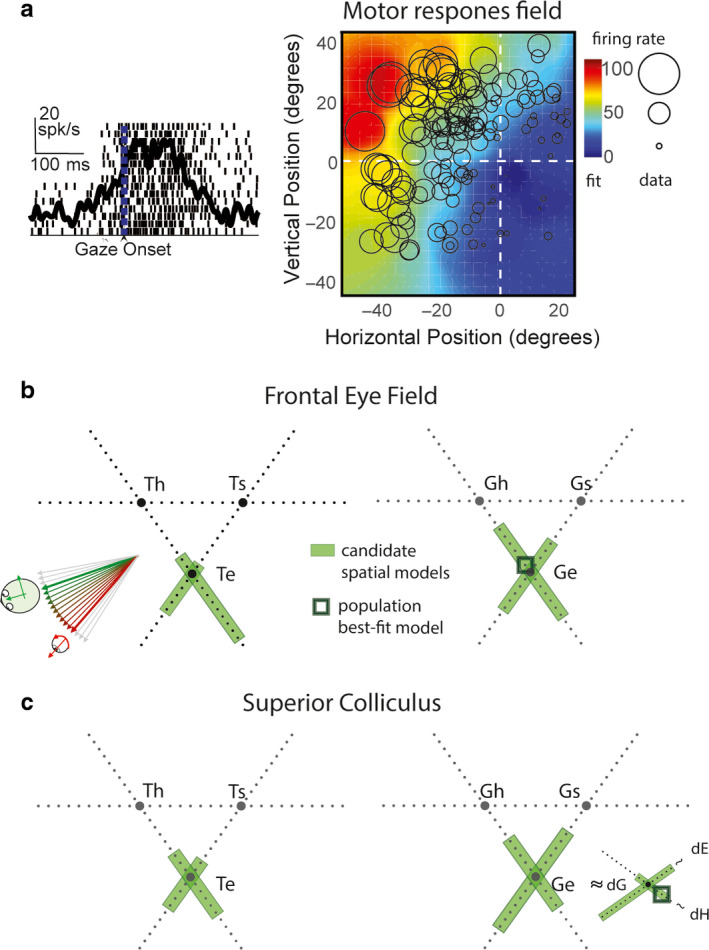
Spatial analysis of motor response fields in FEF and SC. (a) Raster and spike density function aligned on gaze onset (left) and the motor response field plot (right) of a representative FEF motor response. Similar conventions as Figure 4a. (b and c) Spatial analysis of motor response fields of FEF (b) and SC (c) neurons. Similar conventions as Figure 4b,c are used. Motor response was sampled from the entire motor response (Figure 1b). Notice that noneliminated intermediate models (green shades) cluster around eye‐centered T (Te) and G (Ge) models. The population best‐fit for FEF motor activity was an intermediate spatial model close to Ge, and for SC motor activity was an intermediate model close to dG (gaze displacement), which is geometrically very similar to Ge. Adapted from Sajad et al., (2015) and Sadeh et al., (2015)
6. VISUAL RECEPTIVE FIELDS
The current viewpoint is that the visual response in both the FEF and SC can be characterized by a salience or priority map of space (Fernandes, Stevenson, Phillips, Segraves, & Kording, 2013; Krauzlis, Lovejoy, & Zénon, 2013; Thompson & Bichot, 2005; White et al., 2017), but what spatial parameter and reference frame code does this map employ? (note that this is not the same as “retinotopy”, which is the way these signals are anatomically distributed). Most previous studies that have explicitly tested for reference frames suggest that SC and FEF visual responses encode the visual stimulus location fixed in retinal coordinates (e.g. Bruce & Goldberg, 1985; Cassanello & Ferrera, 2007; Lee & Groh, 2012; Schiller & Stryker, 1972; Snyder, 2000; but see Caruso et al., 2018), so this is a good place to test and confirm the new method described above.
DeSouza et al. (2011) were the first to investigate the reference frame of visuomotor responses in the SC in head‐unrestrained conditions using an early version of the method described above (DeSouza et al., 2011). They sampled visuomotor responses during visually guided gaze shifts and found that, overall, variations in combined SC visuomotor responses were best described by target location (and not final position of gaze) in eye‐centered coordinates. However, visual responses were not clearly separated from motor responses in that experiment.
More recently, we gathered data from both the SC and FEF during an oculomotor delayed memory‐guided task, which temporally separates the visual and motor responses intervened by a short memory delay (Sadeh et al., 2015; Sajad et al., 2015). We found that across the complete set of spatial models tested (see Section 2), perhaps not surprisingly, those related to the movement of the eyes (in the head) and the head (on the body) were eliminated. Indeed, the vast majority of visually responsive neurons in both FEF and SC had response fields that exhibited the highest spatial organization (and lowest residuals of fit) when they were plotted based on target position in eye‐centered coordinates (Te; Figure 4a). At the population level (all neurons with visual responses), these fits were significantly better than any other model, and sometimes the preference for eye‐centered coding was statistically significant even at the level of individual neurons.
However, it might be argued that by restricting our fits to canonical models, especially at the population level, one might miss either systematic or variable shifts of individual neuron coding distributions along intermediate frames, away from the canonical models. Therefore, we did a comprehensive testing of intermediate reference frames constructed based on target and gaze endpoint positions (intermediate reference frames between each pair of reference frame, eye‐head, head‐space, and eye‐space). This analysis showed that although single FEF and SC visual neurons showed variable distributions along intermediate points between models, these distributions tended to mainly cluster around Te (Figure 4b,c). Based on these results, we concluded that the visual response in both FEF and SC encodes positions in eye‐centered coordinates.
7. MOTOR RESPONSE FIELDS
The nature of coding of the SC and FEF motor responses has been the subject of more debate than the visual response. Most visual‐motor dissociation tasks suggest that the motor response in FEF and SC encodes saccade direction (e.g. Everling et al., 1999; Everling & Munoz, 2000; Moon et al., 2007; Sato & Schall, 2003), but some have shown evidence for encoding sensory stimulus location (Edelman & Goldberg, 2002; Frens & Van Opstal, 1997; Quessy et al., 2010). Also as mentioned above, it is not known how results from these studies translate to ordinary saccades in which visual‐motor dissociations are absent, and the subject has to directly shift gaze toward the visual stimulus. There are also disagreements about the nature of the spatial code in FEF and SC related to eye‐head gaze behavior. Most head‐unrestrained studies have concluded that gaze (rather than eye or head) is the primary code (Freedman & Sparks, 1997; Guitton & Mandl, 1978; Klier et al., 2001; Knight & Fuchs, 2007; Monteon et al., 2010). Also studies in head‐restrained monkeys that recorded from neck muscle activity have drawn similar conclusions (Corneil, Olivier, & Munoz, 2002; Elsley, Nagy, Cushing, & Corneil, 2007). But some studies have shown evidence for independent eye and head movement coding in these structures (Bizzi & Schiller, 1970; Chen, 2006; Knight, 2012; Walton et al., 2007). Finally, the majority of reference frame studies suggest that an eye‐centered code predominates in FEF and SC (Bruce & Goldberg, 1985; Cassanello & Ferrera, 2007; Klier et al., 2001; Russo & Bruce, 1994; Schiller & Stryker, 1972; Snyder, 2000), but yet again, there are alternative views (Caruso et al., 2018). Some of the disagreements are due to differences in experimental conditions and assumptions about the behavior or neuronal spatial code. For example, if one assumes neurons encode a certain parameter (e.g. target position in many studies) without explicitly testing this, the traditional analysis method of reference frames could yield inaccurate conclusions especially if neurons encode other spatial parameters that show systematic variations relative to the assumed parameter.
We re‐examined this question by applying our model‐fitting approach to motor responses that accompanied head‐unrestrained gaze shifts, following the visual responses (described above) and memory delay (Sadeh et al., 2015; Sajad et al., 2015). We found that the motor response in both FEF and SC, similar to the visual response, showed a strong preference for eye‐centered models. Head‐centered and body/space‐centered models were significantly ruled out at the population level. Importantly, spatial models based on independent eye (in head) and head (in space) position and displacement were also significantly ruled out for both FEF and SC motor responses. Overall, Ge (and very similar model dG) gave the best fits, although Te was not eliminated.
Across the tested intermediate reference frames, for both FEF and SC, similar to the visual response, target and gaze position spatial models based on high degree of eye‐centeredness (but not head‐ and space‐centeredness) were preferred (Figure 5). However, unlike the visual response, the overall best‐fit model for motor response was a model closest to Ge (Figure 5b,c; or gaze displacement, dG, which is a very similar model to Ge; see Figure 5c), previously shown to be a better descriptor of SC motor output (Klier et al., 2001).
8. VISUOMOTOR TRANSFORMATIONS
Thus far, we have described visual and motor response field fits, without considering how the former is transformed into the latter. Visuomotor transformation potentially involves multiple computational stages, each of which can contribute to inaccuracies in gaze behavior (Alikhanian, Carvalho, & Blohm, 2015; Churchland, Afshar, & Shenoy, 2006; Faisal, Selen, & Wolpert, 2008; Gnadt et al., 1991; Ma, Husain, & Bays, 2014; Spaak, Watanabe, Funahashi, & Stokes, 2017; van Beers, 2007; van Bergen, Ma, Pratte, & Jehee, 2015; White, Sparks, & Stanford, 1994; Wimmer et al., 2014). Figure 6a shows a general breakdown of these stages: (a) visual target stimulus location (T) must be integrated with task rules to work out a desired gaze target (Miller & Cohen, 2001). Although we purposefully avoided this in our studies, in certain paradigms task rules are introduced to spatially dissociate stimulus location from the desired gaze location (Munoz & Everling, 2004). (b) Sometimes the gaze target needs to be maintained in working memory for a delayed response (Curtis, Rao, & D'Esposito, 2004; Gnadt et al., 1991). (c) This representation then needs to be relayed to the motor circuitry where the gaze command is generated (Chatham & Badre, 2015; Schall, Purcell, Heitz, Logan, & Palmeri, 2011). (d) This gaze command needs to be decomposed into separate effector commands to rotate the eye in head and the head on the body (Daemi & Crawford, 2015; Gandhi & Sparks, 2007; Guitton, 1992). (e) The separate eye and head movement commands then result in muscle contraction patterns that result in repositioning the gaze (G). Figure 6b shows that the noise in spatial representations associated with each stage (represented by Ɛ stage) can push the spatial code along the error‐space from T toward G, resulting in the overall inaccuracy in gaze behavior (i.e., T‐G disparity). Where along this sequence of information processing do FEF and SC visual and motor responses lie?
FIGURE 6.
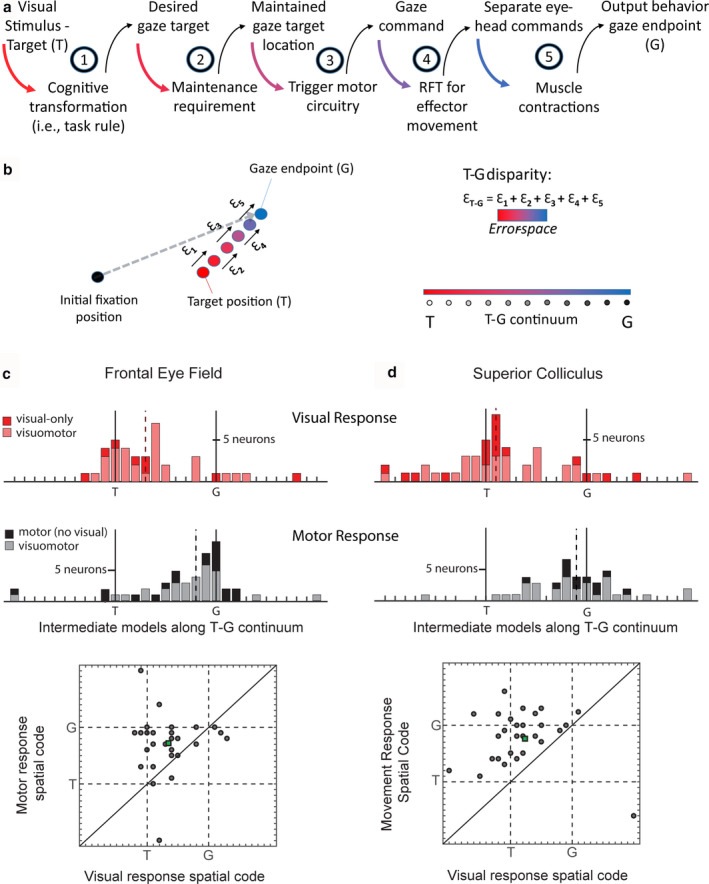
Visuomotor transformations in FEF and SC between visual and motor responses in memory‐guided gaze task. (a) Breakdown of stages in the transformation from target sensory information to output gaze behavior. (b) Red dot: location of the visual target (T); Each process can incrementally add to inaccuracies in spatial representation of target (Ɛ1‐5) resulting in inaccuracy in gaze behavior (gray dotted arrow: gaze vector; blue dot: gaze endpoint). We constructed the T‐G continuum by dividing the error‐space (i.e., T‐G disparity) into equal intervals. This allowed us to explicitly test whether neural activity prefers intermediary positions along this error‐space. Distribution of best‐fit model along the T‐G continuum for visually responsive neurons (c and d top panels) and motor‐responsive neurons (c and d, middle panels). FEF and SC visual responses were sampled as indicated in Figure 1b. Note: visuomotor neurons (pink, c and d top, and gray, c and d bottom) appear on both upper and lower panels. Scatter plots show the best‐fit model distribution of motor response (y‐axis) versus visual response (x‐axis) for individual Visuomotor neurons. Deviation from line of unity indicates change in spatial code along T‐G continuum between visual and motor response in Visuomotor neurons. Adapted from Sajad et al., (2015) and Sadeh et al., (2015)
8.1. Introducing T‐G continuum—transformation of spatial code along the error‐space
To address the question posed in the last paragraph, we created a spatial continuum between Te and Ge, analogous to the idea of intermediate frames of reference, except that Te and Ge are both in the same eye‐centered frame of reference (Figure 6b). What separates these two parameters are variable inaccuracies in gaze behavior. We refer to this spatial continuum “T‐G continuum” as a set of spatial models spanning the error‐space. Accordingly, a change in spatial code from Te toward Ge (henceforth, we will refer to the eye‐centered codes Te and Ge as T and G for simplicity) reflects the incremental accumulation of inaccuracy in spatial representations along the visuomotor pathway, realized as variable errors in gaze behavior.
Figure 6c,d show the results of this analysis for FEF and SC visual (before a memory delay) and motor responses (after a memory delay). As one can see, visual responses clustered around T and motor responses clustered around G. Importantly, the shift from T to G was significant for both brain structures. This was also observed in plots of the motor versus visual T‐G continuum fits for individual Visuomotor neurons. Note that although these data were collected in head‐unrestrained conditions, these particular results would be expected to hold in head‐restrained conditions, because they do not depend on separation of effectors or frames.
Based on these observations, we concluded that the FEF (Sajad et al., 2015) and SC (Sadeh et al., 2015) are involved in the spatial visual‐to‐motor transformations for gaze shifts. Furthermore, they show that this happens both within and between neurons in both structures, suggesting a signal transformation that occurs at the cellular level but is distributed across brain structures.
8.2. Timing of the transformation within and between neurons in memory delay task
With the visual and motor responses in FEF and SC separated by a memory delay, does the transformation from T to G occur before, during, or after the memory delay? Furthermore, what is the differential contribution of different neuron types to this transformation? To address these questions, Sajad et al., (2016) examined the time course of the evolution of the spatial tuning along the T‐G continuum (i.e. error‐space) for FEF neurons by analyzing multiple time steps spanning an early visual period, the memory delay, and the motor response. We found that at the population level, the transition from T to G was characterized as monotonic and gradual through time during the entire visual‐memory‐motor intervals of the task (Figure 7a). A similar analysis of the SC neuronal data from Sadeh et al., 2015, done expressly for this article, revealed the same intermediate spatiotemporal transformation as the FEF (Figure 7b).
FIGURE 7.
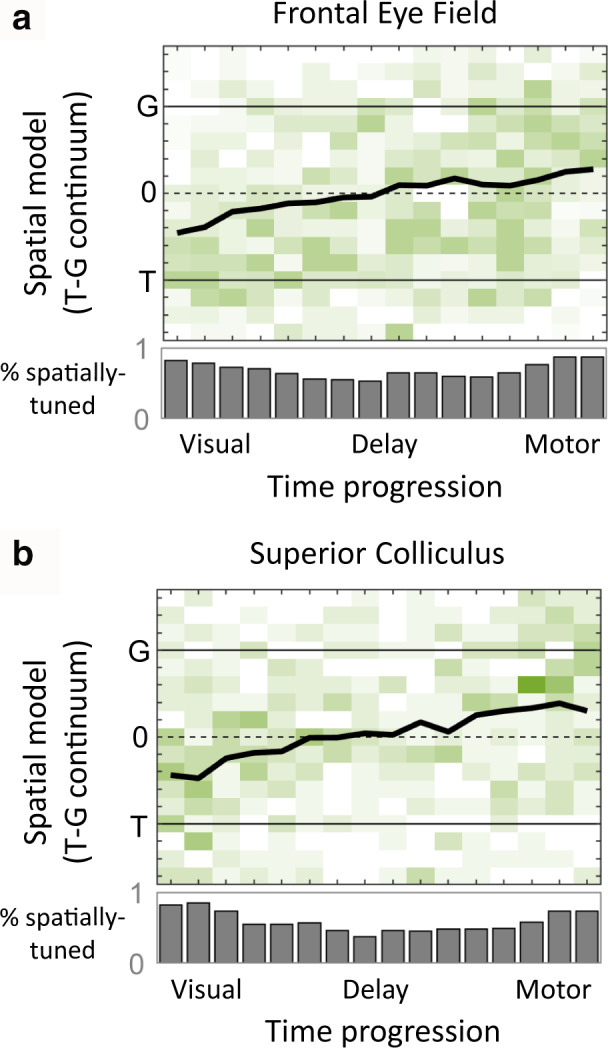
Temporal progression of spatial code during visual‐memory‐motor periods of the memory‐guided task. (a) The time course of T‐to‐G transition across all neurons in the FEF is shown for time intervals spanning visual response onset until saccade time. Green shades represent the best‐fit model for individual neurons. Black traces represent population mean of the best‐fit distribution. Gray histograms indicate the percentage of spatially tuned neurons at each time step. Adapted from Sajad et al., (2016). (b) Same analysis on SC neuronal responses
As described in more detail in the original paper (Sajad et al., 2016), further details emerged when we broke our FEF population down into four distinct subclasses. This revealed a number of fascinating details: Visual neurons encoded T, Visuomotor neurons showed an overall transition like the entire population with a visual code that was close to T but shifted toward G (Figure 7a), neurons with both delay and motor activities had a code that remained fixed between T and G, and neurons with motor‐only activity showed a significant further “jump” to G at the end. Sajad et al., (2016) interpreted this latter jump as evidence for a memory‐motor transformation within the FEF.
Overall, these results demonstrate that FEF and SC spatial codes evolve progressively along almost the entire T‐G range during a memory delay, and that different neuron types can contribute differently to a visual‐memory‐motor transformation, much like a relay team (Cohecelln, Pouget, Heitz, Woodman, & Schall, 2009; Heinzle, Hepp, & Martin, 2007; Lawrence, White, & Snyder, 2005; Markowitz, Curtis, & Pesaran, 2015; Merrikhi et al., 2017; Shin & Sommer, 2012; Spaak et al., 2017; Wurtz, Sommer, Paré, & Ferraina, 2001).
8.3. Rapid transformation during reactive gaze shifts
Does the T‐to‐G transformation described in the previous sections depend on memory‐related processing? Conversely, can a similar transformation be demonstrated in simple saccades made directly to a target with no delay? To address these questions, Sadeh et al. (2020) recorded the activity of SC neurons during a direct visually guided gaze task (reactive task). As expected, gaze behavior was still inaccurate in this task albeit more accurate than the memory‐guided task, likely due to a lack of memory‐dependent processes vulnerable to noise (Figure 6a). This inaccuracy in behavior (i.e., disparity between T and G) allowed us to apply the T‐G continuum analysis, similar to above, to show a T‐to‐G transition both between visually‐ and motor‐responsive neurons and even within individual Visuomotor neurons similar to the memory‐guided gaze task (Figure 8). This time, however, the transformation occurred within the short interval of the response time (i.e. ~200 ms) and followed a similar progression in all neuron types. Thus, even in the absence of a memory period, as the activity evolved from visual‐to‐motor temporal codes, spatial representations evolved from an accurate target representation to one that closely reflects the inaccuracy in gaze endpoint. Overall, these studies suggest that the visuomotor transformation for gaze does not involve a discrete switch between target to gaze coding, but rather an intermediate progression that may or may not involve different neuron types, depending on timing and task details.
FIGURE 8.
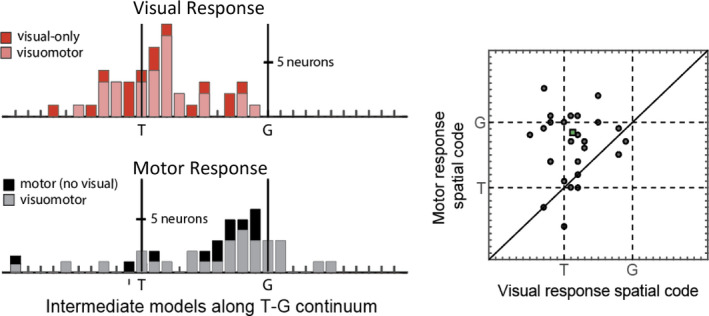
Visuomotor transformations between SC visual and motor response during visually guided (reactive) gaze task. Similar conventions as Figure 6d. Visual response was sampled from 60 to 160 ms relative to target onset, and motor response from −50 to +50 ms relative to gaze onset. Adapted from Sadeh et al., (2020)
9. THEORETICAL IMPLICATIONS: A NEW CONCEPTUAL MODEL FOR GAZE CONTROL
The general conclusion of our FEF and SC findings seems clear: both structures shared very similar spatiotemporal progression of signals and transformations. This suggests extensive sharing of signals between the SC and FEF, likely through their interconnections (Munoz & Schall, 2004; Sommer & Wurtz, 2000, 2001, 2004). This further supports the notion that these two structures behave as a unit in the sensorimotor transformation for gaze shifts (or saccades in head‐restrained conditions), sharing both the desired transformation (designed to land gaze on target) and likely transformation‐related noise, resulting in the variable gaze errors that we measured and used in our analysis. Accordingly, these two structures are largely treated as one unit in the conceptual model that follows (Figure 9).
FIGURE 9.
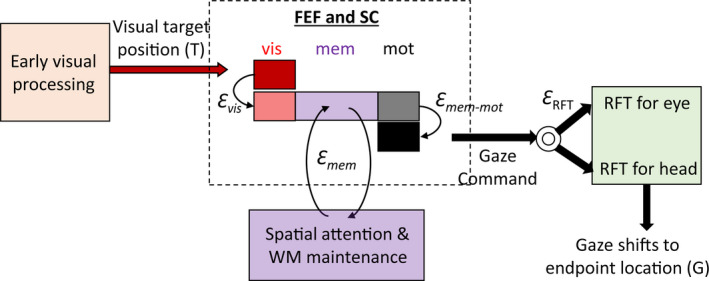
Conceptual model explaining visuomotor transformations in FEF and SC. A schematic of FEF and SC temporal responses during visual, memory, and motor periods (enclosed in dashed box) and relationship with different visuomotor processing stages are shown. Visual neurons (red) encoded the accurate target position in eye coordinates (T). These neurons receive projections from early visual processing areas. Visuomotor neurons (pink) encoded positions that fell close to T but drawn toward the direction that predicted gaze endpoint in eye‐centered coordinates (G). This visual response likely reflects a stage of visual processing which maps, through a noisy gate, visual information into a priority map of movement goals resulting in the accumulation of errors in behavior (Ɛ vis). This position is maintained through recurrent connections between frontal and parietal areas (purple box), which also send projections to FEF and SC. This memory maintenance is susceptible to noise (Ɛ mem), resulting in the diffusion of the attention spotlight (or memorized location). After the GO signal, the most recent memory of the target location is transferred, via a noisy output gate, to the motor circuitry, resulting in additional accumulation of noise (Ɛ mem‐mot). The motor neurons in FEF and SC send this gaze command to downstream structures, where additional processing for the coordination of effectors and appropriate reference frame transformations (RFT) take place. Adapted from Sajad et al., (2015, 2016)
In order to construct the conceptual model illustrated in Figure 9, we synthesized our own results with knowledge derived from the previous literature. Our conceptual model relies on the assumption that visuomotor transformations are inherently noisy (Alikhanian et al., 2015; Arieli, Sterkin, Grinvald, & Aertsen, 1996; Churchland et al., 2006; Faisal et al., 2008; Gnadt et al., 1991; van Bergen et al., 2015; Wimmer et al., 2014).
The model begins with the frontal cortex and SC receiving the true location of the visual stimulus in eye‐centered (i.e. retinal) coordinates. This is based on the observation that visual response of Visual neurons in both FEF and SC was best described by the T model along the T‐G continuum (Sajad et al., 2016). Having access to this accurate visual information can be achieved by direct projections from visual cortex as well as the retina (May, 2006; Perry & Cowey, 1984; Schall et al., 1995).
To guide appropriate behavior, the information about target location needs to be gated to the appropriate memory and motor circuitry to meet the requirements of the task. Such a gating mechanism can be implemented by the corticocortical and cortico‐striato‐thalamic loops, and subcortical circuits through the basal ganglia (Battaglia‐Mayer & Caminiti, 2019; Coe et al., 2019; Krauzlis et al., 2013; Lynch & Tian, 2006; O'Reilly & Frank, 2006). These circuits can integrate various sensory information with learned associations to transfer the spotlight of attention onto relevant locations. One candidate for such a representation in FEF and SC can be the visual response of Visuomotor neurons. In our experiments, the visuospatial representation of Visuomotor neurons, at similar latency to that of T‐coding Visual neurons, was slightly shifted toward G, indicating accumulation of noise. This noise can arise due to the gating that transforms visual input into a movement goal (Figure 9, the noise is labeled Ɛ vis; Chatham & Badre, 2015; O'Reilly & Frank, 2006). Anatomically, this noise can arise from reduction in resolution due to synaptic integration in the basal ganglia circuits (Avery & Krichmar, 2015; Parthasarathy, Schall, & Graybiel, 1992; Zheng & Wilson, 2002). A leading hypothesis suggests that while this noise results in inaccurate behavior, it can offer the required flexibility to perform various cognitive transformations (Faisal et al., 2008; McDonnell & Ward, 2011). In complex tasks that involve stimulus‐response incompatibility (such as the antisaccade task), this noisy gate would transform visual information into a movement goal at a location indicated by the stimulus‐response mapping rule (Boettiger & D'Esposito, 2005; Dash, Yan, Wang, & Crawford, 2015; Everling & Johnston, 2013; Miller & Cohen, 2001; Munoz & Everling, 2004; Sato & Schall, 2003). In simple gaze tasks where the visual stimulus and movement goal are spatially congruent, it would simply transfer activity to the population of neurons that (roughly) represent the same patch of space (Marino et al., 2008; Spaak et al., 2017).
After the movement goal is determined, it needs to be maintained in working memory or directly routed to the motor network depending on the task requirements. It has been shown that the activity in FEF and SC during memory delay reflects the maintained representations in working memory (Funahashi, Bruce, & Goldman‐Rakic, 1989; Fuster & Alexander, 1971; Lundqvist, Herman, & Miller, 2018; Peel, Dash, Lomber, & Corneil, 2017; Sommer & Wurtz, 2001). We observed that there was a transition in spatial representations toward G during the memory delay period in both structures. This confirms models of spatial working memory that describe the diffusion of spatial representations in a random‐walk fashion due to accumulated noise in the population dynamics (Figure 9, label Ɛ mem; Compte, Brunel, Goldman‐Rakic, & Wang, 2000; Wimmer et al., 2014). Our finding that the memory responses did not exactly reach G suggests that this diffusion process does not fully account for gaze endpoint inaccuracy (Churchland et al., 2006; Faisal et al., 2008; Ma et al., 2014).
In our studies on memory‐guided gaze shifts, we only found a strong preference for G code (or very similar codes) in neurons that exclusively fired during the gaze shift, suggesting noise in a memory‐to‐motor transformation (Figure 9, this noise is labeled Ɛ mem‐mot; Ketcham, Hodgson, Kennard, & Stelmach, 2003; Ma et al., 2014; Ploner, Rivaud‐Péchoux, Gaymard, Agid, & Pierrot‐Deseilligny, 1999). We propose that this transformation involves a second noisy gating of maintained movement goal from visually and memory‐responsive neurons to purely motor‐responsive neurons with no memory activity (M‐only neurons), possibly involving striato‐thalamic circuits (Brown, Bullock, & Grossberg, 2004; Chatham & Badre, 2015; Schall et al., 2011). This is in agreement with previous studies that show differential contribution of distinct subpopulations to motor preparation and their differences in anatomical and functional connections (Basso & May, 2017; Cohen et al., 2009; Doubell, Skaliora, Baron, & King, 2003; Markowitz et al., 2015; Merrikhi et al., 2017; Ninomiya, Sawamura, Inoue, & Takada, 2012; Pouget et al., 2009; Ray, Pouget, & Schall, 2009; Redgrave et al., 2010; Segraves & Goldberg, 1987; Weyand & Gafka, 1998). Once the motor network,comprised of FEF and SC M‐only neurons, is triggered to threshold levels, a gaze command is sent to downstream motor structures (Klier et al., 2001; Sparks, 2002).
One might have noticed that in the overall motor response populations, the T‐G code did not quite made it all the way to G (Figures 6, 7, 8), leaving some error unaccounted for. This suggests additional noise in sensorimotor transformations downstream of the FEF and SC, as demonstrated previously (Figure 9; this noise is labeled Ɛ RFT; Alikhanian et al., 2015; Edelman & Goldberg, 2002; Frens & Van Opstal, 1997; Stanford & Sparks, 1994). We also found that the SC motor burst came closer to G in the memory‐guided task compared to reactive gaze shifts. (Sadeh et al., 2018). The most parsimonious explanation for this result is that the unaccounted downstream noise (i.e., Ɛ RFT) was equal in both cases, but would contribute proportionately less to the overall errors when additional memory‐related noise is present.
9.1. Future directions, emerging questions, and new hypotheses
The methodologies, results, and model described in this review can lead to many more questions, such as: (a) How is the T‐to‐G transformation accomplished through the interaction of neurons within and between different layers of SC and FEF microcircuits? (Basso & May, 2017; Bastos et al., 2012; Chandrasekaran, Peixoto, Newsome, & Shenoy, 2017; Heinzleet al., 2007; Massot, Jagadisan, & Gandhi, 2019; Sajad, Godlove, & Schall, 2019; Shin & Sommer, 2012); (b) How do the spatial codes at the individual neuron and population levels change in other visuomotor behaviors, such as express saccades (latency < 100 ms), in which the temporal visual and motor responses entirely overlap (Dorris, Pare, & Munoz, 1997; Isa, 2002)?; (c) how does this methodology extend to other areas of the brain involved in gaze control (Bremmer et al., 2016; Schneider, Dominguez‐Vargas, Gibson, Kagan, & Wilke, 2020)?
Further, the general applicability of the model‐fitting method described here provides the opportunity to investigate other models and other behaviors, so long as there is related spatially tuned activity in the brain and variations in the behavior to distinguish the models. For example, the current review only touches on egocentric models; we have already started applying these methods to investigate the neural coding of allocentric landmarks in the gaze system (Bharmauria, Sajad, Li, et al., 2020a; Bharmauria, Sajad, Yan, Sajad, Yan, Wang, & Crawford, 2020b). We have also started using this method to differentiate gaze, head, and reach transformations in frontal cortex during coordinated eye‐head‐hand reaches (Arora et al., 2019; Nacher et al., 2019). There is no reason to not take this further afield, such as the analysis of activity in areas involved in spatial navigation and spatial memory, including the hippocampus and entorhinal cortex, against ego‐ and allocentric models during complex tasks such as natural viewing and free‐moving navigation (Gulli et al., 2020; Meister & Buffalo, 2018).
Finally, since our T‐G continuum (or potential analogues like a T‐Hand continuum) provides a measure of neural contribution to behavioral noise, these methodologies are applicable to fitting pathological sensorimotor noise (Avery & Krichmar, 2015; Bays & Wolpert, 2007; Burns & Blohm, 2010). Errors in behavior have been commonly investigated to make inference about brain function in healthy and diseased populations. A growing trend in clinical studies is to compare systematic inaccuracies (such as amplitude gain) and (to a lesser extent) variable inaccuracies in movement in diseased populations to gain insight into the nature of their deficits (e.g. Ketcham et al., 2003; Ploner et al., 1999; Thakkar, Schall, Heckers, & Park, 2015). Our methods would allow one to trace this noise to specific neural transformations. For example, it could identify the source of noise for pathological saccades (Chan, Armstrong, Pari, Riopelle, & Munoz, 2005; Le Heron, MacAskill, & Anderson, 2005) or memory‐motor transformations in Parkinson's disease (Ketcham et al., 2003). Such tests are actually being done at this time.
10. GENERAL CONCLUSIONS
The visuomotor transformation for gaze control has been the subject of scientific investigation for decades. While this system is celebrated as a model for understanding general sensorimotor transformations and various cognitive functions, it is extraordinarily difficult to show how its spatial codes evolve through time. Some of this is due to complexity (e.g. accounting for the many possible models in head‐unrestrained behavior) and some ironically due to simplicity (i.e. due to the similarity of visual and motor vectors during ordinary saccades). But solving these technical problems has led us to a methodology with surprising power and versatility, including the ability to test simultaneously between all known models of this system, and track intermediate transformations (especially through the T‐G continuum) through time.
Having applied these methods to the SC and FEF during head‐unrestrained gaze shifts, with or without a memory delay, we find a similar preference for eye‐centered coding in both structures, with the visual response encoding T versus the motor response encoding positions closely described by G (i.e. future gaze position). In the studies reviewed here, we have found a progressive spatiotemporal transition through intermediate T‐G codes, with a memory delay, and a more rapid transition without a delay. This transformation was both local (occurring even within some neuron types) and global, appearing in parallel in these widely separated (but interconnected) brainstem and cortical structures. Importantly, this includes sharing of the neural noise that apparently both allowed us to distinguish T from G, and explains considerable behavioral errors. This does not mean that these structures do the same thing, but that the other functions they support are embedded within fundamentally similar sensorimotor “carrier waves” (Fuster, 2001; Wurtz et al., 2001). Finally, these conclusions likely generalize to other systems. For example, in the reach system, a transition from visual‐to‐motor coding has been observed both at the level of individual neurons, between neurons, and between areas in electrophysiological studies (Bremner & Andersen, 2014; Caminiti, Johnson, Galli, Ferraina, & Burnod, 1991; Cisek & Kalaska, 2005; Fujiwara, Lee, Ishikawa, Kakei, & Izawa, 2017; Kakei, Hoffman, & Strick, 2003; Pesaran, Nelson, & Andersen, 2006; Westendorff et al., 2010), and across lobes at the whole cortex level in neuroimaging studies (e.g. Blohm et al., 2019; Cappadocia, Monaco, Chen, Blohm, & Crawford, 2016; Gallivan & Culham, 2015). A general conclusion from this is that visuomotor transformations are not compartmentalized, but rather involve distributed signals that permeate and underlie many brain functions.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTION
AS and JDC wrote the manuscript. All authors contributed to the primary research (and figures) featured in this review.
ETHICAL STATEMENT
The experiments conducted by the authors featured in this manuscript complied with the guidelines of Canadian Council on Animal Care on the use of laboratory animals and were approved by the York University Animal Care Committee.
ACKNOWLEDGMENTS
We thank Dr. Behrad Noudoost and Dr. Thomas Reppert for helpful comments and suggestions. Requests for materials should be addressed to JDC (e‐mail: jdc@yorku.ca).
Sajad A, Sadeh M, Douglas Crawford J. Spatiotemporal transformations for gaze control. Physiol Rep. 2020;8:e14533 10.14814/phy2.14533
REFERENCES
- Alikhanian, H. , Carvalho, S. R. D. , & Blohm, G. (2015). Quantifying effects of stochasticity in reference frame transformations on posterior distributions. Frontiers in Computational Neuroscience, 9, 82 10.3389/fncom.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, R. A. , Snyder, L. H. , Li, C. S. , & Stricanne, B. (1993). Coordinate transformations in the representation of spatial information. Current Opinion in Neurobiology, 3(2), 171–176. 10.1016/0959-4388(93)90206-E [DOI] [PubMed] [Google Scholar]
- Andersen, R. A. , & Zipser, D. (1988). The role of the posterior parietal cortex in coordinate transformations for visual–motor integration. Canadian Journal of Physiology and Pharmacology, 66(4), 488–501. 10.1139/y88-078 [DOI] [PubMed] [Google Scholar]
- Arieli, A. , Sterkin, A. , Grinvald, A. , & Aertsen, A. D. (1996). Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science, 273(5283), 1868–1871. 10.1126/science.273.5283.1868 [DOI] [PubMed] [Google Scholar]
- Arora, H. K. , Bharmauria, V. , Yan, X. , Sun, S. , Wang, H. , & Crawford, J. D. (2019). Eye‐head‐hand coordination during visually guided reaches in head‐unrestrained macaques. Journal of Neurophysiology, 122(5), 1946–1961. 10.1152/jn.00072.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astruc, J. (1971). Corticofugal connections of area 8 (frontal eye field) in Macaca mulatta . Brain Research, 33(2), 241–256. [DOI] [PubMed] [Google Scholar]
- Avery, M. C. , & Krichmar, J. L. (2015). Improper activation of D1 and D2 receptors leads to excess noise in prefrontal cortex. Frontiers in Computational Neuroscience, 9, 31 10.3389/fncom.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avillac, M. , Deneve, S. , Olivier, E. , Pouget, A. , & Duhamel, J. R. (2005). Reference frames for representing visual and tactile locations in parietal cortex. Nature Neuroscience, 8(7), 941 10.1038/nn1480 [DOI] [PubMed] [Google Scholar]
- Barbas, H. , & Mesulam, M. M. (1981). Organization of afferent input to subdivisions of area 8 in the rhesus monkey. Journal of Comparative Neurology, 200(3), 407–431. 10.1002/cne.902000309 [DOI] [PubMed] [Google Scholar]
- Basso, M. A. , & May, P. J. (2017). Circuits for action and cognition: A view from the superior colliculus. Annual Review of Vision Science, 3, 197–226. 10.1146/annurev-vision-102016-061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos, A. M. , Usrey, W. M. , Adams, R. A. , Mangun, G. R. , Fries, P. , & Friston, K. J. (2012). Canonical microcircuits for predictive coding. Neuron, 76(4), 695–711. 10.1016/j.neuron.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia‐Mayer, A. , & Caminiti, R. (2019). Corticocortical systems underlying high‐order motor control. The Journal of Neuroscience, 39(23), 4404–4421. 10.1523/JNEUROSCI.2094-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays, P. M. , & Wolpert, D. M. (2007). Computational principles of sensorimotor control that minimize uncertainty and variability. The Journal of Physiology, 578(2), 387–396. 10.1113/jphysiol.2006.120121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento, L. A. , & Fallon, J. H. (1975). The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). The Journal of Comparative Neurology, 160, 339–362. 10.1002/cne.901600306 [DOI] [PubMed] [Google Scholar]
- Bharmauria, V. , Sajad, A. , Li, J. , Yan, X. , Wang, H. , & Crawford, J. D. (2020a). Integration of eye‐centered and landmark‐centered codes in frontal eye field gaze responses. Cerebral Cortex. 10.1093/cercor/bhaa090 [DOI] [PubMed] [Google Scholar]
- Bharmauria, V. , Sajad, A. , Yan, X. , Wang, H. , & Crawford, J. D. (2020b). Spatiotemporal coding in the macaque supplementary eye fields: Landmark influence in the target‐to‐gaze transformation. bioRxiv. 10.1101/2020.06.25.172031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi, E. , & Schiller, P. H. (1970). Single unit activity in the frontal eye fields of unanesthetized monkeys during eye and head movement. Experimental Brain Research, 10(2), 151–158. 10.1007/BF00234728 [DOI] [PubMed] [Google Scholar]
- Blohm, G. , Alikhanian, H. , Gaetz, W. , Goltz, H. C. , DeSouza, J. F. , Cheyne, D. O. , & Crawford, J. D. (2019). Neuromagnetic signatures of the spatiotemporal transformation for manual pointing. NeuroImage, 197, 306–319. [DOI] [PubMed] [Google Scholar]
- Blohm, G. , & Crawford, J. D. (2007). Computations for geometrically accurate visually guided reaching in 3‐D space. Journal of Vision, 7(5), 4 10.1167/7.5.4 [DOI] [PubMed] [Google Scholar]
- Blohm, G. , & Crawford, J. D. (2009). Fields of gain in the brain. Neuron, 64(5), 598–600. 10.1016/j.neuron.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Blohm, G. , Keith, G. P. , & Crawford, J. D. (2009). Decoding the cortical transformations for visually guided reaching in 3D space. Cerebral Cortex, 19(6), 1372–1393. 10.1093/cercor/bhn177 [DOI] [PubMed] [Google Scholar]
- Boettiger, C. A. , & D'Esposito, M. (2005). Frontal networks for learning and executing arbitrary stimulus‐response associations. Journal of Neuroscience, 25(10), 2723–2732. 10.1523/JNEUROSCI.3697-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta, A. , Bogadhi, A. R. , & Krauzlis, R. J. (2018). Comparing frontal eye field and superior colliculus contributions to covert spatial attention. Nature Communications, 9(1), 3553 10.1038/s41467-018-06042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud, D. , & Bremmer, F. (1999). Gaze effects in the cerebral cortex: Reference frames for space coding and action. Experimental Brain Research, 128(1–2), 170–180. 10.1007/s002210050832 [DOI] [PubMed] [Google Scholar]
- Bremmer, F. , Kaminiarz, A. , Klingenhoefer, S. , & Churan, J. (2016). Decoding target distance and saccade amplitude from population activity in the macaque lateral intraparietal area (LIP). Frontiers in Integrative Neuroscience, 10, 30 10.3389/fnint.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner, L. R. , & Andersen, R. A. (2014). Temporal analysis of reference frames in parietal cortex area 5d during reach planning. Journal of Neuroscience, 34(15), 5273–5284. 10.1523/JNEUROSCI.2068-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. W. , Bullock, D. , & Grossberg, S. (2004). How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Networks, 17(4), 471–510. 10.1016/j.neunet.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Bruce, C. J. , & Goldberg, M. E. (1985). Primate frontal eye fields. I. Single neurons discharging before saccades. Journal of Neurophysiology, 53(3), 603–635. [DOI] [PubMed] [Google Scholar]
- Bruce, C. J. , Goldberg, M. E. , Bushnell, M. C. , & Stanton, G. B. (1985). Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. Journal of Neurophysiology, 54(3), 714–734. [DOI] [PubMed] [Google Scholar]
- Burns, J. K. , & Blohm, G. (2010). Multi‐sensory weights depend on contextual noise in reference frame transformations. Frontiers in Human Neuroscience, 4, 221 10.3389/fnhum.2010.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti, R. , Johnson, P. B. , Galli, C. , Ferraina, S. , & Burnod, Y. (1991). Making arm movements within different parts of space: The premotor and motor cortical representation of a coordinate system for reaching to visual targets. Journal of Neuroscience, 11(5), 1182–1197. 10.1523/JNEUROSCI.11-05-01182.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappadocia, D. C. , Monaco, S. , Chen, Y. , Blohm, G. , & Crawford, J. D. (2016). Temporal evolution of target representation, movement direction planning, and reach execution in occipital–parietal–frontal cortex: An fmri study. Cerebral Cortex, 27(11), 5242–5260. 10.1093/cercor/bhw304 [DOI] [PubMed] [Google Scholar]
- Caruso, V. C. , Pages, D. S. , Sommer, M. A. , & Groh, J. M. (2018). Beyond the labeled line: Variation in visual reference frames from intraparietal cortex to frontal eye fields and the superior colliculus. Journal of Neurophysiology, 119(4), 1411–1421. 10.1152/jn.00584.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassanello, C. R. , & Ferrera, V. P. (2007). Computing vector differences using a gain field‐like mechanism in monkey frontal eye field. The Journal of Physiology, 582(2), 647–664. 10.1113/jphysiol.2007.128801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioi, A. J. , Gallaway, M. , & Coulter, J. D. (1978). Spinal projections from the midbrain in monkey. Journal of Comparative Neurology, 178(2), 329–345. 10.1002/cne.901780208 [DOI] [PubMed] [Google Scholar]
- Chan, F. , Armstrong, I. T. , Pari, G. , Riopelle, R. J. , & Munoz, D. P. (2005). Deficits in saccadic eye‐movement control in Parkinson's disease. Neuropsychologia, 43(5), 784–796. 10.1016/j.neuropsychologia.2004.06.026 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran, C. , Peixoto, D. , Newsome, W. T. , & Shenoy, K. V. (2017). Laminar differences in decision‐related neural activity in dorsal premotor cortex. Nature Communications, 8(1), 1–16. 10.1038/s41467-017-00715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham, C. H. , & Badre, D. (2015). Multiple gates on working memory. Current Opinion in Behavioral Sciences, 1, 23–31. 10.1016/j.cobeha.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. L. (2006). Head movements evoked by electrical stimulation in the frontal eye field of the monkey: Evidence for independent eye and head control. Journal of Neurophysiology, 95(6), 3528–3542. 10.1152/jn.01320.2005 [DOI] [PubMed] [Google Scholar]
- Churchland, M. M. , Afshar, A. , & Shenoy, K. V. (2006). A central source of movement variability. Neuron, 52(6), 1085–1096. 10.1016/j.neuron.2006.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek, P. , & Kalaska, J. F. (2005). Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron, 45(5), 801–814. 10.1016/j.neuron.2005.01.027 [DOI] [PubMed] [Google Scholar]
- Coe, B. C. , Trappenberg, T. , & Munoz, D. P. (2019). Modeling saccadic action selection: Cortical and basal ganglia signals coalesce in the superior colliculus. Frontiers in Systems Neuroscience, 13 10.3389/fnsys.2019.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. Y. , Pouget, P. , Heitz, R. P. , Woodman, G. F. , & Schall, J. D. (2009). Biophysical support for functionally distinct cell types in the frontal eye field. Journal of Neurophysiology. 101(2), 912–916. 10.1152/jn.90272.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, Y. E. , & Andersen, R. A. (2002). A common reference frame for movement plans in the posterior parietal cortex. Nature Reviews Neuroscience, 3(7), 553–562. 10.1038/nrn873 [DOI] [PubMed] [Google Scholar]
- Colby, C. L. (1998). Action‐oriented spatial reference frames in cortex. Neuron, 20(1), 15–24. 10.1016/S0896-6273(00)80429-8 [DOI] [PubMed] [Google Scholar]
- Compte, A. , Brunel, N. , Goldman‐Rakic, P. S. , & Wang, X. J. (2000). Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cerebral Cortex, 10(9), 910–923. 10.1093/cercor/10.9.910 [DOI] [PubMed] [Google Scholar]
- Corneil, B. D. , Olivier, E. , & Munoz, D. P. (2002). Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. Journal of Neurophysiology, 88(4), 1980–1999. [DOI] [PubMed] [Google Scholar]
- Cowie, R. J. , & Robinson, D. L. (1994). Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. Journal of Neurophysiology, 72(6), 2648–2664. [DOI] [PubMed] [Google Scholar]
- Crawford, J. D. , & Guitton, D. (1997). Visual‐motor transformations required for accurate and kinematically correct saccades. Journal of Neurophysiology, 78(3), 1447–1467. 10.1152/jn.1997.78.3.1447 [DOI] [PubMed] [Google Scholar]
- Crawford, J. D. , Henriques, D. Y. , & Medendorp, W. P. (2011). Three‐dimensional transformations for goal‐directed action. Annual Review of Neuroscience, 34, 309–331. 10.1146/annurev-neuro-061010-113749 [DOI] [PubMed] [Google Scholar]
- Cullen, K. E. , Galiana, H. L. , & Sylvestre, P. A. (2000). Comparing extraocular motoneuron discharges during head‐restrained saccades and head‐unrestrained gaze shifts. Journal of Neurophysiology, 83(1), 630–637. 10.1152/jn.2000.83.1.630 [DOI] [PubMed] [Google Scholar]
- Curtis, C. E. , Rao, V. Y. , & D'Esposito, M. (2004). Maintenance of spatial and motor codes during oculomotor delayed response tasks. Journal of Neuroscience, 24(16), 3944–3952. 10.1523/JNEUROSCI.5640-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemi, M. , & Crawford, J. D. (2015). A kinematic model for 3‐D head‐free gaze‐shifts. Frontiers in Computational Neuroscience, 9, 72 10.3389/fncom.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, S. , Yan, X. , Wang, H. , & Crawford, J. D. (2015). Continuous updating of visuospatial memory in superior colliculus during slow eye movements. Current Biology, 25(3), 267–274. 10.1016/j.cub.2014.11.064 [DOI] [PubMed] [Google Scholar]
- DeCharms, R. C. , & Zador, A. (2000). Neural representation and the cortical code. Annual Review of Neuroscience, 23(1), 613–647. 10.1146/annurev.neuro.23.1.613 [DOI] [PubMed] [Google Scholar]
- Demb, J. B. , & Singer, J. H. (2015). Functional circuitry of the retina. Annual Review of Vision Science, 1, 263–289. 10.1146/annurev-vision-082114-035334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza, J. F. , Keith, G. P. , Yan, X. , Blohm, G. , Wang, H. , & Crawford, J. D. (2011). Intrinsic reference frames of superior colliculus visuomotor receptive fields during head‐unrestrained gaze shifts. Journal of Neuroscience, 31(50), 18313–18326. 10.1523/JNEUROSCI.0990-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, E. C. , Kiesau, M. , & Segraves, M. A. (1995). Acute activation and inactivation of macaque frontal eye field with GABA‐related drugs. Journal of Neurophysiology, 74(6), 2744–2748. 10.1152/jn.1995.74.6.2744 [DOI] [PubMed] [Google Scholar]
- Dorris, M. C. , Pare, M. , & Munoz, D. P. (1997). Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. Journal of Neuroscience, 17(21), 8566–8579. 10.1523/JNEUROSCI.17-21-08566.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubell, T. P. , Skaliora, I. , Baron, J. , & King, A. J. (2003). Functional connectivity between the superficial and deeper layers of the superior colliculus: An anatomical substrate for sensorimotor integration. Journal of Neuroscience, 23(16), 6596–6607. 10.1523/JNEUROSCI.23-16-06596.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman, J. A. , & Goldberg, M. E. (2002). Effect of short‐term saccadic adaptation on saccades evoked by electrical stimulation in the primate superior colliculus. Journal of Neurophysiology, 87(4), 1915–1923. 10.1152/jn.00805.2000 [DOI] [PubMed] [Google Scholar]
- Elsley, J. K. , Nagy, B. , Cushing, S. L. , & Corneil, B. D. (2007). Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. Journal of Neurophysiology, 98(3), 1333–1354. 10.1152/jn.00386.2007 [DOI] [PubMed] [Google Scholar]
- Everling, S. , Dorris, M. C. , Klein, R. M. , & Munoz, D. P. (1999). Role of primate superior colliculus in preparation and execution of anti‐saccades and pro‐saccades. Journal of Neuroscience, 19(7), 2740–2754. 10.1523/JNEUROSCI.19-07-02740.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling, S. , & Johnston, K. (2013). Control of the superior colliculus by the lateral prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1628), 20130068 10.1098/rstb.2013.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling, S. , & Munoz, D. P. (2000). Neuronal correlates for preparatory set associated with pro‐saccades and anti‐saccades in the primate frontal eye field. Journal of Neuroscience, 20(1), 387–400. 10.1523/JNEUROSCI.20-01-00387.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal, A. A. , Selen, L. P. , & Wolpert, D. M. (2008). Noise in the nervous system. Nature Reviews Neuroscience, 9(4), 292 10.1038/nrn2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, H. L. , Stevenson, I. H. , Phillips, A. N. , Segraves, M. A. , & Kording, K. P. (2013). Saliency and saccade encoding in the frontal eye field during natural scene search. Cerebral Cortex, 24(12), 3232–3245. 10.1093/cercor/bht179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders, M. , Tillery, S. I. H. , & Soechting, J. F. (1992). Early stages in a sensorimotor transformation. Behavioral and Brain Sciences, 15(2), 309–320. 10.1017/S0140525X00068813 [DOI] [Google Scholar]
- Franklin, D. W. , Reichenbach, A. , Franklin, S. , & Diedrichsen, J. (2016). Temporal evolution of spatial computations for visuomotor control. Journal of Neuroscience, 36(8), 2329–2341. 10.1523/JNEUROSCI.0052-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, E. G. , & Sparks, D. L. (1997). Eye‐head coordination during head‐unrestrained gaze shifts in rhesus monkeys. Journal of Neurophysiology, 77(5), 2328–2348. 10.1152/jn.1997.77.5.2328 [DOI] [PubMed] [Google Scholar]
- Frens, M. A. , & Van Opstal, A. J. (1997). Monkey superior colliculus activity during short‐term saccadic adaptation. Brain Research Bulletin, 43(5), 473–483. 10.1016/S0361-9230(97)80001-9 [DOI] [PubMed] [Google Scholar]
- Fujiwara, Y. , Lee, J. , Ishikawa, T. , Kakei, S. , & Izawa, J. (2017). Diverse coordinate frames on sensorimotor areas in visuomotor transformation. Scientific Reports, 7(1), 1–9. 10.1038/s41598-017-14579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi, S. (2013). Thalamic mediodorsal nucleus and its participation in spatial working memory processes: Comparison with the prefrontal cortex. Frontiers in Systems Neuroscience, 7, 36 10.3389/fnsys.2013.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi, S. , Bruce, C. J. , & Goldman‐Rakic, P. S. (1989). Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. Journal of Neurophysiology, 61(331–349), 1989. [DOI] [PubMed] [Google Scholar]
- Funahashi, S. , Takeda, K. , & Watanabe, Y. (2004). Neural mechanisms of spatial working memory: Contributions of the dorsolateral prefrontal cortex and the thalamic mediodorsal nucleus. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 409–420. 10.3758/CABN.4.4.409 [DOI] [PubMed] [Google Scholar]
- Fuster, J. M. (2001). The prefrontal cortex—An update: Time is of the essence. Neuron, 30(2), 319–333. [DOI] [PubMed] [Google Scholar]
- Fuster, J. M. , & Alexander, G. E. (1971). Neuron activity related to short‐term memory. Science, 173(3997), 652–654. 10.1126/science.173.3997.652 [DOI] [PubMed] [Google Scholar]
- Gallivan, J. P. , & Culham, J. C. (2015). Neural coding within human brain areas involved in actions. Current Opinion in Neurobiology, 33, 141–149. 10.1016/j.conb.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Gandhi, N. J. , & Katnani, H. A. (2011). Motor functions of the superior colliculus. Annual Review of Neuroscience, 34, 205–231. 10.1146/annurev-neuro-061010-113728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, N. J. , & Sparks, D. L. (2007). Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. Journal of Neurophysiology, 98(1), 360–373. 10.1152/jn.00252.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, J. I. , Chowdhury, R. H. , Perich, M. G. , Miller, L. E. , & Kording, K. P. (2017). Machine learning for neural decoding. arXiv Preprint arXiv:1708.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt, J. W. , Bracewell, R. M. , & Andersen, R. A. (1991). Sensorimotor transformation during eye movements to remembered visual targets. Vision Research, 31(4), 693–715. 10.1016/0042-6989(91)90010-3 [DOI] [PubMed] [Google Scholar]
- Goldberg, M. E. , Colby, C. L. , & Duhamel, J. R. (1990). Representation of visuomotor space in the parietal lobe of the monkey In Cold Spring Harbor symposia on quantitative biology (Vol. 55, pp. 729–739). New York: Cold Spring Harbor Laboratory Press. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic, P. S. , & Porrino, L. J. (1985). The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. Journal of Comparative Neurology, 242(4), 535–560. 10.1002/cne.902420406 [DOI] [PubMed] [Google Scholar]
- Goodale, M. A. (2011). Transforming vision into action. Vision Research, 51(13), 1567–1587. 10.1016/j.visres.2010.07.027 [DOI] [PubMed] [Google Scholar]
- Guitton, D. (1992). Control of eye—Head coordination during orienting gaze shifts. Trends in Neurosciences, 15(5), 174–179. 10.1016/0166-2236(92)90169-9 [DOI] [PubMed] [Google Scholar]
- Guitton, D. , & Mandl, G. (1978). Frontal oculomotor area in alert cat: I. Eye movements and neck activity evoked by stimulation. Brain Research, 149(2), 295–312. [DOI] [PubMed] [Google Scholar]
- Guitton, D. P. M. D. , Munoz, D. P. , & Galiana, H. L. (1990). Gaze control in the cat: Studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. Journal of Neurophysiology, 64(2), 509–531. 10.1152/jn.1990.64.2.509 [DOI] [PubMed] [Google Scholar]
- Gulli, R. A. , Duong, L. R. , Corrigan, B. W. , Doucet, G. , Williams, S. , Fusi, S. , & Martinez‐Trujillo, J. C. (2020). Context‐dependent representations of objects and space in the primate hippocampus during virtual navigation. Nature Neuroscience, 23(1), 103–112. 10.1038/s41593-019-0548-3 [DOI] [PubMed] [Google Scholar]
- Hanes, D. P. , & Schall, J. D. (1996). Neural control of voluntary movement initiation. Science, 274(5286), 427–430. 10.1126/science.274.5286.427 [DOI] [PubMed] [Google Scholar]
- Hanes, D. P. , & Wurtz, R. H. (2001). Interaction of the frontal eye field and superior colliculus for saccade generation. Journal of Neurophysiology, 85(2), 804–815. 10.1152/jn.2001.85.2.804 [DOI] [PubMed] [Google Scholar]
- Harting, J. K. (1977). Descending pathways from the superior colliculus: An autoradiographic analysis in the rhesus monkey (Macaca mulatta). Journal of Comparative Neurology, 173(3), 583–612. 10.1002/cne.901730311 [DOI] [PubMed] [Google Scholar]
- Hawkins, K. M. , Sayegh, P. , Yan, X. , Crawford, J. D. , & Sergio, L. E. (2013). Neural activity in superior parietal cortex during rule‐based visual‐motor transformations. Journal of Cognitive Neuroscience, 25(3), 436–454. 10.1162/jocn_a_00318 [DOI] [PubMed] [Google Scholar]
- Heinzle, J. , Hepp, K. , & Martin, K. A. (2007). A microcircuit model of the frontal eye fields. Journal of Neuroscience, 27(35), 9341–9353. 10.1523/JNEUROSCI.0974-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz, R. P. (2014). The speed‐accuracy tradeoff: History, physiology, methodology, and behavior. Frontiers in Neuroscience, 8, 150 10.3389/fnins.2014.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmbrecht, T. O. , Dal Maschio, M. , Donovan, J. C. , Koutsouli, S. , & Baier, H. (2018). Topography of a visuomotor transformation. Neuron, 100(6), 1429–1445. 10.1016/j.neuron.2018.10.021 [DOI] [PubMed] [Google Scholar]
- Hikosaka, O. , & Wurtz, R. H. (1983). Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory‐contingent visual and saccade responses. Journal of Neurophysiology, 49(5), 1268–1284. [DOI] [PubMed] [Google Scholar]
- Hikosaka, O. , & Wurtz, R. H. (1985). Modification of saccadic eye movements by GABA‐related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. Journal of Neurophysiology, 53(1), 266–291. [DOI] [PubMed] [Google Scholar]
- Huerta, M. F. , Krubitzer, L. A. , & Kaas, J. H. (1986). Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. Subcortical connections. Journal of Comparative Neurology, 253(4), 415–439. 10.1002/cne.902530402 [DOI] [PubMed] [Google Scholar]
- Isa, T. (2002). Intrinsic processing in the mammalian superior colliculus. Current Opinion in Neurobiology, 12(6), 668–677. 10.1016/S0959-4388(02)00387-2 [DOI] [PubMed] [Google Scholar]
- Isa, T. , & Sasaki, S. (2002). Brainstem control of head movements during orienting; organization of the premotor circuits. Progress in Neurobiology, 66(4), 205–241. 10.1016/S0301-0082(02)00006-0 [DOI] [PubMed] [Google Scholar]
- Jamadar, S. D. , Johnson, B. P. , Clough, M. , Egan, G. F. , & Fielding, J. (2015). Behavioral and neural plasticity of ocular motor control: Changes in performance and fMRI activity following antisaccade training. Frontiers in Human Neuroscience, 9, 653 10.3389/fnhum.2015.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay, M. F. , & Sparks, D. L. (1984). Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature, 309(5966), 345 10.1038/309345a0 [DOI] [PubMed] [Google Scholar]
- Johnston, K. , DeSouza, J. F. , & Everling, S. (2009). Monkey prefrontal cortical pyramidal and putative interneurons exhibit differential patterns of activity between prosaccade and antisaccade tasks. Journal of Neuroscience, 29(17), 5516–5524. 10.1523/JNEUROSCI.5953-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas, J. H. (2004). The evolution of the visual system in primates. The Visual Neurosciences, 2, 1563–1572. [Google Scholar]
- Kaas, J. H. , & Huerta, M. F. (1988). The subcortical visual system of primates. Comparative Primate Biology, 4, 327–391. [Google Scholar]
- Kakei, S. , Hoffman, D. S. , & Strick, P. L. (2003). Sensorimotor transformations in cortical motor areas. Neuroscience Research, 46(1), 1–10. 10.1016/S0168-0102(03)00031-2 [DOI] [PubMed] [Google Scholar]
- Kardamakis, A. A. , & Moschovakis, A. K. (2009). Optimal control of gaze shifts. Journal of Neuroscience, 29(24), 7723–7730. 10.1523/JNEUROSCI.5518-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, K. , Brodal, A. , & Hoddevik, G. (1974). The projection of the superior colliculus onto the reticular formation of the brain stem an experimental anatomical study in the cat. Experimental Brain Research, 19(1), 1–19. 10.1007/BF00233392 [DOI] [PubMed] [Google Scholar]
- Keith, G. P. , DeSouza, J. F. , Yan, X. , Wang, H. , & Crawford, J. D. (2009). A method for mapping response fields and determining intrinsic reference frames of single‐unit activity: Applied to 3D head‐unrestrained gaze shifts. Journal of Neuroscience Methods, 180(1), 171–184. 10.1016/j.jneumeth.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Ketcham, C. J. , Hodgson, T. L. , Kennard, C. , & Stelmach, G. E. (2003). Memory‐motor transformations are impaired in Parkinson's disease. Experimental Brain Research, 149(1), 30–39. 10.1007/s00221-002-1332-1 [DOI] [PubMed] [Google Scholar]
- Klier, E. M. , Wang, H. , & Crawford, J. D. (2001). The superior colliculus encodes gaze commands in retinal coordinates. Nature Neuroscience, 4(6), 627–632. 10.1038/88450 [DOI] [PubMed] [Google Scholar]
- Knight, T. A. (2012). Contribution of the frontal eye field to gaze shifts in the head‐unrestrained rhesus monkey: Neuronal activity. Neuroscience, 225, 213–236. 10.1016/j.neuroscience.2012.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, T. A. , & Fuchs, A. F. (2007). Contribution of the frontal eye field to gaze shifts in the head‐unrestrained monkey: Effects of microstimulation. Journal of Neurophysiology, 97(1), 618–634. 10.1152/jn.00256.2006 [DOI] [PubMed] [Google Scholar]
- Krauzlis, R. J. , Lovejoy, L. P. , & Zénon, A. (2013). Superior colliculus and visual spatial attention. Annual Review of Neuroscience, 36, 165–182. 10.1146/annurev-neuro-062012-170249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle, H. , Akert, K. , & Wurtz, R. H. (1976). Projection of area 8 (frontal eye field) to superior colliculus in the monkey. An autoradiographic study. Brain Research, 117(3), 487–492. [DOI] [PubMed] [Google Scholar]
- Lappi, O. (2016). Eye movements in the wild: Oculomotor control, gaze behavior & frames of reference. Neuroscience & Biobehavioral Reviews, 69, 49–68. [DOI] [PubMed] [Google Scholar]
- Lawrence, B. M. , White, R. L. III , & Snyder, L. H. (2005). Delay‐period activity in visual, visuomovement, and movement neurons in the frontal eye field. Journal of Neurophysiology, 94(2), 1498–1508. 10.1152/jn.00214.2005 [DOI] [PubMed] [Google Scholar]
- Le Heron, C. J. , MacAskill, M. R. , & Anderson, T. J. (2005). Memory‐guided saccades in PD: Long delays can improve performance. Experimental Brain Research, 161, 293–298. [DOI] [PubMed] [Google Scholar]
- Leavitt, M. L. , Pieper, F. , Sachs, A. J. , & Martinez‐Trujillo, J. C. (2017). Correlated variability modifies working memory fidelity in primate prefrontal neuronal ensembles. Proceedings of the National Academy of Sciences, 114(12), E2494–E2503. 10.1073/pnas.1619949114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , & Groh, J. M. (2012). Auditory signals evolve from hybrid‐to eye‐centered coordinates in the primate superior colliculus. Journal of Neurophysiology, 108(1), 227–242. 10.1152/jn.00706.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Sajad, A. , Marino, R. , Yan, X. , Sun, S. , Wang, H. , & Crawford, J. D. (2017). Effect of allocentric landmarks on primate gaze behavior in a cue conflict task. Journal of Vision, 17(5), 20 10.1167/17.5.20 [DOI] [PubMed] [Google Scholar]
- Lowe, K. A. , & Schall, J. D. (2018). Functional categories of visuomotor neurons in macaque frontal eye field. eNeuro, 5(5). 10.1523/ENEURO.0131-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist, M. , Herman, P. , & Miller, E. K. (2018). Working memory: Delay activity, yes! Persistent activity? Maybe not. Journal of Neuroscience, 38(32), 7013–7019. 10.1523/JNEUROSCI.2485-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J. C. , Hoover, J. E. , & Strick, P. L. (1994). Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Experimental Brain Research, 100, 181–186. 10.1007/BF00227293 [DOI] [PubMed] [Google Scholar]
- Lynch, J. C. , & Tian, J. R. (2006). Cortico‐cortical networks and cortico‐subcortical loops for the higher control of eye movements. Progress in Brain Research, 151, 461–501. [DOI] [PubMed] [Google Scholar]
- Ma, W. J. , Husain, M. , & Bays, P. M. (2014). Changing concepts of working memory. Nature Neuroscience, 17(3), 347 10.1038/nn.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, R. A. , Rodgers, C. K. , Levy, R. , & Munoz, D. P. (2008). Spatial relationships of visuomotor transformations in the superior colliculus map. Journal of Neurophysiology, 100(5), 2564–2576. 10.1152/jn.90688.2008 [DOI] [PubMed] [Google Scholar]
- Markowitz, D. A. , Curtis, C. E. , & Pesaran, B. (2015). Multiple component networks support working memory in prefrontal cortex. Proceedings of the National Academy of Sciences, 112(35), 11084–11089. 10.1073/pnas.1504172112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Trujillo, J. C. , Medendorp, W. P. , Wang, H. , & Crawford, J. D. (2004). Frames of reference for eye‐head gaze commands in primate supplementary eye fields. Neuron, 44(6), 1057–1066. 10.1016/j.neuron.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Massot, C. , Jagadisan, U. K. , & Gandhi, N. J. (2019). Time‐course of population activity along the dorsoventral extent of the superior colliculus during delayed saccade tasks, bioRxiv, 571307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, P. J. (2006). The mammalian superior colliculus: Laminar structure and connections. Progress in Brain Research, 151, 321–378. [DOI] [PubMed] [Google Scholar]
- Mays, L. E. , & Sparks, D. L. (1980). Dissociation of visual and saccade‐related responses in superior colliculus neurons. Journal of Neurophysiology, 43(1), 207–232. 10.1152/jn.1980.43.1.207 [DOI] [PubMed] [Google Scholar]
- McDonnell, M. D. , & Ward, L. M. (2011). The benefits of noise in neural systems: Bridging theory and experiment. Nature Reviews Neuroscience, 12(7), 415 10.1038/nrn3061 [DOI] [PubMed] [Google Scholar]
- McPeek, R. M. , & Keller, E. L. (2004). Deficits in saccade target selection after inactivation of superior colliculus. Nature Neuroscience, 7(7), 757 10.1038/nn1269 [DOI] [PubMed] [Google Scholar]
- Meister, M. L. , & Buffalo, E. A. (2018). Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. Journal of Neuroscience, 38(10), 2430–2441. 10.1523/JNEUROSCI.2432-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikhi, Y. , Clark, K. , Albarran, E. , Parsa, M. , Zirnsak, M. , Moore, T. , & Noudoost, B. (2017). Spatial working memory alters the efficacy of input to visual cortex. Nature Communications, 8, 15041 10.1038/ncomms15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mohler, C. W. , Goldberg, M. E. , & Wurtz, R. H. (1973). Visual receptive fields of frontal eye field neurons. Brain Research, 61(3), 85–93. 10.1016/0006-8993(73)90543-X [DOI] [PubMed] [Google Scholar]
- Mohler, C. W. , & Wurtz, R. H. (1976). Organization of monkey superior colliculus: Intermediate layer cells discharging before eye movements. Journal of Neurophysiology, 39(4), 722–744. 10.1152/jn.1976.39.4.722 [DOI] [PubMed] [Google Scholar]
- Monteon, J. A. , Constantin, A. G. , Wang, H. , Martinez‐Trujillo, J. , & Crawford, J. D. (2010). Electrical stimulation of the frontal eye fields in the head‐free macaque evokes kinematically normal 3D gaze shifts. Journal of Neurophysiology, 104(6), 3462–3475. 10.1152/jn.01032.2009 [DOI] [PubMed] [Google Scholar]
- Moon, S. Y. , Barton, J. J. S. , Mikulski, S. , Polli, F. E. , Cain, M. S. , Vangel, M. , … Manoach, D. S. (2007). Where left becomes right: A magnetoencephalographic study of sensorimotor transformation for antisaccades. NeuroImage, 36(4), 1313–1323. 10.1016/j.neuroimage.2007.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz, D. P. , & Everling, S. (2004). Look away: The anti‐saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience, 5(3), 218–228. 10.1038/nrn1345 [DOI] [PubMed] [Google Scholar]
- Munoz, D. P. , & Schall, J. D. (2004). Concurrent, distributed control of saccade initiation in the frontal eye field and superior colliculus In Hall W. C. & Moschovakis A. (Eds.), The superior colliculus: New approaches for studying sensorimotor integration, (pp. 55–82). Boca Raton, FL: CRC Press. [Google Scholar]
- Munoz, D. P. , & Wurtz, R. H. (1995). Saccade‐related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. Journal of Neurophysiology, 73(6), 2313–2333. [DOI] [PubMed] [Google Scholar]
- Nacher, V. , Arora, H. K. , Bharmauria, V. , Yan, X. , Sun, S. , Wang, H. , & Crawford, J. D. (2019). Rapid visuomotor transformations and multiple gain field effects in primate ventral premotor (PMv) cortex during head‐unrestrained reaches Program No. 404.15. 2019 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience. [Google Scholar]
- Ninomiya, T. , Sawamura, H. , Inoue, K. I. , & Takada, M. (2012). Segregated pathways carrying frontally derived top‐down signals to visual areas MT and V4 in macaques. Journal of Neuroscience, 32(20), 6851–6858. 10.1523/JNEUROSCI.6295-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani, M. , Kaufman, M. T. , Hatsopoulos, N. G. , & Cheney, P. D. (2017). Perspectives on classical controversies about the motor cortex. Journal of Neurophysiology, 118(3), 1828–1848. 10.1152/jn.00795.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Optican, L. M. (2005). Sensorimotor transformation for visually guided saccades. Annals of the New York Academy of Sciences, 1039(1), 132–148. 10.1196/annals.1325.013 [DOI] [PubMed] [Google Scholar]
- O'Reilly, R. C. , & Frank, M. J. (2006). Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation, 18(2), 283–328. 10.1162/089976606775093909 [DOI] [PubMed] [Google Scholar]
- Paneri, S. , & Gregoriou, G. G. (2017). Top‐down control of visual attention by the prefrontal cortex. Functional specialization and long‐range interactions. Frontiers in Neuroscience, 11, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré, M. , Crommelinck, M. , & Guitton, D. (1994). Gaze shifts evoked by stimulation of the superior colliculus in the head‐free cat conform to the motor map but also depend on stimulus strength and fixation activity. Experimental Brain Research, 101(1), 123–139. 10.1007/BF00243222 [DOI] [PubMed] [Google Scholar]
- Paré, M. , & Guitton, D. (1990). Gaze‐related activity of brainstem omnipause neurons during combined eye‐head gaze shifts in the alert cat. Experimental Brain Research, 83(1), 210–214. 10.1007/BF00232210 [DOI] [PubMed] [Google Scholar]
- Paré, M. , & Hanes, D. P. (2003). Controlled movement processing: Superior colliculus activity associated with countermanded saccades. Journal of Neuroscience, 23(16), 6480–6489. 10.1523/JNEUROSCI.23-16-06480.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy, H. B. , Schall, J. D. , & Graybiel, A. M. (1992). Distributed but convergent ordering of corticostriatal projections: Analysis of the frontal eye field and the supplementary eye field in the macaque monkey. Journal of Neuroscience, 12(11), 4468–4488. 10.1523/JNEUROSCI.12-11-04468.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham, R. (2009). How good is the macaque monkey model of the human brain? Current Opinion in Neurobiology, 19(1), 6–11. 10.1016/j.conb.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel, T. R. , Dash, S. , Lomber, S. G. , & Corneil, B. D. (2017). Frontal eye field inactivation diminishes superior colliculus activity, but delayed saccadic accumulation governs reaction time increases. Journal of Neuroscience, 37(48), 11715–11730. 10.1523/JNEUROSCI.2664-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, V. H. , & Cowey, A. (1984). Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience, 12(4), 1125–1137. 10.1016/0306-4522(84)90007-1 [DOI] [PubMed] [Google Scholar]
- Pesaran, B. , Nelson, M. J. , & Andersen, R. A. (2006). Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron, 51(1), 125–134. 10.1016/j.neuron.2006.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt, M. L. , & Glimcher, P. W. (1998). Response fields of intraparietal neurons quantified with multiple saccadic targets. Experimental Brain Research, 121(1), 65–75. 10.1007/s002210050438 [DOI] [PubMed] [Google Scholar]
- Ploner, C. J. , Rivaud‐Péchoux, S. , Gaymard, B. M. , Agid, Y. , & Pierrot‐Deseilligny, C. (1999). Errors of memory‐guided saccades in humans with lesions of the frontal eye field and the dorsolateral prefrontal cortex. Journal of Neurophysiology, 82(2), 1086–1090. 10.1152/jn.1999.82.2.1086 [DOI] [PubMed] [Google Scholar]
- Pouget, A. , & Snyder, L. H. (2000). Computational approaches to sensorimotor transformations. Nature Neuroscience, 3(11s), 1192 10.1038/81469 [DOI] [PubMed] [Google Scholar]
- Pouget, P. , Stepniewska, I. , Crowder, E. A. , Leslie, M. W. , Emeric, E. E. , Nelson, M. J. , & Schall, J. D. (2009). Visual and motor connectivity and the distribution of calcium‐binding proteins in macaque frontal eye field: Implications for saccade target selection. Frontiers in Neuroanatomy, 3, 2 10.3389/neuro.05.002.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski, J. A. , & Zylberberg, J. (2019). The language of the brain: Real‐world neural population codes. Current Opinion in Neurobiology, 58, 30–36. [DOI] [PubMed] [Google Scholar]
- Quessy, S. , Quinet, J. , & Freedman, E. G. (2010). The locus of motor activity in the superior colliculus of the rhesus monkey is unaltered during saccadic adaptation. Journal of Neuroscience, 30(42), 14235–14244. 10.1523/JNEUROSCI.3111-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. , Pouget, P. , & Schall, J. D. (2009). Functional distinction between visuomovement and movement neurons in macaque frontal eye field during saccade countermanding. Journal of Neurophysiology, 102(6), 3091–3100. 10.1152/jn.00270.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave, P. , Coizet, V. , Comoli, E. , McHaffie, J. G. , Leriche Vazquez, M. , Vautrelle, N. , … Overton, P. G. (2010). Interactions between the midbrain superior colliculus and the basal ganglia. Frontiers in Neuroanatomy, 4, 132 10.3389/fnana.2010.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. A. (1973). Models of the saccadic eye movement control system. Kybernetik, 14(2), 71–83. 10.1007/BF00288906 [DOI] [PubMed] [Google Scholar]
- Russo, G. S. , & Bruce, C. J. (1994). Frontal eye field activity preceding aurally guided saccades. Journal of Neurophysiology, 71(3), 1250–1253. 10.1152/jn.1994.71.3.1250 [DOI] [PubMed] [Google Scholar]
- Sadeh, M. , Sajad, A. , Wang, H. , Yan, X. , & Crawford, J. D. (2015). Spatial transformations between superior colliculus visual and motor response fields during head‐unrestrained gaze shifts. European Journal of Neuroscience, 42(11), 2934–2951. 10.1111/ejn.13093 [DOI] [PubMed] [Google Scholar]
- Sadeh, M. , Sajad, A. , Wang, H. , Yan, X. , & Crawford, J. D. (2018). The influence of a memory delay on spatial coding in the superior colliculus: Is visual always visual and motor always motor? Frontiers in Neural Circuits, 12 10.3389/fncir.2018.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh, M. , Sajad, A. , Wang, H. , Yan, X. , & Crawford, J. D. (2020). Timing determines tuning: A rapid spatiotemporal transformation in superior colliculus neurons during reactive gaze shifts. eNeuro, 7(1). 10.1523/ENEURO.0359-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad, A. , Godlove, D. C. , & Schall, J. D. (2019). Cortical microcircuitry of performance monitoring. Nature Neuroscience, 22(2), 265–274. 10.1038/s41593-018-0309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad, A. , Sadeh, M. , Keith, G. P. , Yan, X. , Wang, H. , & Crawford, J. D. (2015). Visual–motor transformations within frontal eye fields during head‐unrestrained gaze shifts in the monkey. Cerebral Cortex, 25(10), 3932–3952. 10.1093/cercor/bhu279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajad, A. , Sadeh, M. , Yan, X. , Wang, H. , & Crawford, J. D. (2016). Transition from target to gaze coding in primate frontal eye field during memory delay and memory–motor transformation. eNeuro, 3(2). 10.1523/ENEURO.0040-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas, E. , & Abbott, L. F. (2001). Coordinate transformations in the visual system: How to generate gain fields and what to compute with them In Nicolelis M. A. L. (Ed.), Progress in brain research, (Vol. 130, pp. 175–190). Elsevier; 10.1016/S0079-6123(01)30012-2 [DOI] [PubMed] [Google Scholar]
- Sato, T. R. , & Schall, J. D. (2003). Effects of stimulus‐response compatibility on neural selection in frontal eye field. Neuron, 38(4), 637–648. 10.1016/S0896-6273(03)00237-X [DOI] [PubMed] [Google Scholar]
- Schall, J. D. (2015). Visuomotor functions in the frontal lobe. Annual Review of Vision Science, 1, 469–498. 10.1146/annurev-vision-082114-035317 [DOI] [PubMed] [Google Scholar]
- Schall, J. D. (2019). Accumulators, Neurons, and Response Time. Trends in Neurosciences, 42(12), 848–860.. 10.1016/j.tins.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall, J. D. , Morel, A. , King, D. J. , & Bullier, J. (1995). Topography of visual cortex connections with frontal eye field in macaque: Convergence and segregation of processing streams. Journal of Neuroscience, 15, 4464–4487. 10.1523/JNEUROSCI.15-06-04464.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall, J. D. , Purcell, B. A. , Heitz, R. P. , Logan, G. D. , & Palmeri, T. J. (2011). Neural mechanisms of saccade target selection: Gated accumulator model of the visual–motor cascade. European Journal of Neuroscience, 33(11), 1991–2002. 10.1111/j.1460-9568.2011.07715.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall, J. D. , & Thompson, K. G. (1999). Neural selection and control of visually guided eye movements. Annual Review of Neuroscience, 22(1), 241–259. 10.1146/annurev.neuro.22.1.241 [DOI] [PubMed] [Google Scholar]
- Schall, J. D. , Zinke, W. , Cosman, J. D. , Schall, M. S. , Paré, M. , & Pouget, P. (2017). On the evolution of the frontal eye field: Comparisons of monkeys, apes, and humans In Kaas J. (Ed.), Evolution of Nervous Systems 2e, (vol. 4, pp. 249–275). Oxford: Elsevier. [Google Scholar]
- Schiller, P. H. (1984). The superior colliculus and visual function In Brookhart J. M. & Mountcastle V. B. (Eds.), Handbook of physiology (pp. 457–505). Philadelphia, PA: Lippincott Williams and Wilkins. [Google Scholar]
- Schiller, P. H. , Sandell, J. H. , & Maunsell, J. H. (1987). The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. Journal of Neurophysiology, 57(4), 1033–1049. 10.1152/jn.1987.57.4.1033 [DOI] [PubMed] [Google Scholar]
- Schiller, P. H. , & Stryker, M. (1972). Single‐unit recording and stimulation in superior colliculus of the alert rhesus monkey. Journal of Neurophysiology, 35, 915–924. 10.1152/jn.1972.35.6.915 [DOI] [PubMed] [Google Scholar]
- Schlag‐Rey, M. , & Schlag, J. (1984). Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. Journal of Neurophysiology, 51(6), 1149–1174. [DOI] [PubMed] [Google Scholar]
- Schneider, L. , Dominguez‐Vargas, A. U. , Gibson, L. , Kagan, I. , & Wilke, M. (2020). Eye position signals in the dorsal pulvinar during fixation and goal‐directed saccades. Journal of Neurophysiology, 123(1), 367–391. 10.1152/jn.00432.2019 [DOI] [PubMed] [Google Scholar]
- Segraves, M. A. (1992). Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. Journal of Neurophysiology, 68(6), 1967–1985. 10.1152/jn.1992.68.6.1967 [DOI] [PubMed] [Google Scholar]
- Segraves, M. A. , & Goldberg, M. E. (1987). Functional properties of corticotectal neurons in the monkey's frontal eye field. Journal of Neurophysiology, 58(6), 1387–1419. 10.1152/jn.1987.58.6.1387 [DOI] [PubMed] [Google Scholar]
- Shin, S. , & Sommer, M. A. (2012). Division of labor in frontal eye field neurons during presaccadic remapping of visual receptive fields. Journal of Neurophysiology, 108(8), 2144–2159. 10.1152/jn.00204.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A. , & Crawford, J. D. (2005). Distributed population mechanism for the 3‐D oculomotor reference frame transformation. Journal of Neurophysiology, 93(3), 1742–1761. 10.1152/jn.00306.2004 [DOI] [PubMed] [Google Scholar]
- Snyder, L. H. (2000). Coordinate transformations for eye and arm movements in the brain. Current Opinion in Neurobiology, 10(6), 747–754. 10.1016/S0959-4388(00)00152-5 [DOI] [PubMed] [Google Scholar]
- Soechting, J. F. , & Flanders, M. (1992). Moving in three‐dimensional space: Frames of reference, vectors, and coordinate systems. Annual Review of Neuroscience, 15(1), 167–191. 10.1146/annurev.ne.15.030192.001123 [DOI] [PubMed] [Google Scholar]
- Soechting, J. F. , Tillery, S. H. , & Flanders, M. (1990). Transformation from head‐to shoulder‐centered representation of target direction in arm movements. Journal of Cognitive Neuroscience, 2(1), 32–43. [DOI] [PubMed] [Google Scholar]
- Sommer, M. A. , & Wurtz, R. H. (2000). Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. Journal of Neurophysiology, 83(4), 1979–2001. 10.1152/jn.2000.83.4.1979 [DOI] [PubMed] [Google Scholar]
- Sommer, M. A. , & Wurtz, R. H. (2001). Frontal eye field sends delay activity related to movement, memory, and vision to the superior colliculus. Journal of Neurophysiology, 85(4), 1673–1685. 10.1152/jn.2001.85.4.1673 [DOI] [PubMed] [Google Scholar]
- Sommer, M. A. , & Wurtz, R. H. (2004). What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. Journal of Neurophysiology, 91(3), 1381–1402. [DOI] [PubMed] [Google Scholar]
- Spaak, E. , Watanabe, K. , Funahashi, S. , & Stokes, M. G. (2017). Stable and dynamic coding for working memory in primate prefrontal cortex. Journal of Neuroscience, 37(27), 6503–6516. 10.1523/JNEUROSCI.3364-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks, D. L. (1986). Translation of sensory signals into commands for control of saccadic eye movements: Role of primate superior colliculus. Physiological Reviews, 66(1), 118–171. 10.1152/physrev.1986.66.1.118 [DOI] [PubMed] [Google Scholar]
- Sparks, D. L. (1988). Neural cartography: Sensory and motor maps in the superior colliculus. Brain, Behavior and Evolution, 31(1), 49–56. 10.1159/000116575 [DOI] [PubMed] [Google Scholar]
- Sparks, D. L. (2002). The brainstem control of saccadic eye movements. Nature Reviews Neuroscience, 3(12), 952–964. 10.1038/nrn986 [DOI] [PubMed] [Google Scholar]
- Sparks, D. L. , Freedman, E. G. , Chen, L. L. , & Gandhi, N. J. (2001). Cortical and subcortical contributions to coordinated eye and head movements. Vision Research, 41(25–26), 3295–3305. 10.1016/S0042-6989(01)00063-3 [DOI] [PubMed] [Google Scholar]
- Sparks, D. L. , & Mays, L. E. (1990). Signal transformations required for the generation of saccadic eye movements. Annual Review of Neuroscience, 13(1), 309–336. 10.1146/annurev.ne.13.030190.001521 [DOI] [PubMed] [Google Scholar]
- Stanford, T. R. , & Sparks, D. L. (1994). Systematic errors for saccades to remembered targets: Evidence for a dissociation between saccade metrics and activity in the superior colliculus. Vision Research, 34(1), 93–106. 10.1016/0042-6989(94)90260-7 [DOI] [PubMed] [Google Scholar]
- Stanton, G. B. , Deng, S. Y. , Goldberg, E. M. , & McMullen, N. T. (1989). Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. Journal of Comparative Neurology, 282(3), 415–427. 10.1002/cne.902820308 [DOI] [PubMed] [Google Scholar]
- Stanton, G. B. , Goldberg, M. E. , & Bruce, C. J. (1988a). Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. Journal of Comparative Neurology, 271(4), 493–506. 10.1002/cne.902710403 [DOI] [PubMed] [Google Scholar]
- Stanton, G. B. , Goldberg, M. E. , & Bruce, C. J. (1988b). Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. Journal of Comparative Neurology, 271(4), 473–492. 10.1002/cne.902710402 [DOI] [PubMed] [Google Scholar]
- Steenrod, S. C. , Phillips, M. H. , & Goldberg, M. E. (2012). The lateral intraparietal area codes the location of saccade targets and not the dimension of the saccades that will be made to acquire them. Journal of Neurophysiology, 109(2596–2605), 2013 10.1152/jn.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricanne, B. , Andersen, R. A. , & Mazzoni, P. (1996). Eye‐centered, head‐centered, and intermediate coding of remembered sound locations in area LIP. Journal of Neurophysiology, 76, 2071–2076. 10.1152/jn.1996.76.3.2071 [DOI] [PubMed] [Google Scholar]
- Takeichi, N. , Kaneko, C. R. , & Fuchs, A. F. (2007). Activity changes in monkey superior colliculus during saccade adaptation. Journal of Neurophysiology, 97(6), 4096–4107. 10.1152/jn.01278.2006 [DOI] [PubMed] [Google Scholar]
- Thakkar, K. N. , Schall, J. D. , Heckers, S. , & Park, S. (2015). Disrupted saccadic corollary discharge in schizophrenia. Journal of Neuroscience, 35(27), 9935–9945. 10.1523/JNEUROSCI.0473-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, K. G. , & Bichot, N. P. (2005). A visual salience map in the primate frontal eye field. Progress in Brain Research, 147, 249–262. [DOI] [PubMed] [Google Scholar]
- Tweed, D. B. , Haslwanter, T. P. , Happe, V. , & Fetter, M. (1999). Non‐commutativity in the brain. Nature, 399(6733), 261 10.1038/20441 [DOI] [PubMed] [Google Scholar]
- van Beers, R. J. (2007). The sources of variability in saccadic eye movements. Journal of Neuroscience, 27(33), 8757–8770. 10.1523/JNEUROSCI.2311-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen, R. S. , Ma, W. J. , Pratte, M. S. , & Jehee, J. F. (2015). Sensory uncertainty decoded from visual cortex predicts behavior. Nature Neuroscience, 18(12), 1728 10.1038/nn.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton, M. M. , Bechara, B. , & Gandhi, N. J. (2007). Role of the primate superior colliculus in the control of head movements. Journal of Neurophysiology, 98(4), 2022–2037. 10.1152/jn.00258.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K. , & Funahashi, S. (2007). Prefrontal delay‐period activity reflects the decision process of a saccade direction during a free‐choice ODR task. Cerebral Cortex, 17(suppl_1), i88–i100. 10.1093/cercor/bhm102 [DOI] [PubMed] [Google Scholar]
- Westendorff, S. , Klaes, C. , & Gail, A. (2010). The cortical timeline for deciding on reach motor goals. Journal of Neuroscience, 30(15), 5426–5436. 10.1523/JNEUROSCI.4628-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand, T. G. , & Gafka, A. C. (1998). Corticostriatal and corticotectal neurons in area 6 of the cat during fixation and eye movements. Visual Neuroscience, 15(1), 141–151. [DOI] [PubMed] [Google Scholar]
- White, B. J. , Berg, D. J. , Kan, J. Y. , Marino, R. A. , Itti, L. , & Munoz, D. P. (2017). Superior colliculus neurons encode a visual saliency map during free viewing of natural dynamic video. Nature Communications, 8, 14263 10.1038/ncomms14263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. M. , Sparks, D. L. , & Stanford, T. R. (1994). Saccades to remembered target locations: An analysis of systematic and variable errors. Vision Research, 34(1), 79–92. 10.1016/0042-6989(94)90259-3 [DOI] [PubMed] [Google Scholar]
- Wimmer, K. , Nykamp, D. Q. , Constantinidis, C. , & Compte, A. (2014). Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nature Neuroscience, 17(3), 431 10.1038/nn.3645 [DOI] [PubMed] [Google Scholar]
- Wurtz, R. H. , & Albano, J. E. (1980). Visual‐motor function of the primate superior colliculus. Annual Review of Neuroscience, 3(1), 189–226. 10.1146/annurev.ne.03.030180.001201 [DOI] [PubMed] [Google Scholar]
- Wurtz, R. H. , Sommer, M. A. , Paré, M. , & Ferraina, S. (2001). Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Research, 41(25–26), 3399–3412. 10.1016/S0042-6989(01)00066-9 [DOI] [PubMed] [Google Scholar]
- Zhang, M. , & Barash, S. (2000). Neuronal switching of sensorimotor transformations for antisaccades. Nature, 408(6815), 971 10.1038/35050097 [DOI] [PubMed] [Google Scholar]
- Zheng, T. , & Wilson, C. J. (2002). Corticostriatal combinatorics: The implications of corticostriatal axonal arborizations. Journal of Neurophysiology, 87(1007–1017), pmid:11826064. [DOI] [PubMed] [Google Scholar]


