Abstract
Standard treatment for hairy cell leukemia (HCL) is markedly effective, but the constant decrease in disease-free survival, together with the presence of minimal residual disease (MRD), suggests that few if any are cured. HCL cells in MRD are always strongly CD20+ and CD22+, and also CD25 + unless the patient has the poor-prognosis variant HCLv. To target relapsed/refractory HCL, immunotherapy has been developed using anti-CD25 and anti-CD22 recombinant immunotoxins, or the anti-CD20 monoclonal antibody (mAb) rituximab alone or combined with purine analogs. The recombinant immunotoxins contain an Fv fragment of a mAb fused to a truncated form of Pseudomonas exotoxin called PE38. BL22 targeting CD22, in phase I and II testing of relapsed/refractory HCL, achieved 47–61% complete remissions (CRs), several of them ongoing after 9–10 years. A completely reversible form of hemolytic uremic syndrome (HUS) was observed in 12% of patients, several of whom could later achieve a partial remission (PR) or CR with LMB-2 targeting CD25. A higher-affinity version of BL22, termed HA22, CAT-8015, or moxetumomab pasudotox, developed to more effectively treat other hematologic malignancies, also achieves CRs in HCL, and with only non-dose-limiting HUS. In separate randomized trials, rituximab is undergoing phase II testing with cladribine for early HCL and with bendamustine or pentostatin for multiply relapsed HCL.
Keywords: Monoclonal, bendamustine, cladribine, pentostatin, rituximab, LMB-2, BL22, Moxetumomab pasudotox
Need for alternative approaches in hairy cell leukemia
The purine analogs cladribine and pentostatin are each markedly effective in achieving long-term complete remissions (CRs) in patients with hairy cell leukemia (HCL), with CR rates of ~70–90%, most still in CR at 8 years [1,2]. However, despite 20 years of follow-up, there remains no plateau on the disease-free survival curves, suggesting lack of cure with this approach. In fact, only a small percentage of patients followed for 12–28 (median 18) years after diagnosis were reported negative for minimal residual disease (MRD) [3], suggesting that most if not all will relapse given sufficient time. Although third and fourth CRs are achieved with repeated courses of purine analogs, CR rates decrease significantly with each successive course, despite crossing over to the other purine analog [1,4]. Thus, for at least young patients, alternative strategies are needed for relapsed HCL. Moreover, new approaches for first- or second-line treatment are needed, which might in younger patients offer less chance of eventual relapse without effective options.
Intoxication of cells by immunotoxins
Recombinant immunotoxins contain an Fv fragment of a monoclonal antibody (mAb) fused to a toxin, to target enzymatic cell-killing activity to the cell surface receptors, initiating internalization, and eventually producing cell death [5]. Agents with efficacy reported for relapsed/refractory HCL include BL22 (CAT-3888), targeting CD22, and LMB-2, targeting CD25. The Fv in each molecule is fused to a truncated form of Pseudomonas exotoxin called PE38, containing amino acids 253–364 and 381–613 of the toxin. Studies indicate that these molecules kill HCL cells by: (1) binding to their cell-surface antigen, (2) undergoing internalization, (3) unfolding at low pH, (4) undergoing proteolytic cleavage between amino acids 279 and 280 and before the carboxy terminal lysine residue, (5) undergoing reduction of a disulfide bond holding the two fragments together, (6) transporting of the carboxyl terminal portion of the toxin from the transreticular Golgi to the endoplasmic reticulum by the KDEL receptor, (7) translocation to the cytosol, and finally (8) catalytic adenosine diphosphate (ADP) ribosylation of elongation factor II, leading to apoptotic cell death. Thus, recombinant immunotoxins kill target cells selectively without requiring immune mechanisms, and their mechanism is markedly different from chemotherapy including purine analogs.
LMB-2 targeting CD25+ hairy cell leukemia
LMB-2 was the first immunotoxin with reported efficacy in HCL. To target CD25, the variable domains VH and VL of the anti-CD25 mAb anti-Tac were fused together with a peptide linker (G4S)3 and VL was fused to PE38 via a short connector ASGGPE (Figure 1). In a phase I trial conducted in patients with hematologic malignancies, four out of four patients with HCL responded, one completely (CR) and three partially (PR) [6,7]. Responses were observed in other hematologic malignancies, and activity has been observed in a follow-up phase II trial in HCL. Although only 10% of patients with HCL have the CD25-negative variant HCLv, because HCLv is primarily refractory to cladribine, patients with HCLv are overrepresented in the relapsed/refractory population. Thus, CD22, which is strongly positive in essentially all cases of HCL, was targeted.
Figure 1.
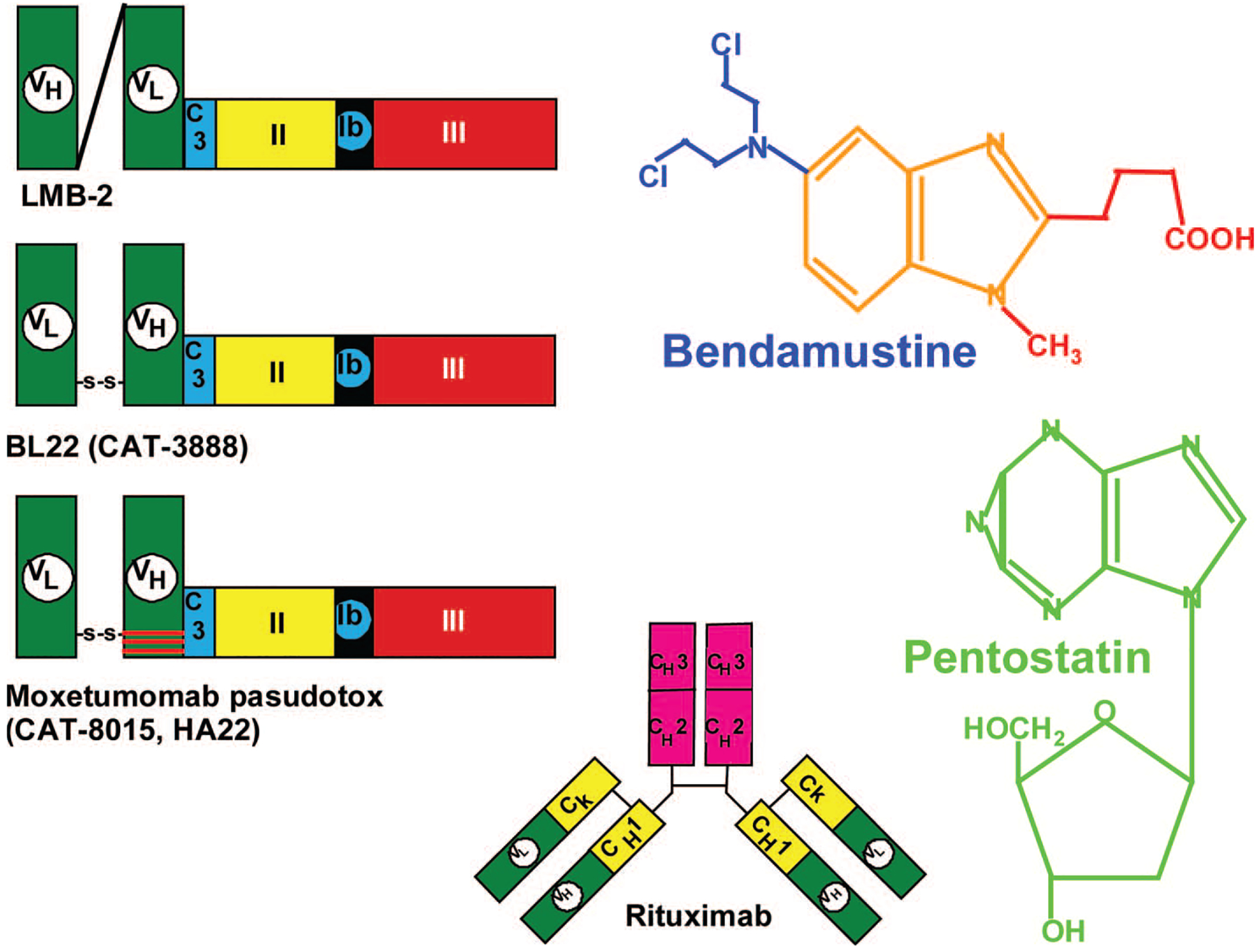
Molecules under investigation for HCL. Recombinant immunotoxins are 63 kDa and contain an Fv domain fused via the C3 connector (ASGGPE, light blue) to amino acids 253–364 and 381–613 of Pseudomonas exotoxin. Domain II (yellow), containing amino acids 253–364, is the translocating domain, domain III (red), containing amino acids 395–613, is the ADP ribosylating domain, and part of domain Ib (amino acids 381–394) separates domains II and III. Anti-Tac(Fv)-PE38 (LMB-2), targeting CD25, contains a (G4S)3 linker between VH and VL, and VL is fused to PE38. In BL22 and HA22, VL and VH are connected by a disulfide bond using cysteine residues replacing Arg44 of VH and Gly100 of VL. HA22, also called moxetumomab pasudotox or CAT-8015, is a high-affinity variant of BL22 containing THW replacing SSY at positions 100, 100a, and 100b of VH. Rituximab is an anti-CD20 chimeric antibody containing murine variable domains and human constant (yellow and pink) domains. Bendamustine and pentostatin are purine analogs, and bendamustine also contains a nitrogen mustard group (blue) which provides alkylator activity.
BL22, targeting CD22
BL22 contains a disulfide bond linking VH and VL, with VH fused to PE38 (Figure 1). In a phase I trial of 31 patients with cladribine relapsed/refractory HCL, BL22 achieved 19 (62%) CRs and six (19%) PRs, an overall response rate (ORR) of 81% [8,9]. In this trial, three patients had HCLv and all had CR, and although two of the three relapsed, both were returned to CR with salvage BL22. The most common serious toxicity was a completely reversible hemolytic uremic syndrome (HUS), in four patients. HUS presented with hematuria, hemoglobinuria, thrombocytopenia, and renal insufficiency by day 8 of cycle 2–3, and completely resolved within several weeks. Other than HUS, dose limiting toxicity (DLT) on the phase I trial was limited to a cytokine release syndrome in one patient who had fever, bone pain, hypotension, and weight gain (vascular leak syndrome; VLS) but no pulmonary edema, resolving in 3 days. The cycle 1 maximum tolerated dose (MTD) was 40 μg/kg every other day for three doses (QOD × 3), and the CR rate was significantly higher when patients began BL22 at least at this dose level. Since HUS was only observed with retreatment at 3–4-week intervals, the phase II trial was designed to test the efficacy of one cycle of 40 μg/kg QOD × 3, retreating only those with stable disease (SD) or PR not achieving resolution of blood counts to the levels needed for CR. With one cycle of BL22 in 36 phase II patients with HCL enrolled, 25% achieved CR and 25% achieved PR. Fifty-six percent of patients were retreated, resulting in final response rates of 47% CRs and 25% PRs. Dose-limiting HUS was observed in only two patients on trial, and in two patients treated by special exemption. It was found that HUS resolved completely without plasmapheresis, which was consistent with documentation of adequate levels of the protease ADAMTS13 in the plasma of patients, thus not requiring removal of an inhibitor. Since the CD22 was present at such high density on the HCL cells (median 44 000 sites/cell), the pharmacokinetics of BL22 was strongly affected by tumor burden. Accordingly, plasma BL22 levels usually increased on the cycles given after patients responded. High levels of neutralizing antibodies that prevented retreatment were observed in four (11%) patients. CR rates were higher in patients with spleen diameters <200 mm than in those with either larger spleens or prior splenectomy (p = 0.019), due to either more advanced disease in the latter group, or more limited tumor penetration of BL22 into bone marrow or splenic tissue. Thus, BL22 activity against relapsed/refractory HCL was confirmed, with best responses achieved before spleens were either removed or allowed to grow to massive size.
Targeting CD22 with higher affinity
Response to BL22 in chronic lymphocytic leukemia (CLL) was more limited than in HCL, attributed to the relatively low CD22 expression in CLL (median 1250 sites/cell) and the significant off-rate of BL22. To allow more toxin molecules binding to CLL cells to become internalized, the affinity was increased and off-rate decreased by mutation of hot-spot (RGYW) residues in the complementarity determining regions (CDRs) [10]. By phage display selection, a mutant was developed with THW replacing SSY at positions 100, 100a, and 100b of the VH CDR3, resulting in 15-fold improvement in binding affinity, leading to improvement in cytotoxicity toward primary CLL as well as HCL cells. In phase I testing of CAT-8015, also called HA22 and moxetumomab pasudotox, the first 28 patients tested achieved a CR rate of 43%, and of the 25 patients receiving 10–50 μg/kg QOD × 3, 48% achieved CR. No DLT was observed in this trial, although two patients with minor laboratory abnormalities (creatinine and platelet abnormalities limited to grade I) were diagnosed as grade 2 HUS. Thus, CAT-8015, a higher-affinity version of BL22, achieved responses in HCL, and may be less toxic than BL22, possibly by decreasing off-target binding and non-specific toxicity.
Use of rituximab in early hairy cell leukemia
While purine analogs alone may not be able to eradicate HCL, since MRD detected after cladribine is always strongly CD20 + (>100 000 sites/cell), it may be possible to eradicate MRD using rituximab, providing it is timed correctly. Used as a single agent, rituximab has achieved 10–60% CR rates in multiple trials, but overall, those patients who needed treatment because of cytopenias and had at least one prior course of purine analog had an ORR of 39%, with 20% CRs. The largest trial of rituximab alone achieved 13% CRs and 13% PRs [11]. However, Ravandi et al. reported a 100% CR rate, most without MRD, in 13 patients with early HCL (0–1 prior courses of purine analog) treated with eight weekly doses of rituximab beginning 1 month after five daily doses of cladribine [12]. To determine whether rituximab + cladribine should replace cladribine alone as the standard treatment of HCL, we initiated a randomized trial in which patients with HCL with 0–1 prior courses of cladribine are treated with five daily doses of cladribine (days 1–5) with eight weekly doses of rituximab begun either on day 1 or at least 6 months later as soon as MRD is detectable in the blood. Although one would expect MRD to be lower at 6 months after cladribine if rituximab were begun on day 1 (the primary endpoint), there are several potential advantages to waiting for 6 months before beginning rituximab, including: (1) since minimum HCL burden is achieved at ~6 months after a 5–7-day course of cladribine, rituximab may be best able to reach all HCL cells at that point, (2) since rituximab requires immune mechanisms for killing, cladribine, which kills B- and T-cells, may block efficacy, (3) infusional reactions from rituximab, which often require steroids, are not observed when delayed at least 6 months, and steroids may partially block rituximab efficacy, and (4) patients who are neutropenic immediately after cladribine might have added toxicity with immediate rituximab due to prolonged B-cell depletion, compared to if rituximab were added after neutropenia resolves. This trial so far shows an ORR of 100% in 22 evaluable patients, and without immediate rituximab most patients have MRD in the blood 6 months after cladribine.
Bendamustine versus pentostatin combined with rituximab for multiply relapsed hairy cell leukemia
The combination of pentostatin and rituximab is reported to have an ORR of 100% with six (86%) CRs in seven patients treated [4], although a prospective trial has not been carried out with this combination. Bendamustine is approved for CLL and is active with rituximab in several hematologic malignancies [13] including CLL. Although it has a purine analog group similar to cladribine, it also contains alkylator activity and its mechanisms of cell death allow non-cross-resistance with purine analogs. To evaluate both regimens of pentostatin + rituximab and bendamustine + rituximab in multiply relapsed HCL, a randomized trial has begun whereby each regimen is evaluated in phase II fashion and the two regimens compared, with a crossover allowed.
Summary
The outlook for patients with HCL dramatically improved after the introduction of purine analogs over 20 years ago, but cure seems elusive for patients young enough to outlive their remissions and become treatment-refractory. New immunotherapeutic approaches include rituximab combined with purine analog, or recombinant immunotoxin either alone or eventually combined with other agents (Table I). These new approaches may offer eradication of HCL in some patients, or at least a dramatic lengthening of treatment-free intervals sufficient to prevent HCL-related deaths even in young patients.
Table I.
HCL-specific clinical trials at NIH.
| Agent(s) | Trial type | Target | Eligibility |
|---|---|---|---|
| HA22 (CAT-8015 or moxetumomab pasudotox) | Phase I | CD22 | Two prior therapies Need for treatment* No/low antibodies Adequate organ function |
| LMB-2 | Phase II | CD25 | <4 year CR/PR to ≥2nd-line cladribine Need for treatment* No/low antibodies Adequate organ function CD25 + HCL Prior BL22 or HA22, or ineligible |
| Cladribine + rituximab | Phase II randomized | CD20 | 0–1 prior courses of cladribine No other purine analog No prior rituximab Need for treatment* HCLv allowed (non-randomized) |
| Bendamustine + rituximab vs. pentostatin + rituximab | Phase II randomized | CD20 | >2 prior courses purine analog Need for treatment* HCLv allowed |
Need for treatment in HCL is generally defined as at least one of the following: neutrophils <1000/μL, hemoglobin <10 g/dL, platelets <100 000/μL, lymphocytes >20 000/μL, or symptomatic splenomegaly. All trials require adequate organ function.
HCL, hairy cell leukemia; NIH, National Institutes of Health; CR, complete remission; PR, partial remission; HCLv, variant HCL.
Acknowledgements
This work was supported in part by the National Cancer Institute, Intramural Program. Work regarding moxetumomab pasudotox (HA22 or CAT-8015) and BL22 (CAT-3888) was supported in part by MedImmune, LLC.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Goodman GR, Burian C, Koziol JA, Saven A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol 2003;21:891–896. [DOI] [PubMed] [Google Scholar]
- 2.Grever MR. Pentostatin: impact on outcome in hairy cell leukemia. Hematol Oncol Clin North Am 2006;20:1099–1108. [DOI] [PubMed] [Google Scholar]
- 3.Sigal DS, Sharpe R, Burian C, Saven A. Very long-term eradication of minimal residual disease in patients with hairy cell leukemia after a single course of cladribine. Blood 2010;115:1893–1896. [DOI] [PubMed] [Google Scholar]
- 4.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol 2009;145:733–740. [DOI] [PubMed] [Google Scholar]
- 5.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med 2007;58:221–237. [DOI] [PubMed] [Google Scholar]
- 6.Kreitman RJ, Wilson WH, Robbins D, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood 1999;94:3340–3348. [PubMed] [Google Scholar]
- 7.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol 2000;18: 1614–1636. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 2001;345: 241–247. [DOI] [PubMed] [Google Scholar]
- 9.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol 2005;23: 6719–6729. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity towards cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res 2002;8:995–1002. [PubMed] [Google Scholar]
- 11.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood 2003;102:810–813. [DOI] [PubMed] [Google Scholar]
- 12.Ravandi F, Jorgensen JL, O’Brien SM, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood 2006;107:4658–4662. [DOI] [PubMed] [Google Scholar]
- 13.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:4473–4479. [DOI] [PubMed] [Google Scholar]


