Abstract
Recombinant immunotoxins, containing an Fv fragment and a bacterial toxin, frequently elicit neutralizing antibodies, nearly always against the toxin. Moxetumomab pasudotox (previously called CAT-8015 or HA22) contains an anti-CD22 Fv fused to PE38, a truncated form of Pseudomonas exotoxin, containing amino acids 253–364 and 381–613. One avenue to reducing immunogenicity is to identify B- and T-cell epitopes and remove them while retaining toxin activity. To determine B-cell epitopes on PE38, 60 monoclonal antibodies against PE38 were tested in a pairwise manner, and seven major epitope groups with 13 subgroups were identified. The locations of many of these epitopes were identified by mutating large surface-exposed residues to alanine. A mutant of moxetumomab pasudotox containing eight epitope-eliminating mutations (HA22–8X) was prepared, and greatly reduced immunogenicity in mice. In parallel, two large sections of PE38 containing lysosomal protease cleavage sites were removed, leaving only amino acids 274–284 and 394–613 of the toxin. The resulting molecule, HA22-LR, retained cytotoxicity toward CD22þ cell lines, killed primary chronic lymphocytic leukemia cells more potently than moxetumomab pasudotox, was much less toxic to mice, and had significantly improved antitumor activity toward murine xenografts. The immunogenicity and activity of recombinant immunotoxins may be optimized by combinations of these approaches.
Keywords: Recombinant immunotoxins, hairy cell leukemia
Immunogenicity of recombinant immunotoxins
Recombinant immunotoxins are composed of a variable fragment (Fv) of a monoclonal antibody (MAb) fused to truncated bacterial toxin [1]. Examples include BL22 (CAT-3888), targeting CD22, and SS1P, targeting mesothelin. Each of these molecules contains PE38, a truncated form of Pseudomonas exotoxin A (PE) containing amino acids 253–364 and 381–613. Recombinant immunotoxins in clinical trials have demonstrated remarkable potency. BL22, for example, has been reported to induce complete remissions in 47–61% of patients with chemoresistant hairy cell leukemia [2,3]. Unfortunately, neutralizing antibodies are frequently produced when recombinant immunotoxins are administered to humans or laboratory animals, and these antibodies nearly always target the bacterial toxin rather than the murine Fv. In the phase II trial of BL22, four (11%) patients produced levels of neutralizing antibodies sufficiently high to prevent retreatment, and others made lower levels of antibodies. In solid tumors, immunogenicity is much higher; nearly all patients treated with SS1P produced significant levels of neutralizing antibodies [4,5]. One avenue for avoiding immunogenicity is to alter the toxin to mutate or remove immunogenic epitopes, which requires a mechanistic knowledge of essential toxin regions.
Intoxication of cells by Pseudomonas exotoxin
The functional domains of native PE include Ia (amino acids 1–252), which binds to cells and is not present in PE38, II (253–364), which is though to mediate translocation, III (400–613), which contains adenosine diphosphate (ADP)-ribosylating activity, and Ib (365–399), which separates domains II and III. The known steps required for PE to kill cells include (1) binding to a cell-surface antigen, (2) internalization into an endocytic vesicle where the toxin unfolds at low pH, (3) proteolytic cleavage by furin between amino acids 279 and 280, leaving a disulfide bridge between Cys265 and Cys287, (4) reduction of the disulfide bond, (5) transport of the carboxyl terminal portion of the toxin from the transreticular Golgi to the endoplasmic reticulum by the KDEL receptor, (6) translocation to the cytosol, and finally (7) catalytic ADP-ribosylation of elongation factor II, leading to apoptotic cell death. Thus, recombinant immunotoxins, unlike vaccines, kill cells directly without help from the immune system. On the contrary, a humoral immune response against recombinant immunotoxins will block their function, and preventing immunogenicity is an important goal for the development of recombinant immunotoxins.
Identification of immunogenic epitopes in PE38
To identify immunogenic epitopes, a panel of 60 anti-PE38 MAbs were isolated and tested pairwise (3600 combinations) in an immune complex capture enzyme-linked immunosorbent assay (ELI-SA). Each experiment utilized one MAb as an indicator and another as a competitor [6]. As shown in Figure 1(A), these experiments identified seven major epitopes. The next step was to determine where on the PE38 molecule these epitopes were located. To accomplish this, point mutations to alanine or glycine were performed in PE38, targeting amino acids predicted to be highly surface-exposed. As shown in Figure 1(B), several of the point mutants reacted with anti-PE38 MAbs with <10% of the affinity of native PE38, disclosing the locations of the immunogenic epitopes. Figure 1(C) shows the location of the epitopes, some of them discontinuous and separated by more than 50 amino acids.
Figure 1.
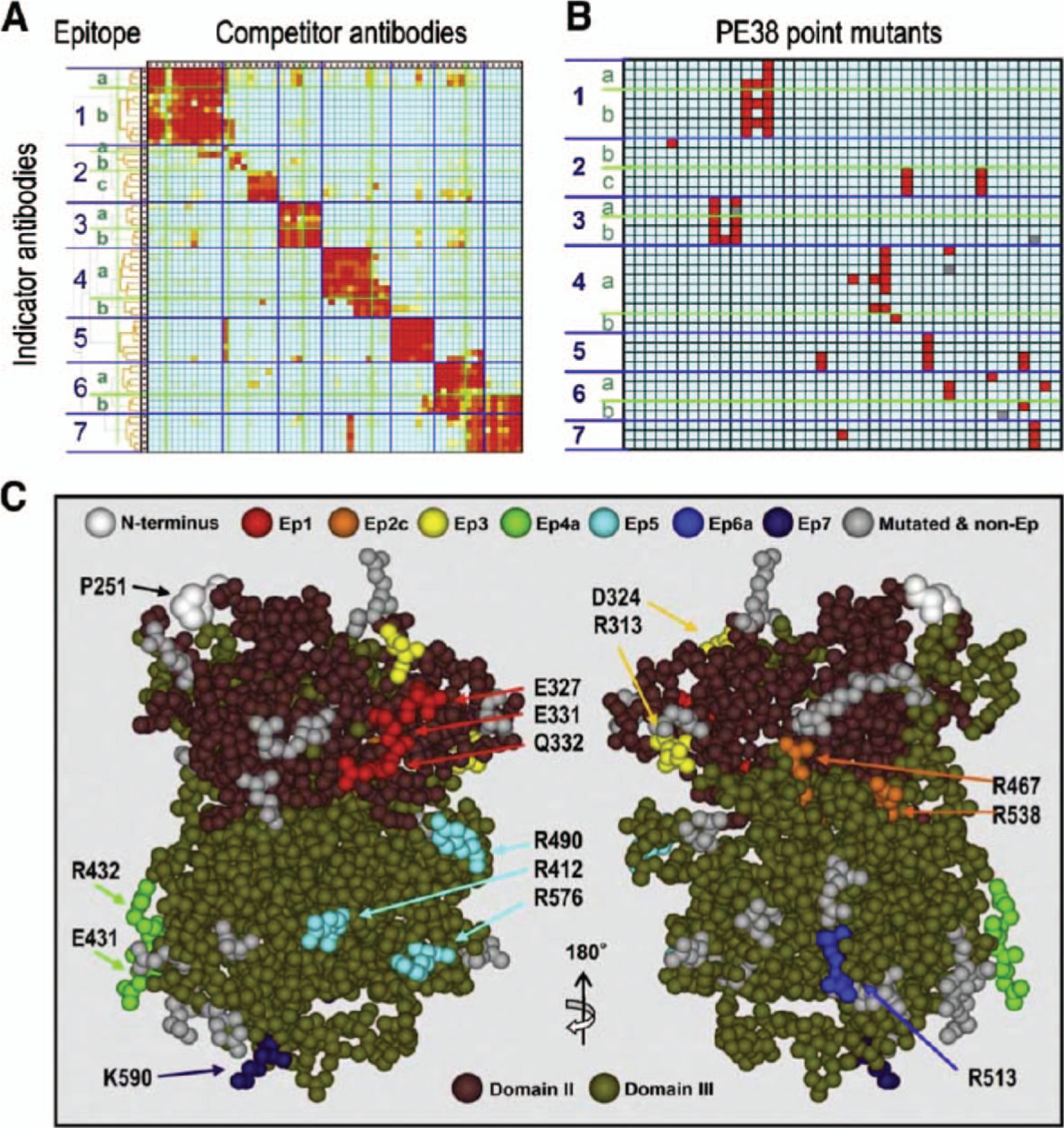
Summary of steps to identify and remove B cell epitopes in PE38. (A) Mutual competitive binding assay of 60 mouse monoclonal antibodies against PE38 produced for B cell epitope analysis leading to deimmunization of PE38. Binding of indicator antibodies (in rows) in the presence of competitor antibodies (in columns) was measured by immunocytochemistry-ELISA. Color code (red to blue) shows the degree of competition from 100 to 0%. (B) Binding of each antibody (in rows) to each point mutant of PE38 (in columns). Mutations were made in highly exposed amino acids in a crystal structure. The binding assay measured the affinity difference between mutant and wild-type PE38; mutations that reduced affinity to the antibody under 10% compared to wild-type are shown in red. (C) Locations of mutated amino acids that decrease binding of PE38 to antibodies in an epitope-specific manner (from data shown in B) are displayed in different colors on a structural model of PE38. Clustered residues shown in different colors indicate the location of each epitope. The two different approaches (topographical epitope mapping and location of epitopes on the crystal structure determined by mutagenesis) are in close agreement. This concordance verifies the epitope locations without analyzing crystal structures of the antigen–antibody complexes. Adapted with permission from The Journal of Immunology [6].
Mutation of the immunogenic epitopes of PE38
Once the seven epitopes were identified, the next step was to evaluate their activity and combine them into a single molecule. Moxetumomab pasudotox, an affinity-optimized anti-CD22 recombinant immunotoxin previously known as HA22 or CAT-8015, was chosen for these experiments. Mutation of key residues within each epitope to alanine, serine, or glycine produced seven point mutants, each with 490% cytotoxic activity: R313A, Q332S, R432G, R467A, R490A, R513A, and K590S [7]. These mutants corresponded to epitopes 3, 1, 4, 2, 5, 6, and 7, respectively. An additional point mutant, E548A, also part of epitope 6, was 17% active. However, when all eight of these mutations were combined in a single molecule, cytotoxic activity was preserved at ~100% [7]. This mutant, termed HA22–8X, was tested against a human CD22+ Burkitt lymphoma CA46 subcutaneous xenograft model, and found to have antitumor activity similar to that of moxetumomab pasudotox. Additional experiments observing the generation of anti-PE antibodies in mice dosed with moxetumomab pasudotox or HA22–8X demonstrated that HA22–8X was significantly less immunogenic than moxetumomab pasudotox [7].
Removal of multiple epitopes by domain deletion
Concurrent with research identifying and eliminating epitopes in PE38, experiments were also investigating the susceptibility of recombinant immunotoxins to lysosomal protease digestion. Research suggests that only a small number of toxin molecules reach the cytosol, and a significant proportion of internalized recombinant immunotoxins traffic unproductively into lysosomes for degradation. If lysosomal degradation of recombinant immunotoxins could be minimized, it might be possible to increase the proportion of recombinant immunotoxins that reach the cytosol. Multiple protease cleavage sites were identified in domains II and Ib, and targeted for removal [8]. To determine whether the sites could be removed en bloc, the entire region, comprised of residues 251–394, was removed from moxetumomab pasudotox and replaced only by amino acids 274–284, which includes the furin cleavage site between Arg279 and Gly280. This strategy has the additional benefit of removing immunogenic epitopes along with protease cleavage sites. The resulting mutant, HA22-LR, had between 22 and 212% of the cytotoxic activity of moxetumomab pasudotox on six different cell lines, with a median relative cytotoxic activity of 102% [8]. When tested on freshly obtained primary chronic lymphocytic leukemia (CLL) cells, however, HA22-LR was more cytotoxic than moxetumomab pasudotox by a median of >16.8-fold [8]. A possible explanation is that in CLL cells, lysosomal proteases are a key impediment for cytotoxic activity, which is circumvented by recombinant immunotoxins lacking protease sites. Alternatively, it is possible that the molecule, because of its smaller size, translocates more efficiently into the cytosol. Another surprising finding from this work was the lack of non-specific toxicity of HA22-LR in mice. Compared to moxetumomab pasudotox, which was 100% lethal to mice at 2 mg/kg, HA22-LR failed to kill any of 10 mice at 20 mg/kg [8]. Antitumor studies in SCID (severe combined immunodeficiency) mice bearing CA46 xenograft tumors confirmed that HA22-LR was much more effective than moxetumomab pasudotox in inducing complete regressions, due to higher doses allowed by its low toxicity.
Summary
In summary, several immunogenic epitopes have been identified on the surface of PE38 that are thought to be targets for neutralizing antibodies. Two methods that have reduced the immunogenicity of recombinant immunotoxins include point mutations and large deletions of epitope-containing regions. The latter method yielded particularly exciting results in view of the greatly increased cytotoxicity toward CLL cells and the greatly diminished animal toxicity, although it raises significant questions about the function of domain II in PE. Further work is proceeding to combine both of these methods to obtain optimally active and non-immunogenic recombinant immunotoxins [9]. Such agents would be particularly appropriate for targeting solid tumors, where immunogenicity is a major obstacle to successful therapy with recombinant immunotoxins.
Acknowledgement
This work was supported in part by the National Cancer Institute, Intramural Program, and MedImmune, LLC.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med 2007;58:221–237. [DOI] [PubMed] [Google Scholar]
- 2.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 2001;345: 241–247. [DOI] [PubMed] [Google Scholar]
- 3.Kreitman RJ, Squires DR, Stetler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol 2005;23: 6719–6729. [DOI] [PubMed] [Google Scholar]
- 4.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144–5149. [DOI] [PubMed] [Google Scholar]
- 5.Kreitman RJ, Hassan R, FitzGerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009;15:5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onda M, Nagata S, Fitzgerald DJ, et al. Characterization of the B cell epitopes associated with a truncated form of pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol 2006;177:8822–8834. [DOI] [PubMed] [Google Scholar]
- 7.Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA 2008;105:11311–11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weldon JE, Xiang L, Chertov O, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood 2009;113: 3792–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen JK, Weldon JE, Xiang L, Beers R, Onda M, Pastan I. A recombinant immunotoxin targeting CD22 with low immunogenicity, low nonspecific toxicity, and high antitumor activity in mice. J Immunother 2010;33:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


