Abstract
Background: The TROFEO trial demonstrated that febuxostat causes greater and more rapid reduction of serum uric acid (s-UA) than topiroxostat. We compared these drugs in patients with chronic kidney disease (CKD) by sub-analysis of the TROFEO trial.
Methods: This sub-analysis targeted patients with an estimated glomerular filtration rate (eGFR) ≤60 mL/min/1.73 m2. The primary endpoint was the s-UA level. Secondary endpoints included creatinine, eGFR, urinary albumin, cystatin-C, oxidized low-density lipoprotein (Ox-LDL), eicosapentaenoic acid/arachidonic acid ratio, lipid biomarkers, high-sensitivity C-reactive protein, and B-type natriuretic peptide (BNP).
Results: There was no significant difference of s-UA between the two groups either before or after treatment. However, s-UA did not exceed 6.0 mg/dL in febuxostat group during the study period, but it exceeded this level in seven patients from topiroxostat group, with the number being significantly higher in topiroxostat group. Serum creatinine (s-Cr) and eGFR were significantly better after 6 months of febuxostat treatment compared with topiroxostat Cystatin-C was significantly lower after 6 months of febuxostat treatment compared with topiroxostat. The Ox-LDL was significantly lower after 3 and 6 months of febuxostat treatment compared with topiroxostat.
Conclusion: Febuxostat had stronger renoprotective and antioxidant effects than topiroxostat in patients with hyperuricemia and CKD.
Keywords: hyperuricemia, febuxostat, topiroxostat, xanthine oxidase, gout
Introduction
Hyperuricemia is associated with increased risk of new-onset kidney disease and progression to chronic kidney disease (CKD).1–3) Xanthine oxidase reductase inhibitors (XOR-I) are used for the treatment of hyperuricemia worldwide. Recently, the effectiveness of novel XOR-I, febuxostat and topiroxostat, was reported.4–6) We previously reported that febuxostat reduced the serum uric acid (s-UA) level more rapidly than allopurinol, had a stronger renoprotective effect, and also showed superior antioxidant and anti-inflammatory effects in a head-to-head comparison of allopurinol and febuxostat (NU-FLASH trial).7,8) We have also performed a comparative study of febuxostat and topiroxostat (TROFEO trial). TROFEO trial was conducted in 55 patients complicated with hyperuricemia who underwent cardiovascular surgery, and a prospective cross-over trial (study ID: UMIN000014771). The primary endpoint was the s-UA level. The secondary endpoints were as follows: serum creatinine (s-Cr), estimated glomerular filtration rate (eGFR), urinary albumin, cystatin-C, oxidized low density lipoprotein (O-LDL), eicosapentaenoic acid/arachidonic acid (EPA/AA) ratio, total cholesterol (T-cho), triglycerides (TGs), LDL, high-density lipoprotein (HDL), remnant-like particles-cholesterol (RLP-cho), high-sensitivity C-reactive protein (hs-CRP), B-type natriuretic peptide (BNP), and adverse reactions. The study result demonstrated that febuxostat caused greater and more rapid reduction of s-UA than topiroxostat, as well as an antioxidant effect superior to topiroxostat.9) However, that study did not compare the two drugs in patients with CKD, so we performed a sub-analysis of CKD patients from the TROFEO trial in the present study (TROFEO CKD trial). All patients in TROFEO trial were those who underwent cardiovascular surgery. Cardiovascular surgery patients are more likely to be a high risk because of complications including CKD. This study was conducted in patients at a high risk.
Methods
Study protocol
In this trial (TROFEO CKD trial), sub-analysis was performed of the patients with an eGFR ≤60 mL/min/1.73 m2 among subjects of the TROFEO trial. The TRFEO trial was outpatients with cardiovascular disease and hyperuricemia in whom s-UA was controlled at 6 mg/dL or lower by treatment with allopurinol or febuxostat. In TROFEO trial, patients were randomized by the envelop method to receive treatment with either febuxostat (Teijin Pharma Ltd., Tokyo, Japan) or topiroxostat (Sanwa Kagaku Kenkyusho Co., Ltd., Aichi, Japan and Fujiyakuhin Co., Ltd., Saitama, Japan) for 6 months, after which they switched to the other medication for another 6 months. Baseline data were obtained prior to switching to febuxostat or topiroxostat and monitoring was continued for 6 months after switching. The details of the study were explained to the patients and informed consent was obtained. Approval of our institutional review board was also obtained and the study was registered with our Hospital Medical Information Network (study ID: UMIN000014771) 9).
Endpoints: The primary endpoint was the s-UA level after treatment. The secondary endpoints were as follows: s-Cr, eGFR, urinary albumin, cystatin-C, Ox-LDL, EPA/AA ratio, T-cho, TG, LDL, HDL, RLP-cho, hs-CRP, BNP, and adverse reactions. s-UA, s-Cr, eGFR, T-cho, TG, LDL, HDL, and LDL/HDL (L/H) were measured before the start of treatment as well as after every month of treatment, whereas urinary albumin, cystatin-C, Ox-LDL, the EPA/AA ratio, and BNP were measured before treatment and after 3 and 6 months of treatment 9).
Statistical analysis
Measured values were expressed as the mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was used to compare parameters between the febuxostat and topiroxostat groups and a p value of less than 0.05 was considered statistically significant.
Results
Patients
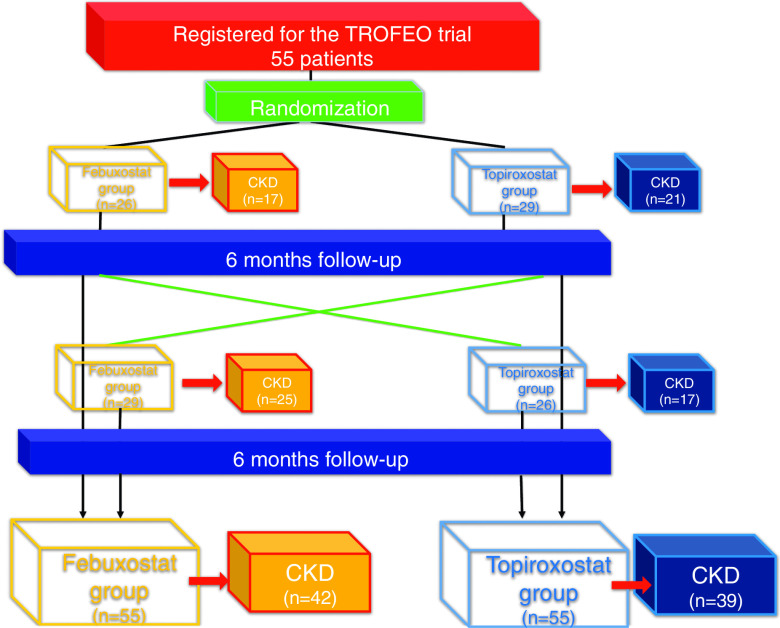
In all, 55 patients were enrolled in TROFEO trial and 42 patients in febuxostat group and 39 patients in topiroxostat group were sub-analyzed in TROFEO for CKD trial (Fig. 1 and Table 1).
Fig. 1. Study population.
Table 1. Patient characteristics.
| Febuxostat | Toxpiroxostat | |
|---|---|---|
| Number | 42 | 39 |
| Age (years) | 70.0 ± 8.0 | 70.8 ± 7.3 |
| Gender (male: female) | 31:11 | 28:11 |
| Basic disease | ||
| Ischemic heart disease | 17 (41%) | 17 (44%) |
| Valvular disease | 15 (36%) | 12 (31%) |
| Aortic disease | 9 (21%) | 9 (23%) |
| Others | 1 (2%) | 1 (2%) |
| Risk factors | ||
| Diabetes mellitus | 15 (36%) | 15 (38%) |
| Hypertension | 35 (83%) | 32 (82%) |
| Dyslipidemia | 29 (69%) | 26 (67%) |
| Cerebrovascular disease | 3 (7%) | 3 (8%) |
| Obesity | 5 (12%) | 5 (13%) |
| Smoking | 13 (31%) | 13 (33%) |
| CKD stage | ||
| G3a | 11 | 15 |
| G3b | 22 | 19 |
| G4 | 9 | 5 |
| Medication | ||
| ARB | 24 (57%) | 21 (54%) |
| ACE inhibitor | 4 (10%) | 4 (10%) |
| Renin inhibitor | 4 (10%) | 4 (10%) |
| Aldosterone blocker | 23 (55%) | 20 (51%) |
| Calcium antagonist | 17 (41%) | 17 (44%) |
| Beta-blocker | 30 (71%) | 28 (72%) |
| Statin | 29 (69%) | 26 (67%) |
| Furosemide | 21 (50%) | 21 (54%) |
| Febuxostat | 16 (38%) | 38 (97%) |
| Toxpiroxostat | 25 (60%) | 0 (0%) |
| Allopurinol | 1 (2%) | 1 (3%) |
ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker; CKD: chronic kidney disease
Primary endpoint
There was no significant difference of s-UA between the two groups either before or after treatment (Fig. 2). However, serum UA did not exceed 6.0 mg/dL in the febuxostat group during the study period, but it exceeded this level in seven patients from the topiroxostat group, with the number being significantly higher in the topiroxostat group (p = 0.004). The dose of febuxostat was 17.3 mg ± 10.5 mg at the initiation of treatment. No patient required dose escalation of febuxostat and the same dose was maintained for 6 months. The dose of topiroxostat was 63.1 ± 37.4 mg at initiation of treatment, while it was 66.1 ± 37.2 mg, 67.2 ± 39.1 mg, 70.3 ± 40.5 mg, 72.3 ± 40.9 mg, 74.4 ± 43.3 mg, and 74.4 ± 43.3 mg after 1, 2, 3, 4, 5, and 6 months, respectively.
Fig. 2. Change of serum uric acid and oxidized LDL.
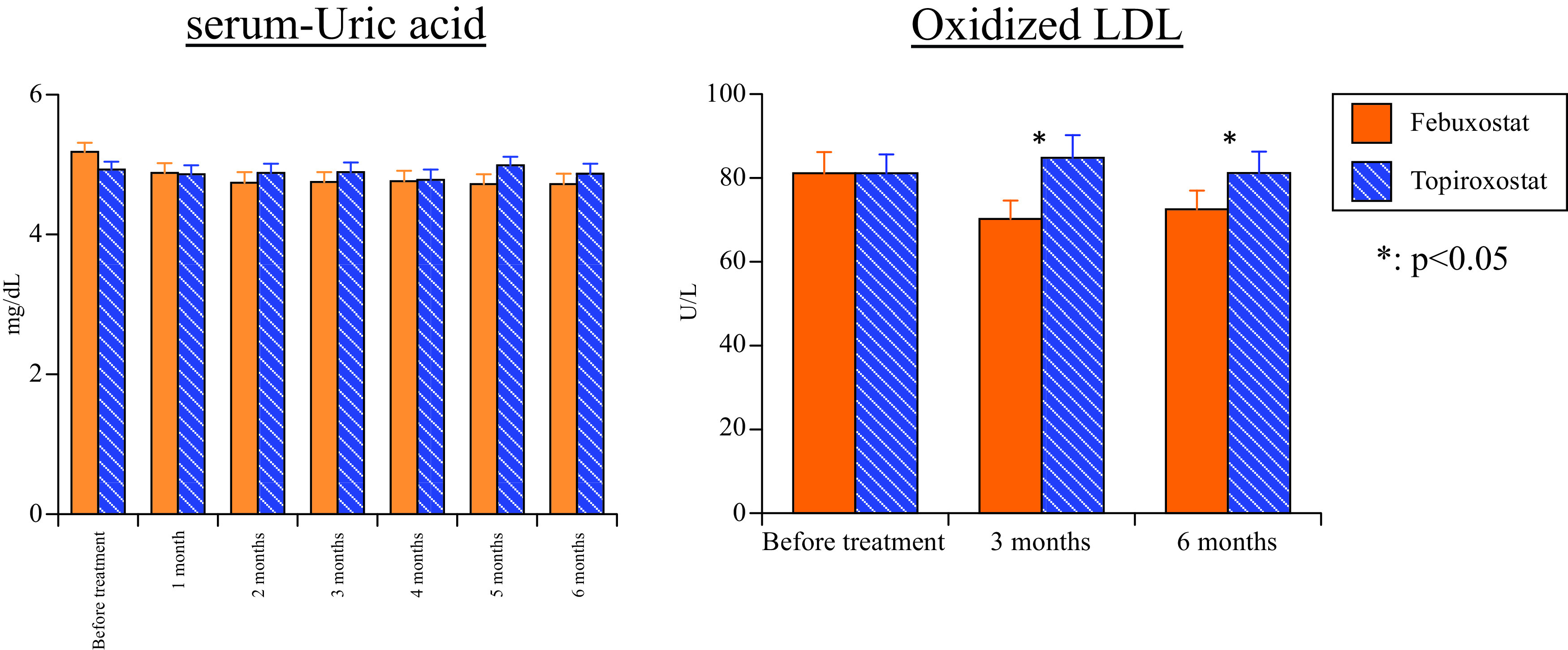
LDL: low-density lipoprotein
Secondary endpoints
Markers related to renal functions (s-Cr, eGFR, urinary albumin, and cystatin C). Although there was no significant difference of s-Cr and eGFR between the two drugs before treatment, s-Cr was significantly lower after 6 months of febuxostat treatment compared with topiroxostat (p = 0.043) and eGFR was significantly higher after 6 months of febuxostat treatment compared with topiroxostat (p = 0.041) (Fig. 3). There was no significant difference of urinary albumin between the two groups either before or after treatment (3 months: p = 0.354, 6 months: p = 0.313) (Fig. 3). Although there was no difference of cystatin-C before treatment with the two drugs, it was significantly lower after 6 months of febuxostat treatment compared with topiroxostat (p = 0.011) (Fig. 3).
Fig. 3. Changes of serum creatinine, eGFR, cystatin C, and urinary albumin.
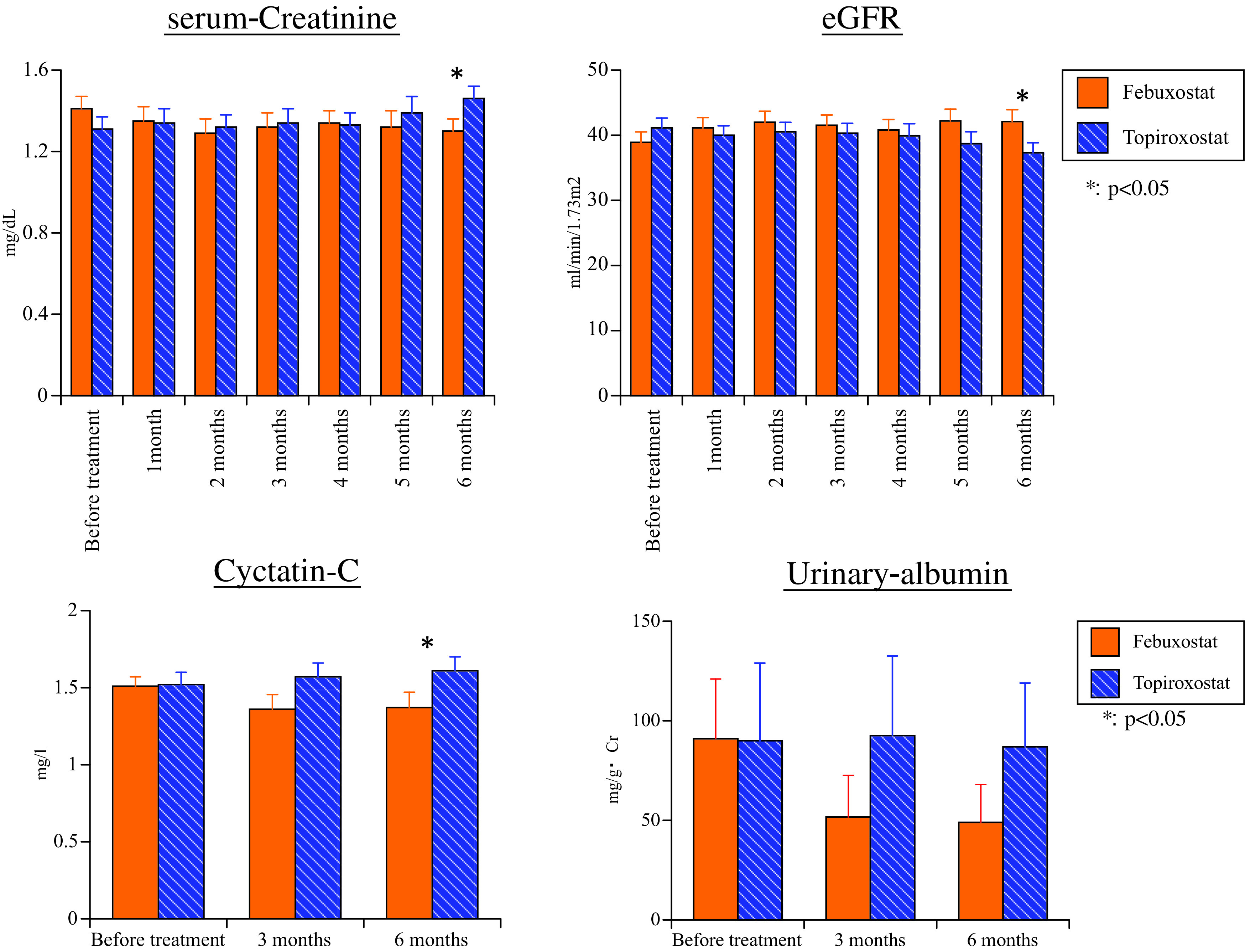
eGFR: estimated glomerular filtration rate
Oxidative stress marker (Ox-LDL): There was no difference of Ox-LDL prior to treatment with the two drugs. Ox-LDL was significantly lower after 3 and 6 months of febuxostat treatment compared with topiroxostat (3 months: p = 0.038, 6 months: p = 0.048) (Fig. 2).
Markers related to lipids and fatty acids (T-cho, TG, LDL, HDL, L/H, RLP-cho, and EPA/AA ratio) (Table 2): There were no significant differences of T-cho, TG, LDL, HDL, and L/H between the two drugs either before or after treatment. There was no significant difference of RLP-cho between the two drugs either before or after treatment. There was no significant difference of EPA/AA ratio between the two drugs either before or after treatment.
Table 2. Changes of each blood test.
| T-cho (mg/dL) | Pre | 1M | 2M | 3M | 4M | 5M | 6M |
|---|---|---|---|---|---|---|---|
| Febuxostat | 159.3 ± 4.7 | 160.5 ± 4.4 | 167.6 ± 4.1 | 165.4 ± 5.5 | 167.9 ± 5.0 | 164.4 ± 4.8 | 159.5 ± 4.5 |
| Topiroxostat | 166.3 ± 5.2 | 163.6 ± 5.2 | 164.0 ± 5.1 | 164.6 ± 4.6 | 167.2 ± 5.0 | 163.1 ± 4.9 | 162.6 ± 5.4 |
| TG (mg/dL) | Pre | 1M | 2M | 3M | 4M | 5M | 6M |
| Febuxostat | 118.8 ± 8.5 | 114.8 ± 7.5 | 116.8 ± 8.3 | 119.0 ± 8.8 | 114.4 ± 6.8 | 117.0 ± 8.0 | 105.0 ± 7.4 |
| Topiroxostat | 135.7 ± 13.3 | 139.2 ± 14.2 | 121.0 ± 9.3 | 139.6 ± 12.7 | 119.4 ± 8.0 | 111.3 ± 7.8 | 122.9 ± 9.1 |
| L/H | Pre | 1M | 2M | 3M | 4M | 5M | 6M |
| Febuxostat | 1.67 ± 0.10 | 1.64 ± 0.10 | 1.64 ± 0.10 | 1.61 ± 0.10 | 1.63 ± 0.10 | 1.61 ± 0.10 | 1.60 ± 0.10 |
| Topiroxostat | 1.74 ± 0.11 | 1.71 ± 0.10 | 1.71 ± 0.10 | 1.75 ± 0.12 | 1.74 ± 0.13 | 1.63 ± 0.11 | 1.66 ± 0.10 |
| RLP-cho(mg/dL) | Pre | 3M | 6M | ||||
| Febuxostat | 4.97 ± 0.58 | – | – | 4.99 ± 0.59 | – | – | 4.14 ± 0.50 |
| Topiroxostat | 4.87 ± 0.59 | – | – | 5.21 ± 0.59 | – | – | 4.57 ± 0.46 |
| EPA/AA | Pre | 3M | 6M | ||||
| Febuxostat | 0.53 ± 0.06 | – | – | 0.60 ± 0.07 | – | – | 0.61 ± 0.07 |
| Topiroxostat | 0.63 ± 0.06 | – | – | 0.62 ± 0.07 | – | – | 0.58 ± 0.06 |
| BNP (pg/mL) | Pre | 3M | 6M | ||||
| Febuxostat | 124.1 ± 29.3 | – | – | 108.4 ± 26.5 | – | – | 110.6 ± 29.9 |
| Topiroxostat | 116.6 ± 0.04 | – | – | 125.3 ± 31.7 | – | – | 149.8 ± 36.4 |
| hs-CRP (mg/dL) | Pre | 1M | 2M | 3M | 4M | 5M | 6M |
| Febuxostat | 0.21 ± 0.06 | 0.38 ± 0.19 | 0.23 ± 0.04 | 0.19 ± 0.03 | 0.28 ± 0.08 | 0.32 ± 0.07 | 0.23 ± 0.05 |
| Topiroxostat | 0.19 ± 0.03 | 0.23 ± 0.05 | 0.33 ± 0.09 | 0.30 ± 0.09 | 0.24 ± 0.07 | 0.41 ± 0.17 | 0.31 ± 0.07 |
BNP: B-type natriuretic peptide; hs-CRP: high-sensitivity C-reactive protein; T-cho: total cholesterol; TG: triglyceride; L/H: high-density lipoprotein/low-density lipoprotein; RLP-cho: remnant-like particles-cholesterol; EPA/AA: eicosapentaenoic acid/arachidonic acid
Inflammatory marker (hs-CRP): There was no significant difference of hs-CRP between the two drugs either before or after treatment (Table 2).
BNP
There was no significant difference of BNP between the two drugs either before or after treatment (Table 2).
Discussion
This study demonstrated that febuxostat has a stronger renoprotective effect and stronger antioxidant effect than topiroxostat base on data for s-Cr, eGFR, cystatin-C, and Ox-LDL. A renoprotective effect of XOR-I, such as an allopurinol, febuxostat, and topiroxostat, has already been reported in CKD patients. Pisano et al. performed a meta-analysis and concluded that XOR-I may represent a promising tool for delaying the progression of CKD 6). Shibagaki et al. showed that treatment with febuxostat increased the eGFR and decreased proteinuria in patients with stage 3b-5 CKD.10) Whelton et al. followed patients using febuxostat for 5 years and showed that the improvement of eGFR was inversely correlated with the reduction of s-UA from baseline; their model projected an improvement of eGFR by 1 mL/min from baseline for every 1 mg/dL decrease in s-UA.4) Cystatin-C is a sensitive biomarker of renal function that is not influenced by food, muscle mass, or exercise.11) Although there have been no investigations of s-UA levels in CKD patients with hyperuricemia after treatment with XOR-I other than our study, febuxostat seems to be a promising drug for long-term use based on the present results, since s-UA was decreased significantly more by febuxostat than topiroxostat after 6 months of treatment and the data for s-Cr and eGFR were also better. Urinary albumin showed no significant difference between the two groups, although it tended to be lower with febuxostat. In a study of hyperuricemic patients with stage 3 CKD who were on treatment with allopurinol, one group of patients was switched to febuxostat and the other group remained on allopurinol. While s-UA was reduced significantly in the febuxostat group after 3 months of treatment, there was no significant difference of s-UA between the two groups.12) In an animal model of cisplatin-induced acute renal injury, febuxostat dose-dependently lowered urinary protein and oxidative stress parameters.13) Hosoya et al. reported that urinary albumin is significantly reduced by topiroxostat compared with untreated patients.5) In addition, a prospective study of topiroxostat in patients with diabetic nephropathy (ETUDE study) demonstrated that urinary albumin was significantly reduced in the high-dose topiroxostat (160 mg daily) group compared with the low-dose topiroxostat group (40 mg daily).14) Furthermore, Nakamura et al. demonstrated dose-dependent reduction of urinary albumin and plasma XOR by topiroxostat, while febuxostat did not show dose-dependent effects in a study comparing febuxostat with topiroxostat in mice.15) In a clinical study, 13 CKD patients switched from febuxostat to topiroxostat and the urinary protein level decreased significantly after switching.16) Since there has been no other clinical study comparing febuxostat and topiroxostat directly apart from ours, it is unclear which drug has a stronger renoprotective effect, although the renoprotective effect of XOR-I has been demonstrated by a large number of reports.
Jalal et al. reported that a decrease in GFR due to arteriosclerosis caused by endothelial injury, inflammation, oxidative stress, and renin–angiotensin system hyperactivity is important for the association between hyperuricemia and onset/progression of CKD.17) In the present study, Ox-LDL was measured as an index for antioxidant effect. There are other index for antioxidant effect measured in clinical research include, in addition to Ox-LDL, such as 8-hydroxy-2′-deoxyguanosine, lipid peroxide, superoxide dismutase, and nitric oxide. Ox-LDL is involved in the formulation and progression of arteriosclerosis and is considered as an influential oxidative stress marker. It is detected at arteriosclerosis lesions together with various lipid oxidation products. It is covered by health insurance in Japan and we selected Ox-LDL as one useful in clinical practice. The study result demonstrated that Ox-LDL was significantly reduced in the febuxostat group compared with the topiroxostat group after 3 months of treatment, suggesting that inhibition of oxidative stress by febuxostat may have had a positive impact on renal function after 6 months.
XOR activity was reported to have a negative impact on the vascular endothelium and the cardiovascular system, while XOR-I have a uric acid lowering effect as well as cardioprotective, renoprotective, and hypotensive effects, along with inhibition of vascular endothelial injury and oxidative stress.18–21) There have been reports that febuxostat and topiroxostat are more active than allopurinol with respect to these effects,22,23) raising the possibility that both drugs may have uses beyond the treatment of gout and hyperuricemia.
All patients in this study were those who underwent cardiovascular surgery and are at a high risk because of complications with CKD and elderly as well as diabetes mellitus (DM), hypertension, or dyslipidemia. This study was conducted in high-risk patients and the study result indicated that for high-risk patients, the use of XOR-I could improve renal functions and oxidative stress as well as uric acid control. Thus, it is considered to improve long-term prognosis.
Conclusion
TRFEO trial demonstrated that febuxostat, compared to topiroxostat, decreased s-UA more rapidly and shown anti-oxidative effect and anti-inflammatory effect. However, there was no difference in renal functions. Compared to TRFEO trial, the patient cohort in this study was limited to CKD patients. Similar to TRFEO trial, reduction of s-UA and anti-oxidative effect were more pronounced in febuxostat than topiroxostat. Moreover, based on the result of s-Cr, eGFR, and cystatin-C, febuxostat was demonstrated to be more potent in renoprotective effects than topiroxostat.
Limitations
Although the effects of treatment should ideally have been investigated in patients classified by CKD stage, the number of patients per stage was too small in the present study. We hope to investigate a larger number of patients stratified by CKD stage in the future. This study selected Ox-LDL as a marker for oxidative stress. 8-hydroxy- 2′-deoxyguanosine, lipid peroxide, superoxide dismutase, and nitric oxide might have provided more robust assessment of oxidative stress. These parameters will be investigated in the future.
Financial Support
Akira Sezai received funding from Daiichi Sankyo Co., Ltd. and Teijin Co., Ltd. and this study was also supported by a Research Grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 21591805) and Nihon University School of Medicine Ozawa Fund Research Grant (2017). Akira Sezai has received lecture fees from Daiichi Sankyo Co., Ltd. The other authors have no conflict of interest associated with this study.
Clinical Trial Registration
UMIN (http://www.umin.ac.jp/), Study ID: UMIN 000014771.
Disclosure Statement
Akira Sezai has received lecture fees from Daiichi Sankyo Co., Ltd. The other authors have no conflicts of interest associated with this study.
References
- 1).Rincon-Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol 2017; 46: 315–22. [DOI] [PubMed] [Google Scholar]
- 2).Li L, Yang C, Zhao Y, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 2014; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Takae K, Nagata M, Hata J, et al. Serum uric acid as a risk factor for chronic kidney disease in a Japanese community- The Hisayama study. Circ J 2016; 80: 1857–62. [DOI] [PubMed] [Google Scholar]
- 4).Whelton A, Macdonald PA, Zhao L, et al. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol 2011; 17: 7–13. [DOI] [PubMed] [Google Scholar]
- 5).Hosoya T, Ohno I, Nomura S, et al. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol 2014; 18: 876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Pisanoo A, Cermaro V, Gembillo G, et al. Xanthine oxidase inhibitors for improving renal function in chronic kidney disease patients: an updated systematic review and meta-analysis. Int J Mol Sci 2017; 18: 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J 2013; 77: 2043–9. [DOI] [PubMed] [Google Scholar]
- 8).Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients with chronic kidney disease (NU-FLASH trial for CKD). J Cardiol 2015; 66: 298–303. [DOI] [PubMed] [Google Scholar]
- 9).Sezai A, Obata K, Abe K, et al. Cross-over trial of febuxostat and topiroxostat for hyperuricemia with cardiovascular disease (TROFEO trial). Circ J 2017; 81: 1707–12. [DOI] [PubMed] [Google Scholar]
- 10).Shibagaki Y, Ohno I, Hosoya T, et al. Safety, efficacy and renal effect of febuxostat in patients with moderate- to-severe kidney dysfunction. Hypertens Res 2014; 37: 919–25. [DOI] [PubMed] [Google Scholar]
- 11).Tanaka M, Matsuo K, Enomoto M, et al. A sol particle homogeneous immunoassay for measuring serum cystatin C. Clin Biochem 2004; 37: 27–35. [DOI] [PubMed] [Google Scholar]
- 12).Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol 2015; 19: 1044–53. [DOI] [PubMed] [Google Scholar]
- 13).Fahmi AN, Shehatou GS, Shebl AM, et al. Febuxostat exerts dose-dependent renoprotection in rats with cisplatin- induced acute renal injury. Naunyn Schniedebergs Arch Pharmacol 2016; 389: 819–30. [DOI] [PubMed] [Google Scholar]
- 14).Kato S, Ando M, Mizukoshi T, et al. Randomized control trial for the assessment of the anti-albuminuric effects of topiroxostat in hyperuricemic patients with diabetic nephropathy (the ETUDE study). Nagoya J Med Sci 2016; 78: 135–42. [PMC free article] [PubMed] [Google Scholar]
- 15).Nakamura T, Murase T, Nampei M, et al. Effects of topiroxostat and febuxostat on urinary albumin excretion and plasma xanthine oxidoreductase activity in db/db mice. Eur J Phamacol 2016; 780: 224–31. [DOI] [PubMed] [Google Scholar]
- 16).Terawaki H, Hooshi H, Kazama JJ. Effect of switching xanthine oxidoreductase inhibitor from febuxostat to topiroxostat on urinary protein excretion. Clin Exp Nephrol 2017;. 21: 356–7. [DOI] [PubMed] [Google Scholar]
- 17).Jalal DI, Chonchol M, Chen W, et al. Uric acid as a target of therapy in CKD. Am J Kidney Dis 2013; 61: 134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 2015; 131: 1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Bove M, Cicero AFG, Borghi C. The effect of xanthine oxidase inhibitors on blood pressure and renal function. Curr Hypertens Rep 2017; 19: 95. [DOI] [PubMed] [Google Scholar]
- 20).Fujimura Y, Yamauchi Y, Murase T, et al. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS ONE 2017; 12: e0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Xu X, Hu J, Song N, et al. Hyperuricemia increases the risk of acute kidney injury: a systematic review and meta-analysis. BMC Nephrol 2017; 18: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Borghi C, Perez-Ruiz F. Urate lowering therapies in the treatment of gout: a systematic review and meta- analysis. Eur Rev Med Pharmacol Sci 2016; 20: 983–92. [PubMed] [Google Scholar]
- 23).Zhang T, Pope JE. Cardiovascular effects of urate- lowering therapies in patients with chronic gout: a systematic review and meta-analysis. Rheumatology (Oxford) 2017; 56: 1144–53. [DOI] [PubMed] [Google Scholar]