Abstract
Single concentrate feeds are mixed together forming compound feeds for cattle. However, knowledge regarding the potential interactions (associative effects) between the feeding values of single feeds in compound feeds is lacking. The main objective of the present study was to evaluate ruminal fermentation characteristics and feeding values of eight industrially produced compound feeds in mash form from their constituent single feeds for dairy cows through in vitro assays. Additivity was given for gas production (GP), digestibility of organic matter (dOM) and utilisable CP at the duodenum (uCP). Additivity of CP fractions (determined using the Cornell Net Carbohydrate and Protein System (CNCPS)) was dependent on the fraction and compound feed type; however, the effective degradation calculated from CP fractions (EDCNCPS) showed additivity. Additivity was not given for intestinal digestibility of rumen-undegraded protein (IDRUP) for five out of eight compound feeds. Precise calculation of metabolisable energy (ME) of compound feeds from ME of single feeds was possible when using the same ME equations for all single and compound feeds. Compound feeds are often provided in pellet form; therefore, our second objective was to evaluate the effects of pelleting on ruminal fermentation characteristics and feeding values of compound feeds. Pelleting affected GP at 24 h (GP24; up to 2.4 ml/200 mg DM), dOM (up to 2.3 percentage point (pp)) and ME (up to 0.3 MJ/kg DM), but these differences were overall small. More considerable effects of pelleting were observed for uCP, which was increased in all compound feeds except the two with the highest CP concentrations. The IDRUP was lower in most compound feeds following pelleting (up to 15 pp). Pelleting also affected CP fractions in a non-systematic way. Overall, the effects of pelleting were not considerable, which could be because pelleting conditions were mild. Our third objective was to compare in situ ruminal CP degradation (EDIN_SITU) of compound feeds with ED using two prediction methods based on CP fractions. EDIN_SITU reference data were obtained from a companion study using the same feeds. Prediction accuracy of EDIN_SITU and EDCNCPS was variable and depended on the compound feed and prediction method. However, future studies are needed as to date not enough data are published to draw overall conclusions for the prediction of EDIN_SITU from CP fractions.
Keywords: additivity, associative effects, in situ prediction, mixed feed, interaction
Implications
Compound feeds are often fed to high-yielding dairy cows, both in mash and pellet form. Estimation of ruminal fermentation characteristics and feeding value of compound feeds from the single feeds contained therein is necessary for efficient feeding; therefore, this was assessed in the present study. Pelleting of compound feeds had only a negligible effect on ruminal fermentation characteristics and feeding values. Predictions of ruminal protein degradation based on CP fractions of the feed were not reliable.
Introduction
Intensive dairy cow farming is reliant on adequate feeding to satisfy the increasing nutritive requirements of cows due to increasing milk yield. Concentrate compound feeds are often included in diets of dairy cows and are either provided with forages in the form of total mixed rations or separately. The additivity of feeding values of single feeds used in compound feeds is commonly assumed based on the presumption that no interactions between single feeds exist.
In vitro methods are widely used for feed evaluation because in vivo evaluations are expensive and laborious, and they require animals (GfE, 2017). To estimate the digestibility of organic matter (dOM) and metabolisable energy (ME), measuring gas production (GP) by the Hohenheim gas test (HGT), as described by Menke and Steingass (1988), is an established assay. An extension of this method known as extended HGT (eHGT; Steingaß and Südekum, 2013) can be used to estimate the utilisable CP at the duodenum (uCP), which is the basis for the calculation of metabolisable protein used in the German protein evaluation system for cows (GfE, 2001). Calsamiglia and Stern (1995) developed a three-step method for estimating the intestinal digestibility of rumen-undegraded protein (ID RUP). These in vitro methods involve the use of ruminally fistulated animals as donors of rumen fluid. Sniffen et al. (1992) described a rapid CP fractionation method to be part of the Cornell Net Carbohydrate and Protein System (CNCPS). Therein, the CP in a feedstuff is separated into fractions by measuring N solubility. In an experiment by Chrenková et al. (2014), CP fractions were correlated with ruminal effective CP degradation (ED) values determined in situ. The CP fractions can be used to estimate ED values, which were found to correlate well with the ED values determined in situ (Shannak et al., 2000). Additivity of feeding values of forages or mixes of forages and concentrates has been investigated utilising GP (Sandoval-Castro et al., 2002; Robinson et al., 2009; Niderkorn et al., 2011) and uCP (Zhao et al., 2005). However, to our knowledge, there has been no research on the additivity of IDRUP. Also, comprehensive data on additivity of multiple single feeds in a compound feed are not available.
Compound feeds for cattle are often in pellet form, and pelleting can increase the availability of CP and starch (ST) or increase indigestible bonds, depending on the intensity of the pelleting process (Svihus and Zimonja, 2011). In compound feeds, these effects can depend on the choice of single feeds, and hence, they should be examined over a wide range of various compound feeds. The objective of the present study was to characterise GP and the related values of dOM and ME as well as uCP, IDRUP and CP fractions of single feeds and the compound feeds produced with them, both in mash and pellet form. Three hypotheses were developed:
-
(I)
Values of GP, dOM, ME, uCP, IDRUP and CP fractions of compound feeds in mash form can be calculated from data obtained for single feeds;
-
(II)
Pelleting significantly affects GP, dOM, ME, uCP, IDRUP and CP fractions of compound feeds;
-
(III)
Ruminal effective degradability of CP determined in situ can be predicted from CP fractions.
Material and methods
Samples of single and compound feeds
Eight compound feeds with different target CP concentrations (16%, 18%, 20%, 22%, 24%, 26%, 28% and 30% CP in DM) were mixed using 12 single feeds: maize, wheat, barley, soya beans, soya bean meal, rapeseed meal, sunflower meal, faba beans, dried distillers’ grains with solubles (DDGS), maize gluten, wheat bran and sugar beet pulp. Between five and seven single feeds were included in each compound feed in different concentrations. Compound feeds were produced in mash and pellet form using standard industrial processes in the feed mill of RKW-Kehl (Kehl, Germany). Production and analysed nutrient concentrations and particle size distribution of all feeds were detailed previously (Grubješić et al., 2019). Targeted CP concentrations were achieved in all compound feeds. Crude protein, ash, ether extract (EE), NDF assayed with a heat stable amylase and expressed exclusive of residual ash (aNDFom) and ADF expressed exclusive of residual ash (ADFom) did not differ more than one percentage point (pp) between calculated concentrations from single feeds and analysed concentrations in mash compound feeds.
Gas production kinetics, metabolisable energy and digestibility of organic matter
In vitro GP kinetics were measured using HGT following the procedure described by Seifried et al. (2016). Approximately 200 ± 5 mg of feed ground through a 1-mm sieve was transferred into graded glass syringes (100 ml volume). Fresh rumen fluid was obtained from two rumen-fistulated Jersey cows, one lactating and one not lactating. The lactating cow was provided a ration consisting of (on DM basis) 41.3% concentrate mix, 20.0% maize silage, 16.3% meadow hay, 15.0% grass silage, 3.6% barley straw, 2.4% mineral mix and 1.4% rapeseed meal. The other cow was provided a ration consisting of 35.4% maize silage, 35.4% grass silage, 24.6% meadow hay, 3.2% barley straw, 1.0% mineral mix and 0.4% urea. Cows had ad libitum access to feed.
The rumen fluid obtained from the two cows was mixed to a 1 : 1 ratio, filtered through two layers of cheesecloth, and a reduced buffer solution was added. Syringes were pre-warmed to 39°C before 30 ml of buffer-rumen fluid mix was poured into each syringe under constant CO2 flow. Each feed was included in five separate HGT runs with two replicated syringes per feed in each run. Additionally, each run contained three syringes without feed samples (blanks) and three syringes with a concentrate standard feed. Cumulative GP was recorded after 2, 4, 6, 8, 12, 24, 48 and 72 h of incubation at 39°C under constant rotation. The following non-linear regression was fitted to the obtained GP data according to Seifried et al. (2016):
| (1) |
where bGP is the potential GP (ml/200 mg DM), cGP the rate of GP (%/h) and t the incubation time (h).
The dOM was calculated using GP at 24 h (GP 24) corrected for the blanks and standard (GP24; ml/200 mg DM) and chemical analysis according to Menke and Steingass (1988):
| (2) |
The ME was calculated using GP24 and specific to the type of feed, as follows:
-
(a)Maize, wheat, barley, faba beans, maize gluten and wheat bran according to Krieg et al. (2017):
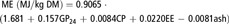
(3) -
(b)Non-cereal feeds (soya beans, soya bean meal, rapeseed meal, sunflower meal, DDGS and sugar beet pulp) according to Menke and Steingass (1988):
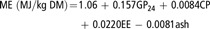
(4) -
(c)Compound feeds according to GfE (2009):

(5)
In equations (2) to (5), CP, ST, EE, ash and ADFom are expressed in g/kg DM.
Utilisable CP at the duodenum
The eHGT method described by Steingaß and Südekum (2013) was used to estimate uCP and was conducted according to Westreicher-Kristen et al. (2015). Some former studies using this approach used the term ‘modified HGT’ and the abbreviation ‘modHGT’. However, the term ‘extended HGT’ and the abbreviation ‘eHGT’ may be more appropriate as this method is not a real modification of the original HGT but an extension (measuring NH3-N after incubation) and can be connected with GP24 measurement to estimate dOM and ME. Samples were incubated similarly to those in the HGT method described above. Donor cows had ad libitum access to a ration consisting of (on DM basis) 25.8% concentrate mix, 24.3% grass silage, 24.3% maize silage, 17.0% hay, 4.4% rapeseed meal, 2.2% barley straw and 2.0% mineral mix. Samples were incubated twice for different times (8 and 24 h), and a standard concentrate sample with known uCP concentration was included to check the variation of uCP results among runs. Each feed sample was incubated in five separate runs per incubation time. Following incubation, all syringes were rapidly frozen to minimise microbial fermentation. The following day, the NH3-N concentration of incubation residues obtained from the syringes was analysed (Vapodest 50; C. Gerhardt GmbH & Co. KG, Königswinter, Germany). The NH3-N concentration was used to estimate the uCP concentration as follows:
| (6) |
where Nsample is the amount of N from the feed sample (mg), NH3-Nsample and NH3-Nblank are the NH3-N content of feed samples and blank incubation residues (mg) and weight is the weight of feed sample inserted into the glass syringe (mg DM). Effective uCP was estimated for theoretical ruminal passage rates (k) of 5 and 8%/h by plotting uCP values (y) against the natural logarithm of the incubation time (x) in a linear regression model and calculating the function values of ln (20) and ln (12.5), respectively (Steingaß and Südekum, 2013).
Intestinal digestibility of rumen-undegraded protein
The three-step enzymatic method of Calsamiglia and Stern (1995) was used to determine IDRUP. Samples of single and compound feeds were ground through a 2-mm screen. The first step was a 16-h in situ incubation in the rumen, and this was conducted using three rumen-fistulated Jersey cows, following the procedure described in Seifried et al. (2016). A minimum of 60 mg of residual N per feed was accumulated for subsequent in vitro simulation of digestibility in the abomasum and duodenum. Two or three samples per feed containing 15 mg of residual N were incubated utilising 10 ml HCl (0.1 N, pH = 1.9), pepsin (1 g/l, Sigma P-7012; Sigma, St. Louis, MO, USA) and pancreatin solution (0.5 M KH2PO4 buffer standardised at pH 7.8 containing 50 ppm of thymol and 3 g/l of pancreatin, Sigma P-7545; Sigma). Trichloroacetic acid was added to stop enzymatic action and precipitate undigested proteins. Samples were centrifuged at 15 000 g for 25 min. Supernatants were analysed for soluble N by the Kjeldahl method (VDLUFA, 2007). Finally, IDRUP was calculated as follows:
| (7) |
where Nsoluble is the amount of soluble N determined in vitro (mg) and Nincubated is the total N that was incubated with pepsin and pancreatin (mg).
CP fractionation
Crude protein fractions were estimated according to the CNCPS (Sniffen et al., 1992): fraction A represented the non-protein N, fraction B the true protein and containing three sub-fractions (B1 to B3) differing in their rate of ruminal degradation and fraction C the acid detergent insoluble N. To calculate CP fractions, non-protein N, buffer-soluble protein, neutral detergent insoluble N and acid detergent insoluble N were determined according to Licitra et al. (1996) for all samples of single and compound feeds. Table values of ruminal degradation rates of CP fractions of single feeds (Fox et al., 2003) were used together with determined CP fractions to calculate EDCNCPS using equation (8) (Fox et al., 2003):
| (8) |
where EDCNCPS is ED calculated from CP fractions, A and B1 to B3 are determined CP fractions, Prot-B1 to Prot-B3 are table values for ruminal degradation rates of CP fractions and k is the ruminal passage rate (5 or 8%/h). The ruminal degradation rate of faba beans was not reported in Fox et al. (2003), and thus, the value for lupins was used instead as they both belong to the legume family and show similar ED values (Goelema et al, 1998). Table values of ruminal degradation rates of compound feeds were not available; therefore, they were calculated from values of the respective single feeds. This calculation included weighting contributions of CP of single feeds to the total CP of the respective compound feed. Ruminal degradation rates were then used together with determined CP fractions to estimate the observed EDCNCPS for mash and pelleted compound feeds.
An alternative prediction equation (Shannak et al., 2000) based on CP fractions and NDF was used to estimate RUP of compound feed as follows:
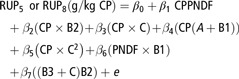 |
(9) |
where RUP5 or RUP8 are RUP values for rumen outflow rates of k = 5 and 8%/h, respectively. The CPPNDF is the CP concentration in PNDF (NDF determined by manual filtration on paper) and all nutrients are given as g/kg DM, whereas A, B and C fractions are given as g/kg CP. Instead of CPPNDF and PNDF, the CP concentration in aNDF as well as aNDF was determined in the present study by the conventional method (VDLUFA, 2007). The general form of the equation is identical for RUP5 and RUP8, and the parameter estimated of β0 to β7 is given in Shannak et al. (2000). The EDCNCPS was then calculated from RUP values for the given rumen outflow as:
| (10) |
The EDCNCPS values of compound feeds (calculated either with equation (8) or with equations (9) and (10)) were compared with measured EDIN_SITU values of a companion study that determined in situ degradation values of the same feeds used in the present study (Grubješić et al., 2019).
Additivity calculation
To evaluate the additivity of all traits of single feeds in a mash compound feed, the expected value of the compound feed was calculated based on weighted contribution of DM (for bGP, cGP, GP24, dOM, ME and uCP) or CP (for IDRUP and CP fractions) from single feeds to the DM and CP contained in the respective compound feed. These values are referred to as ‘calculated’ herein. To calculate the ME values of compound feeds from single feeds, two approaches were used. The ME values of single feeds were determined according to either equations (3) or (4) depending on the feed group or alternatively equation (5) for all single feeds.
Statistical analyses
Calculated and observed values of mash compound feeds, and values of mash and pellet compound feeds, were regressed using procedure REG (version 9.4 of SAS system for Windows SAS Institute, Cary, NC, USA). The REG procedure was also used to calculate if slopes and intercepts were significantly different from 1 and 0, respectively, by determination of 95% CI to detect possible associative effects.
Results
In vitro ruminal fermentation and feeding values of single feeds
Overall, ruminal fermentation characteristics and feeding values of single feeds varied widely (Table 1). The highest GP24 was found in maize (81 ml/200 mg DM) and the lowest in sunflower meal (36 ml/200 mg DM). The highest dOM was found in maize and wheat (96%) and the lowest in sunflower meal (65%). The highest ME was found in soya beans (16.0 MJ/kg DM) followed by maize (14.5 MJ/kg DM) and wheat (14.2 MJ/kg DM) and the lowest in sunflower meal (9.4 MJ/kg DM). The uCP concentration varied between 144 g/kg DM in wheat bran and 279 g/kg DM in soya bean meal for k = 5%/h, and between 158 g/kg DM in maize and wheat bran and 356 g/kg DM in soya bean meal for k = 8%/h. The IDRUP ranged between 18% in wheat bran and 83% in maize and soya bean meal. All CP fractions varied widely between single feeds. This was reflected in the high variability of EDCNCPS values.
Table 1.
In vitro fermentation characteristics and feeding value of single feeds
| Single feed | In vitro gas production | dOM | ME | uCP | IDRUP | CP fractions | EDCNCPS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bGP (ml/200 mg DM) | cGP (%/h) | GP24 (ml/200 mg DM) | (%) | (MJ/kg DM) | k = 5%/h (g/kg DM) | k = 8%/h (g/kg DM) | (%) | A (%) | B1 (%) | B2 (%) | B3 (%) | C (%) | k = 5%/h (%) | k = 8%/h (%) | |
| Maize | 92 | 5.7 | 81 | 96 | 14.5 | 163 | 158 | 83 | 12.5 | 5.9 | 66.6 | 14.9 | 0.0 | 65 | 55 |
| Wheat | 70 | 8.3 | 78 | 96 | 14.2 | 180 | 198 | 35 | 9.8 | 22.2 | 63.5 | 4.4 | 0.0 | 80 | 73 |
| Barley | 81 | 7.7 | 75 | 92 | 13.6 | 162 | 175 | 47 | 11.4 | 16.4 | 62.3 | 5.0 | 5.0 | 76 | 69 |
| Soya beans | 47 | 10.8 | 46 | 79 | 16.0 | 227 | 282 | 71 | 4.5 | 4.8 | 82.7 | 6.4 | 1.6 | 56 | 45 |
| Soya bean meal | 56 | 8.0 | 51 | 92 | 13.3 | 279 | 356 | 83 | 2.9 | 8.4 | 83.8 | 3.6 | 1.2 | 72 | 62 |
| Rapeseed meal | 50 | 8.5 | 45 | 78 | 11.3 | 241 | 280 | 26 | 7.1 | 13.4 | 62.5 | 10.2 | 6.8 | 73 | 65 |
| Sunflower meal | 39 | 8.9 | 36 | 65 | 9.4 | 166 | 205 | 46 | 8.3 | 27.3 | 54.6 | 5.9 | 3.9 | 76 | 69 |
| Faba beans | 70 | 8.3 | 63 | 90 | 12.9 | 202 | 224 | 32 | 12.7 | 42.3 | 38.7 | 4.2 | 2.1 | 80 | 75 |
| DDGS | 51 | 8.7 | 44 | 73 | 11.9 | 275 | 296 | 75 | 20.6 | 0.0 | 44.1 | 11.8 | 23.6 | 55 | 50 |
| Maize gluten | 62 | 6.8 | 54 | 78 | 11.0 | 188 | 196 | 59 | 48.5 | 6.2 | 33.8 | 8.6 | 2.9 | 79 | 75 |
| Wheat bran | 57 | 9.9 | 52 | 72 | 10.9 | 144 | 158 | 18 | 12.5 | 21.1 | 49.4 | 13.6 | 3.4 | 80 | 74 |
| Sugar beet pulp | 78 | 9.4 | 74 | 90 | 13.2 | 182 | 199 | 67 | 39.9 | 0.0 | 29.2 | 24.8 | 6.2 | 82 | 78 |
bGP = potential gas production; cGP = rate of gas production; GP24 = corrected gas production at 24 h; dOM = digestibility of organic matter; ME = metabolisable energy; uCP = utilisable CP for ruminal passage rates (k) of 5 and 8%/h; IDRUP = intestinal digestibility of rumen-undegraded protein; CP fractions = crude protein fractions: A = non-protein nitrogen; B1 = rapidly degradable true protein; B2 = moderately degradable true protein; B3 = slowly degradable true protein, C = undegradable and indigestible true protein, determined using Cornell Net Carbohydrate and Protein System (CNCPS); EDCNCPS = effective protein degradation for ruminal passage rates of 5 and 8%/h, calculated using Fox et al. (2003); DDGS = dried distillers’ grains with solubles.
Additivity of fermentation characteristics and feeding values
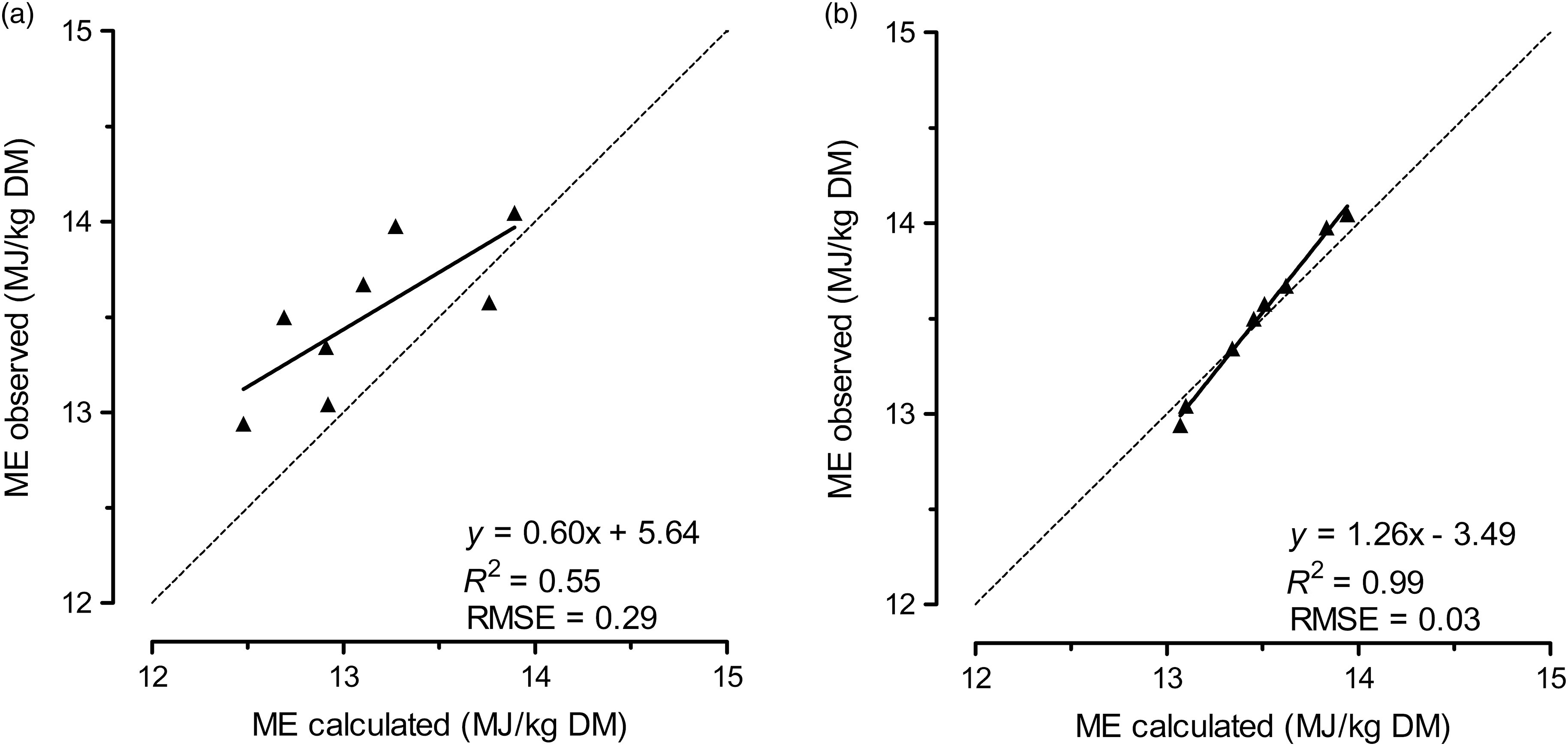
Calculated and observed ruminal fermentation characteristics and nutritional values of the mash compound feeds are presented in Table 2. Estimation of bGP, GP24 and dOM was precise as indicated by the slope of the regression lines (close to 1) and the high R 2 values (Table 3). Observed cGP differed numerically (0.3 to 0.7 pp) from calculated values, and the estimated slope of regression was only 0.68, associated with a large CI. Deviation of calculated and observed ME values was high when the specific equations for each group of feed were used. The comparison between calculated and observed ME showed a low R 2 value of 0.55 with a RMSE of 0.29 (Figure 1). However, when using the same ME equation (equation (5)) for all single and compound feeds, estimated ME of compound feeds from that of single feeds was precise, with an R 2 value of 0.99 (Figure 1).
Table 2.
In vitro fermentation characteristics and feeding value of mash and pellet compound feeds and values calculated from single feeds
| In vitro gas production | dOM | uCP | IDRUP | CP fractions | EDCNCPS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound feed |
bGP (ml/200 mg DM) |
cGP (%/h) | GP24 (ml/200 mg DM) | (%) | k = 5%/h (g/kg DM) | k = 8%/h (g/kg DM) | (%) | A (%) | B1 (%) | B2 (%) | B3 (%) | C (%) | k = 5%/h (%) | k = 8%/h (%) | |
| 1 | Calculated | 77 | 7.0 | 73 | 92 | 181 | 193 | 63 | 12.9 | 12 | 66.3 | 7.9 | 0.9 | 75 | 67 |
| Mash | 81 | 7.3 | 74 | 93 | 173 | 188 | 71 | 17.1 | 7.1 | 68.0 | 7.8 | 0.0 | 75 | 67 | |
| Pellet | 77 | 8.4 | 71 | 91 | 192 | 212 | 73 | 19.4 | 3.0 | 69.9 | 7.7 | 0.0 | 74 | 66 | |
| 2 | Calculated | 69 | 7.9 | 66 | 87 | 190 | 205 | 46 | 16.5 | 11.8 | 54.5 | 10.8 | 6.4 | 76 | 69 |
| Mash | 71 | 8.6 | 65 | 86 | 177 | 195 | 55 | 23.2 | 3.9 | 55.3 | 14.1 | 3.5 | 76 | 69 | |
| Pellet | 70 | 9.2 | 65 | 86 | 185 | 204 | 48 | 21.7 | 5.9 | 54.9 | 14.0 | 3.5 | 76 | 69 | |
| 3 | Calculated | 68 | 8.2 | 62 | 84 | 175 | 193 | 44 | 17.3 | 21.3 | 51.3 | 6.8 | 3.2 | 77 | 70 |
| Mash | 67 | 8.3 | 61 | 83 | 173 | 188 | 49 | 18.1 | 19.5 | 52.9 | 6.3 | 3.2 | 77 | 70 | |
| Pellet | 67 | 9.1 | 62 | 83 | 178 | 198 | 34 | 17.4 | 16.9 | 56.3 | 6.3 | 3.2 | 76 | 69 | |
| 4 | Calculated | 72 | 8.2 | 66 | 89 | 192 | 216 | 59 | 9.3 | 15.3 | 65.3 | 6.0 | 4.1 | 71 | 62 |
| Mash | 73 | 7.5 | 67 | 90 | 186 | 210 | 70 | 8.8 | 14.7 | 67.9 | 5.7 | 2.9 | 71 | 62 | |
| Pellet | 72 | 8.5 | 66 | 89 | 190 | 214 | 63 | 10.1 | 15.6 | 65.5 | 5.8 | 2.9 | 72 | 63 | |
| 5 | Calculated | 68 | 7.6 | 63 | 87 | 200 | 222 | 49 | 9.3 | 18.6 | 59.8 | 7.7 | 4.6 | 73 | 66 |
| Mash | 69 | 8.1 | 62 | 86 | 191 | 209 | 58 | 10.1 | 18.9 | 57.8 | 7.9 | 5.3 | 73 | 66 | |
| Pellet | 68 | 9.0 | 63 | 86 | 190 | 213 | 51 | 9.0 | 17.8 | 62.2 | 5.5 | 5.5 | 72 | 65 | |
| 6 | Calculated | 63 | 8.7 | 58 | 83 | 196 | 225 | 49 | 9.3 | 19.0 | 61.1 | 7.3 | 3.3 | 73 | 66 |
| Mash | 64 | 8.4 | 59 | 84 | 189 | 213 | 49 | 9.0 | 21.1 | 60.0 | 7.4 | 2.5 | 74 | 66 | |
| Pellet | 66 | 8.9 | 61 | 85 | 194 | 217 | 44 | 9.5 | 19.9 | 59.9 | 5.4 | 5.4 | 71 | 64 | |
| 7 | Calculated | 55 | 8.9 | 54 | 81 | 199 | 233 | 50 | 9.3 | 14.1 | 66.4 | 7.1 | 3.0 | 73 | 65 |
| Mash | 59 | 9.2 | 54 | 81 | 195 | 226 | 53 | 9.4 | 14.4 | 64.9 | 9.0 | 2.2 | 73 | 65 | |
| Pellet | 59 | 9.5 | 55 | 81 | 188 | 220 | 41 | 10.5 | 12.6 | 67.5 | 7.1 | 2.4 | 72 | 64 | |
| 8 | Calculated | 62 | 8.7 | 57 | 85 | 203 | 240 | 60 | 7.1 | 17.9 | 67.6 | 5.0 | 2.4 | 73 | 65 |
| Mash | 64 | 8.4 | 59 | 87 | 199 | 237 | 56 | 9.4 | 18.4 | 66.0 | 4.2 | 2.1 | 73 | 65 | |
| Pellet | 63 | 9.2 | 59 | 86 | 198 | 233 | 48 | 7.7 | 17.8 | 68.0 | 4.3 | 2.1 | 72 | 64 | |
bGP = potential gas production; cGP = rate of gas production; GP24 = corrected gas production at 24 h; dOM = digestibility of organic matter; uCP = utilisable CP for ruminal passage rates (k) of 5 and 8%/h; IDRUP = intestinal digestibility of rumen-undegraded protein; CP fractions = crude protein fractions: A = non-protein nitrogen; B1 = rapidly degradable true protein; B2 = moderately degradable true protein; B3 = slowly degradable true protein, C = undegradable and indigestible true protein, determined using Cornell Net Carbohydrate and Protein System (CNCPS); EDCNCPS = Effective protein degradation for ruminal passage rates of 5 and 8%/h, calculated using Fox et al. (2003). Metabolisable energy (ME) values are presented in Figure 1.
Table 3.
Results of simple linear regressions for in vitro fermentation characteristics and feeding values of compound feeds
| Calculated v. observed | Mash v. pelleted | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Slope CI | Intercept | Intercept CI | R 2 | RMSE | Slope | Slope CI | Intercept | Intercept CI | R 2 | RMSE | |
| In vitro gas production | ||||||||||||
| bGP | 0.98 | 0.74 to 1.21 | 3.58 | −11.95 to 19.11 | 0.95 | 1.70 | 0.79 | 0.64 to 0.93 | 13.80 | 3.87 to 23.72 | 0.97 | 1.06 |
| cGP | 0.68 | 0.03 to 1.33 | 2.67 | −2.65 to 7.98 | 0.52 | 0.44 | 0.59 | 0.43 to 0.75 | 4.14 | 2.82 to 5.47 | 0.93 | 0.10 |
| GP24 | 0.96 | 0.78 to 1.14 | 2.72 | −8.52 to 13.95 | 0.97 | 1.19 | 0.82 | 0.67 to 0.98 | 11.27 | 1.63 to 20.91 | 0.97 | 1.00 |
| dOM | 1.10 | 0.73 to 1.40 | −4.95 | −33.43 to 23.53 | 0.91 | 1.25 | 0.77 | 0.59 to 0.94 | 19.84 | 4.66 to 35.02 | 0.95 | 0.74 |
| ME | –1 | – | – | – | – | – | 0.71 | 0.54 to 0.87 | 3.82 | 1.61 to 6.03 | 0.95 | 0.07 |
| uCP | ||||||||||||
| k = 5%/h | 0.97 | 0.61 to 1.33 | −1.05 | −70.00 to 67.89 | 0.88 | 3.73 | 0.37 | −0.11 to 0.85 | 120.09 | 31.12 to 209.07 | 0.38 | 5.16 |
| k = 8%/h | 0.97 | 0.77 to 1.17 | −1.26 | −45.18 to 42.66 | 0.96 | 3.89 | 0.53 | 0.29 to 0.77 | 103.00 | 53.24 to 152.76 | 0.83 | 4.53 |
| IDRUP | 0.95 | 0.23 to 1.66 | 7.86 | −30.08 to 45.80 | 0.64 | 5.53 | 1.42 | 0.99 to 1.85 | −31.27 | −56.26 to −6.28 | 0.92 | 3.94 |
| CP fractions | ||||||||||||
| A | 1.37 | 0.79 to 1.94 | −24.19 | −92.26 to 43.88 | 0.85 | 23.09 | 0.95 | 0.69 to 1.20 | 7.15 | −28.95 to 43.25 | 0.93 | 15.30 |
| B1 | 1.65 | 0.94 to 2.35 | −119.87 | −237.17 to −2.56 | 0.84 | 26.55 | 0.94 | 0.64 to 1.24 | −2.20 | −49.96 to 45.55 | 0.91 | 20.23 |
| B2 | 0.94 | 0.64 to 1.23 | 38.34 | −144.37 to 221.05 | 0.91 | 19.22 | 0.88 | 0.52 to 1.24 | 88.96 | −131.32 to 309.24 | 0.86 | 22.79 |
| B3 | 1.70 | 1.22 to 2.18 | −46.79 | −82.89 to −10.69 | 0.93 | 8.71 | 0.94 | 0.57 to 1.32 | −3.55 | −34.68 to 27.58 | 0.86 | 12.03 |
| C | 0.71 | 0.13 to 1.29 | 2.36 | −19.67 to 24.40 | 0.60 | 10.21 | 0.98 | 0.31 to 1.65 | 4.60 | −15.84 to 25.04 | 0.68 | 10.83 |
| EDCNCPS | ||||||||||||
| k = 5%/h | 1.24 | −0.41 to 2.89 | −12.35 | −134.36 to 109.66 | 0.36 | 3.40 | 0.63 | 0.14 to 1.11 | 31.83 | −7.02 to 70.68 | 0.62 | 2.08 |
| k = 8%/h | 1.41 | −0.20 to 3.02 | −20.20 | −126.68 to 86.28 | 0.43 | 4.22 | 0.65 | 0.19 to 1.10 | 28.54 | −4.71 to 61.79 | 0.67 | 2.55 |
bGP = potential gas production; cGP = rate of gas production; GP24 = corrected gas production at 24 h; dOM = digestibility of organic matter; ME = metabolisable energy; uCP = utilisable CP for ruminal passage rates (k) of 5 and 8%/h; IDRUP = intestinal digestibility of rumen-undegraded protein; CP fractions = crude protein fractions: A = non-protein nitrogen; B1 = rapidly degradable true protein; B2 = moderately degradable true protein; B3 = slowly degradable true protein, C = undegradable and indigestible true protein, determined using Cornell Net Carbohydrate and Protein System (CNCPS); EDCNCPS = effective protein degradation for ruminal passage rates of 5 and 8%/h, calculated using Fox et al. (2003). Observed values refer to values for compound feeds in mash form.
Simple linear regressions of calculated and observed ME values are presented in Figure 1.
Figure 1.
Comparison of calculated and observed metabolisable energy (ME) values of compound feeds using an in vitro ruminal fermentation technique. The ME values of compound feeds were calculated from ME values of single feeds that were determined according to the equations of: (a) Krieg et al. (2017) and Menke and Steingass (1988), respective of the feed group; or (b) GfE (2009) for all single feeds. The dotted line represents the angle bisector.
Observed uCP was numerically lower than calculated in all compound feeds. However, the difference did not exceed 13 g/kg DM. The regression line slopes were close to 1 (0.97 for both k = 5 and 8%/h) and regression equations showed high R 2 values (0.88 and 0.96 for k = 5 and 8%/h, respectively).
Observed IDRUP in compound feeds differed from calculated values between 0 and 11 pp. Regression analysis between calculated and observed IDRUP values showed an R² value of 0.64 and relatively large CIs for the slope and intercept, respectively.
Conformity between calculated and observed CP fractions depended on the specific fraction and the compound feed type. Confidence interval of the slope did not include the value of 1 only for B3 (CI = 1.22 to 2.18), even though the R 2 value for this parameter was high (R 2 = 0.93).
Results of the regression analysis of calculated and observed EDCNCPS values showed small accuracy (R 2 of 0.36 and 0.43 for k = 5 and 8%/h, respectively). However, the slope values included 1 and intercept values included 0 and numerical differences were in most cases not even detectable (Table 2).
Effects of pelleting on ruminal fermentation characteristics and feeding value of compound feeds
Differences between mash and pellet compound feeds in GP24 did not exceed 3 ml/200 mg DM, 3 pp in dOM and 0.3 MJ ME/kg DM (Table 2). However, based on CI ranges (Table 3), the results indicated that pelleting did affect GP characteristics. The slopes and the intercepts for bGP, cGP, GP24, dOM and ME were all significantly different from 1 to 0, respectively, even though the R 2 value was 0.93 or higher. Pelleting numerically increased uCP in compound feeds with lower CP concentration and decreased uCP in compound feeds with higher CP concentration. The slopes and intercepts were significantly different from 1 and 0, respectively, with considerable differences in R 2 values between k = 5%/h (R 2 = 0.38) and k = 8%/h (R 2 = 0.83). Pelleting decreased estimated IDRUP in most compound feeds, with a maximum of 15 pp in compound feed 3. Pelleting increased estimated IDRUP only in compound feed 1, but the difference was negligible (2 pp). Although the CI for the slope of IDRUP included 1 and the R 2 value was high (R 2 = 0.92), the intercept was significantly different from 0. Pelleting did not systematically affect CP fractions in compound feeds (Table 3). Pelleting reduced the EDCNCPS in most compound feeds slightly (up to 3 pp) for both k = 5 and 8%/h.
Prediction of in situ ruminal CP degradation from CP fractions
The EDCNCPS values were smaller than EDIN_SITU for all compound feeds, and the difference was up to 11 and 14 pp for k = 5 and 8%/h, respectively (Figure 2). Calculation of EDCNCPS using individual CP fractions and tabular values for their specific degradation rates resulted in a very low variation from 71% to 77% (k = 5%/h) and 62% to 70% (k = 8%/h), whereas EDIN_SITU of compound feeds showed wider variation from 74% to 88% (k = 5%/h) and 67% to 84% (k = 8%/h). The EDCNCPS based on the regression analysis according to Shannak et al. (2000) resulted in a remarkable higher variability between compound feeds (from 67% to 95% for k = 5%/h and 61% to 86% for k = 8%/h) compared to EDIN_SITU and ranked feeds differently (Figure 2).
Figure 2.
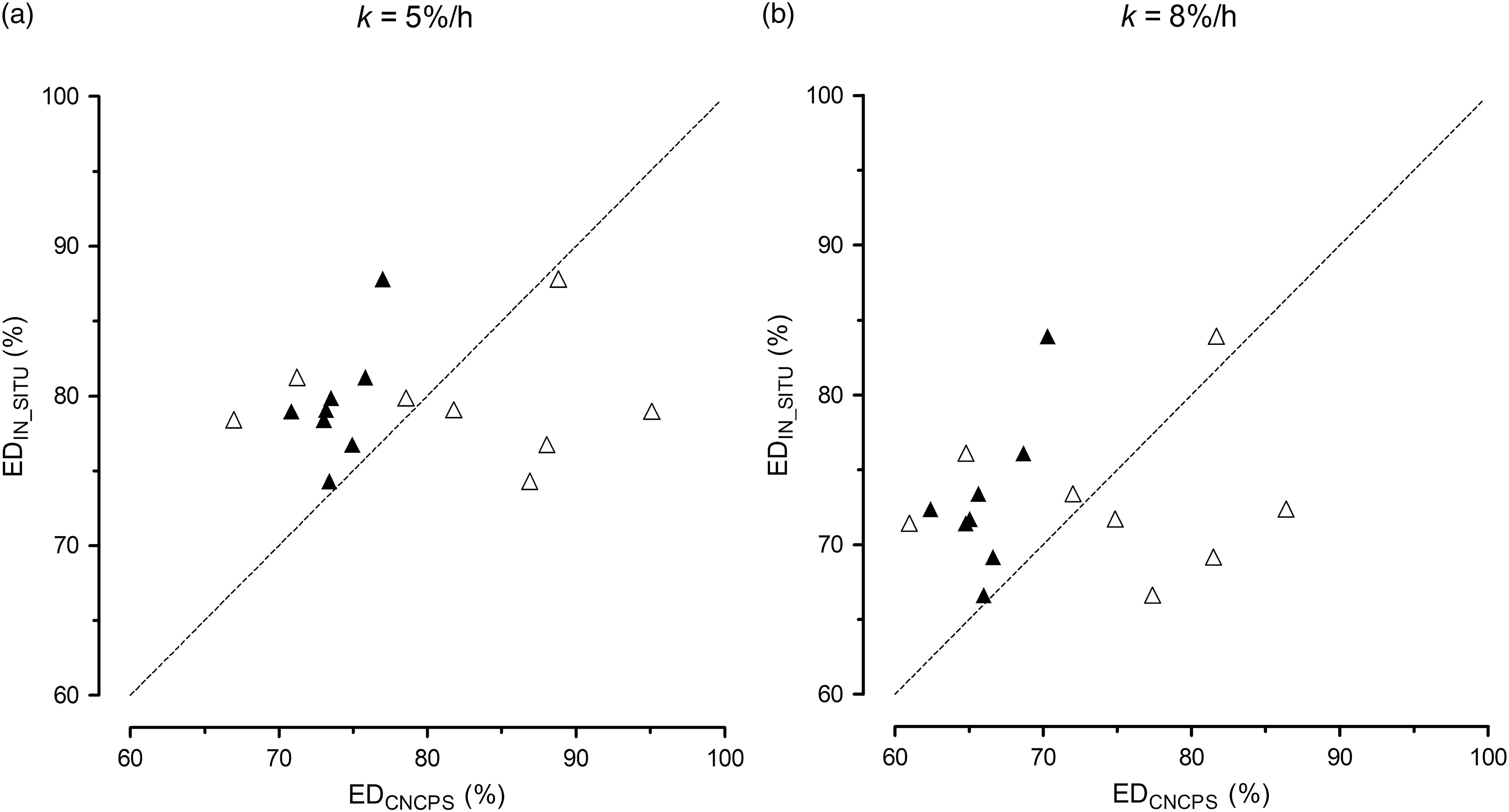
Comparison of ruminal effective protein degradation of compound feeds for ruminal passage rates of 5 and 8%/h based on CP fractions (EDCNCPS) and calculated according to Fox et al. (2003) (▴) or Shannak et al. (2000) (▵) and determined in situ (EDIN_SITU; Grubješić et al., 2019). The dotted line represents the angle bisector.
Discussion
Additivity of ruminal fermentation characteristics and feeding values
It was hypothesised that values of GP, dOM, ME, uCP, IDRUP and CP fractions of compound feeds in mash form can be calculated from single feeds. Based on the results of the present study, this hypothesis can be accepted only in part. The agreement between calculated and observed values was good for bGP, cGP, GP24, dOM and uCP, and thus, we consider these criteria to be additive. The highest deviation of calculated from observed value was 4 ml/200 mg DM for bGP, 0.7%/h for cGP, 2.3 ml/200 mg DM for GP24 and 2 pp for dOM.
For ME calculations, the equations chosen for single feeds largely affected the outcome of the comparison (Figure 1). In the literature, equations to predict ME from GP and nutrient concentrations are often specific to single feeds or groups of feeds because this is associated with high prediction accuracy. However, it does not necessarily hold true across different feed groups and also for feed mixtures (Menke and Steingass, 1988). This caused issues when values for single feeds and related compound feeds were compared in the present study. We argue that the conclusion that additivity does not exist is misleading because it is an artefact of using different equations for different groups of feeds. When equation (5) was used to calculate the ME for all feeds (single and compound), the differences between calculated and observed ME became negligible (Figure 1).
Gas production techniques are underutilised for estimation of potential interactions between feeds (D’Mello, 2000) and energy evaluation, and previous research on additivity has most commonly focused on concentrate–forage mixes. Robinson et al. (2009) noted associative effects (15% to 25%) in mixture of alfalfa hay, barley grain, maize silage and soya bean meal using HGT in early phase of incubation, while they disappeared later. Similarly, Arhab et al. (2010) used mixtures of triticale and barley with a commercial concentrate supplement and found significant differences only in GP up to 8 h. In the present study, the discrepancy between calculated and observed GP at multiple time points can be considered to be negligible with a maximal difference of 2 ml/200 mg DM after 2 and 4 h of incubation. The observed GP24 was similar to the calculated ones and was considered to be additive. Since dOM and ME values are derived from GP24 (Menke and Steingass, 1988), this is an important finding and we presume that the differences between calculated and observed ME of compound feeds were caused only by the choice of ME equation. For the estimation of dOM values, the SD of regression residuals (s y.x) is 3.07% (Menke and Steingass, 1988). The s y.x for estimation of ME values depends on the regression equation used, with a s y.x of 2.92 MJ/kg DM (Menke and Steingass, 1988) or a RMSE value of 1.98 MJ/kg DM (GfE, 2009) for equations (4) and (5), respectively. The RMSE between calculated and observed dOM and ME (equation (5)) of the compound feeds of the present study was 1.15% and 0.09 MJ/kg DM, respectively, and therefore markedly lower compared to the s y.x and the RMSE of the prediction equations. This underlines the assumption that additivity of those values is given.
The uCP consists of the RUP and microbial CP, as defined in the German feed protein evaluation system (GfE, 2001). Calculated uCP values corresponded well with observed values as the slope of regression and intercept was within their CI. A systematic overestimation of uCP was observed (up to 13 g/kg DM). Repeated measurements of observed uCP for each feed and incubation time were close together and showed low SD between runs up to 23 g/kg DM. However, also variation of observed and calculated uCP between compound feeds was low for both rumen outflow rates with a maximal differences of 49 g/kg DM. Due to the small variability between feeds and only eight data points for regression analysis, this systematical overestimation should be interpreted with caution and reference to their biological and practical relevance, which seems negligible. This contrasts with the findings of Zhao et al. (2005), who reported higher differences between calculated and observed uCP values in an experiment using 16 single feeds and 19 mixtures. They noted statistically significant and non-systematic differences between calculated and observed uCP values. However, the authors mentioned the possibility of incomplete incubation of some feeds due to incomplete mixing with the incubation liquid (Zhao et al., 2005). Such an effect was avoided in the present study by the constant motion of the rotary incubator.
Calsamiglia and Stern (1995) highlighted the importance of IDRUP for evaluating feed protein. GfE (2001) and NRC (2001) assumed a constant IDRUP value of 80% for all feeds. In the CNCPS, IDRUP was assumed to be 100% for CP fractions A, B1 and B2, 80% for fraction B3 and zero for fraction C (Fox et al., 2003). A wide range of IDRUP of single and compound feeds was found in the present study. Observed IDRUP in most compound feeds was higher than calculated (up to 11 pp). Accurate estimation of IDRUP from single feeds was thus not possible for all compound feeds of the present study using the three-step method. This is underlined by the analytical tolerance of the determination of IDRUP which was set to maximal 10% relative deviation from the mean value otherwise the procedure was repeated. Relative deviation of replicates varied between 0.04 and 8.11% around the mean value for all feed samples of the present study. However, for five out of eight compound feeds, relative deviations of calculated IDRUP from observed IDRUP exceeded the value of 10%, which represents the analytical tolerance. This indicates that associative effects occurred when analysing IDRUP of compound feeds. Those interactions between single feeds in mixture can occur in any of the three steps. Calculation of the 16 h in situ CP degradation of compound feeds from single feeds showed better additivity than IDRUP with a slight tendency to overestimate CP degradation (1 to 7 pp). Compound feeds that showed higher deviations between calculated and observed in situ degradation also tended to show higher differences between the calculated and observed IDRUP. Hence, associative effects seem more pronounced during the in vitro enzymatic part but play also a role in the first step of in situ incubation. As it seems that majority of the associative effects in the three-step method occurred during the in vitro enzymatic part, the results of the present study should be verified using the mobile bag technique as an alternative to the second step.
To our knowledge, CP fractions have not been previously studied for additivity. In the present study, observed CP fractions of mash compound feeds were often different from those calculated, as indicated by intercept values (for fractions B1 and B3 CI not including 0) and slopes (fraction B3 CI not including 1) and the wide CI range overall.
The accurate determination of CP fractions depends, among others, on accurate CP determination. For some CP fractions, differences between calculated and observed values were higher than analytical tolerances for CP analysis (VDLUFA, 2019). This was the case for the A and B1 fraction of compound feeds 1 and 2, and the B3 fraction of compound feed 2. However, for all other CP fractions and compound feeds, the difference between the calculated and observed values of CP fractions is similar or even lower than the analytical tolerance of CP analysis. In addition, small variability between compound feeds (particularly for CP fraction C) probably lowered the accuracy of regression analysis. Consequently, interpretation of additivity for CP fractions is difficult from the results of the present study and different depending on the specific fraction and feed type. Additivity of EDCNCPS was given for all compound feeds. However, the accuracy of regression analysis may be limited owing to the relatively small sample size (n = 8 compound feeds) of the present study. Therefore, we recommend to examine the additivity of CP fractions of single feeds in compound feeds in further experiments.
Effects of pelleting on ruminal fermentation characteristics and feeding value of compound feeds
The second hypothesis of the present study was that the pelleting process would significantly affect GP, dOM, ME, uCP, IDRUP and CP fractions of compound feeds. Based on the present results, this hypothesis can be rejected. Even though the results of statistical analysis indicated an effect of pelleting on GP and related values of ME and dOM, uCP and IDRUP, the overall numerical differences were negligible.
When heat is excessively applied during the processing of compound feeds, the intestinal digestibility of protein can be reduced owing to the formation of Maillard products which can neither be fermented nor digested (Sniffen et al., 1992). Any optimum of processing conditions would aim to reduce CP degradability in the rumen without affecting IDRUP. The data obtained in situ with the same feeds as used in the present study (Grubješić et al., 2019) indicated that pelleting increased rumen degradation of some compound feeds, thus resulting in less RUP entering the small intestine. However, pelleting increased the share of smaller feed particles compared with the mash feeds, which might have increased the number of feed particles leaving the bags without microbial degradation, and thus overestimated degradation. This conclusion is consistent with the results of the present study. In the present study, pelleting increased uCP (which consists of RUP and microbial CP) of most compound feeds (16%, 18%, 20%, 22%, 24% and 26% of CP in DM) up to 24 g/kg DM. No difference was found in the two compound feeds with the highest CP concentrations (28% and 30% of CP in DM).
In a study using duodenally cannulated animals, Goelema et al. (1998) did not find an effect on intestinal protein digestibility of mixtures of lupins, peas and faba beans after toasting for 3 min at 132°C. This temperature was higher than the one applied in the present study (pelleting exit temperature of up to 80°C to 90°C). The process of toasting is however technologically not equal to pelleting, as factors other than heat (pressure and moisture) also differ and might result in chemical or physical changes of the substrate. In the present study, except for compound feed 1, IDRUP decreased from 6 to 15 pp in all compound feeds by pelleting.
In situ incubations over 16 h were used to generate RUP for in vitro determination of IDRUP, and results showed that degradation after 16 h increased between 1.4 and 6.4 pp in pelleted compound feeds compared to their corresponding mash feeds. It can therefore be assumed that RUP of mash feeds after in situ incubation contained more potentially digestible CP for the in vitro enzymatic steps to determine IDRUP. This is underlined by the calculation of total tract digestibility (TTD) from the summation of 16 h in situ RUP and in vitro IDRUP which showed that differences in TTD between mash and pelleted compound feeds ranged only between 0.2 and 2.2 pp and can therefore be considered to be negligible. The higher rumen-degraded protein of pelleted compound feeds might be attributed to a smaller particle size compared to mash feeds, as explained in the previous sections.
Pelleting did not have a large effect on CP fractions and EDCNCPS values of compound feeds. Heat treatment during the pelleting process can denaturise protein fraction B2 making it insoluble, resulting in increased B2 and C fractions (Licitra et al., 1996). Such an effect was not found in the present study, probably due to the temperature during pelleting not being very high.
Prediction of in situ ruminal CP degradation from CP fractions
The third hypothesis of the present study was that EDIN_SITU could be predicted using CP fractions. Based on the present results, this hypothesis is rejected. Compared with the corresponding EDIN_SITU data (Grubješić et al., 2019), neither the calculation of EDCNCPS using individual CP fractions and tabular values for their specific degradation rates (Fox et al., 2003) nor EDCNCPS using proximate nutrients and CP fractions based on regression analysis (Shannak et al., 2000) showed adequate prediction accuracy for all compound feeds. However, for two (calculated according to Fox et al. (2003)) and three (calculated according to Shannak et al. (2000)) out of eight compound feeds, ED prediction with both methods was similar (differences ≤ 3 pp). Attempts of using CP fractions together with proximate nutrients to estimate in situ ruminal CP degradation of single and compound feeds showed varying success. Titze et al. (2018) reported an overestimation of EDCNCPS of lupins using the approach of Fox et al. (2003), for an average of 10 pp. In the present study, EDCNCPS was generally lower than EDIN_SITU for all compound feeds and prediction accuracy was very variable with differences from 1 to 14 pp. A problem when using the approach of Fox et al. (2003) is the necessity of using tabulated values for the degradation rate of the specific CP fractions. It was not mentioned how degradation rates were obtained, how many samples the provided mean values are based on and how high the range of degradation rates for individual CP fractions of the same feedstuff was. Shannak et al. (2000) derived their prediction equations from selected proximate nutrients and CP fractions for in situ RUP values including 11 dairy compound feeds. Therefore, prediction of ED of compound feeds may be possible with good accuracy. Shannak et al. (2000) found differences between in situ RUP values and respective estimates of up to 79 g/kg CP; however, 8 out of 11 RUP values had differences ≤ 50 g/kg CP. For samples of the present study, EDIN_SITU and EDCNCPS calculated according to Shannak et al. (2000) differed by up to 16 pp and hence 5 out of 8 compound feeds had differences between estimated and in situ RUP ≥ 100 g/kg CP for k = 5 and 8%/h. Poor estimation may result from differences in the assay details because NDF was determined by manual filtration in the study of Shannak et al. (2000), and authors stated that results may deviate from those obtained with the conventional NDF method which was used in the present study. Moreover, NDF values ranged between 212 and 554 g/kg DM in the 11 compound feeds of Shannak et al. (2000) and only between 142 and 255 g/kg DM in the present study. Shannak et al. (2000) also included forages and special by-products in the development of the regression equations, which is another difference to the present study. It is therefore recommended to extend the existing database. More accurate equations may be developed when covering a wider range of feedstuff groups.
Conclusion
We conclude that, when formulating compound feeds for cattle, single feed data for GP24, dOM, ME and uCP are additive, while those for IDRUP are not. Additivity of CP fractions is dependent on the fraction and compound feed type, whereas EDCNCPS is precisely additive. The pelleting process had little effect on ruminal fermentation characteristics and feeding values of compound feeds, probably because heat exposure was moderate. Using CP fractions in the present study did not reliably predict in situ ruminal CP degradation of compound feeds: more studies are needed to extend the database for the development of prediction equations.
Acknowledgements
Goran Grubješić received doctoral scholarships from the Faculty of Agricultural Sciences at the University of Hohenheim and the H. Wilhelm Schaumann Stiftung, Hamburg, which are gratefully acknowledged. The authors are also grateful for the support of Raiffeisen Kraftfutterwerk (RKW) Kehl in providing single feed samples and facilities for mixing and pelleting the compound feeds.
G. Grubješić 0000-0002-5007-6973
N. Titze 0000-0001-5992-6404
J. Krieg 0000-0002-6614-1442
M. Rodehutscord 0000-0003-3156-7889
Declaration of interest
No potential conflict of interest is reported by the authors.
Ethics statement
The use of rumen-cannulated cows for rumen fluid collection was approved by the Regierungspräsidium Stuttgart, Germany, approval number A401-14 TE.
Software and data repository resources
No data were deposited in an official repository.
References
- Arhab R, Laadjimi K, Driss D, Djabri B and Bousseboua H 2010. Evaluation of feed mixture interactions by using in vitro gas production method. Livestock Research for Rural Development 22, Article #217.
- Calsamiglia S and Stern MD 1995. A three-step in vitro procedure for estimating intestinal digestion of protein in ruminants. Journal of Animal Science 73, 1459–1465. [DOI] [PubMed] [Google Scholar]
- Chrenková M, Čerešňáková Z, Weisbjerg MR, Formelová Z, Poláčiková M and Vondráková M 2014. Characterization of proteins in feeds according to the CNCPS and comparison to in situ parameters. Czech Journal of Animal Science 59, 288–295. [Google Scholar]
- D’Mello JPF 2000. Farm animal metabolism and nutrition. CABI Pub., Scottish Agricultural College, Edinburgh, UK. [Google Scholar]
- Fox DG, Tylutki TP, Tedeschi LO, Van Amburgh ME, Chase LE, Pell AN, Overton TR and Russell JB 2003. The net carbohydrate and protein system for evaluating herd nutrition and nutrient excretion: CNCPS version 5.0, model documentation. In Animal Science Mimeo 213, Animal Science Department, Cornell University, Ithaca, NY, USA.
- Gesellschaft für Ernährungsphysiologie (GfE) 2001. Energie-und Nährstoffbedarf landwirtschaftlicher Nutztiere. Nr. 8 Empfehlungen zur Energie-und Nährstoffversorgung der Milchkühe und Aufzuchtrinder. DLG-Verlag Frankfurt am Main, Germany.
- Gesellschaft für Ernährungsphysiologie (GfE) 2009. New equations for predicting metabolisable energy of compound feeds for cattle. In Proceedings of the Society of Nutrition Physiology, 10–12 March 2009, Göttingen, Germany, pp. 143–146.
- Gesellschaft für Ernährungsphysiologie (GfE) 2017. Opinion on the indispensability of animal experiments in animal nutrition research and suitability of alternative methods. Retrieved on 26 January 2020, from https://gfe-frankfurt.de/2017/02/12/tierversuche/
- Goelema JO, Spreeuwenberg MAM, Hof G, van der Poel AFB and Tamminga S 1998. Effect of pressure toasting on the rumen degradability and intestinal digestibility of whole and broken peas, lupins and faba beans and a mixture of these feedstuffs. Animal Feed Science and Technology 76, 35–50. [Google Scholar]
- Grubješić G, Titze N, Krieg J and Rodehutscord M 2019. Determination of in situ ruminal crude protein and starch degradation values of compound feeds from single feeds. Archives of Animal Nutrition 73, 414–429. [DOI] [PubMed] [Google Scholar]
- Krieg J, Seifried N, Steingass H and Rodehutscord M 2017. In situ and in vitro ruminal starch degradation of grains from different rye, triticale and barley genotypes. Animal 11, 1745–1753. [DOI] [PubMed] [Google Scholar]
- Licitra G, Hernandez TM and Van Soest PJ 1996. Standardization of procedures for nitrogen fractionation of ruminant feeds. Animal Feed Science and Technology 57, 347–358. [Google Scholar]
- Menke KH and Steingass H 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research and Development 28, 7–55.
- Niderkorn V, Baumont R, Le Morvan A and Macheboeuf D 2011. Occurrence of associative effects between grasses and legumes in binary mixtures on in vitro rumen fermentation characteristics. Journal of Animal Science 89, 1138–1145. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 2001. Nutrient requirements of dairy cattle, 7th revised edition. National Academy Press, Washington, DC, USA.
- Robinson PH, Getachew G and Cone JW 2009. Evaluation of the extent of associative effects of two groups of four feeds using an in vitro gas production procedure. Animal Feed Science and Technology 150, 9–17. [Google Scholar]
- Sandoval-Castro CA, Capetillo-Leal C, Cetina-Góngora R and Ramirez-Avilés L 2002. A mixture simplex design to study associative effects with an in vitro gas production technique. Animal Feed Science and Technology 101, 191–200. [Google Scholar]
- Seifried N, Steingass H, Hoffmann N and Rodehutscord M 2016. In situ starch and crude protein degradation in the rumen and in vitro gas production kinetics of wheat genotypes. Journal of Animal Physiology and Animal Nutrition 101, 779–790. [DOI] [PubMed] [Google Scholar]
- Shannak S, Südekum KH and Susenbeth A 2000. Estimating ruminal crude protein degradation with in situ and chemical fractionation procedures. Animal Feed Science and Technology 85, 195–214. [Google Scholar]
- Sniffen CJ, O’Connor JD, Van Soest PJ, Fox DG and Russell JB 1992. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Animal Science 70, 3562–3577. [DOI] [PubMed] [Google Scholar]
- Steingaß H and Südekum KH 2013. Proteinbewertung beim Wiederkäuer–Grundlagen, analytische Entwicklungen und Perspektiven. Übersichten zur Tierernährung 41, 51–73.
- Svihus B and Zimonja O 2011. Chemical alterations with nutritional consequences due to pelleting animal feeds: a review. Animal Production Science 51, 590–596. [Google Scholar]
- Titze N, Krieg J, Steingass H and Rodehutscord M 2018. Variation of lupin protein degradation in ruminants studied in situ and using chemical protein fractions. Animal 13, 709–717. [DOI] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 2007. Handbuch der Landwirtschaftlichen Versuchs-und Untersuchungsmethodik Bd. III: Die chemische Untersuchung von Futtermitteln. VDLUFA-Verlag, Darmstadt, Germany. [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 2019. Analysenspielräume (ASR). Version 12 Retrieved on 15 January 2020, from https://www.vdlufa.de/Dokumente/Fachgruppen/FG6/ASR_Version_12_2019.pdf
- Westreicher-Kristen E, Steingass H and Rodehutscord M 2015. Estimation of utilisable crude protein at the duodenum of dried distillers’ grains with solubles using a modified gas test. Archives of Animal Nutrition 69, 351–365. [DOI] [PubMed] [Google Scholar]
- Zhao GY, Li YX, Ren JB, Li YJ and Guo DS 2005. The influence of associative effects on the in vitro-estimated utilizable crude protein (uCP) of feeds for ruminants. Archives of Animal Nutrition 59, 149–154. [DOI] [PubMed] [Google Scholar]


