Abstract
Paternal care, particularly in cases of uncertain paternity, carries significant costs. Extensive research, both theoretical and experimental, has explored the conditions in which paternal care behaviour would be favoured. Common explanations include an adjustment of care with uncertainty in paternity and limited accuracy in parentage assessment. Here, we propose a new explanation that microbes may play a role in the evolution of paternal care among their hosts. Using computational models, we demonstrate that microbes associated with increased paternal care could be favoured by natural selection. We find that microbe-induced paternal care could evolve under wider conditions than suggested by genetic models. Moreover, we show that microbe-induced paternal care is more likely to evolve when considering paternal care interactions that increase microbial transmission, such as feeding and grooming. Our results imply that factors affecting the composition of host microbiome may also alter paternal behaviour.
This article is part of the theme issue ‘The role of the microbiome in host evolution’.
Keywords: microbiome, paternal care, mathematical model, extra-pair mating, sexual conflict, non-genetic inheritance
1. Introduction
When would a father benefit more from caring for its offspring, rather than looking for additional mating opportunities? This question has been broadly addressed both theoretically and experimentally. Paternal care has been frequently observed among avian species [1] (approx. 85%), and also found in mammalian species [2] (approx. 5%), amphibians [3], and many species of fish [4]. Paternal care is most commonly observed alongside maternal care, while exclusive paternal care is rare [4,5]. A male may demonstrate care for its offspring with several types of interactions [4,5], such as feeding, grooming or guarding against predators. It can also provide spousal care for the female while she cares for the young [6,7].
A commonly proposed explanation for the prevalence of paternal care is borrowed from classical evolutionary theory [8]. This explanation suggests that paternal care would be favoured whenever the paternal contribution to offspring survival increases paternal fitness more than pursuing additional mating opportunities [9]. Nevertheless, studies relating paternal effort to the certainty of paternity have obtained mixed results [10,11]. Interestingly, paternal care is also observed in species where a sizable proportion (10–90%) of broods contain extra-pair offspring [12–14]. In some cases, males even knowingly care for unrelated young [15]. When a simple fitness trade-off falls short of justifying substantial paternal investment, alternative explanations have been suggested [16–19].
Here, we consider the potential role of microbes in host paternal care. The microbiome is a significant agent affecting host health and behaviour [20,21], through pathways such as the ‘gut–brain axis’. Numerous studies have demonstrated a possible association between microbes and social behaviour (reviewed in [20–22]), and certain species of the microbiome have been showed to alleviate symptoms of anxiety and depression [23] and improve social interactions [24]. Microbes are highly heritable, through gestation/incubation [25,26] or parental care [27–29]. Microbes can also be transmitted horizontally in a social setting [30], through interactions such as feeding, grooming and copulation [29]. The effect of microbes on host behaviour has given rise to the idea that host manipulation by microorganisms may be driven by natural selection on the microbes [22]. Selection could drive such an effect when the induced behaviour increases microbial fitness, for example, by increasing the rate of microbial transmission or proliferation [22], including host proliferation. Previous theoretical studies suggested that by encouraging host sociality [31] or altruistic behaviour [32], the microbes can help their own propagation.
We integrated the notion of microbe-associated behaviour into mathematical models for the evolution of paternal care. A family is a unit with a high probability of microbial transmission [33], since the members of the family partake in frequent and profound interactions. Caring for the young presents an excellent opportunity from a microbial perspective, since providing care both increases the odds of offspring survival [34] and establishes a higher transmission probability. Therefore, a microbial gene that is associated with host intra-family caring behaviour could be favoured by natural selection even when encouraging care towards genetically unrelated young individuals. The propagation of microbes carrying these genes may have contributed to the maintenance of paternal care even when paternity levels were rather low. Potential candidate microbes, such as Lactobacillus [35] and bifidobacteria [36], have been shown to impact mood or emotional behaviour and could be associated with paternal care.
2. Results
We examine two possible family structures. In every family, there is one female, who is mother to all offspring in the family. In the first family structure, all offspring are fathered by a single male, making them full siblings. In the second family type, we allow mixed paternity within the brood, while still maintaining a social pair structure.
(a). Model I: broods of full siblings
In this model, males adopt one of two pure strategies, either paternal care or lack thereof. Offspring fitness is increased by paternal care [34], due to provision and protection from predation, by a factor of s > 0. The fitness of an offspring whose father does not provide paternal care is ωβ = 1, while an offspring that receives paternal care has increased fitness ωα = 1 + s. Females mate with only one male. A male who provides paternal care can mate with one female and form a social pair. A male who does not provide paternal care can mate with more than one female and is limited only by female availability and receptivity [9]. The two types of males are subject to competition and sexual selection, wherein the caring males suffer a competitive disadvantage relative to the non-caring males (see [37] for discussion). The expected number of matings for both types of males is frequency-dependent [38], governed by the initial male composition in the mating pool. Only a fraction of the males of each type reach mating at all [39], thus the expected number of matings for a young male of the caring type is usually less than 1. Total male mating opportunities are bound by the Fisher condition [40], with a mean of 1. We assume that there is a cost to paternal care [8] and that the expected number of matings for a caring male decreases with paternal care [9].
First, we examine a population of non-caring males where the paternal behaviour is genetically driven. A rare mutant providing paternal care will have a higher fitness than a non-caring male when (at p = 0)
| 2.1 |
where nβ is the expected number of matings for a non-caring male, nα the expected number of matings for a caring male and s the increase in offspring fitness due to paternal care.
Now, we extend the model to include microbes as a reproductive unit that can affect paternal care behaviour. For simplicity, we neglect the effect of host genetic background in the microbe model and assume that host paternal behaviour is determined by its microbes. Let us consider microbes of type α, which are associated with paternal care behaviour, and microbes of type β, which have no effect on paternal care behaviour. Microbes can be transmitted to the offspring from the mother, with probability Tv, or from the father, with probability Tc when the father cares for the offspring. If parental microbes fail to establish in the offspring, it can adopt microbes horizontally by interacting with the general population [41], with probability determined by the population frequencies. In many species, allofeeding behaviour is more common from male to female than vice versa [42,43]. In our model, microbes can also be transmitted from the father to the mother during mating with probability Tm and possibly through allofeeding of the mother, with probability Tn. For simplicity, we assume that females carry the microbe, but it does not affect their behaviour. We assume that each host is inhabited by a single type of microbe at a given time. A transmission probability thus includes the probabilities that a microbe transmits to a new individual, establishes and replaces the resident microbe, encompassing the competition dynamics between different microbial strains. The transmission pathways and transmission probabilities of the two microbes are illustrated in figure 1. We consider a model where the mother cares for the offspring [44,45] and additionally can transmit microbes during gestation [26] and natally [25], so overall maternal transmission is higher than paternal transmission (Tv > Tc). We also assume that paternal care involves more interaction—and potential for microbe transmission—than a singular mating encounter (Tc > Tm). Since the probability of transmitting microbes during mating is asymmetric between the sexes, with a higher probability for male-to-female transmission [46], we neglect the probability of female-to-male transmission. We assume a delay in the effect of the microbes on behaviour and neglect the possibility of a male altering its paternal behaviour due to contracting different microbes at the mating stage. We initially assume that males have full paternity in their brood and relax that assumption later (figure 2).
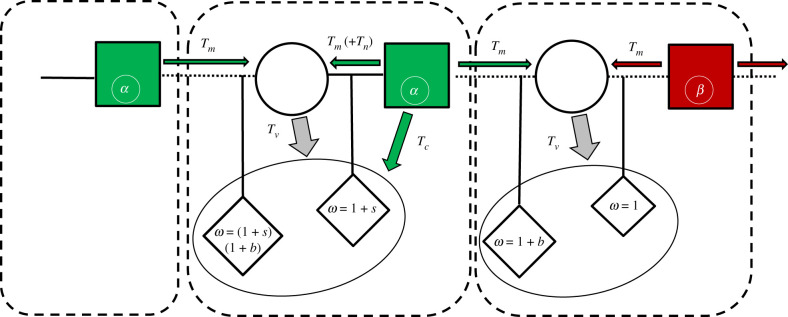
Figure 1.
Model 1 (full siblings): illustration of microbe transmission pathways within the family: (a) where the father carries microbes of type α, inducing paternal care and (b) where the father carries microbes of type β, that have no effect on behaviour. Males carrying β do not care for the offspring and can be involved in n additional matings (illustrated is the case n = 1). Tv, vertical transmission probability through maternal influence (prenatal and postnatal); Tc, probability of transmission through paternal care; Tm, probability of male-to-female microbe transmission during mating; Tn, probability of transmission through male-to-female allofeeding.
Figure 2.
Model II (mixed brood): illustration of microbe transmission pathways within families with extra-pair mating. Males carrying microbes of type β do not care for the offspring, while males carrying microbes of type α care for the offspring in their social nest. All males and females engage in extra-pair mating. Tv, vertical transmission probability through maternal influence (prenatal and postnatal); Tc, probability of transmission through paternal care; Tm, probability of male-to-female transmission during mating; Tn, probability of transmission through male-to-female allofeeding. Offspring sired by an extra-pair mate are 1 + b times more fit than offspring sired by the social mate. Offspring that receive paternal care are 1 + s times more fit than offspring that do not receive it.
We assume the following order of events within the reproductive process: transmission via mating occurs first, second is maternal transmission and finally transmission via paternal care, if it exists.
The condition for the evolution of microbe type α (see electronic supplementary material for full derivation) is given by
| 2.2 |
where nβ is the expected number of matings for a non-caring male (at p = 0), nα the expected number of matings for a caring male (at p = 0), s the increase in offspring fitness due to paternal care, Tv the vertical transmission probability through maternal care, Tc the probability of transmission through paternal care, Tm the probability of male-to-female microbe transmission during mating and Tn the probability of transmission through male-to-female allofeeding.
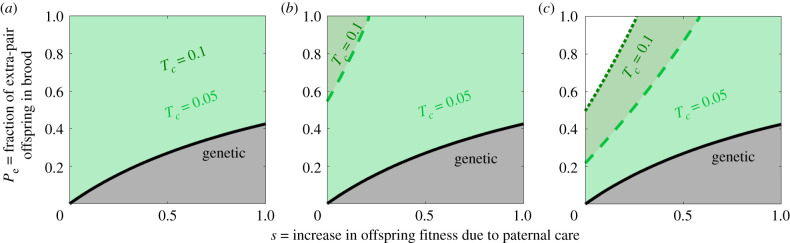
Figure 3 presents the range of fitness costs of caring imposed on the male that allows for the evolution of paternal care in the model. When p = 0, then the number of matings for a mutant of type α is , while the number of matings for type β is . We define the cost of caring, , as the loss of mating success for a rare caring mutant of type α, where γc is a factor governing the cost of caring. In the genetic case, the cost–benefit isocline is given by equation (2.1). The range of conditions where a gene for paternal care evolves is shown by the grey area (figure 3). The conditions where a microbe inducing paternal care evolves can be much wider, shown by the areas below the green lines. The range widens with Tc, the probability of microbe transmission through paternal care, and narrows with Tm, the transmission probability during mating (figure 3). Even when transmission through mating is larger than transmission through care, microbe-induced paternal care fares better than paternal care driven by a host gene (see electronic supplementary material, figure S1). This is in large part due to the maternal transmission of microbes to the offspring, the probability of which is assumed to be stronger than that of maternal genes (T = 0.5). However, reduced maternal transmission also allows microbe-induced paternal care to evolve quite easily (see electronic supplementary material, figure S2). Counterintuitively, the results also demonstrate that microbial genes inducing paternal care behaviour can evolve even in the paradoxical case where paternal ‘care’ decreases offspring fitness (see electronic supplementary material, figure S3).
Figure 3.
Model I (full siblings): microbes can expand the conditions for the evolution of paternal care. Tc, probability of microbe transmission from father to offspring through paternal care; Tm, probability of transmission from male to female during mating. The area below each graph represents the conditions allowing paternal care to evolve in the population. A microbe associated with paternal care behaviour can widen the range of conditions where paternal care prevails, and the effect increases with the transmission probability of the paternal microbes to the offspring during care. Other parameters: Tn = 0, Tv = 0.8. (a) Tm = 0.01, (b) Tm = 0.1 and (c) Tm = 0.2.
Overall, if the ratio of transmission probability through paternal care to the probability through mating is sufficiently large, microbe-induced paternal care widens the range of conditions that allows for the evolution of paternal care behaviour. This is more significant when paternal care carries a substantial cost to the benefactor in terms of mating success or does not provide enough benefit to the beneficiary.
(b). Model II: mixed brood
Now we consider a different social structure, where both males and females can engage in extra-pair mating, but offspring are brought up by social pairs [47–50]. Males of both types are guaranteed to form a social pair. Females mate with one extra-pair male besides their social mate, and a fraction Pe of their brood are sired by the extra-pair mate. This factor is affected by mate guarding, sperm competition and cryptic female choice [51]. We assume that male mating success as an extra-pair sire reduces with paternal care of that male [9]. The more the father invests in its offspring, the fitter they will be, but the father may have fewer extra-pair progeny. The fitness of an offspring cared for by its social father is increased by a factor of 1 + s. The fitness of an extra-pair offspring is increased by a factor of 1 + b, due to direct or indirect benefits gained from extra-pair mating [52,53]. Note that this extra-pair offspring can still benefit from the care of its social father.
Let ωxyz be the fitness of an offspring with a social father of type x, a mother of type y and a biological father of type z (denoted by ωxy if the social father x is also the biological father). From our assumptions, ωαβα = ωαββ = ωααα = ωααβ = (1 + b) ∗ (1 + s), ωβαα = ωβαβ = ωββα = ωβββ = (1 + b), while ωαa = ωαβ = (1 + s) and ωβa = ωββ = 1.
We denote the expected number of extra-pair matings for a caring and non-caring male as nα, nβ, respectively. We define , where γc is a factor governing the cost of caring, s is the increase in offspring fitness from paternal care, and p is the population frequency of α, the microbe associated with paternal care. Thus, when a rare mutant microbe inducing paternal care emerges in a population of males carrying microbes of type β, it will obtain matings. On the other hand, if the population comprises only caring males, they will obtain, on average, one extra-pair mating opportunity.
We find the conditions for the evolution of an α host gene, coding for paternal care and similarly for the evolution of microbes of type α, inducing host paternal care (see electronic supplementary material, for mathematical derivations).
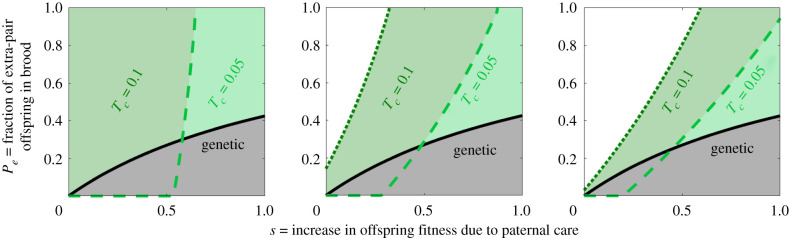
Figure 4 shows the maximal fraction of extra-pair offspring in brood, Pe, that allows for a gene or a microbe of type α (paternal care) to evolve. A high degree of extra-pair paternity in the population has a dual effect in the same direction. First, it allows for more opportunities to breed as an extra-pair sire. Males of type β have greater mating success as extra-pair sires than males of type α, thus could potentially gain more from the benefits to offspring stemming from extra-pair mating. We examine the effect of extra-pair mating benefits (b) in electronic supplementary material, figure S4. Second, it reduces the genetic relatedness of the social father to the offspring in its nest, and thus, the fitness benefits it receives from paternal care. This effect is stronger in the genetic case, since from a microbial perspective, paternal care for a genetically unrelated young individual contributes the same fitness benefits as for a genetically related one.
Figure 4.
Model II (mixed brood): the evolution of paternal care in the face of extra-pair offspring in the brood. The figure represents the maximal fraction of extra-pair offspring in brood, Pe, that allows for the evolution of paternal care induced by either genes or microbes. The solid lines represent the genetic case and the dashed and dotted lines represent the microbial cases. Generally, in the microbial case, paternal care evolves under wider conditions. The range narrows with an increase in extra-pair paternity in both the genetic and microbial cases. However, the effect is reduced when the transmission probability through paternal care (Tc) is high. The different plots (a), (b) and (c) represent different values of transmission through mating (Tm). In both the microbial and the genetic cases, as s increases, this allows for paternal care under higher degrees of extra-pair paternity. Other parameters: b = 0.5, Tn = 0, Tv = 0.8, C = 0.9; (a) Tm = 0.01, (b) Tm = 0.1 and (c) Tm = 0.2.
The increase in offspring fitness due to paternal care (s) affects the evolution of paternal care in an intricate manner, as both the benefits and the costs are increased with paternal investment. When the paternal contribution is sufficiently high, microbe-induced paternal care can evolve even when paternal care is subject to substantial costs (see electronic supplementary material, figure S5 for an example of diminished costs, where the evolution of microbe-induced paternal care is unlimited by paternity or paternal contribution).
The dynamics between the two microbe types (α and β) are strongly affected by the ratio between transmission probability through paternal care (Tc) and transmission probability through mating (Tm). Generally, a higher Tc allows for a wider range of conditions in which microbe-induced paternal care can evolve (see electronic supplementary material, figure S6 for extreme values of Tm). Figure 5 demonstrates the asymmetric contagiousness case, when microbes of type α have a lower transmission probability than microbes of type β in mating interactions (and through maternal transmission (. In this case, when considering the extra-pair behaviour, microbes of type α have diminished success both due to the males' reduced mating opportunities and due to the lower transmission probability through mating. Hence, when the fraction of extra-pair offspring in the brood (Pe) is high, the disparity in fitness increases in favour of microbes of type β. To counteract these costs and allow the care-inducing microbe to evolve, paternal care must provide a significant benefit. When the fitness benefit of paternal care (s) or transmission probability through care (Tc) are sufficiently high, microbe-induced paternal care allows for a wider range of costs than when driven by host genes. We examined microbe-induced paternal care with transmission disadvantage for Model 1 as well (see electronic supplementary material, figure S7).
Figure 5.
Model 2 (mixed brood): the evolution of paternal care in the face of extra-pair offspring in the brood, asymmetric transmission. Here, microbes of type α have a lower transmission probability than microbes of type β in mating interactions and through maternal transmission. When the fitness benefit of paternal care (s) or transmission probability through care (Tc) are sufficiently high, microbe-induced paternal care allows for a wider range of costs than when driven by host genes. Otherwise, the substantially diminished success of microbes of type α via extra-pair behaviour hinders their evolution. The different plots represent different values of transmission through mating (Tm). Other parameters: b = 0.5, Tn = 0, Tv = 0.8, C = 0.9; (a) Tm = 0.01, (b) Tm = 0.1 and (c) Tm = 0.2 · .
In conclusion, microbe-induced paternal care allows for the evolution of paternal care behaviour even in the face of the loss of paternity due to extra-pair mating, and when paternal investment results in significantly reduced mating success.
3. Discussion
In this work, we present a novel perspective into the benefits of paternal care under low levels of paternity and when paternal investment is costly. Our findings suggest that paternal care evolves more easily when induced by the microbes’ genes than by the host's genes. Our model predicts that microbe-induced paternal care would more easily evolve when parent–offspring interactions lead to a significant increase in offspring fitness, and when the probability of microbial transmission is high [41,54–57] (high Tc, probability of paternal transmission of microbes via care, resulting from interactions corresponding to feeding, grooming). We demonstrate that microbe-induced paternal care can explain the maintenance of paternal care even under high levels of extra-pair mating, when the cost of caring is high, and even in some cases when the microbe inducing paternal care has a transmission disadvantage.
Previous work has discussed possible adaptive explanations for male care behaviour when paternity is uncertain, and care is costly [16]. Suggested benefits include acquisition of skills that produce future direct fitness benefits [58]; increased opportunities for successful parenthood in the following year [17]; and increased chances of mating [19] or territory acquisition [58]. Additionally, since female choice is a key factor in determining male reproductive success, sexual selection may act directly to favour paternal care [8,59,60]. It has also been suggested that males overestimate the likelihood of paternity [61,62], which helps preserve a stable level of paternal care [16,63,64]. However, when the cost of caring is high, and the expected level of paternity is low, selection is expected to favour more suspicious males that reduce their paternal investment with increased risk of extra-pair mating [16,64–68]. Nevertheless, in some organisms, paternal care has been observed even in these cases [13,14,69]. We propose that microbial evolutionary benefits could contribute to the maintenance of paternal care. We expect that microbe-induced paternal care could play a more significant role in circumstances where the classic fitness trade-off may fall short of explaining the observed degree of paternal investment, such as in adoption [15], as well as in species where extra-pair paternity is common [48].
Thus, microbe-induced paternal care could mediate some of the costs associated with female extra-pair mating behaviour. The benefits of extra-pair mating for females [50,52,70,71] may be in obtaining a higher quality or more compatible sire [70] and bet-hedging by increasing the genetic diversity of offspring [72]. Extra-pair mating can also result in significant costs to the female. The possible costs include loss of care by social mate [73], male sexual aggression [74], increased sibling competition [75,76] and the risk of contracting sexually transmitted pathogens [77]. As demonstrated by our results, microbe-induced care by the social mate prevails under a wider range of paternity levels in comparison to care driven by host genes. Within-brood aggression between half-siblings [75] could also be mitigated by microbes, since relatedness among the microbes of the sibling is expected to be significant even if their genetic relatedness is not high [78,79].
Our model can be extended in several ways. We examined two extremes: paternal care governed exclusively by host genes or exclusively by microbial genes. However, the evolution of paternal care is likely driven by selection on reproductive units in both levels, possibly leading to intermediate levels of paternal care between host and microbial optimum. Additionally, host manipulation is unlikely to have arisen by a single mutation and more plausibly would evolve gradually through small cumulative changes. It is possible to consider that when host genes and microbial genes experience conflicting selective pressures, selection on the host would drive the evolution of resistance genes to the microbial influence. In this case, we expect the host–microbe coevolution to generate oscillatory rock–paper–scissors evolutionary dynamics, that can allow the long-term maintenance of paternal care. Similar dynamics have been suggested by some of us with respect to microbe-induced cooperation and host resistance [80]. It should also be noted that host manipulation does not have to require novel complex behaviour [81] but may more commonly take place through influencing pre-existing host behaviours [82,83]. Another extension would be allowing more female strategies. We assumed a constant level of maternal care. Yet, studies show that females may reduce their care if the male provides sufficiently intensive care or increase their care to compensate for lack of male care [84–86]. This behaviour lowers the return on paternal investment in terms of benefits to offspring fitness, effectively increasing the cost/benefit ratio. We may also consider microbial genes that contribute to the maintenance of maternal care. We predict that in many cases, microbial evolutionary interests would be to promote high levels of maternal investment, as the offspring is very likely to carry microbes of the same type as his mother, especially in cases where paternal involvement is meagre or lacking.
Our model joins the rank of previous models concerning the role of different non-genetic elements in the evolution of social traits [32,87–89]. Recent evidence suggests that microbes hold a significant role in shaping host evolution [21,32,90,91]. However, it is worth noting that the assumptions presented here are not limited to the microbiome and apply to any class of non-genetic elements that are capable of both vertical and horizontal/oblique [92] transfer and of influencing complex behavioural phenotypes. Examples of such elements may include epigenetic states [93,94] and culture [87,95–98]. Paternal care is a unique case of cooperation. The general outline of our model can also be applied to cooperation among genetic relatives with varying degrees of relatedness, cooperative breeding and eusociality [58,99].
Our theoretical results suggest a new evolutionary explanation, involving microbial regulation, to paternal care in the face of significant costs. Our results call for empirical testing of our predictions: that microbes are involved in the regulation of paternal behaviour and that factors that affect the composition of the host microbiome dramatically (e.g. antibiotics [100,101]) may also alter paternal behaviour.
Supplementary Material
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
Y.G. and L.H. designed the study and formulated the model. Y.G. and O.L.-E. derived the analytical equations and implemented the code. Y.G. and L.H. analysed the results and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This project was supported by the Clore Foundation Scholars Programme (O.L.-E.), the Israeli Science Foundation 2064/18 (L.H.) and by the Minerva Center on Lab Evolution (L.H.).
References
- 1.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383. ( 10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleiman D, Malcolm J. 1981. The evolution of male parental investment in mammals. In Parental care in mammals (eds Gubernick DJ, Klopier PH), pp. 347–387. New York, NY: Plenum Publishing. [Google Scholar]
- 3.Brown JL, Morales V, Summers K. 2010. A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am. Nat. 175, 436–446. ( 10.1086/650727) [DOI] [PubMed] [Google Scholar]
- 4.Ridley M. 1978. Paternal care. Anim. Behav. 26, 904–932 . ( 10.1016/0003-3472(78)90156-2) [DOI] [Google Scholar]
- 5.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Shellman-Reeve J. 1990. Dynamics of biparental care in the dampwood termite, Zootermopsis nevadensis (Hagen): response to nitrogen availability. Behav. Ecol. Sociobiol. 26, 389–397. ( 10.1007/BF00170895) [DOI] [Google Scholar]
- 7.Freeman H. 1983. Behavior in adult pairs of captive snow leopards (Panthera uncia). Zoo Biol. 2, 1–22. ( 10.1002/zoo.1430020102) [DOI] [Google Scholar]
- 8.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell BG.), pp. 136–179. Piscataway, NJ: Aldine Transaction. [Google Scholar]
- 9.Magrath MJL, Komdeur J. 2003. Is male care compromised by additional mating opportunity? Trends Ecol. Evol. 18, 424–430. ( 10.1016/S0169-5347(03)00124-1) [DOI] [Google Scholar]
- 10.Sheldon BC. 2002. Relating paternity to paternal care. Phil. Trans. R. Soc. Lond. B 357, 341–350. ( 10.1098/rstb.2001.0931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Møller AP, Birkhead TR. 1993. Certainty of paternity covaries with paternal care in birds. Behav. Ecol. Sociobiol. 33, 261–268. ( 10.1007/BF02027123) [DOI] [Google Scholar]
- 12.Brouwer L, Griffith SC. 2019. Extra-pair paternity in birds. Mol. Ecol. 28, 4864–4882. ( 10.1111/mec.15259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouwman KM, Lessells CM, Komdeur J. 2005. Male reed buntings do not adjust parental effort in relation to extrapair paternity. Behav. Ecol. 16, 499–506. ( 10.1093/beheco/ari021) [DOI] [Google Scholar]
- 14.Kamel SJ, Grosberg RK. 2012. Exclusive male care despite extreme female promiscuity and low paternity in a marine snail. Ecol. Lett. 15, 1167–1173. ( 10.1111/j.1461-0248.2012.01841.x) [DOI] [PubMed] [Google Scholar]
- 15.Riedman ML. 1982. The evolution of alloparental care and adoption in mammals and birds. Q. Rev. Biol. 57, 405–435. ( 10.1086/412936) [DOI] [Google Scholar]
- 16.Griffin AS, Alonzo SH, Cornwallis CK. 2013. Why do cuckolded males provide paternal care? PLoS Biol. 11, e1001520 ( 10.1371/journal.pbio.1001520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum S, Vigilant L, Kuzawa CW, Stoinski TS. 2018. Caring for infants is associated with increased reproductive success for male mountain gorillas. Sci. Rep. 8, 1–8. ( 10.1038/s41598-018-33380-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisenden BD. 1999. Alloparental care in fishes. Rev. Fish Biol. Fish. 9, 45–70. ( 10.1023/A:1008865801329) [DOI] [Google Scholar]
- 19.Kerhoas D, Kulik L, Perwitasari-Farajallah D, Agil M, Engelhardt A, Widdig A. 2016. Mother-male bond, but not paternity, influences male-infant affiliation in wild crested macaques. Behav. Ecol. Sociobiol. 70, 1117–1130. ( 10.1007/s00265-016-2116-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cryan JF, Dinan TG. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. ( 10.1038/nrn3346) [DOI] [PubMed] [Google Scholar]
- 21.Vuong HE, Yano JM, Fung TC, Hsiao EY. 2017. The microbiome and host behavior. Annu. Rev. Neurosci. 40, 21–49. ( 10.1146/annurev-neuro-072116-031347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF. 2019. Microbiota and the social brain. Science 366, eaar2016 ( 10.1126/science.aar2016) [DOI] [PubMed] [Google Scholar]
- 23.Foster JA, McVey Neufeld K-A. 2013. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. ( 10.1016/j.tins.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 24.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. 2017. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 15, 1–14. ( 10.1186/s12916-016-0771-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mändar R, Mikelsaar M. 1996. Transmission of mother's microflora to the newborn at birth. Neonatology 69, 30–35. ( 10.1159/000244275) [DOI] [PubMed] [Google Scholar]
- 26.Funkhouser LJ, Bordenstein SR. 2013. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 11, e1001631 ( 10.1371/journal.pbio.1001631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banning JL, Weddle AL, Wahl Iii GW, Simon MA, Lauer A, Walters RL, Harris RN. 2008. Antifungal skin bacteria, embryonic survival, and communal nesting in four-toed salamanders, Hemidactylium scutatum. Oecologia 156, 423–429. ( 10.1007/s00442-008-1002-5) [DOI] [PubMed] [Google Scholar]
- 28.Gunderson AR, Forsyth MH, Swaddle JP. 2009. Evidence that plumage bacteria influence feather coloration and body condition of eastern bluebirds Sialia sialis. J. Avian Biol. 40, 440–447. ( 10.1111/j.1600-048x.2008.04650.x) [DOI] [Google Scholar]
- 29.Kulkarni S, Heeb P. 2007. Social and sexual behaviours aid transmission of bacteria in birds. Behav. Processes. 74, 88–92. ( 10.1016/j.beproc.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 30.Koch H, Schmid-Hempel P. 2011. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl Acad. Sci. USA 108, 19 288–19 292. ( 10.1073/pnas.1110474108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stilling RM, Bordenstein SR, Dinan TG, Cryan JF. 2014. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Front. Cell Infect. Microbiol. 4, 147 ( 10.3389/fcimb.2014.00147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewin-Epstein O, Aharonov R, Hadany L. 2017. Microbes can help explain the evolution of host altruism. Nat. Commun. 8, 1–7. ( 10.1038/ncomms14040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothschild D, et al. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature, 555, 210–215. ( 10.1038/nature25973) [DOI] [PubMed] [Google Scholar]
- 34.Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16 050–16 055. ( 10.1073/pnas.1102999108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt C. 2015. Mental health: thinking from the gut. Nature 518, S12–S15. ( 10.1038/518S13a) [DOI] [PubMed] [Google Scholar]
- 37.Stiver KA, Alonzo SH. 2009. Parental and mating effort: is there necessarily a trade-off? Ethology 115, 1101–1126. ( 10.1111/j.1439-0310.2009.01707.x) [DOI] [Google Scholar]
- 38.Kokko H, Jennions MD. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919–948. ( 10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 39.Ahnesjö I, Kvarnemo C, Merilaita S. 2001. Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behav. Ecol. 12, 397–401. ( 10.1093/beheco/12.4.397) [DOI] [Google Scholar]
- 40.Jennions MD, Fromhage L. 2017. Not all sex ratios are equal: the Fisher condition, parental care and sexual selection. Phil. Trans. R. Soc. B 372, 20160312 ( 10.1098/rstb.2016.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tung J, et al. 2015. Social networks predict gut microbiome composition in wild baboons. eLife 4, e05224 ( 10.7554/eLife.05224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Noordwijk MA, Van Schaik CP. 2009. Intersexual food transfer among orangutans: do females test males for coercive tendency? Behav. Ecol. Sociobiol. 63, 883–890. ( 10.1007/s00265-009-0728-3) [DOI] [Google Scholar]
- 43.Poiani A. 1992. Feeding of the female breeder by male helpers in the bell miner Manorina melanophrys. Emu 92, 233–237. ( 10.1071/mu9920233) [DOI] [Google Scholar]
- 44.Smiseth PT, Moore AJ. 2004. Behavioral dynamics between caring males and females in a beetle with facultative biparental care. Behav. Ecol. 15, 621–628. ( 10.1093/beheco/arh053) [DOI] [Google Scholar]
- 45.Hunt J, Simmons LW. 2002. Behavioural dynamics of biparental care in the dung beetle Onthophagus taurus. Anim. Behav. 64, 65–75. ( 10.1006/anbe.2002.3036) [DOI] [Google Scholar]
- 46.White J, Mirleau P, Danchin E, Mulard H, Hatch SA, Heeb P, Wagner RH. 2010. Sexually transmitted bacteria affect female cloacal assemblages in a wild bird. Ecol. Lett. 13, 1515–1524. ( 10.1111/j.1461-0248.2010.01542.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westneat DF, Sherman PW, Morton ML. 1990. The ecology and evolution of extra-pair copulations in birds. Curr. Ornithol. 7, 331–369. [Google Scholar]
- 48.Petrie M, Kempenaers B. 1998. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 13, 52–57. ( 10.1016/S0169-5347(97)01232-9) [DOI] [PubMed] [Google Scholar]
- 49.Griffith SC, Owens IPF, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 50.Westneat DF, Stewart IRK. 2003. Extra-pair paternity in birds: causes, correlates and conflict. Annu. Rev. Ecol. Evol. Syst. 34, 365–396. ( 10.1146/annurev.ecolsys.34.011802.132439) [DOI] [Google Scholar]
- 51.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 52.Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B. 2014. Female extra-pair mating: adaptation or genetic constraint? Trends Ecol. Evol. 29, 456–464. ( 10.1016/j.tree.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 53.Jennions MD, Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Philos. Soc. 75, 21–64. ( 10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 54.Ezenwa VO, Ghai RR, McKay AF, Williams AE. 2016. Group living and pathogen infection revisited. Curr. Opin. Behav. Sci. 12, 66–72. ( 10.1016/j.cobeha.2016.09.006) [DOI] [Google Scholar]
- 55.Moeller AH, Foerster S, Wilson ML, Pusey AE, Hahn BH, Ochman H. 2016. Social behavior shapes the chimpanzee pan-microbiome. Sci. Adv. 2, e1500997 ( 10.1126/sciadv.1500997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song SJ, et al. 2013. Cohabiting family members share microbiota with one another and with their dogs. eLife 2, 458 ( 10.7554/eLife.00458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.VanderWaal KL, Atwill ER, Isbell LA, McCowan B. 2014. Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis). J. Anim. Ecol. 83, 406–414. ( 10.1111/1365-2656.12137) [DOI] [PubMed] [Google Scholar]
- 58.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. ( 10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 59.Kokko H, Brooks R, Jennions MD, Morley J. 2003. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270, 653–664. ( 10.1098/rspb.2002.2235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moller AP. 2000. Male parental care, female reproductive success, and extrapair paternity. Behav. Ecol. 11, 161–168. ( 10.1093/beheco/11.2.161) [DOI] [Google Scholar]
- 61.Neff BD. 2003. Decisions about parental care in response to perceived paternity. Nature 422, 716–719. ( 10.1038/nature01528) [DOI] [PubMed] [Google Scholar]
- 62.Lissåker M, Svensson O. 2008. Cannibalize or care? The role of perceived paternity in the sand goby, Pomatoschistus minutus. Behav. Ecol. Sociobiol. 62, 1467–1475. ( 10.1007/s00265-008-0576-6) [DOI] [Google Scholar]
- 63.Westneat DF, Sherman PW. 1993. Parentage and the evolution of paternal care. Behav. Ecol. 4, 66–77. ( 10.1093/beheco/4.1.66) [DOI] [Google Scholar]
- 64.Kokko H. 1998. Should advertising parental care be honest? Proc. R. Soc. Lond. B 265, 1871–1878. ( 10.1098/rspb.1998.0515) [DOI] [Google Scholar]
- 65.Manica A. 2004. Parental fish change their cannibalistic behaviour in response to the cost-to-benefit ratio of parental care. Anim. Behav. 67, 1015–1021. ( 10.1016/j.anbehav.2003.09.011) [DOI] [Google Scholar]
- 66.Houston AI, McNamara JM. 2002. A self-consistent approach to paternity and parental effort. Phil. Trans. R. Soc. Lond. B 357, 351–362. ( 10.1098/rstb.2001.0925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamilton WD. 1964. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17–52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 68.Houston AI. 1995. Parental effort and paternity. Anim. Behav. 50, 1635–1644. ( 10.1016/0003-3472(95)80017-4) [DOI] [Google Scholar]
- 69.Bose APH, Houpt N, Rawlins M, Miller JS, Juanes F, Balshine S. 2020. Indirect cue of paternity uncertainty does not affect nest site selection or parental care in a Pacific toadfish. Behav. Ecol. Sociobiol. 74, 1–10. ( 10.1007/s00265-019-2778-5) [DOI] [Google Scholar]
- 70.Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. 2003. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature 425, 714–717. ( 10.1038/nature01969) [DOI] [PubMed] [Google Scholar]
- 71.Kempenaers B, Dhondt AA. 1993. Why do females engage in extra-pair copulations? A review of hypotheses and their predictions. Belg. J. Zool. 123, 93–103. [Google Scholar]
- 72.Fox CW, Rauter CM. 2003. Bet-hedging and the evolution of multiple mating. Evolut. Ecol. Res. 5, 273–286. [Google Scholar]
- 73.Arnqvist G, Kirkpatrick M. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 165(Suppl. 5), S26–S37. ( 10.1086/429350) [DOI] [PubMed] [Google Scholar]
- 74.Valera F, Hoi H, Krištín A. 2003. Male shrikes punish unfaithful females. Behav. Ecol. 14, 403–408. ( 10.1093/beheco/14.3.403) [DOI] [Google Scholar]
- 75.Briskie JV, Naugler CT, Leech SM. 1994. Begging intensity of nestling birds varies with sibling relatedness. Proc. R. Soc. Lond. B 258, 73–78. ( 10.1098/rspb.1994.0144) [DOI] [Google Scholar]
- 76.Brante A, Fernández M, Viard F. 2013. Non-random sibling cannibalism in the marine gastropod Crepidula coquimbensis. PLoS ONE 8, e67050 ( 10.1371/journal.pone.0067050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheldon BC. 1993. Sexually transmitted disease in birds: occurrence and evolutionary significance. Phil. Trans. R. Soc. Lond. B 339, 491–497. ( 10.1098/rstb.1993.0044) [DOI] [PubMed] [Google Scholar]
- 78.Mosites E, et al. 2017. Microbiome sharing between children, livestock and household surfaces in western Kenya. PLoS ONE 12, e0171017 ( 10.1371/journal.pone.0171017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brito IL, et al. 2019. Transmission of human-associated microbiota along family and social networks. Nat. Microbiol. 4, 964–971. ( 10.1038/s41564-019-0409-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewin-Epstein O, Hadany L. 2020. Host–microbiome coevolution can promote cooperation in a rock–paper–scissors dynamics. Proc. R. Soc. B 287, 20192754 ( 10.1098/rspb.2019.2754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hughes DP, Andersen SB, Hywel-Jones NL, Himaman W, Billen J, Boomsma JJ. 2011. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 11, 13 ( 10.1186/1472-6785-11-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCurdy DG. 1999. Evidence that the parasitic nematode Skrjabinoclava manipulates host Corophium behavior to increase transmission to the sandpiper, Calidris pusilla. Behav. Ecol. 10, 351–357. ( 10.1093/beheco/10.4.351) [DOI] [Google Scholar]
- 83.Alcock J, Maley CC, Aktipis CA. 2014. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 36, 940–949. ( 10.1002/bies.201400071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kokko H. 1999. Cuckoldry and the stability of biparental care. Ecol. Lett. 2, 247–255. ( 10.1046/j.1461-0248.1999.00075.x) [DOI] [Google Scholar]
- 85.Houston AI, Székely T, McNamara JM. 2005. Conflict between parents over care. Trends Ecol. Evol. 20, 33–38. ( 10.1016/j.tree.2004.10.008) [DOI] [PubMed] [Google Scholar]
- 86.Harrison F, Barta Z, Cuthill I, Szekely T. 2009. How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800–1812. ( 10.1111/j.1420-9101.2009.01792.x) [DOI] [PubMed] [Google Scholar]
- 87.Feldman MW, Cavalli-Sforza LL, Peck JR. 1985. Gene-culture coevolution: models for the evolution of altruism with cultural transmission. Proc. Natl Acad. Sci. USA 82, 5814–5818. ( 10.1073/pnas.82.17.5814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonduriansky R, Day T. 2018. Extended heredity: a new understanding of inheritance and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 89.Bonduriansky R, Day T. 2013. Nongenetic inheritance and the evolution of costly female preference. J. Evol. Biol. 26, 76–87. ( 10.1111/jeb.12028) [DOI] [PubMed] [Google Scholar]
- 90.Morimoto J, Simpson SJ, Ponton F. 2017. Direct and trans-generational effects of male and female gut microbiota in Drosophila melanogaster. Biol. Lett. 13, 20160966 ( 10.1098/rsbl.2016.0966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. 2012. Animal behavior and the microbiome. Science 338, 198–199. ( 10.1126/science.1227412) [DOI] [PubMed] [Google Scholar]
- 92.Ram Y, Liberman U, Feldman MW. 2019. Vertical and oblique cultural transmission fluctuating in time and in space. Theor. Popul. Biol. 125, 11–19. ( 10.1016/j.tpb.2018.11.001) [DOI] [PubMed] [Google Scholar]
- 93.Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 94.Jablonka E, Lamb MJ, Zeligowski A. 2014. Evolution in four dimensions, revised edn Cambridge, MA: MIT Press. [Google Scholar]
- 95.Bartsch C, Weiss M, Kipper S. 2015. Multiple song features are related to paternal effort in common nightingales. BMC Evol. Biol. 15, 1–8. ( 10.1186/s12862-015-0390-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murray CM, Gilby IC, Mane SV, Pusey AE. 2008. Adult male chimpanzees inherit maternal ranging patterns. Curr. Biol. 18, 20–24. ( 10.1016/j.cub.2007.11.044) [DOI] [PubMed] [Google Scholar]
- 97.Cavalli-Sforza LL, Feldman M. 1981. Cultural transmission and evolution: a quantitative approach, Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 98.Rushton JP, Littlefield CH, Lumsden CJ. 1986. Gene-culture coevolution of complex social behavior: human altruism and mate choice. Proc. Natl Acad. Sci. USA 83, 7340–7343. ( 10.1073/pnas.83.19.7340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clutton-Brock T. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. ( 10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 100.Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. 2010. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 5, e9836 ( 10.1371/journal.pone.0009836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, Guigo R, Knight R, Guarner F. 2010. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 20, 1411–1419. ( 10.1101/gr.107987.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.