Abstract
Across the tree of life, hosts have evolved mechanisms to control and mediate interactions with symbiotic partners. We suggest that the evolution of physical structures that allow hosts to spatially separate symbionts, termed compartmentalization, is a common mechanism used by hosts. Such compartmentalization allows hosts to: (i) isolate symbionts and control their reproduction; (ii) reward cooperative symbionts and punish or stop interactions with non-cooperative symbionts; and (iii) reduce direct conflict among different symbionts strains in a single host. Compartmentalization has allowed hosts to increase the benefits that they obtain from symbiotic partners across a diversity of interactions, including legumes and rhizobia, plants and fungi, squid and Vibrio, insects and nutrient provisioning bacteria, plants and insects, and the human microbiome. In cases where compartmentalization has not evolved, we ask why not. We argue that when partners interact in a competitive hierarchy, or when hosts engage in partnerships which are less costly, compartmentalization is less likely to evolve. We conclude that compartmentalization is key to understanding the evolution of symbiotic cooperation.
This article is part of the theme issue ‘The role of the microbiome in host evolution’.
Keywords: compartmentalization, symbiosis, cooperation, punishment, mutualism
1. Introduction
Across the tree of life, hosts have evolved key adaptations to control and mediate interactions with symbiotic partners. In the vast majority of these partnerships, a host is interacting simultaneously with multiple—potentially competing—symbionts [1–4]. Simultaneously interacting with multiple partners can be beneficial, especially if there is variation in the benefits conferred [5]. Multiple partners can help to buffer against variable environments and changing conditions [6]. However, it can also entail costs, with conflicts among symbionts being potentially harmful to the host [4,7–12]. One problem is that interacting with multiple partners creates a potential tragedy of the commons where less beneficial individuals can share in the collective benefits the host provides, while paying lower costs. We consider such partners to be ‘cheaters' if they benefit from non-reciprocating behaviours and are evolutionarily derived from mutualists (see [13,14]). A major question in symbiosis research is how such conflicts among symbionts, and between host and symbionts, are avoided.
Vertical transmission, where symbionts are passed on from one generation to the next, can help align the fitness interests of hosts and symbionts, by increasing relatedness among symbionts sharing a host [11,15,16]. Empirical research has demonstrated how vertical transmission effectively limits symbiont diversity, reduces conflicts and can drive higher levels of dependency [17–19]. However, symbiotic partnerships involving multiple, unrelated and horizontally transmitted partner species are likewise pervasive [1,20,21], raising the question of how conflict among competing symbionts [8] is avoided when relatedness among symbionts is low, and symbionts are transmitted horizontally.
A common mechanism for helping to control symbionts is compartmentalization, where physical structures are used to separate microbes in space. These structures allow hosts to stabilize cooperation in ways that would be impossible in the absence of such structures [22]. Here, we argue that compartmentalization can help stabilize cooperation by allowing hosts to: (i) isolate symbionts and control their reproduction; (ii) reward cooperative symbionts and punish or stop interactions with non-cooperative symbionts; and (iii) reduce direct conflict among different symbiont strains in a single host. We highlight the diverse functions that compartmentalization serve and illustrate its ubiquity. Finally, we ask: how can multi-partner mutualisms be explained where compartmentalization has not evolved?
2. Compartmentalization as a means to isolate symbionts across specific tissues and control their reproduction
Containing the growth and spread of microbes to specific structures can help hosts to harness their benefits, and prevent microbial parasitism [23–27]. Many, but not all, endosymbiotic partnerships have parasitic ancestry—and by recruiting symbionts from the environment, hosts face heightened risk of infections [28–33]. Host control is essential to ensure that microbes do not invade all host tissues. In evolving the ability to restrict a microbe to particular tissues, hosts are able to limit direct negative effects, and gain benefits of partnerships. Many of these cases represent strict forms of compartmentalization (table 1). By strict compartmentalization, we mean that symbionts—often as a single genotype—are fully enveloped by well-defined, physical compartments. By physically isolating symbionts in this way, hosts can regulate symbiont growth, for example, through strict controls on their reproduction. Symbionts can also be enclosed in more fluid compartments where the boundaries are less discrete or permanent, or in partial compartments, where only part of the symbiont is enclosed. Below we describe these types of symbiont containment and controls, starting with highly compartmentalized examples and moving to less compartmentalized examples.
Table 1.
Examples of compartments divided into four main types according to their how hosts enclose their partners. These are: (i) strict compartmentalization, where symbionts, often single genotypes, are fully enveloped by well-defined, (semi) permanent boundaries; (ii) fluid compartmentalization, where the boundaries of the compartment are less discrete or permanent; (iii) partial compartmentalization, where only part of the symbiont—such as the nutrient exchange structure—is compartmentalized; and (iv) compartmentalization of non-microbial partners, whereby the enclosed mutualist is itself a large macro-organism. (?), tests of these mechanisms are not yet known.
| host | symbiont | name of compartment | containment to prevent spread within host tissue | control reproduction | mediate discrimination | control resource allocation | reduce within-host conflict | references |
|---|---|---|---|---|---|---|---|---|
| 1. host–microbe mutualisms—strict compartmentalization | ||||||||
| cicadas, mealy bugs | Sulcia, Hodgkinia, Ophiocordyceps, Moranella endobia, Tremblaya princeps | bacteriocytes within bacteriomes | yes | yes | ? | ? | yes | [32,34] |
| aphids | Buchnera, Serratia | bacteriocyte | yes | yes | no (?) | ? | yes | [35,36] |
| tortoise leaf beetles (Cassida) | Stammera | extracellular bacterium housed in specialized organs connected to the foregut | yes | yes | ? | ? | no (?) | [37] |
| sepiolid squid | Vibrio bacteria | crypts | yes | ? | yes | ? | yes | [38,39] |
| legumes, Parasponia | Rhizobia | symbiosomes within nodules (legumes) intracellular fixation threads in nodule cells (Parasponia) |
yes | yes, in some legume lineages | yes | yes | yes | [40–42] |
| scleratinian corals | Symbionidium | symbiosome (host vacuole) | yes | yes | ? | yes | no | [43,44] |
| Paracatenula flatworm | Alphaproteobacteria | trophosome | yes | yes | no (?) | yes | no (?) | [45,46] |
| Trichoplax sp. H2 | Grellia incantans and Ruthmannia eludens | rough endoplasmic reticulum of the host's internal fibre cells [G. incantans]; ventral epithelial cells [Ruthmannia eludens] | probably | ? | ? | ? | probably | [47] |
| 2. host–microbe mutualisms—fluid compartmentalization | ||||||||
| shield bug | Gammaproteobacteria | symbiont capsules, jelly secretions, faecal droplets | no | yes | no (?) | no (?) | no (?) | [24,48] |
| humans | skin microbiome | defined niches, such as hair follicles | yes | ? | no (?) | no (?) | no (?) | [49] |
| bean bug | Burkholderia | gut crypts | yes (?) | no | no (?) | ? | yes, by reducing physical contact | [50] |
| lichenicolous fungi (i.e. lichens) | algae, yeast, bacterial biome | thallus | yes | yes | no (?) | ? | probably | [51] |
| vertebrates | gut microbiome | lamina propria, Peyer's patches | yes | ? | no (?) | ? | ? | [52] |
| honeybee, termite | gut microbiome | midgut, ileum, rectum | yes | ? | no (?) | ? | ? | [53,54] |
| attine ants | Pseudonocardia bacteria | specialized cuticular structures, including crypts and tubercles | no | yes | no (?) | no (?) | yes (?) | [55,56] |
| 3. host–microbe mutualisms—partial compartmentalization | ||||||||
| land plants | arbuscular mycorrhizal fungi | root cell containing arbuscule | no | no | yes | yes | yes | [57] |
| 4. compartmentalization in non-microbial mutualisms | ||||||||
| Yucca | yucca moth | entire flower | no | yes | yes | — | ? | [58] |
| Ficus | fig-wasp | entire inflorescence (fig) | no | yes | yes | — | ? | [59,60] |
| Glochidion | Epicephala moths | entire flower | no | yes | yes | — | ? | [61] |
(a). Compartmentalization as a containment mechanism
Symbiosomes are the compartments created by scleratinian corals to host Symbionidium symbionts [43] (table 1). These compartments create a favourable environment to modulate symbiont physiology and promote photosynthesis [62], while simultaneously allowing the host to control the algal symbiont's growth [63]. Coral host compartments are generally symbiont-specific, and hosts use precise cell–cell recognition to coordinate partner entry ([64] but see [65]). Hosts have evolved mechanisms to preferentially expel highly proliferating cells over non-proliferating cells, effectively regulating population densities on a small spatial scale. This means that factors that increase symbiont division rates, such as elevated temperature, simultaneously increase expulsion rates [44].
In insects, containment of microbes in specific structures called bacteriocytes allow hosts to mediate control over symbiont spread and reproduction [66] (table 1 and figure 1a). Many—but not all—insect lineages that rely on microbial symbionts have evolved bacteriocytes, suggesting it is a common evolutionary solution for mediating symbiotic interactions [67]. In theory, bacteriocytes allow hosts to precisely control where and when microbes spread and reproduce, and thus the evolution of these structures can provide a direct benefit to hosts. While bacteriocytes tend to be highly specific, hosting only certain symbiont lineages, the occupancy of these compartments can change over evolutionary time in some insect lineages [68,69]. For example, in Japanese cicadas, recurrent losses of the bacterial symbionts Hodgkinia have been mirrored by recruitment of fungal symbiont lineages from the genus Ophiocordyceps. In these cases, fungal symbionts are located in the same bacteriome compartments that had hosted Hodgkinia—or in some cases—adjacent to it [32].
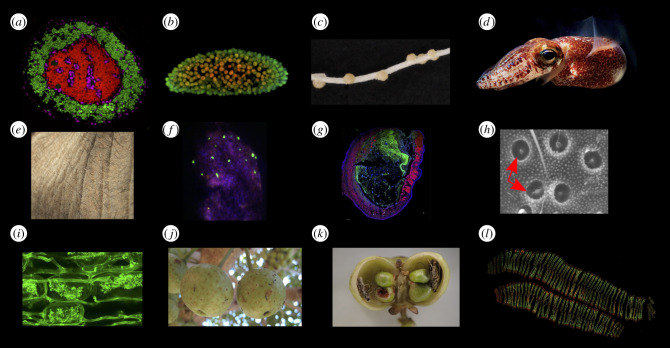
Figure 1.
Diversity of host compartmentalization in symbioses across the tree of life. (a–d) Strict symbiont compartmentalization in which symbionts are fully enclosed by well-defined, permanent compartment boundary typically with one genotype per compartment. (a) Nested bacteriocyte in cicadas. Green shows Sulcia symbionts; red shows Hodgkinia symbionts; magenta shows cicada nuclei. (b) Z-projection of confocal sections depicting the chlorophyll autofluorescence of Chlorella endosymbionts within one P. bursaria cell, with colours representing the intensity of fluorescence and therefore the position of the Chlorella in the Z-plane. (c) Lotus (legume) nodules harbour thousands of Rhizobia bacteria. (d) Hawaiian bobtail squid harbours luminescent bacteria in individual crypts. (e–h) Fluid symbiont compartmentalization in which symbionts are enclosed by less or non-discrete or permanent boundaries. (e) Vertebrate skin harbours a diverse microbiome in more open compartments. (f) Fluorescence micrograph of two bacterial gut symbionts (Leptomonas pyrrhocoris (green) and Coriobacterium glomerans (red)) in a faecal droplet from the firebug Dysdercus fasciatus. Counterstaining of DNA with DAPI (blue). (g) Representative fluorescence in situ hybridization (FISH) image of a distal colon section in a mouse fed with a conventional diet. The picture shows all bacteria stained with EUB 338 I–III (green), the eukaryotic cells stained with EUK-516 (pink) and DNA stained with DAPI (blue). (h) In fungus-farming ants, open crypts occur below the ant's thorax, allowing the development of nested populations of antibiotic-producing bacteria. (i) Partial compartmentalization with only the nutrient exchange structure compartmentalized. Arbuscular of an AM fungus. (j,k) Compartmentalization in mutualisms with non-microbial partners. In the fig/fig-wasp (j) and in the Glochidion/Epicephala moth mutualism (k), each inflorescence (fig) or flower (Glochidion) acts as a compartment that can be shed if the moth consumes too many seeds and does not pollinate. (l) Absence of compartmentalization. Confocal laser scanning microscopy image of deep-sea mussel Bathymodiolus azoricus whole gill cross-section based on direct gene FISH in which up to 16 symbionts can coexist. Genetic marker (red: Alexa Fluor 647), sulfur-oxidizing symbionts (SOX) 16S rRNA (Atto488; green). Photo credit: (a) James Van Leuven and John McCutcheon; (b) Ewan Minter; (c–e,i,j) Wikipedia; (f) Hassan Salem; (g) Alessandra Riva; (h) Hongjie Li; (k) Shixiao Luo; (l) Miguel Angel.
The physical confinement of microbes to specific host tissue can mediate how partners interact, and even drive the evolution of metabolic dependencies between those partners. As a result, once independent partners may begin to function collectively as a single metabolic unit [66]. In the mouthless catenulid flatworm genus Paracatenula, up to 50% of the body volume of some worm species can be composed of symbionts. Over 500 Myr of evolution, hosts have evolved to depend on nutritional symbionts to such a degree that they have lost their mouths and digestive tracts. Housed in compartments known as trophosomes—Paracatenula symbionts, similar to some insect symbionts, have reduced genomes and are passed directly from parent to offspring [45] (table 1). In insects, metabolic dependencies tend to be associated with spatially explicit arrangements of the interacting microbes similar to organelles [70,71]. Metabolic co-dependencies are particularly evident in nested symbioses, whereby insects—for example, mealy bugs—confine symbionts, which in turn harbour their own symbionts [34] (figure 1a).
If insect bacteriocytes represent the extreme of compartmentalization, the ‘selective assortment’ of symbionts into chemical and physical microenvironments is a less drastic form of confinement practiced by some hosts. It is more fluid, meaning the boundaries of the compartment or not as discrete or permanent as for strict compartmentalization (table 1), but likewise successful. Historically, the gut (with the exception of ruminants) was considered an open pipe system, devoid of structured compartments. Recent higher resolution mapping has challenged this view by uncovering spatially explicit sorting of symbiont communities [72,73] (figure 1g). In the vertebrate gut microbiome, for example, bacteria are often contained in compartments characterized by diverse microenvironments differing in their pH, food resources, and O2 and H2 gradients [4,74–77]. In the mammal gut, this compartmentalization by microenvironment is reinforced by the immune system. Bacterial recognition via Toll-like receptor signalling from the innate immunity system is key in removing the few bacteria which cross the containment barrier (intestinal lumen into the mucosa) [52]. This function of killing the ‘escaped bacteria’ from the immune system can be compensated by the adaptive immune system if the innate immunity system fails [52]. These mechanisms drive the compartmentalization of bacteria in the gut lumen, which is pivotal for the maintenance of the host–microbiome symbiosis.
Gut compartments in invertebrates likewise play important roles in mediating the benefits of microbial consortia [53,54]. In the honey bee microbiome, microbes degrade distinct molecules in spatially explicit patterns, with major sugar fermenters such as Gilliamella apicola in the centre of the lumen, Lactobacillus spp. in the distal rectum producing short-chain fatty acids (SCFA), and Snodgrassella alvi in the hindgut wall using acetate to maintain a stable O2 gradient. In other insects, such as termites, gut compartments become even more physically and chemically defined as hosts must employ specific microbes to break down complex materials, such as lignocellulose [78]. In contrast with the strict compartmentalization observed in insect bacteriomes and bacteriocytes, these gut compartments are more fluid in time and space (table 1).
Even in externally open environments, such as skin microbiomes, hosts rely on ‘compartmentalized control’ for skin immunity (figure 1e). Here, compartmentalization consists of confining commensal microbes to defined niches, such as hair follicles and sebaceous glands. This spatially explicit confinement allows symbionts to mediate local immunity of the host's skin [49].
(b). Compartmentalization facilitates control of partner reproduction
By spatially restricting partners to specific tissues, hosts can control their reproduction. The most effective route is via strict vertical transmission of symbiont lineages in specific compartments [4,8,66,67]. Bacteriocytes are extremely effective in facilitating the transmission of obligate symbionts in ways that resemble the transmission of organelles [79,80]. While in many cases, symbiont transmission is directly coupled to the host's reproductive organs (e.g. [81,82]), specialized external compartments and compartmentalizing behaviours can also be used to control partner reproduction, especially in cases in which symbionts are hosted extracellularly [83]. This includes attine ant genera that extracellularly harbour their bacterial symbionts in specialized cuticular crypts [55,56], and also symbionts of Pyrrhocoridae bugs, whose reproduction is controlled via egg smearing, symbiont capsules and jelly secretions, respectively ([83] and references therein; figure 1f). In these cases, compartmentalizing the symbiont in a specific secretion results in the ability to transmit symbiont lineages with comparable effectiveness as intracellular symbiont transmission [83–86]. Recent work has shown that it is not strictly the mode of transmission that stabilizes cooperation, but rather the outcome of transmission: namely genetic uniformity (high symbiont relatedness) of communities within hosts [16,87].
Compartmentalization can likewise facilitate manipulation of a partner's life cycle as a means to control reproduction. In the legume–Rhizobium nitrogen-fixing symbioses, plant hosts can manipulate individual bacterium housed in autonomous compartments called nodules (table 1) [88]. Some legume hosts are able to induce extreme cell swelling that forces terminal differentiation in their nitrogen-fixing rhizobial symbionts. By supressing Rhizobia reproduction in nodule-specific compartments, the host is able to actively rewire investment away from symbiont reproduction, and towards N2 fixation (see §3). This mechanism has evolved multiple times across the legumes [40,89].
In extreme cases of life cycle manipulation, the host can use physical confinement to prevent symbionts from entering a reproductive stage at all. This is a necessary adaptation in the symbiosis between Japanese cicadas and Ophiocordyceps fungi: reproduction by Ophiocordyceps fungi has the potential to directly kill hosts [32]. In other cases, hosts have evolved means to link life cycle manipulation to environmental conditions. The protist host Paramecium bursaria, which compartmentalizes an individual algal cell within a peri-algal vacuole, controls the growth of its Chorella algal symbiont according to light levels [33] (figure 1b). The host has evolved means to limit reproduction via acidification of the peri-algal vacuole in low light levels where the partner fails to provide significant benefits [90]. Host regulation of symbiont load may likewise operate via host-triggered symbiont division [91,92] (see §3). Similar mechanisms operate in the cnidarian–dinoflagellate symbiosis, whereby hosts can constrain symbiont cell division by acidifying the symbiosome [93].
3. Host control over compartments: discrimination followed by punishments and rewards
Compartmentalization offers the possibility to spatially screen and monitor competing genotypes [94]. In the absence of such fine-tuned discrimination, hosts can only evaluate the collective, rather than individual, performance of their partners. This can result in selfish genotypes spreading at the expense of cooperating genotypes [7,15,16]. Below, we discuss how hosts have evolved monitoring platforms to identify differences and discriminate among symbionts (§3a). We then show how hosts use this information to selectively allocate resources to the best partners, while punishing cheaters (§3b).
(a). Compartments allow for discrimination among partners
Discrimination is used in the literature to describe both (i) pre-infection mechanisms of partner choice (i.e. filtering step) and (ii) the post-infection ability of the host to monitor individual partner benefits. Here, we illustrate how compartments are used in the legume/Rhizobia and bobtail squid/Vibrio symbioses for pre-infection and post-infection discrimination, and highlight research gaps in these fields.
Legume plants can bear tens to hundreds, even thousands, of nodules along their root systems, but each nodule is generally colonized by a single Rhizobia [95] (figure 1c). This strict compartmentalization is highly controlled and initiated in legume roots when root hairs curl to encompass a single bacterium to form a nodule [96]. Pre-infection compatibility during these processes is insured by complex signalling and cell membrane receptors [97]. This is a form of partner choice that results in only a specific subset of rhizobia getting into nodules. However, some legume hosts, such as the common bean Phaseolus vulgaris, are more promiscuous than others, allowing for several types of rhizobia to form intracellular infections [98,99]. This could increase the chance of infection by less-beneficial rhizobia. An open question is whether the costs of monitoring these more diverse partnerships in nodules are high, and acts as a selection pressure driving legume hosts to evolve specificity in compartmentalization, as is more commonly seen [99]. Hosts allowing promiscuous infections could also face a stronger selection pressure to evolve effective post-infection discrimination (see §3b).
The mechanisms by which hosts monitor individual nodules, once formed, are still largely unknown. New tools, such as reporter plasmids that facilitate high-throughput measurement of N2 fixation in individual nodules, recently allowed researchers to simultaneously monitor 84 different Rhizobium leguminosarum strains in pea hosts [100]. These types of datasets will greatly improve our understanding of the mechanisms of host monitoring because rhizobia fitness can be more precisely linked with N2 symbiont-provided benefits.
Like in many host–symbiont relationships, symbionts are often under strong selection to subvert host discrimination mechanisms. Rhizobia have evolved a diverse set of tools to prevent hosts from regulating the nodulation process [101]. These include the ability to hyper-proliferate within nodule tissue [102], and to form nodules even when nitrogen from the environment is readily available [103]. In response, hosts can evolve resistance to counter-manipulations, escalating the arms race [102,104,105].
Despite being hugely divergent in their habitat, function and phylogenetic histories, sepiolid squid and legume plants have converged on surprisingly similar physical structures for symbiont control. Like legumes, squid house their bioluminescent symbiont bacteria (Vibrio fischeri) in specialized nutrient-rich epithelial compartments, known as light organs, where the symbionts reach densities of 1011 cm–3 [38,106] (figure 1d). During nocturnal hunting, the squid use light produced by bacteria in these crypts as counter illumination, potentially to hide from predators swimming at a lower depth [107].
Similar to nodules, each crypt is typically, but not always, colonized by a single bacterium, which forms a population of light-producing bacteria within the same day. However, squid hosts get to this single genotype stage in a very different way than legumes. Rather than using strict pre-infection discrimination, squid hosts use a winnowing process that involves a range of morphological, immunological and biochemical adaptations. This consists of hosts creating a selective environment in which only certain partners succeed [108,109]. Daily ventilation of the entire duct system removes approximately 90% of all Vibrio cells present, and constant environmental sampling by the host is thought to act as a selective filter to ensure that compartments are highly controlled [110], and non-luminescent bacteria are eliminated [38,111]. It has been hypothesized that discrimination against symbiont defectors can be detected upon contact, such that Vibrio dark mutants fail to trigger light organ swelling, a key step in the initiation of the symbiosis [39,112,113] (see §3b). Similar to rhizobia, Vibrio have evolved mechanisms to influence the selection process—for example, the ability of the symbionts to form biofilms and hyperaggregate can quantitatively influence how they interact with squid hosts [114].
(b). Compartmentalization drives rewards and punishments
Hosts monitor symbionts to gather information on the benefits they provide (see §3a). In theory, this ability to discriminate among simultaneously competing symbionts allows for a subsequent reaction in which hosts selectively reward and punish partners with greater effectiveness. This can include the preferential parcelling of resources (the ‘carrot’) or the selective punishment of cheats (the ‘stick’). In reality, it can be difficult to biologically separate the monitoring/discrimination step of symbiont control from selective rewarding and punishing.
In the legume–Rhizobia symbiosis, hosts use discrimination to selectively choose which symbionts form a nodule, as well as using both rewards and punishment to mediate rhizobial success inside the nodule. Past work has shown how legumes can couple resource allocation (i.e. sugars) to N2 fixation rate [88,115,116], and also suffocate rhizobia that fail to fix N2 [41]. In the sepiolid squid–Vibrio symbiosis, hosts rely on high levels of reactive oxygen species (ROS) that can be neutralized by light-producing reactions, allowing squids to link light benefits with symbiont survival and reproduction [106]. In the Paramecium bursaria–Chorella symbiosis, the host actively regulates nutrient exchange, such that Ca2+ from the host inhibits amino acid uptake into Chlorella, whereas host glucose increases the uptake—this is thought to act as a selective reward system for productive symbionts within a single host ([33,117] and references therein).
The ability to link resource allocation to symbiotic performance is facilitated when a host is able to fully enclose their symbiont partner [118], as found in legume nodules, squid crypts and peri-algal vacuoles of Paramecium. But in some cases, the host only compartmentalizes the structure of the symbiont where nutritional exchanges occur (table 1 and figure 1l). We call this partial compartmentalization. In the symbiosis between land plants and arbuscular mycorrhizal (AM) fungi, a plant root will be simultaneously colonized by multiple strains of AM fungi. These fungi form a hyphal network which extends into the soil foraging for soil nutrients, while also penetrating the host plants' root tissue. Within the plant, part of the hyphae of each strain will be confined to a membrane-bound host root compartment [57,119,120]. These compartments are called arbuscules and are formed when the plasma membrane of the host cell invaginates and proliferates around developing intracellular fungal structures [121]. Arbuscules are the primary sites of nutrient transfer, and their compartmentalization facilitates the discrimination among competing fungal strains [122]. There is evidence that the arbuscules providing less nutrients generally degenerate faster than more profitable arbuscules, leading to the hypothesis that hosts use arbuscules to monitor symbiotic quality and regulate symbiont success [123]. While the system potentially allows hosts to mediate carbon allocation at the level of individual root cells, it does not fully enclose the fungal network. This means the fungus is free to actively move resources to areas of higher plant demand, where it can potentially gain better returns [124], or even find and colonize a less-discriminating host plant [125].
In all these examples, compartmentalization allows hosts to discriminate among partners based on symbiont performance, rather than relying solely on symbiont identity. This may be fundamentally important for the stability of many horizontally transmitted symbioses. Pre-infection mechanisms (i.e. ‘partner choice’) generally rely on signalling cues as indicator of partner quality. Signals, however, are notoriously vulnerable to exploitation or cheating mimicking signals of cooperative competitors [126,127]. A system that uses compartmentalization to monitor and preferentially allocate resources will be more robust to cheating or exploitation [15,105,128]. A critical test of the robustness of post-infection sanctions was demonstrated in a recent experiment whereby pea hosts were colonized by isogenic lines of Rhizobia that differed in the expression level of the nitrogen-fixing gene nifH. The work showed that pea hosts were unable to identify non-fixing Rhizobia at the pre-infection stage, but successfully discriminated against the non-fixing strain after nodule formation by decreasing sugar allocation to nodules formed by this ineffective partner [116].
Scaling up to mutualisms between plants and pollinators, compartmentalization likewise mediates partner rewards. In nursery pollination systems, plants are pollinated exclusively by specific lineages of pollinators in exchange for oviposition within flowers [61,129,130]. The hatching larvae, which are obligately dependent on the host plant, then consume a subset of the resulting seeds. There is a tension between pollinators and host plants because when a pollinator lays too many eggs on a single flower, a high proportion of seeds will be eaten by pollinator larvae, hampering plant reproduction. In figs, hosts appear to control the number of wasps entering each reproductive compartment (i.e. enclosed fig inflorescence), with numbers of foundress wasps per fruit more clearly reflecting the reproductive interests of the figs compared to the wasps [129]. In figs, Yucca and Glochidion there is some evidence that hosts can selectively abort flowers with high egg loads (table 1) [58,59,61,131,132]. This type of compartment-level sanctioning by host plants results in a direct cost to pollinating insects: moths suffer fitness losses as high as 62% in the Glochidion mutualism if they oviposit into pre-infested flowers [61]. Now future work needs to explore the extent to which this behaviour is based on pre-adaptations versus adaptive responses directly to cheating pollinators. These examples highlight that compartmentalization does not only occur in microbial symbioses (see this section and Conclusion for outstanding questions).
(c). Imperfect discrimination can still be effective
Above, we have focused on the effectiveness of discrimination at the compartment level. However, discrimination is rarely absolute. It more likely follows a continuum of precision and effectiveness. Biologically, this can mean that hosts allocate resources into coarse features (also called ‘modules') where symbiotic services are generated rather than directly allocating resources to individual symbionts [133]. While precise sanctioning may provide a selective advantage, it is not universal. This could be because precise sanctioning is costly, or because it is physically difficult, as in the case of the fig–fig-wasp mutualism [60]. This implies that there could be an evolutionarily optimum level well below ‘maximum precision’.
The degree to which symbionts are effectively compartmentalized is likely related to the direct benefits the host receives by controlling (or not controlling) the partner. Parasponia plants in the Cannabaceae family are the only known non-legume host to form N2-fixing symbioses with rhizobia. The symbiosis has been called ‘a delicate balance between mutual benefits and parasitic colonization’, largely because of the inability of the host to compartmentalize and control growth of certain inefficient rhizobial strains [134]. In contrast with legume nodules, in which rhizobia are housed in transient organelle-like structures (figure 1c), rhizobia remain in intracellular fixation threads in Parasponia nodule cells and nodules appear more like lateral roots [128]. However imperfect the discrimination, there is evidence that hosts can mediate the success of the intracellular rhizobia when necessary, like under high nitrogen conditions [42]. Such imperfect discrimination has likewise been identified in the mycorrhizal mutualism [120,122,125].
4. Compartmentalization can prevent within-host conflict
Hosts interact with multitudes of microbes. The coexistence of diverse consortiums is often crucial to nutritional and defensive functions of the host [135–138]. However, mixing of symbiont lineages can create within-host conflict. Within-host conflict means that competitive interactions among the symbionts themselves are detrimental to the host, for example, by driving an overall reduction in symbiont populations. This has been shown, for example, in competition among AM fungi colonizing a single root system: competitive interactions led to both: (i) competitors investing more in accessing host root resources (i.e. which benefits the symbiont) compared to growth strategies dedicated to nutrient foraging (i.e. which benefits the host) and (ii) an overall reduction in mycorrhizal fungal biomass [139]. A theory by Frank [7,8] raised the issue that symbiont mixing can lead to conflict, but also stressed the idea that hosts would not be under strong selection to reduce symbiont diversity, because the benefits of reducing diversity would not be immediate in many systems. However, in cases where hosts realize an immediate benefit to reducing or restricting diversity, these processes can be a strong selection pressure (e.g. [140]).
This is further illustrated in examples where hosts use compartments to create selective habitats in which only the effective mutualists thrive. In the bean bug Riptortus, highly specialized crypts in the posterior midgut create a selective environment where mutualistic Burkholderia bacteria can outcompete non-symbiotic bacteria, effectively functioning as an organ for symbiont sorting [50]. Similarly, African fungus-growing termites actively propagate single variants of their Termitomyces symbiont, even though cultures are initially started from genetically variable spores from the habitat. In these cases, the ‘compartments’ are selective habitats created by the host to help mediate competition among symbionts by facilitating the colonization of the preferred symbiont over the less-preferred symbiont [19] (table 1).
In theory, compartmentalization is a powerful way to avoid within-host symbiont conflicts. But as discussed above (see §3c), imperfect compartmentalization may be common. In legumes, for example, when rhizobial densities in soil are high, nodules can contain more than one founding bacterium, creating ‘mixed nodules’ of multi-genotypes [115,141,142]. New work suggests that mixed nodules are even more common that previously assumed: one study found mixed occupancy in approximately 20% of nodules, with some nodules containing up to six different strains [100].
The problem of mixed nodules is twofold. First, competition within a nodule may divert resources away from N2 fixation and towards competitive antagonism [143]; this potentially includes the production of rhizobial warfare compounds such as bacteriocins [144]. Second, compartmentalization in mixed nodules is less effective because low-quality strains can benefit from sharing a nodule with a high-quality N2 fixer, facilitating their spread [145,146]. Again, this is similar to secondary invasion of squid crypts by Vibro, meaning that strict segregation of symbiont lineages is not always possible [147]. In the case of legumes, the reduction of effectiveness in symbiosis is due to within-host conflict, whereas in squids, the conflict is between the host and the symbiont.
There is evidence, however, in both legume and squid systems that hosts can target mixed compartments, and eliminate competing strains and poor partners. Emerging evidence based on micrographs suggests that certain legumes (e.g. Lotus) can target individual non-N2-fixing bacteroids in mixed nodules containing both fixing and non-fixing genotypes [146]. There is also evidence that squids can detect and eliminate low-quality strains from mixed crypts [111]. So-called dark mutants that fail to produce light can initially colonize squid crypts, but they decline exponentially, reaching undetectable levels within weeks [148]. The mechanistic basis of this process is not yet understood, but the finding underscores the idea that precision in segregation is under strong selection.
5. Compartmentalization is not always the solution
Compartmentalization is a very powerful ‘divide and conquer’ strategy for dealing with multiple partners. However, it is not always the best—or only—evolutionary solution. Hosts could maintain symbiont quality by providing a specific environment to selectively cultivate high-quality symbionts [149–153]. In microbiomes, this mechanism works by allowing symbionts to directly compete in ways which winnow out less-effective partners [154]. This suggests that within-host conflict (see §4) can be positive for hosts in some systems and contexts rather than negative. While the symbionts are still restricted to specific crypts or structures over which the host has a high level of control, it is the within-host symbiont competition itself that drives selection for the ‘optimal’ symbiont. Are there general patterns for explaining cases where compartmentalization has not evolved in symbiotic partnerships?
First, compartmentalization is only likely to evolve if hosts realize a direct and immediate benefit of compartmentalizing their partners [7,8,155]. Second, there are cases where simultaneously hosting multiple partners may be good because partners provide different types of benefits, which are important in different environments or across ontogeny [5,6]. For instance, in the whistle-thorn acacia (Vachellia drepanolobium), four species of symbiotic ants provide distinct mutualistic services across ontogeny, yet they cannot coexist on the same tree [156]. Here, conflict between multiple partner is not supressed by hosts, and competition among partners may even be positive because it allows for selection of the most effective symbiont under those certain conditions. Other examples include fungus-cultivating ambrosia beetles, whereby direct competition among fungal strains helps hosts select for more effective symbionts [150], and toxic warfare among symbionts competing in the guts of bees potentially leading to more beneficial gut communities [157].
Third, there are cases in which the host does not experience costs associated with multiple partners. This is likely because partners are not directly fed by the host, and thus do not represent a cost. In deep-sea Bathymodiolus mussels, individuals can host as many as 16 coexisting strains of intracellular, sulfur-oxidizing symbionts [21]. The strains, which differ in key functions, feed from the environment (i.e. sulfur seeps) and despite their intracellular nature, are low cost for mussel hosts. More generally, low-cost partnerships do not face the same selection pressures as those where hosts are providing a large portion of their resources (e.g. 10–20% of fixed carbon to rhizobia) to maintaining symbiotic partners.
6. Conclusion
Compartmentalization is widespread and fundamental to cooperative partnerships. It allows hosts to: (i) isolate symbionts, preventing parasitic invasion, and manipulate symbiont reproduction; (ii) discriminate between cooperative and non-cooperative symbionts, enabling rewards and punishment or cessation of the interaction; and (iii) reduce within-host conflict. We expect compartmentalization to evolve only when it provides an immediate, direct benefit to the host [7,8]. Consequently, our framework suggests a number of future research directions. First, we know very little about the selective pressures governing when and what type of compartment evolves—for instance, when do we see strict versus fluid compartments evolving (table 1)? Based on our current understanding of the distribution of compartment types, we would expect strict compartmentalization to evolve where strict control over reproduction and/or controlled resource allocation is selected for. Second, it is unclear whether the precision of compartmentalization (symbiont genotype versus more modular) depends more on the costs to the host of increased precision, or on the physical constraints of evolving precision in compartmentalized symbioses. Third, our treatment of compartmentalization as a mechanism to reduce within-host conflict has focused on symbiosis with microbes. How compartmentalization operates more generally across mutualisms with macro-organisms, beyond our pollination examples, remains unknown. Answering these questions remains a major task.
Acknowledgements
We thank Zachary Phillips and two anonymous referees for their very useful feedback.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to conception; G.C. and E.T.K. led the writing with substantial edits from all coauthors.
Competing interests
We declare we have no competing interests.
Funding
Research was supported by European Research Council ERC 335542 (to E.T.K.) and the Ammodo Foundation (E.T.K.). G.C. is supported by a NERC Independent Research Fellowship (grant no. NE/S014470/1).
References
- 1.Schloissnig S, et al. 2013. Genomic variation landscape of the human gut microbiome. Nature 493, 45–50. ( 10.1038/nature11711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronstein JL. (ed.). 2015. Mutualism. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Martin FM, Uroz S, Barker DG. 2017. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356, eaad4501 ( 10.1126/science.aad4501) [DOI] [PubMed] [Google Scholar]
- 4.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. 2017. The evolution of the host microbiome as an ecosystem on a leash. Nature 548, 43–51. ( 10.1038/nature23292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batstone RT, Carscadden KA, Afkhami ME, Frederickson ME. 2018. Using niche breadth theory to explain generalization in mutualisms. Ecology 99, 1039–1050. ( 10.1002/ecy.2188) [DOI] [PubMed] [Google Scholar]
- 6.Chomicki G, Weber M, Antonelli A, Bascompte J, Kiers ET. 2019. The impact of mutualisms on species richness. Trends Ecol. Evol. 34, 698–711. ( 10.1016/j.tree.2019.03.003) [DOI] [PubMed] [Google Scholar]
- 7.Frank SA. 1994. Genetics of mutualism: the evolution of altruism between species. J. Theor. Biol. 170, 393–400. ( 10.1006/jtbi.1994.1200) [DOI] [PubMed] [Google Scholar]
- 8.Frank SA. 1996. Host–symbiont conflict over the mixing of symbiotic lineages. Proc. R. Soc. Lond. B 263, 339–344. ( 10.1098/rspb.1996.0052) [DOI] [PubMed] [Google Scholar]
- 9.Frank SA. 1996. Host control of symbiont transmission: the separation of symbionts into germ and soma. Am. Nat. 148, 1113–1124. ( 10.1086/285974) [DOI] [Google Scholar]
- 10.Frank SA. 1997. Models of symbiosis. Am. Nat. 150, S80–S99. ( 10.1086/286051) [DOI] [PubMed] [Google Scholar]
- 11.Frank SA. 2003. Repression of competition and the evolution of cooperation. Evolution 57, 693–705. ( 10.1111/j.0014-3820.2003.tb00283.x) [DOI] [PubMed] [Google Scholar]
- 12.Foster KR, Wenseleers T. 2006. A general model for the evolution of mutualisms. J. Evol. Biol. 19, 1283–1293. ( 10.1111/j.1420-9101.2005.01073.x) [DOI] [PubMed] [Google Scholar]
- 13.Ghoul M, Griffin AS, West SA. 2014. Toward an evolutionary definition of cheating. Evolution 68, 318–331. ( 10.1111/evo.12266) [DOI] [PubMed] [Google Scholar]
- 14.Chomicki G, Kiers ET, Renner SS In press. The evolution of mutualistic dependence. Annu. Rev. Ecol. Evol. Syst. 51 ( 10.1146/annurev-ecolsys-110218-024629) [DOI] [Google Scholar]
- 15.West SA, Kiers ET, Simms EL, Denison RF. 2002. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. Lond. B 269, 685–694. ( 10.1098/rspb.2001.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeks A, dos Santos M, West SA. 2019. Transmission, relatedness, and the evolution of cooperative symbionts. J. Evol. Biol. 32, 1036–1045. ( 10.1111/jeb.13505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher RM, Henry LM, Cornwallis CK, Kiers ET, West SA. 2017. The evolution of host-symbiont dependence. Nat. Commun. 8, 15973 ( 10.1038/ncomms15973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs JL, Wilcox TP. 2005. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc. R. Soc. B 273, 425–429. ( 10.1098/rspb.2005.3346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aanen DK, Henrik H, Debets AJ, Kerstes NA, Hoekstra RF, Boomsma JJ. 2009. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 326, 1103–1106. ( 10.1126/science.1173462) [DOI] [PubMed] [Google Scholar]
- 20.Engel P, Stepanauskas R, Moran NA. 2014. Hidden diversity in honey bee gut symbionts detected by single-cell genomics. PLoS Genet. 10, e1004596 ( 10.1371/journal.pgen.1004596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansorge R, Romano S, Sayavedra L, Porras MÁG, Kupczok A, Tegetmeyer HE, Dubilier N, Petersen J. 2019. Functional diversity enables multiple symbiont strains to coexist in deep-sea mussels. Nat. Microbiol. 4, 2487–2497. ( 10.1038/s41564-019-0572-9) [DOI] [PubMed] [Google Scholar]
- 22.Maynard SJ, Szathmáry E. 1995. The major transitions in evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Yamamura N. 1993. Vertical transmission and evolution of mutualism from parasitism. Theor. Pop. Biol. 44, 95–109. ( 10.1006/tpbi.1993.1020) [DOI] [Google Scholar]
- 24.Salem H, Onchuru TO, Bauer E, Kaltenpoth M. 2015. Symbiont transmission entails the risk of parasite infection. Biol. Lett. 11, 20150840 ( 10.1098/rsbl.2015.0840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King KC, et al. 2016. Rapid evolution of microbe-mediated protection against pathogens in a worm host. ISME J. 10, 1915–1924. ( 10.1038/ismej.2015.259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro JW, Turner PE. 2018. Evolution of mutualism from parasitism in experimental virus populations. Evolution 72, 707–712. ( 10.1111/evo.13440) [DOI] [PubMed] [Google Scholar]
- 27.Tso GHW, et al. 2018. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595. ( 10.1126/science.aat0537) [DOI] [PubMed] [Google Scholar]
- 28.Law R, Dieckmann U. 1998. Symbiosis through exploitation and the merger of lineages in evolution. Proc. R. Soc. Lond. B 265, 1245–1253. ( 10.1098/rspb.1998.0426) [DOI] [Google Scholar]
- 29.Spatafora JW, Sung GH, Sung JM, Hywel-Jones NL, White JF Jr. 2007. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 16, 1701–1711. ( 10.1111/j.1365-294X.2007.03225.x) [DOI] [PubMed] [Google Scholar]
- 30.Sachs JL, Skophammer RG, Regus JU. 2011. Evolutionary transitions in bacterial symbiosis. Proc. Natl Acad. Sci. USA 108, 10 800–10 807. ( 10.1073/pnas.1100304108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zug R, Hammerstein P. 2015. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 90, 89–111. ( 10.1111/brv.12098) [DOI] [PubMed] [Google Scholar]
- 32.Matsuura Y, Moriyama M, Łukasik P, Vanderpool D, Tanahashi M, Meng XY, McCutcheon JP, Fukatsu T. 2018. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc. Natl Acad. Sci. USA 115, E5970–E5979. ( 10.1073/pnas.1803245115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sørensen ME, Lowe CD, Minter EJ, Wood AJ, Cameron D, Brockhurst MA. 2019. The role of exploitation in the establishment of mutualistic microbial symbioses. FEMS Microbiol. Lett. 366, fnz148 ( 10.1093/femsle/fnz148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husnik F, McCutcheon JP. 2016. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc. Natl Acad. Sci. USA 113, E5416–E5424. ( 10.1073/pnas.1603910113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braendle C, Miura T, Bickel R, Shingleton AW, Kambhampati S, Stern DL. 2003. Developmental origin and evolution of bacteriocytes in the aphid–Buchnera symbiosis. PLoS Biol. 1, 70–76. ( 10.1371/journal.pbio.0000021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monnin D, Jackson R, Kiers ET, Bunker M, Ellers J, Henry LM. 2020. Parallel evolution in the integration of a co-obligate aphid symbiosis. Curr. Biol. 30, 1949–1957. ( 10.1016/j.cub.2020.03.011) [DOI] [PubMed] [Google Scholar]
- 37.Salem H, et al. 2017. Drastic genome reduction in an herbivore's pectinolytic symbiont. Cell 171, 1520–1531. ( 10.1016/j.cell.2017.10.029) [DOI] [PubMed] [Google Scholar]
- 38.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182, 4578–4586. ( 10.1128/jb.182.16.4578-4586.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyashiro T, Ruby EG. 2012. Shedding light on bioluminescence regulation in Vibrio fischeri. Mol. Microbiol. 84, 795–806. ( 10.1111/j.1365-2958.2012.08065.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oono R, Denison RF. 2010. Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteroids. Plant Phys. 154, 1541–1548. ( 10.1104/pp.110.163436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiers ET, Rousseau RA, West SA, Denison RF. 2003. Host sanctions and the legume–rhizobium mutualism. Nature 425, 78–81. ( 10.1038/nature01931) [DOI] [PubMed] [Google Scholar]
- 42.Dupin SE, Geurts R, Kiers ET. 2020. The non-legume Parasponia andersonii mediates the fitness of nitrogen-fixing rhizobial symbionts under high nitrogen conditions. Front. Plant Sci. 10, 1779 ( 10.3389/fpls.2019.01779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fransolet D, Roberty S, Plumier JC. 2012. Establishment of endosymbiosis: the case of cnidarians and Symbiodinium. J. Exp. Mar. Biol. Ecol. 420, 1–7. ( 10.1016/j.jembe.2012.03.015) [DOI] [Google Scholar]
- 44.Baghdasarian G, Muscatine L. 2000. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 199, 278–286. ( 10.2307/1543184) [DOI] [PubMed] [Google Scholar]
- 45.Gruber-Vodicka HR, et al. 2011. Paracatenula, an ancient symbiosis between thiotrophic Alphaproteobacteria and catenulid flatworms. Proc. Natl Acad. Sci. USA 108, 12 078–12 083. ( 10.1073/pnas.1105347108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, et al. 2020. Genomic, transcriptomic, and proteomic insights into the symbiosis of deep-sea tubeworm holobionts. ISME J. 14, 135–150. ( 10.1038/s41396-019-0520-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruber-Vodicka HR, Leisch N, Kleiner M, Hinzke T, Liebeke M, McFall-Ngai M, Hadfield MG, Dubilier N. 2019. Two intracellular and cell type-specific bacterial symbionts in the placozoan Trichoplax H2. Nat. Microbiol. 4, 1465–1474. ( 10.1038/s41564-019-0475-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karamipour N, Fathipour Y, Mehrabadi M. 2016. Gammaproteobacteria as essential primary symbionts in the striped shield bug, Graphosoma lineatum (Hemiptera: Pentatomidae). Sci. Rep. 6, 33168 ( 10.1038/srep33168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naik S, et al. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119. ( 10.1126/science.1225152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoh H, Jang S, Takeshita K, Ohbayashi T, Ohnishi N, Meng XY, Mitani Y, Kikuchi Y. 2019. Host–symbiont specificity determined by microbe–microbe competition in an insect gut. Proc. Natl Acad. Sci. USA 116, 22 673–22 682. ( 10.1073/pnas.1912397116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawksworth DL, Grube M. 2020. Lichens redefined as complex ecosystems. New Phytol. ( 10.1111/nph.16630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slack E, et al. 2009. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science 325, 617–620. ( 10.1126/science.1172747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA. 2017. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl Acad. Sci. USA 114, 4775–4780. ( 10.1073/pnas.1701819114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng H, Perreau J, Powell JE, Han B, Zhang Z, Kwong WK, Tringe SG, Moran NA. 2019. Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc. Natl Acad. Sci. USA 116, 25 909–25 916. ( 10.1073/pnas.1916224116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311, 81–83. ( 10.1126/science.1119744). [DOI] [PubMed] [Google Scholar]
- 56.Li H, Sosa-Calvo J, Horn HA, Pupo MT, Clardy J, Rabeling C, Schultz TR, Currie CR. 2018. Convergent evolution of complex structures for ant–bacterial defensive symbiosis in fungus-farming ants. Proc. Natl Acad. Sci. USA 115, 10 720–10 725. ( 10.1073/pnas.1809332115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison MJ, Ivanov S. 2017. Exocytosis for endosymbiosis: membrane trafficking pathways for development of symbiotic membrane compartments. Curr. Opin. Plant Biol. 38, 101–108. ( 10.1016/j.pbi.2017.04.019) [DOI] [PubMed] [Google Scholar]
- 58.Pellmyr O, Huth CJ. 1994. Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372, 257–260. ( 10.1038/372257a0) [DOI] [Google Scholar]
- 59.Jandér KC, Herre EA. 2010. Host sanctions and pollinator cheating in the fig tree–fig wasp mutualism. Proc. R. Soc. B 277, 1481–1488. ( 10.1098/rspb.2009.2157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jandér CK, Herre EA, Simms EL. 2012. Precision of host sanctions in the fig tree–fig wasp mutualism: consequences for uncooperative symbionts. Ecol. Lett. 15, 1362–1369. ( 10.1111/j.1461-0248.2012.01857.x) [DOI] [PubMed] [Google Scholar]
- 61.Goto R, Okamoto T, Kiers ET, Kawakita A, Kato M. 2010. Selective flower abortion maintains moth cooperation in a newly discovered pollination mutualism. Ecol. Lett. 13, 321–329. ( 10.1111/j.1461-0248.2009.01425.x) [DOI] [PubMed] [Google Scholar]
- 62.Barott KL, Venn AA, Perez SO, Tambutté S, Tresguerres M. 2015. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl Acad. Sci. USA 112, 607–612. ( 10.1073/pnas.1413483112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkerson FP, Kobayashi D, Muscatine L. 1988. Mitotic index and size of symbiotic algae in Caribbean reef corals. Coral Reefs 7, 29–36. ( 10.1007/BF00301979) [DOI] [Google Scholar]
- 64.Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. 2006. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 8, 1985–1993. ( 10.1111/j.1462-5822.2006.00765.x) [DOI] [PubMed] [Google Scholar]
- 65.Silverstein RN, Correa AM, Baker AC. 2012. Specificity is rarely absolute in coral–algal symbiosis: implications for coral response to climate change. Proc. R. Soc. B 279, 2609–2618. ( 10.1098/rspb.2012.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Douglas AE. 2016. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 14, 731 ( 10.1038/nrmicro.2016.151) [DOI] [PubMed] [Google Scholar]
- 67.Douglas AE. 2010. The symbiotic habit. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Koga R, Bennett GM, Cryan JR, Moran NA. 2013. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 15, 2073–2081. ( 10.1111/1462-2920.12121) [DOI] [PubMed] [Google Scholar]
- 69.Koga R, Moran NA. 2014. Swapping symbionts in spittlebugs: evolutionary replacement of a reduced genome symbiont. ISME J. 8, 1237 ( 10.1038/ismej.2013.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCutcheon JP, Von Dohlen CD. 2011. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr. Biol. 21, 1366–1372. ( 10.1016/j.cub.2011.06.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.López-Madrigal S, Latorre A, Porcar M, Moya A, Gil R. 2013. Mealybugs nested endosymbiosis: going into the ‘matryoshka’ system in Planococcus citri in depth. BMC Microbiol. 13, 74 ( 10.1186/1471-2180-13-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weimer PJ. 2015. Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front. Microbiol. 6, 296 ( 10.3389/fmicb.2015.00296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Welch JLM, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. 2016. Biogeography of a human oral microbiome at the micron scale. Proc. Natl Acad. Sci. USA 113, E791–E800. ( 10.1073/pnas.1522149113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caldara M, Friedlander RS, Kavanaugh NL, Aizenberg J, Foster KR, Ribbeck K. 2012. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 22, 2325–2330. ( 10.1016/j.cub.2012.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336, 1268–1273. ( 10.1126/science.1223490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. 2013. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799. ( 10.1038/nrmicro3109) [DOI] [PubMed] [Google Scholar]
- 77.Hacquard S, et al. 2015. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 17, 603–616. ( 10.1016/j.chom.2015.04.009) [DOI] [PubMed] [Google Scholar]
- 78.Mikaelyan A, Meuser K, Brune A. 2017. Microenvironmental heterogeneity of gut compartments drives bacterial community structure in wood and humus-feeding higher termites. FEMS Microbiol. Ecol. 93, fiw210 ( 10.1093/femsec/fiw210) [DOI] [PubMed] [Google Scholar]
- 79.Koga R, Meng XY, Tsuchida T, Fukatsu T. 2012. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte–embryo interface. Proc. Natl Acad. Sci. USA 109, E1230–E1237. ( 10.1073/pnas.1119212109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keeling PJ, McCutcheon JP. 2017. Endosymbiosis: the feeling is not mutual. J. Theor. Biol. 434, 75–79. ( 10.1016/j.jtbi.2017.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feldhaar H, Straka J, Krischke M, Berthold K, Stoll S, Mueller MJ, Gross R. 2007. Nutritional upgrading for omnivorous carpenter ants by the endosymbiont Blochmannia. BMC Biol. 5, 48 ( 10.1186/1741-7007-5-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perlmutter JI, Bordenstein SR. 2020. Microorganisms in the reproductive tissues of arthropods. Nat. Rev. Genet. 18, 97–111. ( 10.1038/s41579-019-0309-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salem H, Florez L, Gerardo N, Kaltenpoth M. 2015. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc. R. Soc. B 282, 20142957 ( 10.1098/rspb.2014.2957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4, e337 ( 10.1371/journal.pbio.0040337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kikuchi Y, Hosokawa T, Nikoh N, Meng XY, Kamagata Y, Fukatsu T. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7, 2 ( 10.1186/1741-7007-7-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaiwa N, et al. 2014. Symbiont-supplemented maternal investment underpinning host's ecological adaptation. Curr. Biol. 24, 2465–2470. ( 10.1016/j.cub.2014.08.065) [DOI] [PubMed] [Google Scholar]
- 87.Kenkel CD, Bay LK. 2018. Exploring mechanisms that affect coral cooperation: symbiont transmission mode, cell density and community composition. PeerJ 6, e6047 ( 10.7717/peerj.6047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sachs JL, Quides KW, Wendlandt CE. 2018. Legumes versus rhizobia: a model for ongoing conflict in symbiosis. New Phytol. 219, 1199–1206. ( 10.1111/nph.15222) [DOI] [PubMed] [Google Scholar]
- 89.Mergaert P, et al. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium–legume symbiosis. Proc. Natl Acad. Sci. USA 103, 5230–5235. ( 10.1073/pnas.0600912103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lowe CD, Minter EJ, Cameron DD, Brockhurst MA. 2016. Shining a light on exploitative host control in a photosynthetic endosymbiosis. Curr. Biol. 26, 207–211. ( 10.1016/j.cub.2015.11.052) [DOI] [PubMed] [Google Scholar]
- 91.Takahashi T, Shirai Y, Kosaka T, Hosoya H. 2007. Arrest of cytoplasmic streaming induces algal proliferation in green paramecia. PLoS ONE 2, e1352 ( 10.1371/journal.pone.0001352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Minter EJ, Lowe CD, Sørensen ME, Wood AJ, Cameron DD, Brockhurst MA. 2018. Variation and asymmetry in host-symbiont dependence in a microbial symbiosis. BMC Evol. Biol. 18, 108 ( 10.1186/s12862-018-1227-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davy SK, Allemand D, Weis VM. 2012. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 76, 229–261. ( 10.1128/MMBR.05014-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiers ET, Denison RF. 2008. Sanctions, cooperation, and the stability of plant-rhizosphere mutualisms. Annu. Rev. Ecol. Evol. Syst. 39, 215–236. ( 10.1146/annurev.ecolsys.39.110707.173423) [DOI] [Google Scholar]
- 95.Denison RF, Kiers ET. 2011. Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr. Biol. 21, R775–R785. ( 10.1016/j.cub.2011.06.018) [DOI] [PubMed] [Google Scholar]
- 96.Parniske M. 2018. Uptake of bacteria into living plant cells, the unifying and distinct feature of the nitrogen-fixing root nodule symbiosis. Curr. Opin. Plant Biol. 44, 164–174. ( 10.1016/j.pbi.2018.05.016) [DOI] [PubMed] [Google Scholar]
- 97.Kawaharada Y, et al. 2015. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523, 308–312. ( 10.1038/nature14611) [DOI] [PubMed] [Google Scholar]
- 98.Arthikala MK, Sánchez-López R, Nava N, Santana O, Cárdenas L, Quinto C. 2014. RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol. 202, 886–900. ( 10.1111/nph.12714) [DOI] [PubMed] [Google Scholar]
- 99.Wang Q, Liu J, Zhu H. 2018. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 9, 313 ( 10.3389/fpls.2018.00313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendoza-Suárez MA, Geddes BA, Sánchez-Cañizares C, Ramírez-González RH, Kirchhelle C, Jorrin B, Poole PS. 2020. Optimizing Rhizobium-legume symbioses by simultaneous measurement of rhizobial competitiveness and N2 fixation in nodules. Proc. Natl Acad. Sci. USA 117, 9822–9831. ( 10.1073/pnas.1921225117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma WB, Penrose DM, Glick BR. 2002. Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 48, 947–954. ( 10.1139/w02-100) [DOI] [PubMed] [Google Scholar]
- 102.Price PA, Tanner HR, Dillon BA, Shabab M, Walker GC, Griffitts JS. 2015. Rhizobial peptidase hrrP cleaves host-encoded signaling peptides and mediates symbiotic compatibility. Proc. Natl Acad. Sci. USA 112, 15 244–15 249. ( 10.1073/pnas.1417797112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen HP, Miwa H, Obirih-Opareh J, Suzaki T, Yasuda M, Okazaki S. 2019. Novel rhizobia exhibit superior nodulation and biological nitrogen fixation even under high nitrate concentrations. FEMS Microbiol. Ecol. 96, fiz184 ( 10.1093/femsec/fiz184) [DOI] [PubMed] [Google Scholar]
- 104.Yasuda M, Miwa H, Masuda S, Takebayashi Y, Sakakibara H, Okazaki S. 2016. Effector-triggered immunity determines host genotype-specific incompatibility in legume-Rhizobium symbiosis. Plant Cell Physiol. 57, 1791–1800. ( 10.1093/pcp/pcw104) [DOI] [PubMed] [Google Scholar]
- 105.Gano-Cohen KA, Wendlandt CE, Stokes PJ, Blanton MA, Quides KW, Zomorrodian A, Adinata ES, Sachs JL. 2019. Interspecific conflict and the evolution of ineffective rhizobia. Ecol. Lett. 22, 914–924. ( 10.1111/ele.13247) [DOI] [PubMed] [Google Scholar]
- 106.Nyholm SV, McFall-Ngai M. 2004. The winnowing: establishing the squid–Vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642. ( 10.1038/nrmicro957) [DOI] [PubMed] [Google Scholar]
- 107.Nyholm SV, Nishiguchi MK. 2008. The evolutionary ecology of a sepiolid squid-vibrio association: from cell to environment. Vie Milieu Paris 58, 175. [PMC free article] [PubMed] [Google Scholar]
- 108.McFall-Ngai M. 2014. Divining the essence of symbiosis: insights from the squid-vibrio model. PLoS Biol. 12, e1001783 ( 10.1371/journal.pbio.1001783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kremer N, Schwartzman J, Augustin R, Zhou L, Ruby EG, Hourdez S, McFall-Ngai MJ. 2014. The dual nature of haemocyanin in the establishment and persistence of the squid–vibrio symbiosis. Proc. R. Soc. B 281, 20140504 ( 10.1098/rspb.2014.0504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwartzman JA, Ruby EG. 2016. Stress as a normal cue in the symbiotic environment. Trends Microbiol. 24, 414–424. ( 10.1016/j.tim.2016.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tong D, Rozas NS, Oakley TH, Mitchell J, Colley NJ, McFall-Ngai MJ. 2009. Evidence for light perception in a bioluminescent organ. Proc. Natl Acad. Sci. USA 106, 9836–9841. ( 10.1073/pnas.0904571106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montgomery MK, McFall-Ngai M. 1994. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120, 1719–1729. [DOI] [PubMed] [Google Scholar]
- 113.Ruby EG, McFall-Ngai MJ. 1999. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri . Trends Microbiol. 7, 414–420. ( 10.1016/S0966-842X(99)01588-7) [DOI] [PubMed] [Google Scholar]
- 114.Koehler S, Gaedeke R, Thompson C, Bongrand C, Visick KL, Ruby E, McFall-Ngai M. 2019. The model squid–vibrio symbiosis provides a window into the impact of strain and species-level differences during the initial stages of symbiont engagement. Environ. Microbiol. 21, 3269–3283. ( 10.1111/1462-2920.14392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sachs JL, Russell JE, Lii YE, Black KC, Lopez G, Patil AS. 2010. Host control over infection and proliferation of a cheater symbiont. J. Evol. Biol. 23, 1919–1927. ( 10.1111/j.1420-9101.2010.02056.x) [DOI] [PubMed] [Google Scholar]
- 116.Westhoek A, Field E, Rehling F, Mulley G, Webb I, Poole PS, Turnbull LA. 2017. Policing the legume-Rhizobium symbiosis: a critical test of partner choice. Sci. Rep. 7, 1419 ( 10.1038/s41598-017-01634-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sørensen ME, Wood AJ, Minter EJ, Lowe CD, Cameron DD, Brockhurst MA. 2020. Comparison of independent evolutionary origins reveals both convergence and divergence in the metabolic mechanisms of symbiosis. Curr. Biol. 30, 328–334. ( 10.1016/j.cub.2019.11.053) [DOI] [PubMed] [Google Scholar]
- 118.Kiers ET, West SA. 2016. Evolution: welcome to symbiont prison. Curr. Biol. 26, R66–R68. ( 10.1016/j.cub.2015.12.009) [DOI] [PubMed] [Google Scholar]
- 119.Öpik M, Davison J, Moora M, Zobel M. 2013. DNA-based detection and identification of Glomeromycota: the virtual taxonomy of environmental sequences. Botany 92, 135–147. ( 10.1139/cjb-2013-0110) [DOI] [Google Scholar]
- 120.Werner GD, Kiers ET. 2015. Partner selection in the mycorrhizal mutualism. New Phytol. 205, 1437–1442. ( 10.1111/nph.13113) [DOI] [PubMed] [Google Scholar]
- 121.Balestrini R, Bonfante P. 2014. Cell wall remodeling in mycorrhizal symbiosis: a way towards biotrophism. Front. Plant Sci. 5, 237 ( 10.3389/fpls.2014.00237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Noë R, Kiers ET. 2018. Mycorrhizal markets, firms, and co-ops. Trends Ecol. Evol. 33, 777–789. ( 10.1016/j.tree.2018.07.007) [DOI] [PubMed] [Google Scholar]
- 123.Limpens E, Geurts R. 2014. Plant-driven genome selection of arbuscular mycorrhizal fungi. Mol. Plant Pathol. 15, 531–534. ( 10.1111/mpp.12149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whiteside MD, et al. 2019. Mycorrhizal fungi respond to resource inequality by moving phosphorus from rich to poor patches across networks. Curr. Biol. 29, 2043–2050. ( 10.1016/j.cub.2019.04.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grman E. 2012. Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93, 711–718. ( 10.1890/11-1358.1) [DOI] [PubMed] [Google Scholar]
- 126.Edwards DP, Yu DW. 2007. The roles of sensory traps in the origin, maintenance, and breakdown of mutualism. Behav. Ecol. Sociobiol. 61, 1321–1327. ( 10.1007/s00265-007-0369-3) [DOI] [Google Scholar]
- 127.van't Padje A, Whiteside MD, Kiers ET. 2016. Signals and cues in the evolution of plant–microbe communication. Curr. Opin. Plant Biol. 32, 47–52. ( 10.1016/j.pbi.2016.06.006) [DOI] [PubMed] [Google Scholar]
- 128.Behm JE, Geurts R, Kiers ET. 2014. Parasponia: a novel system for studying mutualism stability. Trends Plant Sci. 19, 757–763. ( 10.1016/j.tplants.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 129.Herre EA. 1989. Coevolution of reproductive characteristics in 12 species of New World figs and their pollinator wasps. Experientia 45, 637–647. ( 10.1007/BF01975680) [DOI] [Google Scholar]
- 130.Pellmyr O. 2003. Yuccas, yucca moths, and coevolution: a review. Ann. Miss. Bot. Gard. 90, 35–55. ( 10.2307/3298524) [DOI] [Google Scholar]
- 131.Addicott JF, Bao T. 1999. Limiting the costs of mutalism: multiple modes of interaction between yuccas and yucca moths. Proc. R. Soc. Lond. B 266, 197–202. ( 10.1098/rspb.1999.0622) [DOI] [Google Scholar]
- 132.Shapiro J, Addicott JF. 2004. Re-evaluating the role of selective abscission in moth/yucca mutualisms. Oikos 105, 449–460. ( 10.1111/j.0030-1299.2004.12681.x) [DOI] [Google Scholar]
- 133.Steidinger BS, Bever JD. 2016. Host discrimination in modular mutualisms: a theoretical framework for meta-populations of mutualists and exploiters. Proc. R. Soc. B 283, 20152428 ( 10.1098/rspb.2015.2428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Op den Camp RH, Polone E, Fedorova E, Roelofsen W, Squartini A, Op den Camp HJ, Bisseling T, Geurts R. 2012. Nonlegume Parasponia andersonii deploys a broad rhizobium host range strategy resulting in largely variable symbiotic effectiveness. Mol. Plant Microbe Interact. 25, 954–963. ( 10.1094/MPMI-11-11-0304) [DOI] [PubMed] [Google Scholar]
- 135.Ursell LK, Treuren WV, Metcalf JL, Pirrung M, Gewirtz A, Knight R. 2013. Replenishing our defensive microbes. Bioessays 35, 810–817. ( 10.1002/bies.201300018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith AH, et al. 2015. Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol. Ecol. 24, 1135–1149. ( 10.1111/mec.13095) [DOI] [PubMed] [Google Scholar]
- 137.Pallister T, et al. 2017. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep. 7, 13670 ( 10.1038/s41598-017-13722-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valdes AM, Walter J, Segal E, Spector TD. 2018. Role of the gut microbiota in nutrition and health. BMJ 361, k2179 ( 10.1136/bmj.k2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Engelmoer DJ, Behm JE, Kiers ET. 2014. Intense competition between arbuscular mycorrhizal mutualists in an in vitro root microbiome negatively affects total fungal abundance. Mol. Ecol. 23, 1584–1593. ( 10.1111/mec.12451) [DOI] [PubMed] [Google Scholar]
- 140.Poulsen M, Boomsma JJ. 2005. Mutualistic fungi control crop diversity in fungus-growing ants. Science 307, 741–744. ( 10.1126/science.1106688) [DOI] [PubMed] [Google Scholar]
- 141.Denison RF. 2000. Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 156, 567–576. ( 10.1086/316994) [DOI] [PubMed] [Google Scholar]
- 142.Gage DJ. 2002. Analysis of infection thread development using Gfp and DsRed-expressing Sinorhizobium meliloti. J. Bacteriol. 184, 7042–7046. ( 10.1128/JB.184.24.7042-7046.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wielbo J, Kuske J, Marek-Kozaczuk M, Skorupska A. 2010. The competition between Rhizobium leguminosarum bv. viciae strains progresses until late stages of symbiosis. Plant Soil 337, 125–135. ( 10.1007/s11104-010-0510-3) [DOI] [Google Scholar]
- 144.Oresnik IJ, Twelker S, Hynes MF. 1999. Cloning and characterization of a Rhizobium leguminosarum gene encoding a bacteriocin with similarities to RTX toxins. Appl. Environ. Microbiol. 65, 2833–2840. ( 10.1128/AEM.65.7.2833-2840.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kier ET, Ratcliff WC, Denison RF. 2013. Single-strain inoculation may create spurious correlations between legume fitness and rhizobial fitness. New Phytol. 198, 4–6. ( 10.1111/nph.12015) [DOI] [PubMed] [Google Scholar]
- 146.Regus JU, Quides KW, O'Neill MR, Suzuki R, Savory EA, Chang JH, Sachs JL. 2017. Cell autonomous sanctions in legumes target ineffective rhizobia in nodules with mixed infections. Am. J. Bot. 104, 1299–1312. ( 10.3732/ajb.1700165) [DOI] [PubMed] [Google Scholar]
- 147.Soto W, Punke EB, Nishiguchi MK. 2012. Evolutionary perspectives in a mutualism of sepiolid squid and bioluminescent bacteria: combined usage of microbial experimental evolution and temporal population genetics. Evolution 66, 1308–1321. ( 10.1111/j.1558-5646.2011.01547.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koch EJ, Miyashiro T, McFall-Ngai MJ, Ruby EG. 2014. Features governing symbiont persistence in the squid–vibrio association. Mol. Ecol. 23, 1624–1634. ( 10.1111/mec.12474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scheuring I, Yu DW. 2012. How to assemble a beneficial microbiome in three easy steps. Ecol. Lett. 15, 1300–1307. ( 10.1111/j.1461-0248.2012.01853.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ranger CM, et al. 2018. Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl Acad. Sci. USA 115, 4447–4452. ( 10.1073/pnas.1716852115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chomicki G, Renner SS. 2016. Obligate plant farming by a specialized ant. Nat. Plants 2, 16181 ( 10.1038/nplants.2016.181) [DOI] [PubMed] [Google Scholar]
- 152.Chomicki G, Renner SS. 2019. Farming by ants remodels nutrient uptake in epiphytes. New Phytol. 223, 2011–2023. ( 10.1111/nph.15855) [DOI] [PubMed] [Google Scholar]
- 153.Chomicki G, Kadereit G, Renner SS, Kiers ET. 2020. Tradeoffs in the evolution of plant farming by ants. Proc. Natl Acad. Sci. USA 117, 2535–2543. ( 10.1073/pnas.1919611117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schluter J, Foster KR. 2012. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 10, e1001424 ( 10.1371/journal.pbio.1001424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.West SA, Kiers ET, Pen I, Denison RF. 2002. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J. Evol. Biol. 15, 830–837. ( 10.1046/j.1420-9101.2002.00441.x) [DOI] [Google Scholar]
- 156.Palmer TM, Doak DF, Stanton ML, Bronstein JL, Kiers ET, Young TP, Goheen JR, Pringle RM. 2010. Synergy of multiple partners, including freeloaders, increases host fitness in a multispecies mutualism. Proc. Natl Acad. Sci. USA 107, 17 234–17 239. ( 10.1073/pnas.1006872107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steele MI, Kwong WK, Whiteley M, Moran NA. 2017. Diversification of type VI secretion system toxins reveals ancient antagonism among bee gut microbes. MBio 8, e01630-17 ( 10.1128/mBio.01630-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.