Abstract
Honey bees are both important pollinators and model insects due to their highly developed sociality and colony management. To better understand the molecular mechanisms underlying honey bee colony management, it is important to investigate the expression of genes putatively involved in colony physiology. Although quantitative real-time PCR (qRT-PCR) can be used to quantify the relative expression of target genes, internal reference genes (which are stably expressed across different conditions) must first be identified to ensure accurate normalisation of target genes. To identify reliable reference genes in honey bee (Apis mellifera) colonies, therefore, we evaluated seven candidate genes (ACT, EIF, EF1, RPN2, RPS5, RPS18 and GAPDH) in samples collected from three honey bee tissue types (head, thorax and abdomen) across all four seasons using three analysis programmes (NormFinder, BestKeeper and geNorm). Subsequently, we validated various normalisation methods using each of the seven reference genes and a combination of multiple genes by calculating the expression of catalase (CAT). Although the genes ranked as the most stable gene were slightly different on conditions and analysis methods, our results suggest that RPS5, RPS18 and GAPDH represent optimal honey bee reference genes for target gene normalisation in qRT-PCR analysis of various honey bee tissue samples collected across seasons.
Subject terms: Molecular biology, Transcription, Entomology
Introduction
The Western honey bee, Apis mellifera L., plays an important role as a pollinator1. In addition, the honey bee is considered to be a key model insect due to its relatively complex behaviours, including sociality, labour division and colony management2. Previous studies have demonstrated that endocrine system status and gene expression are important factors for flexible honey bee colony management, which involves colonies seasonally regulating their labour division and population dynamics3–5. In order to extend our understanding of the molecular mechanisms that underlie the regulation of honey bee colony physiology, information on the physiological functions of the genes putatively associated with colony management can be determined by analysing their expression profiles among different seasons and honey bee tissues6,7.
In quantitative real-time PCR (qRT-PCR), gene-specific mRNA (or cDNA) is quantified; this method has been used extensively because of its relative speed, sensitivity, replicability and accuracy8,9. Therefore, qRT-PCR would be an ideal method for analysing the expression patterns of honey bee genes putatively involved in the plasticity of colony molecular physiology in samples collected across different seasons and tissues. However, because qRT-PCR results are highly sensitive to the initial amount of RNA content in the amplification reaction, the interpretation of target gene expression levels among various conditions would result in appreciable errors without the use of a reliable internal standard7–10. Therefore, prior to analysing target gene expression levels among conditions, reference genes are required for accurate normalisation of data to compensate for differences in the amount of RNA in various honey bee samples; these internal reference genes must show similar transcript levels across various conditions8,9,11,12.
Given the importance of accurate normalisation in qRT-PCR assays, reference genes have been identified and validated in various insect species8,13,14. According to previous studies of the honey bee, widely used reference genes were validated at different developmental stages6, in the brains after a bacterial challenge15, different ages and social roles7,10,16. In particular, seasonal expression stabilities of candidate reference genes have been compared between forager and nurse head in our previous study7. However, reference genes have yet to be compared among different honey bee tissues collected across all four seasons. In the present study, therefore, we aimed to identify the most reliable references genes among honey bee tissues types and across seasons. Specifically, we collected workers during the four seasons (i.e., spring, summer, autumn and winter) and prepared RNA samples from three tissues (head, thorax and abdomen). We then chose five candidate genes, which have been widely used as reference genes for target gene normalisation in qRT-PCR assays6,17–19: β-actin (ACT), eukaryotic translation initiation factor (EIF), elongation factor 1 (EF1), 26S proteasome non-ATPase regulatory (RPN2) and 40S ribosomal protein S5 (RPS5). Moreover, two genes, 40S ribosomal protein S18 (RPS18) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), previously identified as optimal reference genes in the honey bee head, were added in the present study7. Subsequently, we used three analysis programmes (NormFinder, BestKeeper and geNorm) to evaluate the expression stabilities of total seven candidate reference genes. In addition, the seven reference genes and a combination of multiple references were validated by normalising catalase (CAT) expression.
Results
Amplification specificity and efficiency
Prior to performing qRT-PCR, amplification specificity and efficiency were investigated. All PCR products amplified with each primer set showed a single band in 1% agarose gels and a shark single peak detected in the melting curve by RT-PCR. Furthermore, given that the forward and reverse primers for EF1, RPS18 and CAT were designed based on two different exons containing an intron, a single band on the agarose gel and a single peak in RT-PCR observed for EF1, RPS18 and CAT further suggested no genomic DNA contamination (Supplementary Fig. S1). In our analysis of PCR efficiencies, all seven candidate genes had linear regression coefficients (R2) > 0.997 and amplification efficiencies of 92–109% (Table 1).
Table 1.
Information on the seven candidate reference genes and the target gene (CAT), including gene name, GenBank accession number, sequences, size, GC percentage, melting temperature of primers and amplicons.
| Gene | Primers | Amplicons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | Full gene name | Accession no | Sequence (5′ → 3′) | Size (bp) | GC (%) | TM (°C) | Size (bp) | GC (%) | Efficiency (%) | |
| ACT | Beta-actin | AB023025 | For. GTATGCCAACACTGTCCTTTCTG | 23 | 48 | 62.9 | 96 | 46.9 | 103 | 0.999 |
| Rev. ATGGTGGATGGTGCTAGGGC | 20 | 60 | 62.5 | |||||||
| EIF | Eukaryotic translation initiation factor 3 subunit C | XM_006564593 | For. GCTGCACATGAATTTGATGCAAGAA | 25 | 40 | 62.5 | 124 | 40.3 | 109 | 0.999 |
| Rev. CCGCGACAACATGTTCTCTCATA | 23 | 48 | 62.9 | |||||||
| EF1 | Elongation factor 1-alpha F2 | NM_001014993 | For. GTCGTGGTTATGTTGCTGGTGAT | 23 | 48 | 62.9 | 177 (456)a | 38.4 | 92 | 0.998 |
| Rev. CGCATTTCTCTTTGATATCAGCGAA | 25 | 40 | 62.5 | |||||||
| RPN2 | 26S proteasome non-ATPase regulatory subunit 1 | LOC727029 | For. GTATGCGTTAGGACTTATTCATGCA | 25 | 40 | 62.5 | 105 | 44.8 | 106 | 0.999 |
| Rev. CAACCTCCATGACGAACCATCT | 22 | 50 | 62.1 | |||||||
| RPS5 | 40S ribosomal protein S5 | XM_006570237 | For. GATGTTTCTCCGTTACGACGAGT | 23 | 48 | 62.9 | 114 | 44.7 | 92 | 0.999 |
| Rev. GAGTTCATCGGCTAAACATTCGG | 23 | 48 | 62.9 | |||||||
| RPS18b | 40S ribosomal protein S18 | XM_625101 | For. GATTCCCGATTGGTTTTTGAATAG | 24 | 38 | 60.3 | 152 (446)a | 35.5 | 107.6 | 0.999 |
| Rev. AACCCCAATAATGACGCAAACC | 22 | 45 | 60.1 | |||||||
| GAPDHb | Glyceraldehyde-3-phosphate dehydrogenase | XM_393605 | For. CACCTTCTGCAAAATTATGGCG | 22 | 45 | 60.1 | 188 | 43.1 | 95.5 | 0.997 |
| Rev. ACCTTTGCCAAGTCTAACTGTTAA | 24 | 38 | 60.3 | |||||||
| CAT | Apis mellifera catalase | NM_001178069 | For. CTTGGCCCAAACAATCTGCAAT | 22 | 45.5 | 60.3 | 151 (521)a | 37.7 | 98 | 0.999 |
| Rev. GACATTCTCTAGGCCCACCA | 20 | 55 | 60.5 | |||||||
aNumbers in bracket indicate the size (bp) of PCR products amplified with genomic DNA.
bSequence information of primers were obtained from previous study7.
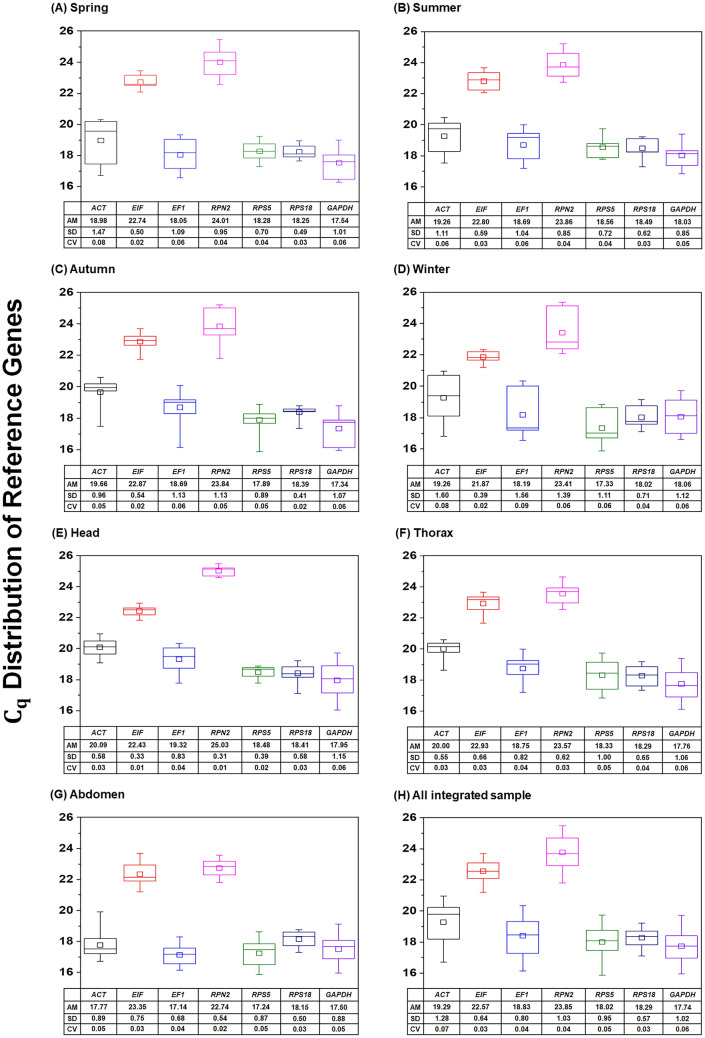
Cq distributions of reference genes
Expression levels, as indicated by Cq values, of the seven candidate reference genes in honey bee transcript samples prepared from four seasons and three tissue types were analysed (Fig. 1). Based on arithmetic means (AM) and standard deviation (SD) values, coefficient of variation (CV) values were calculated as follows: CV = SD/AM. Across the four seasons, EIF showed the lowest variability with a coefficient of variation (CV) of 0.02–0.03 (Fig. 1A–D). Among the three tissue types, the CV values of RPN2 were the lowest of the seven genes (0.01 in the head, 0.03 in the thorax and 0.02 in the abdomen) (Fig. 1E–G). EIF was also the most stable gene in the head and thorax (Fig. 1E,F). As shown in Fig. 1H, by comparison of the seven genes’ Cq values obtained from all samples among the various seasons and tissue types, EIF and RPS18 were the least variable gene (CV = 0.03).
Figure 1.
Box plot comparisons of Cq values for the seven candidate reference genes in honey bee samples. Samples were prepared from four seasons (A–D), three tissue types (E–G) and an integration of all samples (H). The horizontal lines in the box indicate the 25th, 50th and 75th percentile values. The square symbol in the big box shows the mean median. The error bars denote the maximum and minimum values.
Analysis of expression stability using three program
NormFinder analysis
Based on the expression variation of candidate genes, NormFinder identifies the optimal reference gene by calculating stability values20,21. According to average stability values (mean values), which were arithmetically calculated for the four seasons, RPN2 was the most stable gene (mean value = 0.021) (Fig. 2A). In a comparison of all seven genes’ stability values, RPS5 was the most stable gene in spring. RPS18 was the most stable gene in summer, whereas RPN2 was the most stable gene in autumn and winter (Fig. 2A, Table 2). In gene stability analysis of the three tissue types, RPN2 was the most stable (mean stability = 0.009) (Fig. 2B). In the stability analysis of specific tissue types, RPS5 was highest ranked gene in the head (least stable), while the most stable expression levels in thorax and abdomen were EIF and RPN2, respectively (Fig. 2B, Table 2). When the stability values of genes were calculated by combining all four seasons and three tissue types, the stability rank from the most (lowest value) to least (highest value) stable was as follows: RPN2 > EIF = RPS5 > EF1 > RPS18 > ACT > GAPDH (Fig. 2C, Table 2).
Figure 2.
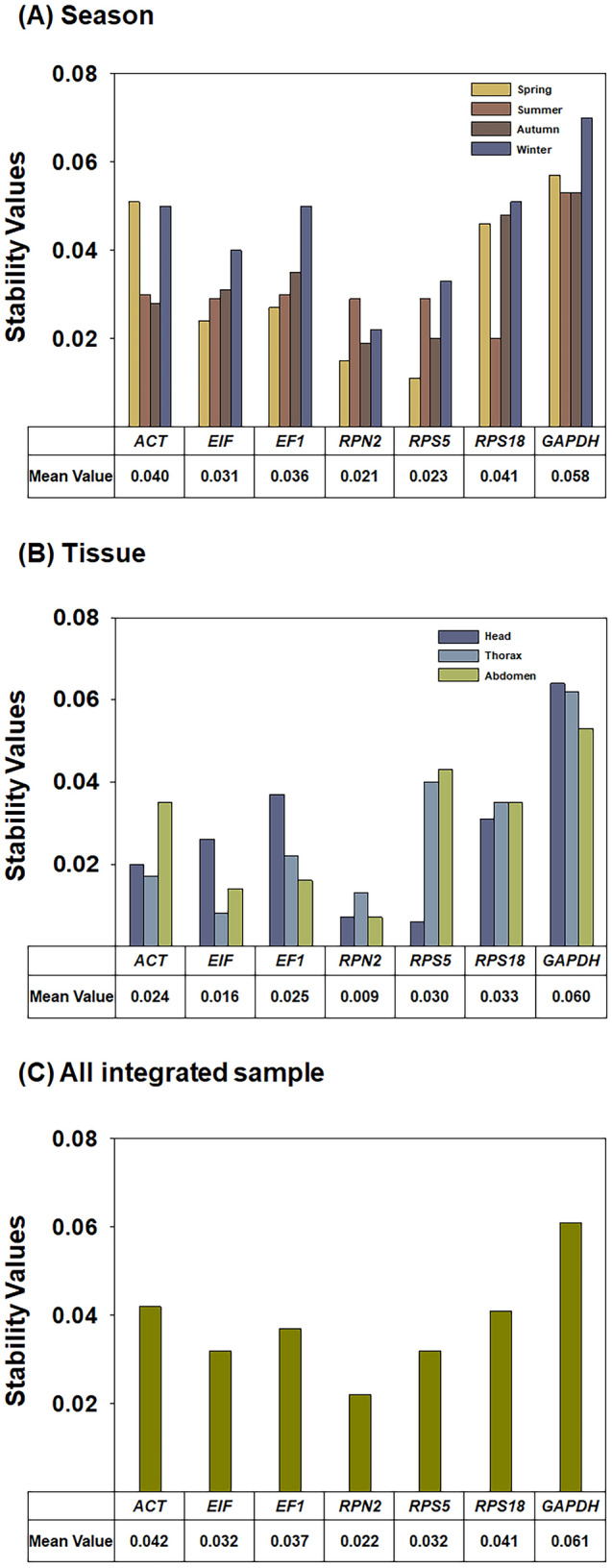
Expression stability values of the seven candidate honey bee reference genes calculated by NormFinder. Average stability values (mean values) were arithmetically calculated from honey bee samples prepared from four seasons (A), three tissue types (B) and an integration of all samples (C).
Table 2.
Summary of gene expression stability values analysed by Cq distribution in NormFinder, BestKeeper and geNorm.
| Rank | Spring | Summer | Autumn | Winter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cq (CV)a | NormFinder (SV)b | BestKeeper (SD)c | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | |
| 1 | EIF (0.02)* | RPS5 (0.011)* | EIF (0.41)* | EIF (0.207)** | RPS18 (0.03)* | RPS18 (0.020)* | RPS18 (0.48)* | EF1 (0.32)** | RPS18 (0.02)* | RPN2 (0.019)* | RPS18 (0.24)* | RPS5 (0.324)** | EIF (0.02)* | RPN2 (0.022)* | EIF (0.31)* | RPN2 (0.265)** |
| 2 | RPS18 (0.03)* | RPN2 (0.015)* | RPS18 (0.43)* | RPS5 (0.23)** | EIF (0.03)* | RPS5 (0.029)* | EIF (0.51)* | ACT (0.346)** | EIF (0.02)* | RPS5 (0.020)* | EIF (0.38)* | EF1 (0.347)** | RPS18 (0.04)* | RPS5 (0.033)* | RPS18 (0.59)* | EF1 (0.302)** |
| 3 | RPS5 (0.04)* | EIF (0.024)* | RPS5 (0.58)* | RPS18 (0.238)** | RPS5 (0.04)* | RPN2 (0.029)* | RPS5 (0.58)* | RPS18 (0.422)** | RPS5 (0.05)* | ACT (0.028)* | RPS5 (0.61)* | ACT (0.377)** | RPN2 (0.06)* | EIF (0.040)* | GAPDH (0.92)* | RPS5 (0.347)** |
| 4 | RPN2 (0.04)* | EF1 (0.027)* | RPN2 (0.77)* | EF1 (0.429)** | RPN2 (0.04)* | EIF (0.029)* | GAPDH (0.68)* | RPS5 (0.567)* | ACT (0.05)* | EIF (0.031)* | ACT (0.69)* | RPN2 (0.512)* | RPS5 (0.06)* | EF1 (0.050)* | RPS5 (0.93)* | RPS18 (0.498)** |
| 5 | EF1 (0.06)* | RPS18 (0.046)* | GAPDH (0.79)* | RPN2 (0.566)* | GAPDH (0.05)* | EF1 (0.030)* | RPN2 (0.69)* | EIF (0.65)* | RPN2 (0.05)* | EF1 (0.035)* | EF1 (0.80)* | RPS18 (0.632)* | GAPDH (0.06)* | ACT (0.050)* | RPN2 (1.21) | ACT (0.63)* |
| 6 | GAPDH (0.06)* | ACT (0.051)* | EF1 (0.92)* | ACT (0.704)* | EF1 (0.06)* | ACT (0.030)* | EF1 (0.91)* | RPN2 (0.74)* | EF1 (0.06)* | RPS18 (0.048)* | RPN2 (0.86)* | EIF (0.709)* | ACT (0.08)* | RPS18 (0.051)* | EF1 (1.32) | GAPDH (0.773)* |
| 7 | ACT (0.08)* | GAPDH (0.057)* | ACT (1.27) | GAPDH (0.987)* | ACT (0.06)* | GAPDH (0.053)* | ACT (0.95)* | GAPDH (0.925)* | GAPDH (0.06)* | GAPDH (0.053)* | GAPDH (0.88)* | GAPDH (0.917)* | EF1 (0.09)* | GAPDH (0.070)* | ACT (1.36) | EIF (0.914)* |
| Rank | Head | Thorax | Abdomen | All integrated sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | Cq (CV) | NormFinder (SV) | BestKeeper (SD) | geNorm (M value) | |
| 1 | RPN2 (0.01)* | RPS5 (0.006)* | RPN2 (0.26)* | RPS5 (0.118)** | EIF (0.03)* | EIF (0.008)* | ACT (0.40)* | EIF (0.172)** | RPN2 (0.02)* | RPN2 (0.007)* | RPN2 (0.44)* | RPS18 (0.234)** | RPS18 (0.03)* | RPN2 (0.022)* | RPS18 (0.47)* | ACT (0.252)** |
| 2 | EIF (0.01)* | RPN2 (0.007)* | EIF (0.27)* | RPN2 (0.123)** | RPN2 (0.03)* | RPN2 (0.013)* | RPN2 (0.50)* | RPS18 (0.19)** | RPS18 (0.03)* | EIF (0.014)* | RPS18 (0.44)* | RPN2 (0.236)** | EIF (0.03)* | EIF (0.032)* | EIF (0.53)* | EF1 (0.254)** |
| 3 | RPS5 (0.02)* | ACT (0.020)* | RPS5 (0.34)* | RPS18 (0.141)** | ACT (0.03)* | ACT (0.017)* | EIF (0.53)* | EF1 (0.205)** | EIF (0.03)* | EF1 (0.016)* | EF1 (0.54)* | EIF (0.256)** | RPN2 (0.04)* | RPS5 (0.032)* | RPS5 (0.76)* | RPS18 (0.276)** |
| 4 | RPS18 (0.03)* | EIF (0.026)* | RPS18 (0.42)* | ACT (0.241)** | RPS18 (0.04)* | EF1 (0.022)* | RPS18 (0.56)* | RPN2 (0.27)** | EF1 (0.04)* | RPS18 (0.035)* | EIF (0.61)* | EF1 (0.281)** | EF1 (0.04)* | EF1 (0.037)* | GAPDH (0.82)* | RPN2 (0.348)** |
| 5 | ACT (0.03)* | RPS18 (0.031)* | ACT (0.45)* | GAPDH (0.35)** | EF1 (0.04)* | RPS18 (0.035)* | EF1 (0.63)* | ACT (0.328)** | ACT (0.05)* | ACT (0.035)* | ACT (0.66)* | GAPDH (0.344)** | RPS5 (0.05)* | RPS18 (0.041)* | RPN2 (0.92)* | EIF (0.399)** |
| 6 | EF1 (0.04)* | EF1 (0.037)* | EF1 (0.67)* | EF1 (0.425)** | RPS5 (0.05)* | RPS5 (0.040)* | RPS5 (0.83)* | RPS5 (0.398)** | GAPDH (0.05)* | RPS5 (0.043)* | GAPDH (0.69)* | ACT (0.415)** | GAPDH (0.06)* | ACT (0.042)* | EF1 (1.03) | RPS5 (0.421)** |
| 7 | GAPDH (0.06)* | GAPDH (0.064)* | GAPDH (0.96)* | EIF (0.494)** | GAPDH (0.06)* | GAPDH (0.062)* | GAPDH (0.83)* | GAPDH (0.526)* | RPS5 (0.05)* | GAPDH (0.053)* | RPS5 (0.70)* | RPS5 (0.513)* | ACT (0.07)* | GAPDH (0.061)* | ACT (1.09) | GAPDH (0.46)** |
aCV refers to the coefficient of variation analysed by Cq distribution as indicated in Fig. 1
bSV refers to the stability values analysed by NormFinder.
cSD indicates the standard deviation of Cq values analysed by BestKeeper.
*Stability values of the genes below the criteria CV < 1 (Cq distribution analysis), SV < 0.15 (NormFinder), SD < 1.0 (BestKeeper) and M < 1.5 (geNorm).
**Stability values of the genes below the criterion M < 0.5 (geNorm).
BestKeeper analysis
Genes that show low SD (usually < 1) and CV values can be chosen as the more stable reference genes in the BestKeeper algorithm22,23. Based on SD and CV values, BestKeeper highlighted EIF (in spring and winter) and RPS18 (in summer and autumn) as the most appropriate reference gene with the least Cq variation (Table 3). Across the three tissue types, according to SD and CV scores, RPN2 was the top ranked gene in the head and abdomen (least stable), while ACT was identified as the optimal reference gene in the thorax (Table 3). Although the stability of genes in the head, thorax and abdomen were variable, all seven genes had SD < 1.0 in all tissues, which suggests that any of the genes could be used as a reference gene for normalisation of target gene expression in the head, thorax or abdomen of honey bees (Table 3). When the Cq values of the seven genes were combined across seasons and tissue types, BestKeeper revealed that RPS18, EIF, RPS5, GAPDH and RPN2 had SD values < 1.0; thus, these genes are perhaps the best candidates as reference genes (Table 3, see All integrated sample).
Table 3.
Gene expression stability values of the seven candidate reference genes analysed by BestKeeper.
| Rank | Spring | Summer | Autumn | Winter | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SDa | CVb | GM (Cq)c | CD (R2)d | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | |
| 1 | EIF | 0.41 | 1.82 | 22.74 | 0.75 | 0.02 | RPS18 | 0.48 | 2.62 | 18.48 | 0.80 | 0.01 | RPS18 | 0.24 | 1.33 | 18.38 | − 0.03 | 0.95 | EIF | 0.31 | 1.41 | 21.86 | 0.53 | 0.14 |
| 2 | RPS18 | 0.43 | 2.34 | 18.24 | 0.19 | 0.63 | EIF | 0.51 | 2.22 | 22.79 | 0.55 | 0.12 | EIF | 0.38 | 1.67 | 22.87 | 0.55 | 0.12 | RPS18 | 0.59 | 3.27 | 18.01 | 0.39 | 0.30 |
| 3 | RPS5 | 0.58 | 3.18 | 18.27 | 0.90 | 0.00 | RPS5 | 0.58 | 3.12 | 18.55 | 0.72 | 0.03 | RPS5 | 0.61 | 3.39 | 17.87 | 0.93 | 0.00 | GAPDH | 0.92 | 5.12 | 18.03 | 0.37 | 0.33 |
| 4 | RPN2 | 0.77 | 3.19 | 24.00 | 0.88 | 0.00 | GAPDH | 0.68 | 3.75 | 18.01 | 0.36 | 0.35 | ACT | 0.69 | 3.50 | 19.63 | 0.84 | 0.00 | RPS5 | 0.93 | 5.39 | 17.30 | 0.88 | 0.00 |
| 5 | GAPDH | 0.79 | 4.48 | 17.51 | 0.52 | 0.15 | RPN2 | 0.69 | 2.89 | 23.84 | 0.65 | 0.06 | EF1 | 0.80 | 4.26 | 18.66 | 0.92 | 0.00 | RPN2 | 1.21 | 5.17 | 23.37 | 0.91 | 0.00 |
| 6 | EF1 | 0.92 | 5.10 | 18.02 | 0.97 | 0.00 | EF1 | 0.91 | 4.86 | 18.67 | 0.95 | 0.00 | RPN2 | 0.86 | 3.61 | 23.81 | 0.91 | 0.00 | EF1 | 1.32 | 7.28 | 18.13 | 0.93 | 0.00 |
| 7 | ACT | 1.27 | 6.67 | 18.93 | 0.97 | 0.00 | ACT | 0.95 | 4.94 | 19.23 | 0.98 | 0.00 | GAPDH | 0.88 | 5.09 | 17.31 | 0.68 | 0.05 | ACT | 1.36 | 7.08 | 19.20 | 0.93 | 0.00 |
| Rank | Head | Thorax | Abdomen | All integrated sample | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SDa | CVb | GM (Cq)c | CD (R2)d | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | Gene | SD | CV | GM (Cq ) | CD (R2) | P value | |
| 1 | RPN2 | 0.26 | 1.03 | 25.02 | 0.74 | 0.01 | ACT | 0.40 | 1.99 | 20.00 | 0.77 | 0.00 | RPN2 | 0.44 | 1.95 | 22.74 | 0.86 | 0.00 | RPS18 | 0.47 | 2.57 | 18.28 | 0.42 | 0.01 |
| 2 | EIF | 0.27 | 1.21 | 22.43 | − 0.16 | 0.63 | RPN2 | 0.50 | 2.11 | 23.56 | 0.81 | 0.00 | RPS18 | 0.44 | 2.42 | 18.15 | 0.33 | 0.29 | EIF | 0.53 | 2.35 | 22.56 | 0.57 | 0.00 |
| 3 | RPS5 | 0.34 | 1.85 | 18.47 | 0.78 | 0.00 | EIF | 0.53 | 2.30 | 22.92 | 0.91 | 0.00 | EF1 | 0.54 | 3.13 | 17.13 | 0.91 | 0.00 | RPS5 | 0.76 | 4.22 | 17.99 | 0.83 | 0.00 |
| 4 | RPS18 | 0.42 | 2.27 | 18.40 | 0.45 | 0.14 | RPS18 | 0.56 | 3.06 | 18.28 | 0.53 | 0.08 | EIF | 0.61 | 2.71 | 22.34 | 0.87 | 0.00 | GAPDH | 0.82 | 4.64 | 17.71 | 0.43 | 0.01 |
| 5 | ACT | 0.45 | 2.25 | 20.08 | 0.68 | 0.02 | EF1 | 0.63 | 3.35 | 18.74 | 0.91 | 0.00 | ACT | 0.66 | 3.73 | 17.76 | 0.81 | 0.00 | RPN2 | 0.92 | 3.85 | 23.75 | 0.84 | 0.00 |
| 6 | EF1 | 0.67 | 3.44 | 19.31 | 0.68 | 0.02 | RPS5 | 0.83 | 4.55 | 18.30 | 0.81 | 0.00 | GAPDH | 0.69 | 3.93 | 17.48 | 0.39 | 0.21 | EF1 | 1.03 | 5.62 | 18.37 | 0.93 | 0.00 |
| 7 | GAPDH | 0.96 | 5.33 | 17.92 | 0.61 | 0.03 | GAPDH | 0.83 | 4.70 | 17.73 | 0.42 | 0.17 | RPS5 | 0.70 | 4.05 | 17.22 | 0.67 | 0.02 | ACT | 1.09 | 5.65 | 19.25 | 0.89 | 0.00 |
aSD indicates the standard deviation of Cq values.
bCV refers to the coefficient of variation value.
cGM represents the geometric mean of Cq values.
dCD indicates the coefficient of determination value.
geNorm analysis
The average expression stability values (M values) of the seven candidates were also determined using geNorm across the different seasons and tissue types (Fig. 3). M ≤ 0.5 has been suggested as the criterion for appropriate reference gene selection21,24. In the seasonal comparison, the M values of EF1 were < 0.5 in each of the seasons, whereas the other six genes had M ≥ 0.5 in at least one season (Fig. 3A); consequently, EF1 was perhaps the most suitable reference gene for target gene normalisation when analysing seasonal gene expression trends in honey bees. When the M scores of candidate reference genes were compared across tissue types, all genes had M ≤ 0.5 with the exception of GAPDH and RPS5 in the thorax and abdomen, respectively (Fig. 3B); hence, ACT, EIF, EF1, RPN2 and RPS18 may be useful as reference genes for gene expression analysis in different honey bee tissue types. When the Cq values of the seven genes obtained from different seasons and tissue types were combined, the M values of all genes were < 0.5 (Fig. 3C), suggesting that any one of the seven genes could be a reference gene according to geNorm analysis.
Figure 3.
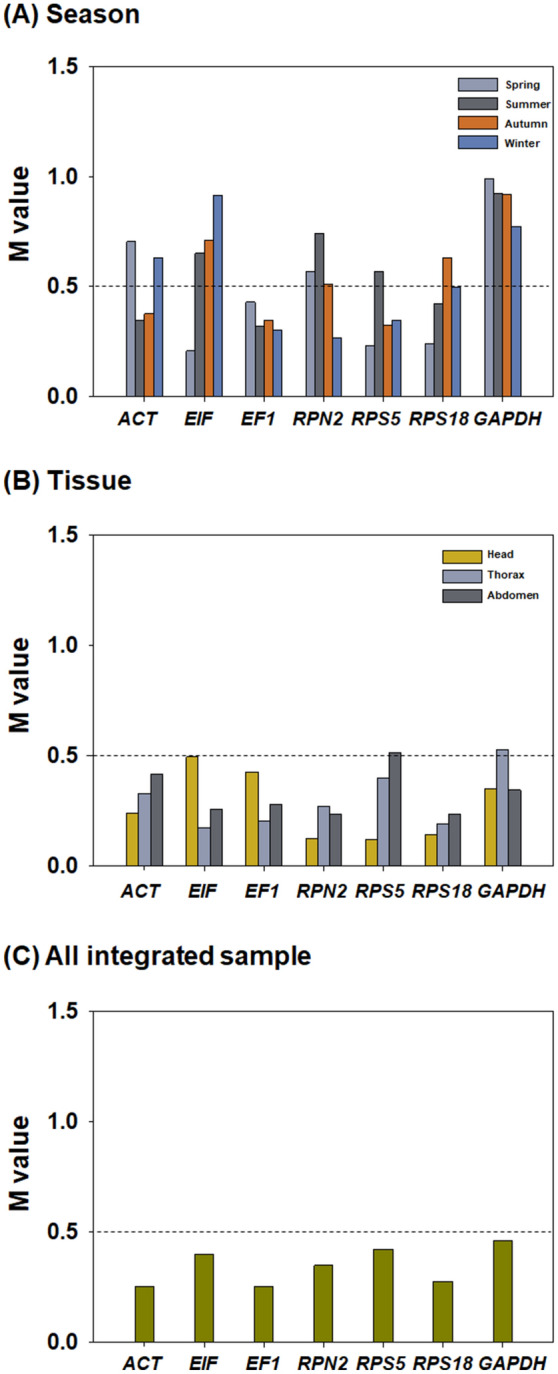
Expression stability values (M) of the seven candidate honey bee reference genes calculated by geNorm. Samples were prepared from four seasons (A), three tissue types (B) and an integration of all samples (C). The dotted lines indicate the M = 0.5 value, which is the criterion for appropriate reference gene selection.
In additional analysis, geNorm was used to calculate pairwise variation (Vn/Vn+1) values that would indicate the optimal number of reference genes for target gene normalisation. According to previous studies, 0.15 is a suitable cutoff value in pairwise variation analysis25. In seasonal analysis, the V2/V3 values of spring, autumn and winter bees were 0.079, 0.14 and 0.144, respectively, indicating that a combination of two genes (EIF + RPS5 for spring; RPS5 + EF1 for autumn; RPN2 + EF1 for winter) would be sufficient for target gene normalisation in these seasons (Fig. 4A), based on the ranks of gene expression stability analysed by geNorm (shown in Fig. 3A, Table 2). In contrast, only V5/V6 of summer was under the 0.15 cutoff value (V5/V6 = 0.14) (Fig. 4A), implying that at least five reference genes should be combined for normalising target gene expression in summer bee. Similarly, pairwise variation analysis demonstrated that V2/V3 values were lower than the cutoff value in head, thorax and abdomen, which also suggests that two genes (RPS5 + RPN2 for the head; EIF + RPS18 for the thorax; and RPS18 + RPN2 for the abdomen) would be sufficient for calculating gene expression (Fig. 4B). When all the samples were combined, pairwise variation analysis revealed that V2/V3 values were higher than V3/V4, V4/V5, V5/V6 and V6/V7 values; however, V2/V3 was 0.102, which was still under the 0.15 cutoff value. This finding supports the conclusion that two genes, ACT and EF1, were the optimal normalisation factors for gene expression analysis (Fig. 4C).
Figure 4.
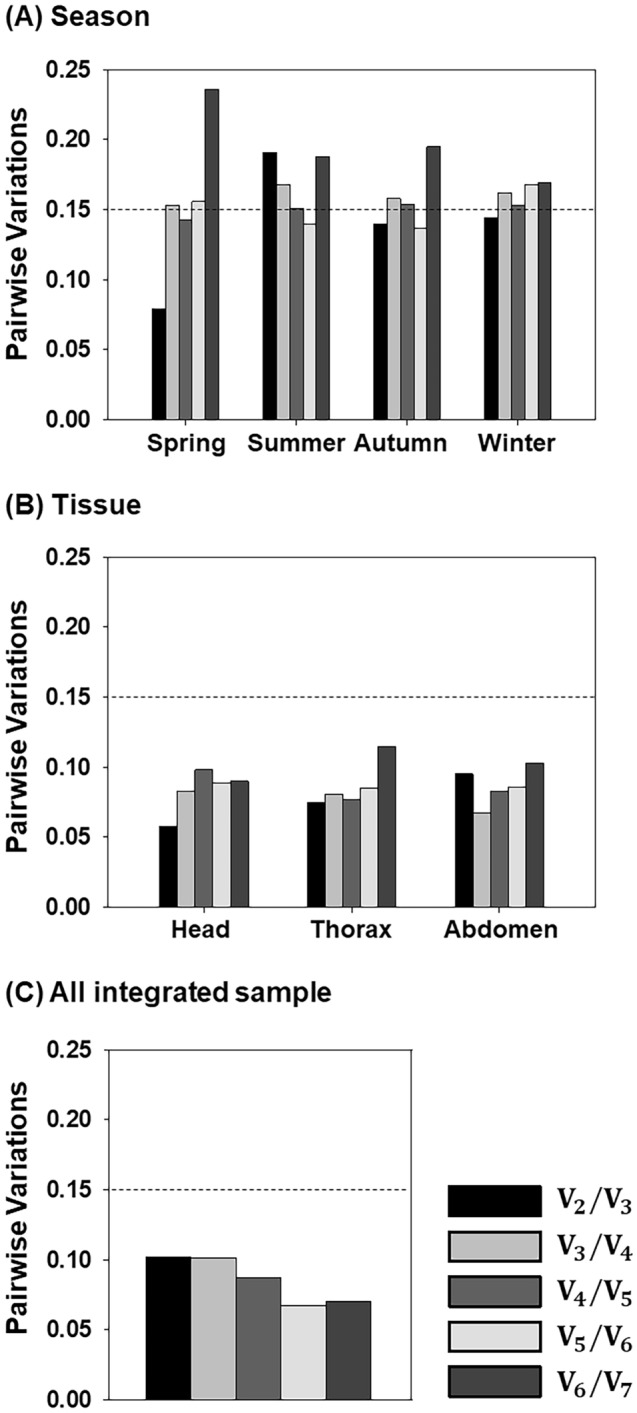
geNorm pairwise variation analysis was used to determine the optimal number of references for target gene normalisation. Pairwise variation values (Vn/Vn+1) were calculated from honey bee samples prepared from four seasons (A), three tissue types (B) and an integration of all samples (C). The dotted lines indicate where the pairwise variation = 0.15, which was the cutoff value used to indicate the optimal number of reference genes.
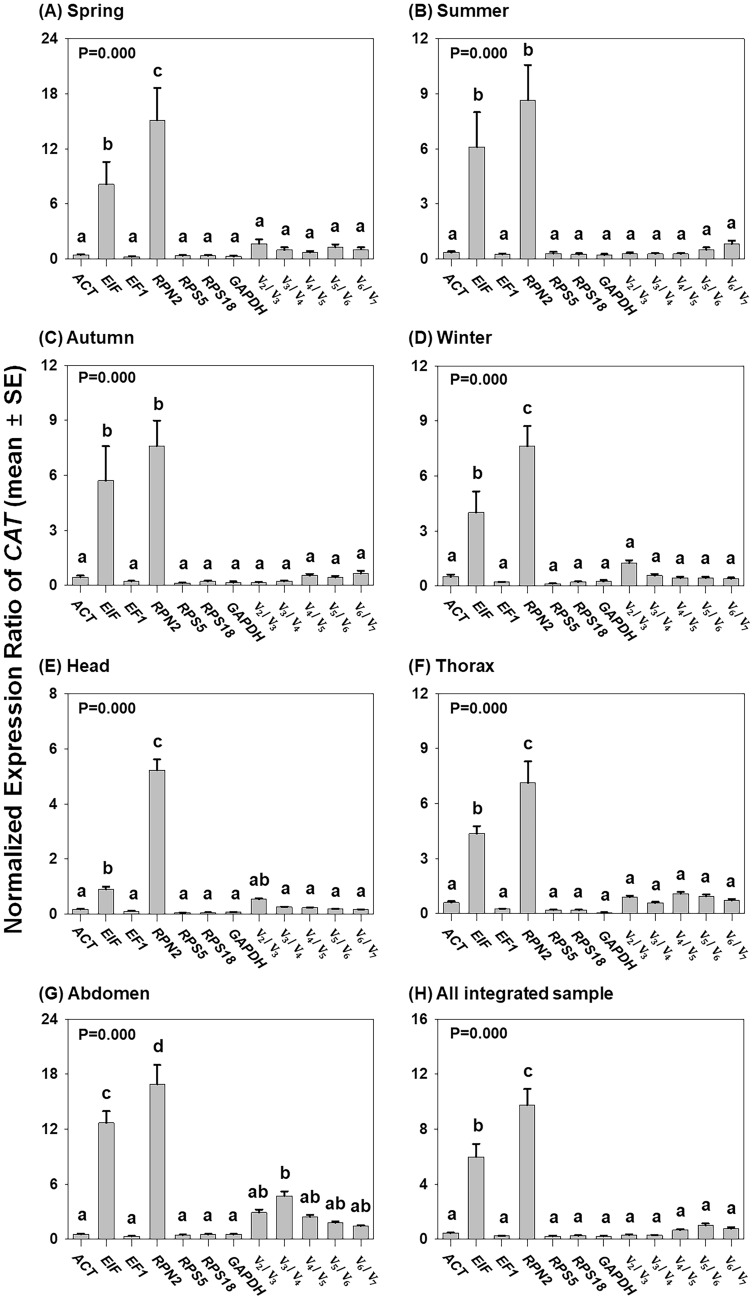
Reference gene validation
Since geNorm pairwise analysis suggested application of multiple reference genes for target gene normalisation (Fig. 4), we compared expression levels of CAT (as the target gene) normalised with each of the seven reference genes and multiple reference genes across the four seasons and three tissue types (Fig. 5). In seasonal analysis, the number of selected reference genes did not alter the expression levels of CAT (Fig. 5A–D). Furthermore, CAT expression levels normalised with a single gene (i.e., ACT, EF1, RPS5, RPS18 or GAPDH) were not significantly different from CAT expression levels normalised with multiple reference genes (P = 1.000 for spring, summer and autumn; P = 0.868 for winter) (Fig. 5A–D). These results indicate that a single gene, namely ACT, EF1, RPS5, RPS18 or GAPDH, could be used as a reference when analysing seasonal expression trends of target genes in honey bees. In a comparison of CAT expression in the three tissue types, when ACT, EF1, RPS5, RPS18 or GAPDH were used as single reference genes, CAT expression levels were not significantly different from those obtained with a combination of multiple reference genes in the head (P = 0.169), thorax (P = 0.720) and abdomen (P = 0.379) analysis (Fig. 5E–G). We also compared the overall expression levels of CAT normalised with a single candidate reference gene and a multiple gene combination (Fig. 5H). Analysis showed that the expression levels of CAT normalised with any number of multiple gene combinations were not significantly different. In addition, each of the ACT, EF1, RPS5, RPS18 and GAPDH normalisations had expression levels of CAT that were statistically similar to those obtained with multiple gene combinations (P = 0.981) (Fig. 5H). This suggests that it would be possible to use a single gene, ACT, EF1, RPS5, RPS18 or GAPDH, as the optimal reference gene for target gene normalisation across different seasons and honey bee tissue types.
Figure 5.
Comparison of expression levels of CAT in honey bee samples normalised with a single gene from the seven references and a combination of multiple reference genes. Samples were prepared from four seasons (A–D), three tissue types (E–G) and the integration of all samples (H). The expression levels of CAT normalised with different methods were statistically analysed with a one-way ANOVA followed by Tukey’s multiple comparison post-hoc test and different letters indicate significantly different values (P < 0.05). Data are presented as mean values ± SE.
Discussion
In order to find optimal reference genes for qRT-PCR assay in the honey bee across four seasons and three tissue types, we evaluated the expression stabilities of seven candidate reference genes, ACT, EIF, EF1, RPN2, RPS5, RPS18 and GAPDH, using three analysis programmes: NormFinder, BestKeeper and geNorm. In addition, pairwise variation analysis in geNorm was used to identify the optimal number of honey bee reference genes required for normalisation of target gene expression. The normalisation methods, including individual reference genes or combinations of multiple genes, were also validated by analysing CAT expression in the various samples.
Consistent with observations in previous studies7,12,13, the three algorithms, NormFinder, BestKeeper and geNorm, produced different results when ranking gene stability in the present study; therefore, the combined use of all algorithms would ensure more credible results13. Considering the cutoff values in each algorithm, most of the seven candidate genes could be deemed acceptable for use as reference genes when analysing gene expression in different conditions in honey bees. Although most previous studies did not set a cutoff value for gene stability in NormFinder analysis6–8,14–16,21,22, several recent studies have suggested values < 0.15 as a suitable cutoff20,26,27. Based on this criterion, all seven genes were suitable reference genes according to NormFinder. This result is supported by the distribution of Cq values, which indicated that all seven candidate genes were stably expressed with CV values < 1, which is considered to indicate low variance28. In BestKeeper analysis, all seven genes were also determined to be appropriate reference genes for analysis of honey bee gene expression in summer, autumn and among the three tissue types as indicated by SD values < 122,23. In contrast, some genes (ACT in spring; RPN2, EF1 and ACT in winter; EF1 and ACT in integrated sample) can not be suggested to be an optimal reference gene due to their SD values > 1. When considering M ≤ 0.5 as the criterion for suitable reference gene selection in geNorm analysis21,24, all seven genes were acceptable references only in the head and the integration of all samples. In contrast, when using M ≤ 1.5 as the criterion, which has been widely suggested as an acceptable level for reference gene selection22,25, all seven genes could be regarded as reference genes across seasons and tissue types. Taken together, our combined analyses suggest that EIF, RPS5, RPS18 and GAPDH would be most suitable as optimal reference genes for the normalisation of target gene expression in honey bee samples prepared from a variety of tissues across seasons.
In addition to the measurement of gene expression stabilities, geNorm can be used to adjunctively analyse pairwise variation values for possible selection of multiple reference genes. In the present study, a combination of two, three, four, five or six genes did not affect target gene normalisation in the three tissue types and integrated sample based on Vn/Vn+1 < 0.15, which was usually used as a cutoff value in geNorm pairwise variation analysis in previous studies21,22,25. However, across the seasons, V2/V3 values for spring, autumn and winter were < 0.15, whereas summer sample exhibited V5/V6 < 0.15, showing that combination of two reference genes is suggested for normalization of target gene expression in spring, autumn and winter, while five genes are needed in summer sample.
Regardless of the optimal number of reference genes suggested for accurate normalisation of target gene expression levels by geNorm pairwise analysis, V2/V3 is suggested as minimum number of reference genes; therefore, at least two genes are required as an internal control when all Vn/Vn+1 values are < 0.1521,29. However, in order to reduce the financial and technical burden in experiments, the selection of one reference gene might be suitable if target gene expression levels obtained with a single reference are not significantly different from those obtained with multiple reference genes10,12. In the present study, across seasons and tissue types, the expression levels of CAT with normalisation by either EIF or RPN2 were found to be significantly higher than those normalised with ACT, EF1, RPS5, RPS18 or GAPDH. In addition, the Cq values of EIF and RPN2 were relatively higher than those of the other five genes. However, across conditions, expression levels of CAT with ACT, EF1, RPS5, RPS18 or GAPDH normalisation were statistically similar to those with multiple reference gene combinations. This suggests that single reference gene selection of ACT, EF1, RPS5, RPS18 or GAPDH could be a possible alternative to a combination of multiple reference genes, despite geNorm pairwise analysis suggesting that multiple genes should be used based on Vn/Vn+1 values. Therefore, when our analyses are taken together, each of ACT, EF1, RPS5, RPS18 and GAPDH is suggested to be suitable as reference gene for qRT-PCR analysis. Among these five genes, although other analysis revealed that expression stability values of all five genes were below the criteria, SD values of ACT and EF1 were over 1.0, the cutoff line in BestKeeper. Therefore, in conclusion, all the stability values of RPS5, RPS18 and GAPDH were below the cutoff values in each of the analysis methods used. Thus, we suggest that RPS5, RPS18 and GAPDH are the most appropriate reference gene for accurate normalisation of target gene expression in honey bee samples prepared from various tissues and seasons.
Methods
Sample preparation and total RNA extraction
The honey bee colonies used in this study (an Italian hybrid) were maintained in the experimental apiary of College of Ecology and Environmental Science, Kyunpook National University, Sangju, Gyeongsangbuk-do, Rep. of Korea (36° 22′ 24. 41″ N, 128° 08′ 24.24″ E). Nurse bees were collected from three different colonies in spring (March 28, 2018), summer (June 27, 2017) and autumn (September 28, 2017) based on the ages and behaviours of bees; however, nurse bees were obtained from the central region of the colony in winter (December 27, 2017), following the previous study5. The collected nurses were immediately frozen with liquid nitrogen and stored at − 70 °C until RNA extraction.
For tissue analysis, the head, thorax and abdomen were separated from five bees and pooled as a single replication in a tube containing TRI reagent. RNA samples were prepared from three biological replicates. Each tissue sample was completely homogenised with a bullet blender and total RNA was extracted using the Direct-zol RNA Miniprep Plus kit (ZYMO RESEARCH, Irvine, CA, USA). Samples were treated with DNase I during the RNA extraction procedure to eliminate genomic DNA contamination following the manufacturer’s protocol10. The purity and quantity of the extracted RNAs were measured in triplicate using a SpectraMax QuickDrop spectrophotometer. The prepared RNA was then stored at − 70 °C until further use.
Primer design and cloning
The sequence information of seven genes was obtained from the NCBI database (https://www.ncbi.nlm.nih.gov) and primers for the seven reference genes and the target gene were designed, following the previous study7. The primer sets for EF1, RPS18 and CAT were designed based on two different exons to amplify genomic DNA containing introns if samples were contaminated by genomic DNA; therefore, they produced larger PCR products (Table 1). Amplification specificity and the lack of genomic DNA contamination were confirmed with gel electrophoresis.
For subcloning, total RNA was used as a template for the reverse transcription PCR reaction with a DiaStar OneStep RT-PCR kit (SOLGENT, Daejeon, Korea) using the following protocol: 50 °C for 30 min; 95 °C for 15 min; 35 cycles of 95 °C for 20 s, 58 °C for 40 s and 72 °C for 30 s; and 5 min at 72 °C. The gene-specific primers were used for each gene amplification (Table 1). They were then subcloned into the pGEM-T easy vector (PROMEGA, Madison, MU, USA). The plasmid-containing positive inserts were submitted for sequencing reactions using the M13 universal primers with an ABI PRISM 3730XL analyser.
Quantitative real-time PCR
For cDNA synthesis, the amount of total RNA was standardised to 1 μg. The first strand of cDNA was synthesised with total RNA, extracted from different tissues over the four seasons using ReverTra Ace reverse transcriptase (TOYOBO, Osaka, Japan), by priming with oligo (dT) following the manufacturer’s protocol.
For the qRT-PCR assay, we used the CFX Connect Real-Time PCR detection system (BIO-RAD, Hercules, CA, USA) with CYBR GREEN methodology. The PCR efficiency of each gene was calculated from the given slope after running standard curves generated with four points of twofold serial dilutions of cDNA using the following formula: E = 2−1/slope. qRT-PCR reactions were performed in duplicate (technical replicates) using the following protocol: 95 °C for 1 min; and then 40 cycles of 95 °C for 15 s, 58 °C for 15 s and 72 °C for 30 s. Quantification cycle (Cq) values of the seven candidate reference genes and the target gene (CAT) were obtained at the same fluorescence threshold (0.1).
To validate the selected reference genes after determination of their gene stabilities, the expression level of the target gene (CAT) was analysed. The Cq values for reference genes and CAT were obtained for each sample and then normalised by a relative quantification method adapted from the original concept of 2−ΔΔCq30. Reference genes were selected based on the stability rank of genes analysed by geNorm (Fig. 2) when multiple references were used for normalisation and the mean Cq value was used for analysis.
Data analysis
The Cq distribution of genes across various seasons and tissues was analysed with Origin Pro 9.0 and the AM, SD and CV values were obtained (CV = SD/AM).
To evaluate the expression stability of the seven candidate reference genes, three freely-available software programs, namely NormFinder (version 0.953)20, BestKeeper (version 1)31 and geNorm (version 3.1)25, were used in this study. NormFinder calculates the stability values of each candidate gene based on the overall variation to evaluate the systematic error introduced for gene normalisation, wherein lower stability values indicate more stable genes20. BestKeeper determines the suitable reference genes by calculating the geometric mean of the genes’ Cq values and then the SD: lower SD values signify more stable genes. BestKeeper also calculates the correlation (R2) of each candidate gene with other genes. Thereafter, highly correlated candidate genes are combined to evaluate P values. Based on the results of BestKeeper, the candidate genes with relatively high R2 values but low SDs, CVs and P values are considered to be more stable genes. The geNorm automatically calculates an M value for each putative reference gene based on the geometric mean of all studied genes: more stable genes are indicated by lower M values. In addition, geNorm compares the pairwise variation (V) of a gene with the other genes; pairwise variation (Vn/Vn+1) is calculated to estimate the optimal number of reference genes required for accurate normalisation. A pairwise variation value below 0.15 indicates that an additional reference gene does not improve normalisation of target gene expression levels25.
SPSS for Windows (version 23.0) was used for statistical analysis of CAT expression (Fig. 5). The expression patterns of CAT among the four seasons and three tissue types, normalised with a single gene or with multiple reference genes, were statistically analysed using a one-way ANOVA followed by Tukey’s multiple comparison post-hoc test (Fig. 5A–H).
Supplementary information
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2017R1C1B2008699).
Author contributions
Y.H.K. designed the experiments and wrote the manuscript. J.H.J. conducted the experiments and analysis and wrote the manuscript. K.M. conducted the experiments and analysis. Y.K. conducted the experiments and analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70965-4.
References
- 1.Leonhardt SD, Gallai N, Garibaldi LA, Kuhlmann M, Klein AM. Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl. Ecol. 2013;14:461–471. doi: 10.1016/j.baae.2013.06.003. [DOI] [Google Scholar]
- 2.Lee SH, Kim YH. Comparative proteome analysis of honey bee workers between overwintering and brood-rearing seasons. J. Asia-Pac. Entomol. 2017;20:984–995. doi: 10.1016/j.aspen.2017.07.011. [DOI] [Google Scholar]
- 3.Huang ZY, Robinson GE. Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J. Comp. Physiol. B. 1995;165:18–28. doi: 10.1007/bf00264682. [DOI] [PubMed] [Google Scholar]
- 4.Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 1982;28:61–68. doi: 10.1016/0022-1910(82)90023-3. [DOI] [Google Scholar]
- 5.Kim YH, Kim JH, Kim K, Lee SH. Expression of acetylcholinesterase 1 is associated with brood rearing status in the honey bee, Apis mellifera. Sci. Rep. 2017;7:39864. doi: 10.1038/srep39864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lourenco AP, Mackert A, Cristino AD, Simoes ZLP. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie. 2008;39:372–385. doi: 10.1051/apido:2008015. [DOI] [Google Scholar]
- 7.Moon K, Lee SH, Kim YH. Validation of quantitative real-time PCR reference genes for the determination of seasonal and labor-specific gene expression profiles in the head of Western honey bee, Apis mellifera. PLoS ONE. 2018;13:e0200369. doi: 10.1371/journal.pone.0200369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai Y, et al. Identification and validation of reference genes for quantitative real-time PCR in Drosophila suzukii (Diptera: Drosophilidae) PLoS ONE. 2014;9:e106800. doi: 10.1371/journal.pone.0106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling D, Salvaterra PM. Robust RT-qPCR data normalization: Validation and selection of internal reference genes during post-experimental data analysis. PLoS ONE. 2011;6:e17762. doi: 10.1371/journal.pone.0017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon K, Lee SH, Kim YH. Evaluation of reference genes for quantitative real-time PCR to investigate seasonal and labor-specific expression profiles of the honey bee abdomen. J. Asia-Pac. Entomol. 2018;21:1350–1358. doi: 10.1016/j.aspen.2018.10.014. [DOI] [Google Scholar]
- 11.Kim Y, et al. Validation of reference genes for quantitative real-time polymerase chain reaction in Drosophila melanogaster exposed to two chemicals. Entomol. Res. 2019;49:277–283. doi: 10.1111/1748-5967.12365. [DOI] [Google Scholar]
- 12.Kim Y, Kim Y, Kim YH. Evaluation of reference genes for gene expression studies using quantitative real-time PCR in Drosophila melanogaster after chemical exposures. J. Asia-Pac. Entomol. 2020;23:385–394. doi: 10.1016/j.aspen.2020.01.008. [DOI] [Google Scholar]
- 13.Wang Z, et al. Evaluation and validation of reference genes for quantitative real-time PCR in Helopeltis theivora Waterhouse (Hemiptera: Miridae) Sci. Rep. 2019;9:13291. doi: 10.1038/s41598-019-49479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponton F, Chapuis MP, Pernice M, Sword GA, Simpson SJ. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 2011;57:840–850. doi: 10.1016/j.jinsphys.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Scharlaken B, et al. Reference gene selection for insect expression studies using quantitative real-time PCR: The head of the honeybee, Apis mellifera, after a bacterial challenge. J. Insect. Sci. 2008;8:1–10. doi: 10.1673/031.008.3301. [DOI] [Google Scholar]
- 16.Reim T, Thamm M, Rolke D, Blenau W, Scheiner R. Suitability of three common reference genes for quantitative real-time PCR in honey bees. Apidologie. 2013;44:342–350. doi: 10.1007/s13592-012-0184-3. [DOI] [Google Scholar]
- 17.Antunez K, et al. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia) Environ. Microbiol. 2009;11:2284–2290. doi: 10.1111/j.1462-2920.2009.01953.x. [DOI] [PubMed] [Google Scholar]
- 18.McQuillan HJ, Barron AB, Mercer AR. Age- and behaviour-related changes in the expression of biogenic amine receptor genes in the antennae of honey bees (Apis mellifera) J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2012;198:753–761. doi: 10.1007/s00359-012-0745-y. [DOI] [PubMed] [Google Scholar]
- 19.Richard FJ, Schal C, Tarpy DR, Grozinger CM. Effects of instrumental insemination and insemination quantity on Dufour's gland chemical profiles and vitellogenin expression in honey bee queens (Apis mellifera) J. Chem. Ecol. 2011;37:1027–1036. doi: 10.1007/s10886-011-9999-z. [DOI] [PubMed] [Google Scholar]
- 20.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 21.Liu CH, et al. Reference gene selection for quantitative real-time RT-PCR normalization in the half-smooth tongue sole (Cynoglossus semilaevis) at different developmental stages, in various tissue types and on exposure to chemicals. PLoS ONE. 2014;9:e91715. doi: 10.1371/journal.pone.0091715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan HJ, et al. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010;399:257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Chechi K, Gelinas Y, Mathieu P, Deshaies Y, Richard D. Validation of reference genes for the relative quantification of gene expression in human epicardial adipose tissue. PLoS ONE. 2012;7:e32265. doi: 10.1371/journal.pone.0032265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julian GS, Oliveira RW, Tufik S, Chagas JR. Analysis of the stability of housekeeping gene expression in the left cardiac ventricle of rats submitted to chronic intermittent hypoxia. J. Bras. Pneumol. 2016;42:211–214. doi: 10.1590/S1806-37562015000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan M, Pereg L. Evaluation of reference genes for gene expression analysis using quantitative RT-PCR in Azospirillum brasilense. PLoS ONE. 2014;9:e98162. doi: 10.1371/journal.pone.0098162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ospina R, Marmolejo-Ramos F. Performance of some estimators of relative variability. Front. Appl. Math. 2019;5:00043. doi: 10.3389/fams.2019.00043. [DOI] [Google Scholar]
- 29.Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006;6:27. doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.