Abstract
Background
S100A9, which is expressed in prostate cancer, has been reported in association with prostate cancer progression. However, the role of S100A9 in prostate cancer metastasis is largely unknown. The aim of this study was to investigate the effect of S100A9 on prostate cancer cell invasion and the involved mechanisms.
Materials and methods
Integrin β1 expression in PC-3 and DU-145 cells was determined by quantitative real-time polymerase chain reaction (PCR) (qRT-PCR) and Western blot. Cellular invasion was measured by transwell invasion assay. Western blot was used to determine protein expression. Concentrations of S100A9 and fibronectin were analyzed by enzyme-linked immunosorbent assay. The protein interaction was detected by immunoprecipitation. The NF-κB activity was measured by luciferase reporter assay. The DU-145 cells metastasis in vivo was determined in mice xenograft models after S100A9 overexpression.
Results
S100A9 promoted prostate cancer cells invasion, integrin β1 expression and fibronectin secretion. Further investigation evidenced that S100A9 interacted with Toll-like receptor 4 (TLR4) and activated NF-κB, which was responsible for tumor cell invasion, integrin β1 up-regulation and focal adhesion kinase (FAK) phosphorylation. Furthermore, integrin β1 inhibition led to decreased FAK phosphorylation and reduced tumor cell invasion. Overexpression of S100A9 increased xenograft tumor micro-metastases, integrin β1 expression and induced NF-κB and FAK activation in vivo.
Conclusion
Our study demonstrated that S100A9 promotes prostate cancer cell invasion, and one of the underlying molecular mechanisms is that S100A9 activates integrin β1/FAK through TLR4/NF-κB signaling leading to metastasis of prostate cancer cell.
Keywords: S100A9, prostate cancer, metastasis, integrin β1, FAK
Introduction
Prostate cancer is the second most frequently diagnosed cancer in men in the world.1 It has been reported that metastasis is the main cause of mortality in prostate cancer patients. Prostate cancer cell can migrate to the many organs such as bone, liver, lung and lymph nodes.2 Although many efforts have been made to investigate the involvement of certain molecules and of chromosomal aberrations in prostate metastasis, the molecular mechanisms underlying the migration and invasion of prostate cancer cell are still poorly understood.
S100A9, a damage-associated molecular pattern (DAMP) molecule, is expressed in many types of cancer, including prostate cancer.3–5 S100A9 has two pattern recognition receptors, TLR4 and RAGE, which mediate the pathologic effects of S100A9. S100A9 has been reported to interact with TLR4 and promotes endotoxin-induced lethality and systemic autoimmunity.6–8 Interaction of S100A9 with TLR4 also promotes premetastatic niches in lungs.9 S100A9 has been shown to promote tumor growth via RAGE ligation.10,11
S100A8/A9 has been reported to be involved in inflammation-associated cancer.12 A study showed that S100A9 might be useful as a prognostic marker for prostate cancer recurrence.4 It has been reported that high density of S100A9 positive inflammatory cells in prostate tumor tissue is associated with poor outcome.13 S100A9 is found to be involved in early tumor metastatic processes. Previous study showed that S100A9 facilitated disseminating tumor cells adhesion to enable tumor cell settles in distant organs.9 S100A8/A9 are involved in H-Ras-mediated human breast epithelial cell invasion and migration.14 Moreover, S100A8/A9 promote B16F10 melanoma cell migrate to the lung through interacting with RAGE.15 Despite increasing evidences showed that S100A9 plays an important role in tumor cell metastatic processes, little is known about the direct effect of S100A9 on prostate cancer cell invasion and metastasis.
In the present study, we demonstrated that S100A9 interacts with TLR4 and promotes invasion of prostate cancer cells through NF-κB activation, β1 integrin up-regulation and FAK phosphorylation. Overall, our results showed that S100A9 regulates prostate cancer metastasis and may serve as a potential target for anti-metastasis therapy.
Materials and methods
Cell line and animals
The prostate cancer cell line PC-3 and DU-145 were purchased from the China Center for Type Culture Collection (Wuhan, China). Nude mice (male BALB/c nu/nu, 6–8 weeks old) were obtained from SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All animal studies (including the mice euthanasia procedure) were performed in compliance with the Accreditation of Laboratory Animal Care International (AAALAC) and Institutional Animal Care and Use Committee (IACUC) of Jining Hospital of Traditional Chinese Medicine guidelines and approved by the IACUC of Jining Hospital of Traditional Chinese Medicine.
Antibodies and reagents
The recombinant human S100A9 (9254-S9), human S100A9 ELISA Kit (DY5578) were purchased from R&D Systems (R&D Systems, Shanghai, China). Rabbit anti-human antibodies against S100A9 (72590), TLR4 (2246), integrin β1 (34971), FAK (3285), p-FAK (3281), RAGE (6996), NF-κB (8242), Phospho-NF-κB p65 (Ser536) antibody (3031) were purchased from Cell Signaling Technology (Danvers, MA, USA). β1 integrin inhibitory antibody MAB 13 (552828) and the isotype control antibody rat IgG2aκ (553927) were purchased from (BD Biosciences-CN, Shanghai, China).
The small interfering RNAs (si RNAs) against TLR4 (sc-40260), integrin β1(sc-35674) for experiments using targeted siRNA transfection were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). BAY 11–7082 (481487), PF562271(PZ0387), mouse anti-human antibody against fibronectin (F6140) and human fibronectin ELISA Kit (RAB1049) were purchased from (Sigma-Aldrich, Shanghai, China). Lipofectamine 2000 (11,668,019) was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Cell culture
PC-3 cells were maintained in RPMI-1640. DU-145 cells were cultured in Dulbecco’s Minimal Essential Medium (DMEM). Media were supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 lg/ml streptomycin. The cells were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Blocking integrin β1 activity
For blocking β1 integrin function, MAB 13 (50 µg/ml) and rat IgG2aκ (50 µg/ml) as the isotype control were used.
Matrigel invasion assay
A chamber of Matrigel-coated 24-well culture inserts (Transwell; Corning, Corning, NY, USA) was used for the transwell migration assay. Cells (2.5×104) were seeded in the upper chamber and suspended in serum-free DMEM or RPMI1640 culture medium. In the lower chamber s, 5% FBS was added as a chemoattractant. After incubation for 24 h, the transmembrane cells were washed briefly with PBS and dried for 5 min, then fixed with methanol and stained with hematoxylin-eosin for 10min. The transmembrane cells were counted under high-power (×100) microscope fields. Mean values were obtained from six randomly selected fields for each well. The experiment was repeated three times.
Plasmid construction and cell transfection
A full-length human cDNA of S100A9 was synthesized by Bioworld (Nanjing, China). The product was then sub-cloned into the pcDNA3.1vector (Invitrogen, Carlsbad, CA, USA) to construct the plasmid pcDNA-S100A9. pcDNA-S100A9 plasmid was transfected into DU-145 cells using Lipofectamine 2000 according to the manufacture’s protocol. 48 h after transfection, transfectants were selected in culture medium supplemented with 400 μg/ml G418. G418-resistant monoclones were picked and expanded in the selection medium.
For silencing specific gene expression, cells were treated with TLR4 siRNA, integrin β1 siRNA. Briefly, 2×105 PC-3 or DU-145 cells were seeded into 6-well plate with 2 ml antibiotic-free normal growth medium containing FBS. TLR4 siRNA, integrin β1 siRNA, or control siRNA was performed according to the manufacture’s protocol.
Quantitative RT-PCR
Total RNA was extracted from cells with TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative real-time PCR analyses were performed by Applied Biosystems using the Takara RNA PCR kit. The mRNA of GAPDH was used as internal control. The relative quantification of integrin β1 mRNA expression was achieved by the 2−ΔΔCt method. The sequences of the primers were as follows: integrin β1, Forward: 5ʹ-GCCTTACATTAGCACAACACC-3ʹ, Reverse: 5ʹ-CATCTCCAGCAAAGTGAAACC-3ʹ. GAPDH, Forward: 5ʹ-TCATTGACCTCAACTACATGGTTT-3ʹ Reverse: 5ʹ-GAAGATGGTGATGGGATTTC-3ʹ. The relative expression of integrin β1 was calculated using 2−ΔΔCt method.
Western blot analysis
The whole-cell extracts were prepared using RIPA lysis buffer (Beyotime, China) with phenylmethanesulfonyl fluoride and protease inhibitor cocktail (Roche, USA). Proteins were quantified by BCA assay. Cell lysates were separated by 8–12% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Bio-Rad, USA). After being blocked using 5% non-fat milk for 1 h at room temperature, membranes were incubated with the indicated primary antibodies overnight at 4 °C and probed with horseradish peroxidase-conjugated secondary antibodies (1:1000). The bands were visualized using a ChemicDocXRS system (Bio-Rad, USA). Protein levels were normalized to the β-actin.
Luciferase reporter assay
The NF-κB activity in cells was detected with a NF-κB luciferase reporter plasmid (Invitrogen, Carlsbad, CA, USA). The cells were co-transfected with NF-κB luciferase reporter plasmid or Renilla luciferase pRL-TK plasmid (Promega, Madison, WI, USA) with or without TLR4 si RNA performing in Lipofectamine 2000 reagent. then the cells were treated with or without S100A9 for 48 h, the reporter activity was measured using the dual-luciferase reporter assay system (Promega). Luciferase activity was normalized for transfection efficiency using the corresponding Renilla luciferase activity.
Immunoprecipitation
Cells were lysed in IP buffer containing1 mM DL-dithiothreitol (DTT), 100 mmol/L NaCl,1 mM MgCl2 (Life Technology, USA) and protease inhibitor cocktails (Cell Signaling Technology, USA).After centrifuge at 15,000 g for 30 min at 4 °C, cell lysates were incubated with specific antibody or control IgG overnight, and then with Protein A/G-Sepharose (Amersham Biosciences, Piscataway, NJ) beads for 4 h at 4 °C. After washing, the immunoprecipiated were subjected to 10% SDS polyacrylamide gel electrophoresis and detected by Western blot.
Enzyme-linked immunosorbent assay
Human S100A9 or fibronectin in supernatants of the prostate tumor cell line was quantified using human S100A9 or fibronectin ELISA Kit. A standard curve was included in each assay, and samples and standards run in triplicate. Absorbance was read at 450 nm using the VICTOR3 1420 Multilabel Plates Counter.
Mice xenograft models
1×106 DU-145 pcDNA3.1 or pcDNA-S100A9 transfected DU-145 cells were injected into mouse tail veins (n=8 for each group). The mice were killed at the indicated time. Lungs were surgically retrieved from mice. Micrometastatic tumors in the lungs were counted. Tissues were embedded in paraffin, sectioned, stained with H&E.
Immunohistochemistry
Tissue sections were prepared and subjected to immunohistochemical analysis. Anti-human S100A9, integrin β1 antibody was used as primary antibodies. HRP-conjugated secondary Ab was used as secondary antibody. Images were obtained using an Olympus-IX71 microscope at 20×10 magnification.
Statistical analysis
The values given are means ± S.E.M. The significance of difference between the experimental groups and controls was assessed by Student’s t-test and one-way analysis of variance (ANOVA). All statistical analyses were performed using SPSS 19.0 software. The difference was significant if the p-value was <0.05.
Results
S100A9 promotes prostate cancer cell invasion and β1 integrin expression via TLR4
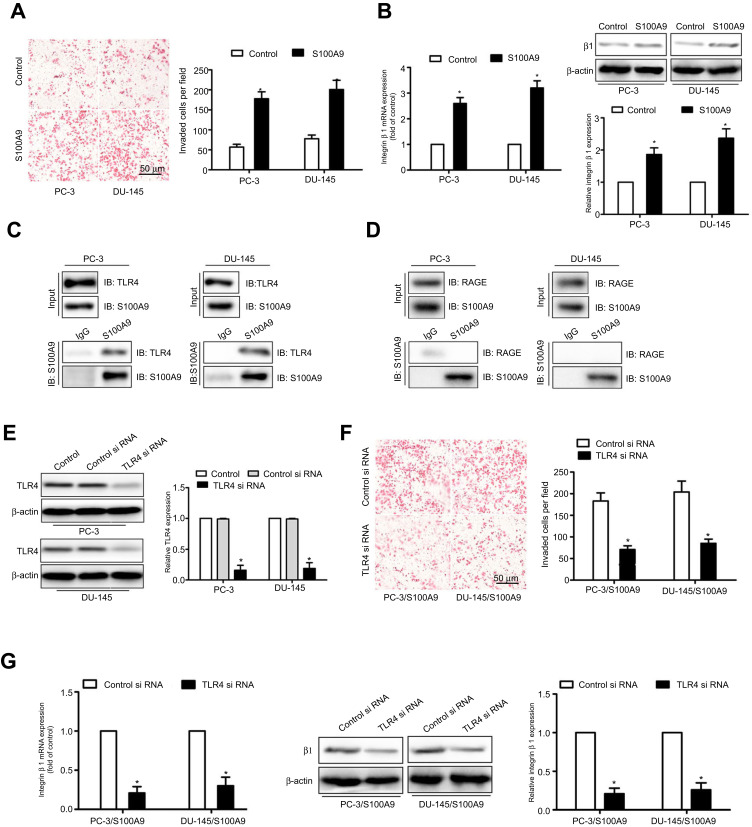
To evaluate the influence of extracellular S100A9 on the migration of prostate cancer cells, we treated PC-3 or DU-145 cells with recombinant S100A9, matrigel invasion assay showed that S100A9 promoted tumor cell invasion (Figure 1A). Previous study reported that integrin β1 mediated the migration of prostate cancer cells.16 We found that S100A9 increased prostate cancer cell integrin β1 expression (Figure 1B).
Figure 1.
S100A9 promotes prostate cancer cell invasion and β1 integrin expression through interaction with TLR4. PC-3 and DU-145 cells were treated with S100A9 (20 µg/ml) for 48 h. (A) PC-3 and DU-145 cells invasion was measured by transwell invasion assay. (B) The mRNA and protein levels of β1 integrin were determined by qPCR or Western blot. PC-3 and DU-145 cells were treated with S100A9 (20 µg/ml) for 48 h. (C, D) Cell extracts were immunoprecipitated (IP) with control mouse IgG, mouse anti-S100A9 antibody. Immunoblot (IB) was used to detect S100A9, TLR4 and RAGE. PC-3 and DU-145 cells were transfected with TLR4 or control siRNA. (E) TLR4 expression was examined by Western blot after 48 h siRNA transfection. PC-3 and DU-145 cells were transfected with TLR4 or control siRNA followed by stimulation with S100A9. (F) Tumor cell invasion was measured by transwell invasion assay. (G) The mRNA and protein levels of β1 integrin were determined by qPCR or Western blot. Scale bar 50 μ. Magnifcation×200. Data are represented as the mean ± S.E.M. *p<0.05.
S100A8/A9 has been reported to mediate signaling through TLR4 and promote premetastatic niches in lungs.8,17 Therefore, we examined whether TLR4 was involved in S100A9-mediated prostate cancer cell invasion. To validate whether TLR4 provided binding sites for S100A9 on prostate cancer cells, we performed co-immunoprecipitation assays and found that S100A9 physically interacted with TLR4 (Figure 1C). S100A8/A9 acts as a ligand for RAGE and TLR4. The co-immunoprecipitation assays showed that S100A9 did not physically interact with RAGE in PC-3 and DU-145 cells (Figure 1D). Transfection of tumor cell with TLR4 siRNA, the expression of TLR4 was validated by Western blot (Figure 1E). We found TLR4 si RNA markedly inhibited prostate cancer cell invasion (Figure 1F) and reduced integrin β1 expression (Figure 1G). These data suggest that TLR4 mediates the effects of S100A9 on prostate cancer cell invasion and β1 integrin expression.
TLR4/Nf-κb mediates s100a9-promoted prostate cancer cell β1 integrin expression and invasion
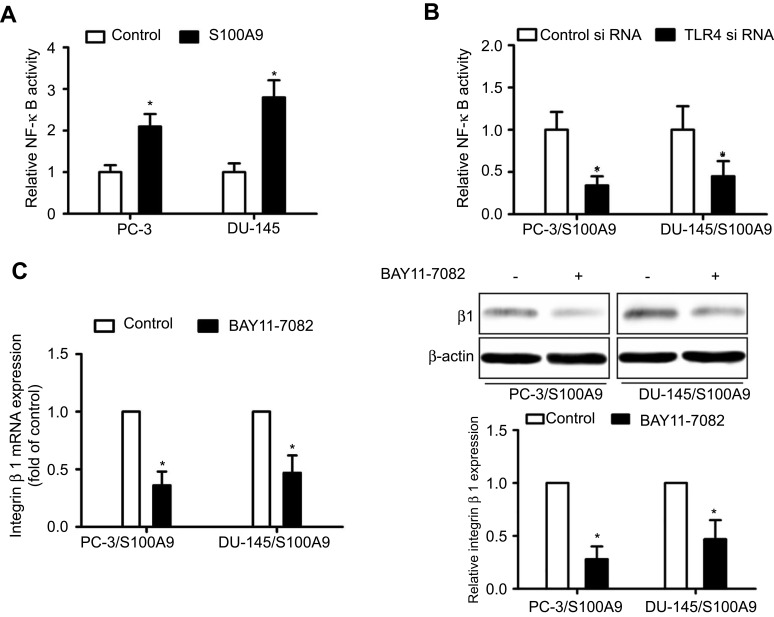
S100A9 can activate NF-κB through binging to TLR4.8 We examined whether S100A9 induced NF-κB activation. Luciferase reporter assay showed that S100A9 promoted NF-κB transcriptional activity, whereas tumor cells transfected with TLR4-siRNA exhibited decreased NF-κB transcriptional activity (Figure 2A and B). In addition, NF-κB inhibitor BAY11-7082 reduced β1 integrin expression increased by S100A9 (Figure 2C). Taken together, our results suggested that TLR4/NF-κB mediates β1 integrin up-regulation induced by S100A9.
Figure 2.
NF-κB mediates S100A9-induced prostate cancer cell β1 integrin up-regulation. PC-3 and DU-145 cells transfected with or without TLR4 siRNA or control siRNA, were transfected with NF-κB-luciferase reporter plasmid, and treated with S100A9 (20 µg/ml) for 48 h. (A, B) Activity of NF-κB was detected by measuring the relative activity of luciferase. PC-3 and DU-145 cells were treated with or without BAY11-7082 (5µM) for 30 min. Then cells were treated with or without S100A9 (20 µg/ml) for 48 h. (C) The mRNA and protein levels of β1 integrin were determined by qPCR or Western blot. Data are represented as the mean ± S.E.M. *p<0.05.
S100A9 induces prostate cancer cell fibronectin secretion and activating integrin β1/FAK signaling
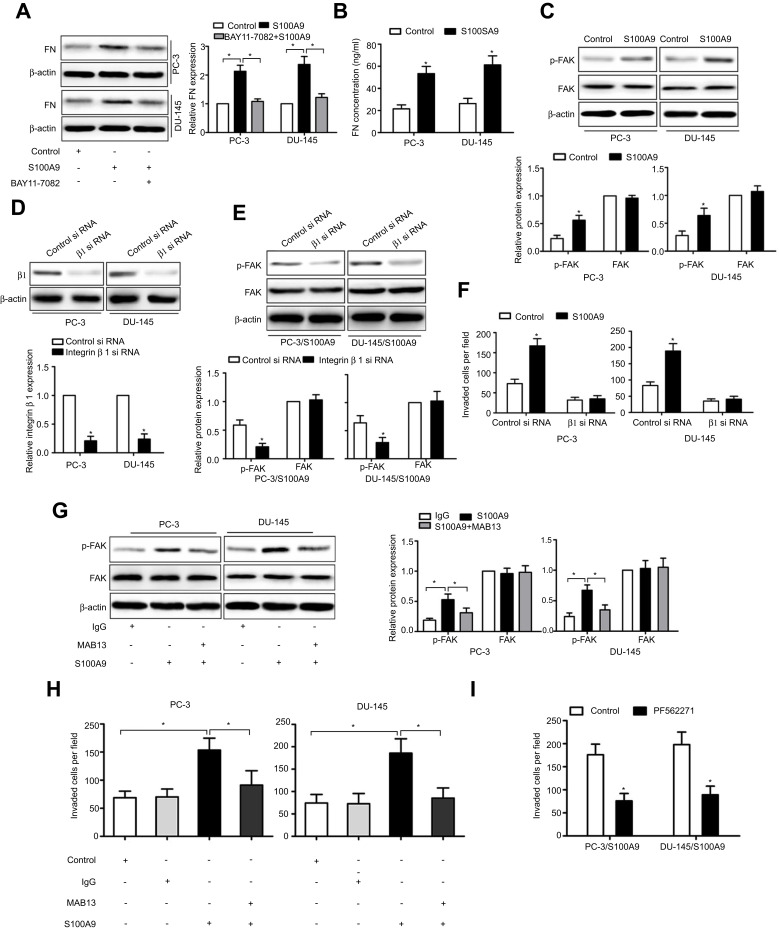
As S100A9 increased integrin β1 expression, we further investigated whether S100A9 affected integrin β1 activation. β1 integrin can be activated by binding to its major receptor fibronectin (FN).18,19 We found that S100A9 promoted tumor cell fibronectin expression, whereas BAY11-7082 reduced fibronectin expression induced by S100A9 (Figure 3A). ELISA assay showed that S100A9 induced fibronectin secretion (Figure 3B). The results implied that S100A9 promoted fibronectin expression through NF-κB and activated integrin β1. Focal-adhesion kinase (FAK) is involved in signaling downstream of integrins, NF-κB activation and play important roles in integrin-mediated signal transductions and tumor metastasis.20–22 The phosphorylation of FAK can be initiated by integrin β1 signaling.23 We investigated whether S100A9 affected FAK activation. The results showed that S100A9 increased tumor cell FAK phosphorylation (Figure 3C). We knockdown tumor cell integrin β1 expression with integrin β1 si RNA (Figure 3D) and found that integrin β1 knockdown inhibited S100A9-induced FAK phosphorylation and tumor cell invasion (Figure 3E and F).
Figure 3.
S100A9 promotes prostate cancer cell invasion via integrin β1/FAK signaling. PC-3 and DU-145 cells were treated with or without BAY11-7082 (5µM) for 30 min. Then cells were treated with or without S100A9 (20 µg/ml) for 48 h. (A) Fibronectin expression was determined by Western blot. PC-3 and DU-145 cells were treated with or without S100A9 (20 µg/ml) for 48 h. (B) Supernatant fibronectin (FN) concentration was determined by ELISA. PC-3 and DU-145 cells were treated with or without S100A9 (20 µg/ml) for 30 min. (C) The phosphorylation of FAK was measured by Western blot. PC-3 and DU-145 cells were transfected with control siRNA or integrin β1-specific siRNA for 48 h. Then cells were treated with or without S100A9 (20 µg/ml) for 30 min, the expression of integrin β1 (D) or phosphorylation of FAK (E) was measured by Western blot. PC-3 and DU-145 cells were transfected with control siRNA or integrin β1-specific siRNA for 48 h. Then cells were treated with or without S100A9 (20 µg/ml) for 48 h. (F) The invasion activity were measured by transwell invasion assay. (G) PC-3 and DU-145 cells were treated with β1 integrin functional blocking antibody MAB13 (50 μg/mL) or control IgG (50 μg/mL) for 30 min and then treated with or without S100A9 (20 µg/ml) for 30 min and the phosphorylation of FAK was measured by Western blot. (H) PC-3 and DU-145 cells were treated with MAB13 (50 μg/mL) or control IgG (50 μg/mL) for 30 min and then treated with or without S100A9 (20 µg/ml) for 48 h for invasion. (I) PC-3 and DU-145 cells were pretreated for 30 min with FAK inhibitor, PF562271 (100 nM) followed by stimulation with S100A9 (20 µg/ml) for 48 h for invasion. Data are represented as the mean ± S.E.M. *p<0.05.
We further used MAB13, an inhibitory antibody of integrin β1 to treat prostate cancer cells. The contribution of S100A9 to tumor cell FAK phosphorylation and invasion is reversed in the presence of MAB13 (Figure 3G and H), indicating that β1 integrin is the mediator of S100A9-induced prostate tumor cell invasion and FAK activation. Moreover, FAK inhibitor, PF562271 inhibited S100A9-induced tumor cell invasion (Figure 3I). These results showed that S100A9 promoted prostate cancer cell invasion via integrin β1/FAK signaling.
S100A9 promotes prostate cancer cell metastasis in vivo
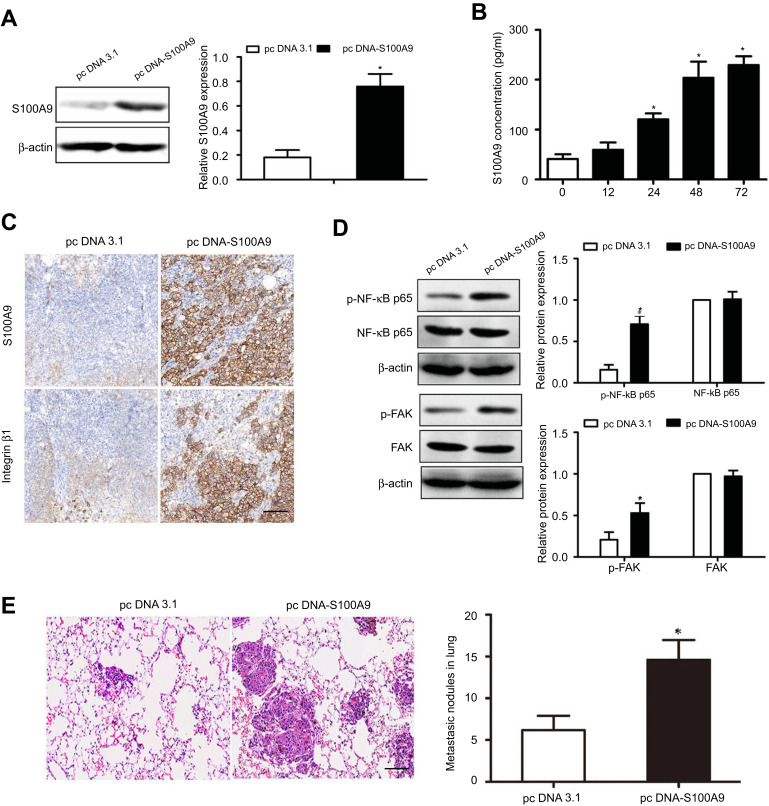
To investigate the effect of S100A9 on prostate cancer cell metastasis in vivo. DU-145 cells were stably transfected with pcDNA 3.1 or pcDNA-S100A9 (Figure 4A). The levels of S100A9 in supernatants of DU-145 cells after S100A9 transfection were measured by ELISA (Figure 4B). We then generated xenograft tumors in the nude mice. The results showed that S100A9 overexpression resulted in the increased integrin β1 expression and higher levels of p-FAK, p-NF-kB p65 by Western blot and IHC analysis in lung metastatic tumors (Figure 4C and D). In addition, the result showed that S100A9 overexpression in DU-145 cells significantly promoted tumor metastasis in vivo, as evidenced by the increased micrometastases in the lung (Figure 4E).
Figure 4.
S100A9 induces prostate cancer cell metastasis in vivo. DU-145 cells were transfected with pcDNA3.1 or pc DNA-S100A9 plasmid. (A, B) The expression or secretion of S100A9 was determined by Western blot and ELISA. The cells were injected to nude mice via tail vein. 30 days after inoculation, nude mice were sacrificed. (C) Expressions of S100A9 and integrin β1 in xenograft tumors were detected by immunohistochemistry. (D) Expressions of FAK, p-FAK, NF-kB p65 and p-NF-kB p65 in xenograft tumors were determined by Western blot. (E) Micrometastatic tumors in the lungs of mouse xenografts were counted and subjected to H and E staining. Scale bar 50 μ. Magnifcation×200. Data are represented as the mean ± S.E.M.*p<0.05.
Discussion
Previous studies have reported that S100A8/A9 promoted prostate cancer progression.4,13,24 However, the effect and mechanism of S100A9 on prostate cancer cell invasion and metastasis remain unclear. In this study, we found that S100A9 promotes prostate cancer cell invasion and integrin β1 expression in vitro and in vivo. The effect of S100A9 was mediated by direct binding of TLR4 and NF-κB activation. Furthermore, we found that integrin β1/FAK signaling mediated the function of S100A9. Inhibition of integrin β1/FAK partially abolished the metastatic capacity of tumor cell induced by S100A9, suggesting that there are other signaling pathways participate in the S100A9-promoted prostate cancer cell metastasis.
S100A8/A9 are danger associated molecular pattern ligands, which not only expressed in cancer stromal cells but also in a variety of different cancer cells. Extracellular S100A8/A9 in tumor microenvironment exerts biological function in tumor progression. In the present study, we used recombinant S100A9 protein to treat prostate cancer cell and found S100A9 promoted tumor cell invasion. Our finding is consistent with the previous studies, showing that S100A9 promoted tumor cell invasion.25,26 However, a recent study reported that recombinant S100A8/A9 protein (0.1–10,000 ng/ml) had no effect on prostate cancer cell line PC-3 migration. It may be the concentration of S100A9 is low, S100A9 cannot exert its biological effects on tumor cell migration.
S100A8/A9 act as a ligand for RAGE and TLR4.27 S100A8/A9 not only binds to RAGE, but also TLR4 on tumor cell. It has been reported that S100A8/A9 interaction with TLR4 promotes premetastatic niches in lungs.17 RAGE is the principal receptor of S100A8/A9 on tumor cells and plays an important role in S100A9-induced prostate cancer proliferation and migration.25 The receptor activated may depend on the cell types, ligand or receptor concentration and distinct epitopes on RAGE and TLRs. Which receptor played prominent roles in S100A9 mediated tumor-promoting effects is debatable. In the present study, we found that S100A9 interacted with TLR4 but not RAGE in PC-3 and DU-145 cells. Moreover, knockdown of prostate cancer cell TLR4 abolished S100A9 promoting effects on tumor cell invasion and integrin β1 expression further confirmed that TLR4 is the principal receptor mediated S100A9 effects. TLR4 signaling has been shown to activate NF-κB and result in up-regulation of integrin αvβ3 in NSCLC cells.22 We found that S100A9 interacted with prostate tumor cells TLR4 and activated NF-κB, which promoted integrin β1 expression.
Integrin β1 is a transmembrane receptor for extracellular matrix proteins that promotes tumor cell adhesion and migration. Increasing amounts of evidence showed that integrin β1 promotes prostate cancer bone metastasis.28–30 Fibronectin is the natural ligand for integrin β1 receptor31 and can activate integrin β1. It has been reported that integrin α5β1 facilitates PC-3 cells adhesion and interacts with fibronectin.32 In this study, our results showed that S100A9 induced PC-3 and DU-145 cells fibronectin expression, suggesting that S100A9 indirectly activated integrin β1 by promoting fibronectin secretion. FAK is one of the most important downstream signaling targets stimulated by integrin β1 and is a crucial signaling molecule to cell motility mediated by integrin β1.33–35 We found that S100A9 increased FAK phosphorylation, which further confirmed integrin β1 activation induced by S100A9. A study has reported that S100A8/9 activated integrin β2 through TLR4/Rap1 activation.36 Our results suggested that S100A9 up-regulated integrin β1 expression though TLR4/NF-κB signaling and indirectly activated integrin β1. However, whether S100A9 can directly induce integrin β1 activation is currently not entirely understood. Future studies will be necessary to further clarify this issue and work out the precise molecular mechanism of S100A9-induced integrin β1 activation in prostate tumor cells.
Conclusion
Our study demonstrated that S100A9 promotes prostate cancer cell invasion through TLR4/NF-κB/integrin β1/FAK signaling. Thus, S100A9 may be a promising molecular target for developing cancer therapeutics.
Disclosure
The authors declare no competing financial interests in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132(6):931–939. [DOI] [PubMed] [Google Scholar]
- 3.Hermani A, Hess J, De Servi B, et al. Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res. 2005;11(14):5146–5152. doi: 10.1158/1078-0432.CCR-05-0352 [DOI] [PubMed] [Google Scholar]
- 4.Grebhardt S, Veltkamp C, Ströbel P, Mayer D. Hypoxia and HIF-1 increase S100A8 and S100A9 expression in prostate cancer. Int J Cancer. 2012;131(12):2785–2794. doi: 10.1002/ijc.27592 [DOI] [PubMed] [Google Scholar]
- 5.Ghavami S, Chitayat S, Hashemi M, et al. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625(1–3):73–83. doi: 10.1016/j.ejphar.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 6.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 Activate Key Genes and Pathways in Colon Tumor Progression. Mol Cancer Res. 2011;9(2):133–148. doi: 10.1158/1541-7786.MCR-11-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 8.Loser K, Vogl T, Voskort M, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16(6):713–717. doi: 10.1038/nm1110-1167 [DOI] [PubMed] [Google Scholar]
- 9.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8(12):1369–1375. doi: 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- 10.Ghavami S, Rashedi I, Dattilo BM, et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83(6):1484–1492. doi: 10.1189/jlb.1107745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt C, Riehl A, Durchdewald M, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205(2):275–285. doi: 10.1084/jem.20070679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebhardt C, Németh J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 2006;72(11):1622–1631. doi: 10.1016/j.bcp.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 13.Tidehag V, Hammarsten P, Egevad L, et al. High density of S100A9 positive inflammatory cells in prostate cancer stroma is associated with poor outcome. Eur J Cancer. 2014;50(10):1829–1835. doi: 10.1016/j.ejca.2014.03.278 [DOI] [PubMed] [Google Scholar]
- 14.Moon A, Yong HY, Song JI, et al. Global gene expression profiling unveils S100A8/A9 as candidate markers in H-ras-mediated human breast epithelial cell invasion. Mol Cancer Res. 2008;6(10):1544–1553. doi: 10.1158/1541-7786.MCR-08-0035 [DOI] [PubMed] [Google Scholar]
- 15.Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem. 2010;285(14):10822–10831. doi: 10.1074/jbc.M109.083550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi R, Goihberg E, Ren W, Pilichowska M, Mathew P. Proteolytic fragments of fibronectin function as matrikines driving the chemotactic affinity of prostate cancer cells to human bone marrow mesenchymal stromal cells via the α5β1 integrin. Cell Adh Migr. 2017;11(4):305–315. doi: 10.1080/19336918.2016.1212139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. doi: 10.1038/ncb1794 [DOI] [PubMed] [Google Scholar]
- 18.Lugano R, Vemuri K, Yu D, et al. CD93 promotes β1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J Clin Invest. 2018;128(8):3280–3297. doi: 10.1172/JCI97459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci. 2011;124(Pt 3):369–383. doi: 10.1242/jcs.069567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Wang X, Li X, et al. CD68+HLA-DR+ M1-like macrophages promote motility of HCC cells via NF-κB/FAK pathway. Cancer Lett. 2014;345(1):91–99. doi: 10.1016/j.canlet.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LL, Liu J, Lei S, Zhang J, Zhou W, Yu HG. PTEN inhibits the invasion and metastasis of gastric cancer via downregulation of FAK expression. Cell Signal. 2014;26(5):1011–1120. doi: 10.1016/j.cellsig.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Luo J, Li Y, et al. HMGB1 induces human non-small cell lung cancer cell motility by activating integrin αvβ3/FAK through TLR4/NF-κB signaling pathway. Biochem Biophys Res Commun. 2016;480(4):522–527. doi: 10.1016/j.bbrc.2016.10.052 [DOI] [PubMed] [Google Scholar]
- 23.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grebhardt S, Müller-Decker K, Bestvater F, Hershfinkel M, Mayer D. Impact of S100A8/A9 expression on prostate cancer progression in vitro and in vivo. J Cell Physiol. 2014;229(5):661–671. doi: 10.1002/jcp.v229.5 [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Duan L, Ye L, et al. S100A9 promotes the proliferation and invasion of HepG2 hepatocellular carcinoma cells via the activation of the MAPK signaling pathway. Int J Oncol. 2013;42(3):1001–1010. doi: 10.3892/ijo.2013.1796 [DOI] [PubMed] [Google Scholar]
- 26.Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006;312(2):184–197. doi: 10.1016/j.yexcr.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 27.Duan L, Wu R, Ye L, et al. S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/β-catenin pathway. PLoS One. 2013;8(4):e62092. doi: 10.1371/journal.pone.0062092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin JK, Tien PC, Cheng CJ, et al. Talin1 phosphorylation activates β1 integrins: a novel mechanism to promote prostate cancer bone metastasis. Oncogene. 2015;34(14):1811. doi: 10.1038/onc.2014.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YC, Jin JK, Cheng CJ, et al. Targeting constitutively activated beta1 integrins inhibits prostate cancer metastasis. Mol Cancer Res. 2013;11(4):405–417. doi: 10.1158/1541-7786.MCR-12-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sottnik JL, Daignault-Newton S, Zhang X, et al. Integrin alpha2beta1 (alpha2beta1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30(5):569–578. doi: 10.1007/s10585-012-9561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028 [DOI] [PubMed] [Google Scholar]
- 32.Stachurska A, Elbanowski J, Kowalczynska HM. Role of alpha5beta1 and alphavbeta3 integrins in relation to adhesion and spreading dynamics of prostate cancer cells interacting with fibronectin under in vitro conditions. Cell Biol Int. 2012;36(10):883–892. doi: 10.1042/CBI20110342 [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Wang Z, Li G, et al. CXCL1 from tumor associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017;385:28–38. doi: 10.1016/j.canlet.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 34.Zeng B, Zhou M, Wu H, Xiong Z. SPP1 promotes ovarian cancer progression via Integrin β1/FAK/AKT signaling pathway. Onco Targets Ther. 2018;11:1333–1343. doi: 10.2147/OTT.S154215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Zhou Q, Yu Z, et al. Cancer-associated fibroblast-derived lumican promotes gastric cancer progression via the integrin β1-FAK signaling pathway. Int J Cancer. 2017;141(5):998–1010. doi: 10.1002/ijc.30801 [DOI] [PubMed] [Google Scholar]
- 36.Pruenster M, Kurz AR, Chung KJ, et al. Extracellular MRP8/14 is a regulator of b2 integrin-dependent neutrophil slow rolling and adhesion. Nat Commun. 2015;6:6915. doi: 10.1038/ncomms7915 [DOI] [PMC free article] [PubMed] [Google Scholar]