Abstract
Study Design
A retrospective case control study.
Purpose
The purpose of this study was to compare the surgical outcomes of multilevel lateral lumbar interbody fusion (LIF) and multilevel posterior lumbar interbody fusion (PLIF) in the surgical treatment of adult spinal deformity (ASD) and to evaluate the sagittal plane correction by combining LIF with posterior-column osteotomy (PCO).
Overview of Literature
The surgical outcomes between multilevel LIF and multilevel PLIF in ASD patients remain unclear.
Methods
We retrospectively reviewed 31 ASD patients who underwent multilevel LIF combined with PCO (LIF group, n=14) or multilevel PLIF (PLIF group, n=17) and with a minimum 2-year follow-up. In the comparison between LIF and PLIF groups, their mean age at surgery was 69.4 vs. 61.8 years while the mean follow-up period was 29.2 vs. 59.3 months. We evaluated the transition of pelvic incidence–lumbar lordosis (PI–LL) and disc angle (DA) in the LIF group, in fulcrum backward bending (FBB), after LIF and after posterior spinal fusion (PSF) with PCO. The spinopelvic radiographic parameters were compared between LIF and PLIF groups.
Results
Compared with the PLIF group, the LIF group had less blood loss and comparable surgical outcomes with respect to radiographic data, health-related quality of life scores and surgical time. In the LIF group, the mean DA and PI–LL were unchanged after LIF (DA, 5.8°; PI–LL, 15°) compared with the values using FBB (DA, 4.3°; PI–LL, 15°) and improved significantly after PSF with PCO (DA, 8.1°; PI–LL, 0°).
Conclusions
In the surgical treatment of ASD, multilevel LIF is less invasive than multilevel PLIF and combination of LIF and PCO would be necessary for optimal sagittal correction in patients with rigid deformity.
Keywords: Posterior column osteotomy, Adult spinal deformity, Multilevel lateral lumbar interbody fusion, Multilevel posterior lumbar interbody fusion
Introduction
Adult spinal deformity (ASD) surgery aims to improve the spinal alignment and balance, thereby improving pain and functional symptoms. Preservation or restoration of sagittal global balance has been shown to be the most important predictor of surgical outcomes. The pathology of ASD involves rigid sagittal malalignment secondary to degeneration; therefore, three-column spinal osteotomy may be necessary for corrective surgery. Traditionally, anterior spinal fusion is a common form of corrective surgery for ASD that provides excellent deformity correction [1]. However, traditional open anterior surgery is associated with increased risk of major complications due to its surgical invasiveness [2]. Instead of traditional open anterior surgery, more recent options for treatment of ASD are multilevel posterior lumbar interbody fusion (PLIF) and three-column osteotomy including pedicle subtraction osteotomy and posterior vertebral column resection [3]. Several authors have reported good surgical outcomes following PLIF, but the incidence of perioperative complications is relatively high [4,5]. In ASD surgery, multilevel PLIF is necessary for optimal correction, but the procedure may cause potential perioperative complications including neural compromise and excessive bleeding from epidural vessels. Minimally invasive lateral lumbar interbody fusion (LIF) was first described by Ozgur et al. [6] as a safe and effective surgery for degenerative lumbar disease and since then has gained increased popularity. Besides, previous studies showed that LIF combined with percutaneous screw fixation was effective for coronal-plane deformities [7], but only modest improvements in lumbar lordosis (LL) have been reported (3°–8°) [7,8]. Thus, combination of percutaneous pedicle screwing and multilevel LIF has limited indications for corrective surgery in relatively flexible ASD [9]. We performed LIF combined with traditional open posterior spinal fusion (PSF) on patients with ASD. However, the advantage of multilevel LIF and PSF for ASD remain unclear. To clarify the advantages of LIF, we compared the surgical outcomes, including radiographic data and postoperative health-related quality of life (HRQOL) scores, between multilevel LIF and multilevel PLIF with thoraco-pelvic fixation in patients with ASD. Additionally, we evaluated the corrective effectiveness of posterior-column osteotomy (PCO) for optimal restoration of LL in multilevel LIF.
Materials and Methods
1. Patient population
We retrospectively reviewed the medical records of 58 consecutive patients with ASD who underwent corrective surgery by a single surgeon at a single institution and had a minimum of 2-year follow-up period. All eligible patients were older than 40 years and had at least one of the following parameters: coronal lumbar curve of >30°, pelvic incidence–lumbar lordosis (PI–LL) of >20°, sagittal vertical axis (SVA) of >95 mm or pelvic tilt of >30°. We excluded 18 patients with ASD who underwent three-column osteotomy, two patients who underwent only PSF and seven patients with upper-instrumented vertebrae at L2. All 31 included patients were matched. We performed multilevel LIF and PSF on 14 patients and multilevel PLIF and PSF on 17 patients. In the comparison between LIF and PLIF groups, the mean patient age at surgery was 69.4 versus 61.8 years (p=0.0552) and their mean follow-up period was 39.6 versus 71.8 months (p<0.01). The patient demographics are shown in Table 1.
Table 1.
Patients’ demographic data
| Characteristic | LIF group | PLIF group | p-value |
|---|---|---|---|
| No. of cases | 14 | 17 | - |
| Age (yr) | 69.4 (43–81) | 61.8 (42–77) | 0.06 |
| Gender (male:female) | 2:12 | 0:17 | - |
| Follow-up periods (mo) | 39.6 (24–51) | 59.3 (24–115) | <0.01 |
| Total no. of fused levels | 45 (LIF: 32, TLIF: 13) | 50 | - |
| LIF | L1/2 (4), L2/3 (13), L3/4 (11), L4/5 (4) | - | - |
| PLIF | L3/4 (1), L4/5 (6), L5/S1 (6) | L1/2 (10), L2/3 (13), L3/4 (5), L4/5 (14), L5/S1 (8) | - |
| No. of UIV cases | T9 (4), T10 (10) | T9 (8), T10 (9) | - |
| No. of LIV cases | L5 (1), P (13) | L5 (5), P (12) | - |
| No. of fused vertebrae | 8.2 (8–9) | 8.2 (7–9) | 0.85 |
| Blood loss (mL) | 683 (200–1,120) | 1,574 (370–2,700) | <0.01 |
| Operation time (min) | 504 (412–629) | 515 (395–720) | 0.71 |
Values are presented as number or mean (range).
LIF, lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; LIV, lower-instrumented vertebra; UIV, upper-instrumented vertebra.
In regard to our basic strategy for treating ASD, we evaluated the pre-operative spinal flexibility in patients using fulcrum backward bending (FBB) films [10,11]. In the patients with PI–LL of <25° on FBB film, we performed intervertebral release procedures including PLIF and LIF. In the patients with PI–LL of >25° on FBB film, we performed three-column osteotomy such as pedicle subtraction osteotomy or posterior vertebral column resection.
2. Surgical procedure in the lateral lumbar interbody fusion group
All operative procedures were performed by a senior spine surgeon at a single institute. First, LIF was performed on the patient in the lateral decubitus position on a standard operating table. We performed mini-open LIF approach as follows: (1) made a slightly larger skin incision than previously described [6] and exposed the psoas muscle to for direct observation, (2) split the psoas muscle gently and exposed the target disc clearly, and (3) positioned the retractor (The MaXcess; NuVasive Inc., San Diego, CA, USA). We used a cage with a lordotic angle of 10° for all discs. Second, PCO and PSF with instrumentation were performed on the patient in the prone position on an Axis Jackson surgical table (Mizuho OSI, Union City, CA, USA). For PCO, we performed grade 2 osteotomy on all segments that received LIF and grade 1 osteotomy on the other segments [12]. The aims of PCO were to prevent foraminal stenosis after spinal correction, accelerate bone union and acquire spinal flexibility.
3. Radiographic measurements
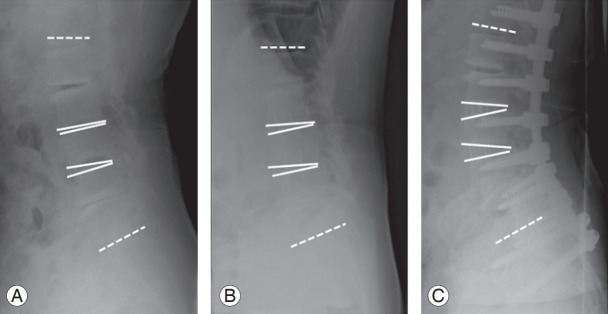
The following radiographic spinopelvic parameters were assessed with standing anteroposterior and lateral X-rays preoperatively, 1 week postoperatively and at the final follow-up: coronal vertical axis, Cobb angle of lumbar curve, SVA, thoracic kyphosis (T5–12), LL (T12–S1), pelvic tilt, and PI. Subsequently, we used these data to compare the radiographic spinopelvic parameters between LIF and PLIF groups. In the LIF group, we measured the PI–LL and disc angle (DA) at the level of the intervertebral disc undergoing LIF, in the FBB, after LIF in the prone position, and after PCO and PSF in the prone position (Fig. 1). We assessed changes in the PI–LL and DA values to analyze the effectiveness of additional PCO after LIF.
Fig. 1.
We measured the PI–LL and disc angle at the level undergoing LIF on FBB films (A), plain radiographs immediately after LIF in the prone position (B), and plain radiographs after PCO and PSF in the prone position (C). PI–LL, pelvic incidence–lumbar lordosis; LIF, lateral lumbar interbody fusion; FBB, fulcrum backward bending; PCO, posterior-column osteotomy; PSF, posterior spinal fusion.
4. Clinical outcomes
We evaluated clinical outcomes based on the Japanese edition of the Scoliosis Research Society 22-item outcomes metric (SRS-22) and the physical and mental component summary scores (PCS and MCS, respectively) on the 36-item Short-Form Health Survey (SF-36) preoperatively and at the final follow-up. All data were statistically analyzed using analysis of variance, Wilcoxon signed-rank test or a paired or unpaired t-test, as appropriate (JMP ver. 11.0; SAS Institute Inc., Cary, NC, USA). The level of significance for all tests was defined as p<0.05. This research was approved by the institutional review board of the authors’ affiliated institution (IRB approval no., 1801102), and informed consent was obtained from all individual participants included in the study.
Results
1. Surgical data
Our findings showed that both LIF and PLIF groups had comparable operation time (p=0.71), 504 minutes (range, 412 to 629 minutes), and 515 minutes (range, 395 to 720 minutes), respectively. On the other hand, there was a significantly lower blood loss lower in the LIF than PLIF group (p<0.01), 683 g (range, 200 to 1,120 g) versus 1,574 g (range, 370 to 2,700 g). The average number of fused vertebrae was 8.2 (range, 8 to 9) in the LIF group and 8.2 (range, 7 to 9) in the PLIF group (p=0.85). In addition, both groups demonstrated comparable average number of levels fused (LIF versus PLIF group: 3.2 versus 2.9, respectively). Two surgery-related complications occurred in the PLIF group: one dural tear and one rod fracture. Neither complication resulted in adverse sequelae; however, additional surgery time was needed to change the rod. No early complications, such as a neurological deficit, infection, or epidural hematoma, were observed in either group. One patient in each group sustained an upper-instrumented vertebral fracture; however, these fractures were asymptomatic and did not lead to proximal junctional kyphosis. The surgical data are summarized in Table 1.
2. Clinical outcomes
All domains of the SRS-22 survey improved significantly at the final follow-up (p<0.05). Comparison of the preoperative and final follow-up values revealed the following: activity (2.3 versus 3.5), pain (2.3 versus. 4.2), image (1.9 versus 3.8), and mental (2.3 versus 3.4) in the LIF group and activity (3.1 versus 3.9), pain (3 versus 4.1), image (2.3 versus 3.9), and mental (3.2 versus 4.0) in the PLIF group. The satisfaction score was 4.2 in the LIF group and 4.3 in the PLIF group. Also, results of the SRS-22 survey indicated comparable surgical outcomes in both groups. Comparison of the preoperative and final follow-up values on the SF-36 survey revealed that LIF group demonstrated significant improvement in PCS and MCS (p<0.05), PCS of 23.5 versus 32.0 and MCS of 42.0 versus 51.7. In contrast, the preoperative and final follow-up values in the PLIF group were as follows, respectively: PCS of 28.5 versus 41.4 and MCS of 48.6 versus 51.4. The PCS of PLIF group showed significant improvement at the final follow-up, but the MCS did not change. Clinical outcomes of both groups are summarized in Table 2. Comparative analysis of the clinical outcomes between the two groups revealed significant differences in two domains including function/activity and mental health of SRS-22. However, all clinical outcome data at final follow-up did not show any significant differences. The results of comparative analysis are summarized in Table 3.
Table 2.
Surgical outcome data: Japanese version of the SRS-22 and health-related quality of life outcomes measured with the SF-36
| Variable | LIF group |
PLIF group |
||||
|---|---|---|---|---|---|---|
| Preoperative | Latest FU | p-value* | Preoperative | Latest FU | p-value* | |
| SRS-22 | ||||||
| Function/activity | 2.3 | 3.5 | <0.01 | 3.1 | 3.9 | <0.01 |
| Pain | 2.3 | 4.2 | <0.01 | 3 | 4.1 | <0.01 |
| Self-image | 1.9 | 3.6 | <0.01 | 2.3 | 3.9 | <0.01 |
| Mental health | 2.3 | 3.4 | 0.024 | 3.2 | 4 | <0.01 |
| Satisfaction | - | 4.2 | - | - | 4.3 | - |
| SF36 | ||||||
| PCS | 23.5 | 32.0 | 0.08 | 28.5 | 41.4 | <0.01 |
| MCS | 42.0 | 51.7 | 0.06 | 48.6 | 51.4 | 0.35 |
SRS-22, Scoliosis Research Society 22-item outcomes metric; SF-36, 36-item Short-Form Health Survey; LIF, lateral lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; FU, follow-up; PCS, physical component summary score; MCS, mental component summary score.
p <0.05.
Table 3.
Comparison of surgical outcomes with the SRS-22 and SF-36 summarized as physical and mental component scores between the LIF and PLIF groups
| Variable | Category | LIF group | PLIF group | p-value |
|---|---|---|---|---|
| SRS-22 | ||||
| Function/activity | Preoperative | 2.3 | 3.1 | 0.04 |
| Latest FU | 3.5 | 3.9 | 0.10 | |
| Pain | Preoperative | 2.3 | 3.0 | 0.07 |
| Latest FU | 4.2 | 4.1 | 0.53 | |
| Self-image | Preoperative | 1.9 | 2.3 | 0.14 |
| Latest FU | 3.6 | 3.9 | 0.23 | |
| Mental health | Preoperative | 2.3 | 3.2 | 0.02 |
| Latest FU | 3.4 | 4.0 | 0.06 | |
| Satisfaction | Latest FU | 4.2 | 4.3 | 0.65 |
| SF-36 | ||||
| PCS | Preoperative | 23.5 | 28.5 | 0.55 |
| Latest FU | 32.0 | 41.4 | 0.09 | |
| MCS | Preoperative | 42.0 | 48.6 | 0.18 |
| Latest FU | 51.7 | 51.4 | 0.93 |
Values are presented as mean value.
SRS-22, Scoliosis Research Society 22-item outcomes metric; SF-36, 36-item Short-Form Health Survey; LIF, lateral lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; FU, follow-up; PCS, physical component summary score; MCS, mental component summary score.
3. Radiographic evaluation
All radiographic parameters in both groups improved significantly at the final follow-up (Table 4). Comparative analysis of the two groups showed that majority of the parameters were statistically equivalent. The mean values of the preoperative radiographic parameters in LIF and PLIF groups were as follows, respectively: LL, 6.1° versus 22° (p=0.024); pelvic tilt, 32° versus 30° (p=0.6); PI, 48° versus 53° (p=0.082); coronal vertical axis, 20 versus 37 mm (p=0.077); SVA, 93 versus 84 mm (p=0.59); and Cobb angle, 45° versus 58° (p=0.032). Comparison of the LIF and PLIF groups revealed a preoperative mean PI–LL of 42° versus 31° (p=0.11), a decrease in the LL to 33° versus 38° (p=0.21) on FBB films, a PI–LL on FBB films of 15° versus 15° (p=0.92), a decrease in the PI–LL to 3.8° versus 6.5° postoperatively (p=0.33), an improvement in the pelvic tilt to 23° versus 23° at the final follow-up (p=0.82) and an improvement in the SVA to 17 versus 41 mm at the final follow-up (p=0.076). The complete results of the comparative analysis are shown in Table 5.
Table 4.
Radiographic spinopelvic values in the LIF and PLIF groups
| Variable | LIF group |
PLIF group |
||||
|---|---|---|---|---|---|---|
| Preoperative | Latest FU | p-value | Preoperative | Latest FU | p-value | |
| CVA (mm) | 20 (0 to 61) | 6.6 (0 to 40) | 0.034 | 37 (0 to 111) | 18 (0 to 50) | 0.025 |
| Cobb (°) | 45 (0 to 67) | 14 (0 to 29) | <0.01 | 58 (40 to 86) | 20 (10 to 45) | <0.01 |
| SVA (mm) | 93 (20 to 173) | 17 (−45 to 66) | <0.01 | 84 (0 to 175) | 41 (0 to 160) | 0.010 |
| PT (°) | 32 (5 to 45) | 23 (12 to 36) | <0.01 | 30 (16 to 50) | 23 (15 to 34) | <0.01 |
| TK (°) | 13 (−15 to 34) | 34 (20 to 58) | <0.01 | 17 (−10 to 38) | 32 (5 to 59) | <0.01 |
| LL (°) | 6.1 (−28 to 44) | 44 (26 to 58) | <0.01 | 22 (−9 to 43) | 46° (35 to 64) | <0.01 |
| PI (°) | 48 (27 to 60) | - | - | 53 (43 to 64) | - | - |
| PI–LL (°) | 42 (−6 to 83) | 3.8 (−17 to 18) | <0.01 | 31 (8 to 59) | 6.5° (−4 to 19) | <0.01 |
| LL (FBB, °) | 33 (15 to 48) | - | - | 38 (20 to 67) | - | - |
Values are presented as mean (range).
LIF, lateral lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; FU, follow-up; CVA, coronal vertical axis; SVA, sagittal vertical axis; PT, pelvic tilt; TK, thoracic kyphosis; LL, lumbar lordosis; PI, pelvic incidence; FBB, fulcrum backward bending.
Table 5.
Comparison of spinopelvic parameters between the LIF and PLIF groups
| Variable | Category | LIF group | PLIF group | p-value |
|---|---|---|---|---|
| CVA (mm) | Preoperative | 20 | 37 | 0.08 |
| Latest FU | 6.6 | 18 | 0.03 | |
| Cobb (°) | Preoperative | 45 | 58 | 0.03 |
| Latest FU | 14 | 20 | 0.10 | |
| SVA (mm) | Preoperative | 93 | 84 | 0.59 |
| Latest FU | 17 | 41 | 0.08 | |
| PT (°) | Preoperative | 32 | 30 | 0.60 |
| Latest FU | 23 | 23 | 0.82 | |
| TK (°) | Preoperative | 13 | 17 | 0.35 |
| Latest FU | 34 | 32 | 0.54 | |
| LL (°) | Preoperative | 6.1 | 22 | 0.02 |
| Latest FU | 44 | 46 | 0.44 | |
| PI (°) | Preoperative | 48 | 53 | 0.08 |
| PI–LL (°) | Preoperative | 42 | 31 | 0.11 |
| Latest FU | 3.8 | 6.5 | 0.33 | |
| LL (FBB, °) | Preoperative | 33 | 38 | 0.21 |
| PI–LL (FBB, °) | Preoperative | 15 | 15 | 0.92 |
Values are presented as mean value.
LIF, lateral lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; CVA, coronal vertical axis; FU, follow-up; SVA, sagittal vertical axis; PT, pelvic tilt; TK, thoracic kyphosis; LL, lumbar lordosis; PI, pelvic incidence; FBB, fulcrum backward bending.
In the LIF group, we evaluated changes in the DA and PI–LL and discovered that the mean DA values on FBB films and sagittal computed tomographic views were 4.3° (range, 0° to 11°) and 3.3° (range, −1° to 9°), respectively. The mean DA after LIF, in the prone position, was 5.8° (range, 0° to 12°), but there was no significant difference when comparing the preoperative values on FBB films and those after LIF in the prone position (p=0.053). The mean DA and PI–LL values after PCO and PSF in the prone position and on sagittal computed tomographic views postoperatively were 8.1° (range, 0° to 16°) and 10.4° (range, 4° to 21°), and we observed significant differences between values after LIF versus after PCO and PSF (p=0.013). In contrast, the mean PI–LL on FBB films was 16° (range, −7° to 46°). The mean PI–LL in the prone position was not significantly changed after LIF (p=0.94); however, the mean PI–LL after PCO and PSF was 0° (range, −16° to 19°), and there was a significant difference between these values after LIF versus after PCO and PSF (p<0.01). Changes in the DA and PI–LL are summarized in Table 6.
Table 6.
Effects of LIF and PCO/PSF on segmental disc angle and PI–LL
| Variable | Preoperative |
After LIF |
After PCO/PSF |
|||
|---|---|---|---|---|---|---|
| Standing position | FBB film | CT sag. | X-ray at prone position | X-ray at prone position | CT sag. | |
| Disc angle (°) | - | 4.3 (0 to 11) | 3.3 (−1 to 9) | 5.8 (0 to 12) | 8.1 (0 to 16) | 10.4 (4 to 21) |
| p-value | - | - | - | 0.053 (vs. FBB film) | 0.013 (vs. after LIF) | <0.01 (vs. after LIF) |
| <0.01 (vs. CT sag.) | ||||||
| PI–LL (°) | 42 (−6 to 83) | 15 (−6 to 37) | - | 15 (−3 to 42) | 0 (−16 to 19) | - |
| p-value | - | - | - | 0.94 (vs. FBB film) | <0.01 (vs. after LIF) | <0.01 (vs. after LIF) |
Values are presented as mean (range).
LIF, lateral lumbar interbody fusion; PCO, posterior-column osteotomy; PSF, posterior spinal fusion; PI, pelvic incidence; LL, lumbar lordosis; FBB, fulcrum backward bending; CT sag., sagittal computed tomographic view.
Discussion
Since the first report by Ozgur et al. [6], minimally invasive LIF has been proven safe and effective for degenerative lumbar disease. Several studies have indicated the advantages of LIF for degenerative lumbar disease including indirect decompression [13,14], less invasive than transforaminal LIF (TLIF)/PLIF [15] and good corrective ability [16]. Application of transpsoas approach and large-footprint interbody fusion cage in LIF provides better anterior column support and restores intervertebral disc height. The advantages of LIF has led to its introduction to ASD surgery. Preliminary reports of PSF combined with LIF indicated better correction and acceptable complications [17,18]. Although previous studies of LIF in ASD surgery were case series, three studies evaluated the clinical outcomes of LIF combined with PSF for ASD compared with a combination of LIF and open PSF for ASD [19,20]. Strom et al. [19] indicated that LIF could reduce perioperative complications and blood loss, and provide better correction. Theologis et al. [21] reported that LIF combined with PSF could provide better coronal and sagittal correction, despite more complications were observed during the 6-month follow-up. Park et al. [20] showed that LIF could provide adequate indirect decompression and better sagittal correction in a 2-year follow-up. These three comparative studies clearly demonstrated the advantages of LIF; however, the surgical procedure in the control group of each study was PSF with L5/S1 interbody fusion. To the best of our knowledge, no studies have evaluated the surgical outcomes of ASD surgery performed through a posterior approach with a comparison between multilevel LIF and multilevel TLIF/PLIF. The purpose of our study was to evaluate the efficacy of supplementary LIF combined with PSF versus PSF with PLIF/TLIF for ASD during a 2-year follow-up.
In our series, the preoperative key radiographic parameters (SVA, PI–LL, and pelvic tilt) and preoperative HRQOL scores were equivalent. In both groups, we performed surgery in comparable fusion areas and interbody fusion segments. The key radiographic parameters in both groups improved significantly after surgery and the results were well-maintained until the f follow-up. We found no significant differences in the radiographic parameters between the two groups. HRQOL scores using the SRS-22 and SF-36 surveys improved significantly at the final follow-up, and the results were equivalent between the two groups. With respect to the surgical data, the operation time was comparable, but the blood loss in the PLIF group was significantly lower. The major advantage of LIF is reduced epidural bleeding, as evidenced by the lower blood loss observed in our study, it was speculated that the reduced blood loss could be attributed to indirect decompression [13]. Therefore, our data indicate that LIF is less invasive than PLIF and provides comparable correction.
The pathology of ASD involves loss of spinal flexibility with degenerative changes wherein morphological disc degeneration, osteophytes, and degenerated facet joints can be observed [22-24]. Therefore, three-column osteotomy is necessary for surgical correction of ASD. When the intervertebral disc has enough flexibility for correction, PCO including Ponte osteotomy [25] or Smith-Peterson osteotomy [26] can provide 5° to 15° of segmental correction. Schwab et al. [12] established a comprehensive spinal osteotomy classification, noting that a grade 1 or 2 osteotomy can provide 5° to 10° of segmental correction. In contrast, our results showed that PLIF could provide 6.8° of segmental sagittal correction per level in corrective surgery for patients with degenerative lumbar kyphoscoliosis [11]. An advantage of PLIF is that three-column osteotomy and simultaneous adequate neural decompression can be achieved through a single posterior approach. However, LIF can provide an anterior- and middle-column release that includes the disc and osteophytes connected to each vertebra as well as indirect neural decompression. However, LIF cannot release the posterior column including the facet joints and posterior ligamentous complex; additional PCO should be mandatory for rigid deformity. In fact, our data indicated no significant difference between DA on FBB films and DA immediately after performing LIF. The DA increased significantly after PCO and PSF, resulting in an optimal LL. These correction maneuvers are shown in Fig. 2. According to our data, LIF cannot provide optimal correction in the rigid intervertebral segments due to lesser flexibility of the anterior longitudinal ligament and locked facet joints (Fig. 3). Therefore, additional PCO should be performed at segments undergoing LIF because combination of LIF and PCO provides enough three-column release for optimal correction.
Fig. 2.
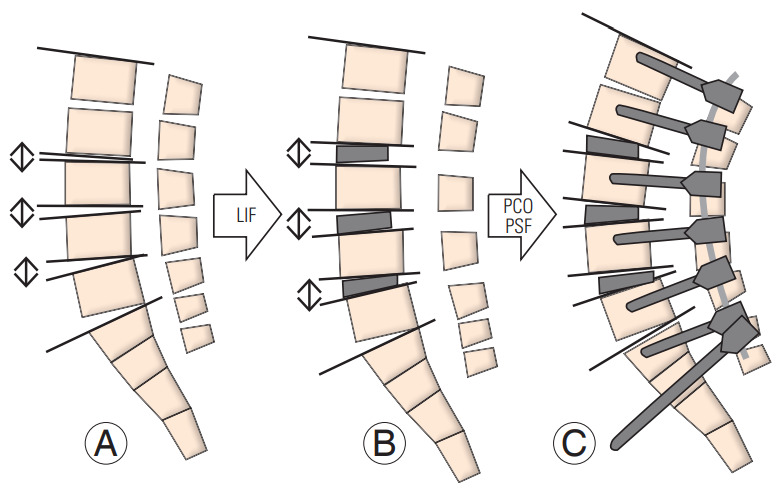
Schema describing the mechanism of sagittal correction in the LIF group. (A) Before surgery. (B) The LIF cage does not provide optimal lordosis but increases the disc height. (C) Optimal lordosis can be achieved with the addition of PCO and PSF. LIF, lateral lumbar interbody fusion; PCO, posterior-column osteotomy; PSF, posterior spinal fusion.
Fig. 3.
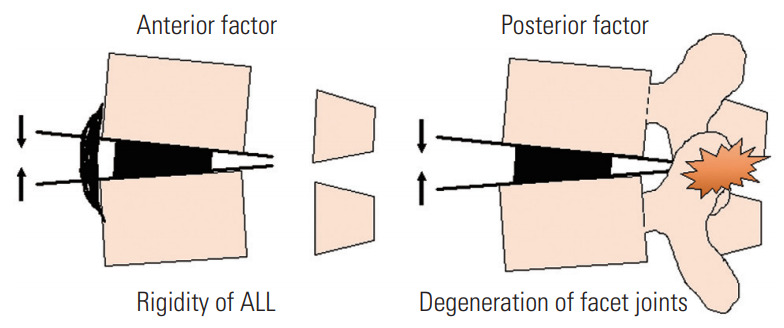
Schema showing why LIF incapable of providing optimal lumbar lordosis. Less flexibility in the ALL obstructs anterior opening of the disc space and degenerated facet joints obstruct posterior column closing. These factors may prevent improved segmental lordosis with LIF. LIF, lateral lumbar interbody fusion; ALL, anterior longitudinal ligament.
Regarding the shape of physiological LL, previous reports indicate that lordosis in lumbosacral area (L4–S1) occupies approximately 60%–80% of total LL in normal population [27,28]. Therefore, realignment surgery in ASD is essential in restoring lordosis in lumbosacral area (L4–S1). However, LIF cannot be applied at the lumbosacral area (L5–S1) because of anatomical reason such as vascular location. In the LIF group, we performed additional PLIF at the lumbosacral area for optimal correction. From our perspectives on the concept of realignment surgery in ASD, we should select a suitable procedure or combination of procedures for optimal correction and try to reduce the operative invasiveness.
There are several limitations in our study. Firstly, the main limitation is the small number of patients and short-term follow-up. Future work should involve a larger sample size and longer follow-up period. Secondly, the preoperative radiographic parameters of lumbar cobb angle and LL showed significant differences between the two groups. Therefore, those results cannot not be compared fairly between the groups. However, regardless of the differences in the preoperative radiographic parameters, both groups could achieve optimal spinopelvic alignment after surgery and provide good clinical outcomes. Hence, we suggest that multilevel LIF with PCO could be a better surgical procedure than multilevel PLIF with regard to its less invasiveness.
Conclusions
We conclude that multilevel LIF with PCO is less invasive than multilevel PLIF in the surgical correction of ASD and addition of PCO to LIF would be necessary for optimal sagittal correction in patients with rigid deformity.
Acknowledgments
We thank Jane Charbonneau, DVM and Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Pateder DB, Kebaish KM, Cascio BM, Neubaeur P, Matusz DM, Kostuik JP. Posterior only versus combined anterior and posterior approaches to lumbar scoliosis in adults: a radiographic analysis. Spine (Phila Pa 1976) 2007;32:1551–4. doi: 10.1097/BRS.0b013e318067dc0e. [DOI] [PubMed] [Google Scholar]
- 2.Dorward IG, Lenke LG, Bridwell KH, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: a matched cohort analysis. Spine (Phila Pa 1976) 2013;38:E755–62. doi: 10.1097/BRS.0b013e31828d6ca3. [DOI] [PubMed] [Google Scholar]
- 3.Bergin PF, O’Brien JR, Matteini LE, Yu WD, Kebaish KM. The use of spinal osteotomy in the treatment of spinal deformity. Orthopedics. 2010;33:586–94. doi: 10.3928/01477447-20100625-22. [DOI] [PubMed] [Google Scholar]
- 4.Zanirato A, Damilano M, Formica M, et al. Complications in adult spine deformity surgery: a systematic review of the recent literature with reporting of aggregated incidences. Eur Spine J. 2018;27:2272–84. doi: 10.1007/s00586-018-5535-y. [DOI] [PubMed] [Google Scholar]
- 5.De Kunder SL, van Kuijk SM, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17:1712–21. doi: 10.1016/j.spinee.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976) 2013;38:1853–61. doi: 10.1097/BRS.0b013e3182a43f0b. [DOI] [PubMed] [Google Scholar]
- 8.Baghdadi YM, Larson AN, Dekutoski MB, et al. Sagittal balance and spinopelvic parameters after lateral lumbar interbody fusion for degenerative scoliosis: a case-control study. Spine (Phila Pa 1976) 2014;39:E166–73. doi: 10.1097/BRS.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand N, Kong C, Fessler RG. A staged protocol for circumferential minimally invasive surgical correction of adult spinal deformity. Neurosurgery. 2017;81:733–9. doi: 10.1093/neuros/nyx353. [DOI] [PubMed] [Google Scholar]
- 10.Cheung KM, Luk KD. Prediction of correction of scoliosis with use of the fulcrum bending radiograph. J Bone Joint Surg Am. 1997;79:1144–50. doi: 10.2106/00004623-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura A, Namikawa T, Kato M, et al. Posterior corrective surgery with a multilevel transforaminal lumbar interbody fusion and a rod rotation maneuver for patients with degenerative lumbar kyphoscoliosis. J Neurosurg Spine. 2017;26:150–7. doi: 10.3171/2016.7.SPINE16172. [DOI] [PubMed] [Google Scholar]
- 12.Schwab F, Blondel B, Chay E, et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery. 2014;74:112–20. doi: 10.1227/NEU.0000000000000182o. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331–7. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 14.Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16:329–33. doi: 10.3171/2012.1.SPINE11528. [DOI] [PubMed] [Google Scholar]
- 15.Ohba T, Ebata S, Haro H. Comparison of serum markers for muscle damage, surgical blood loss, postoperative recovery, and surgical site pain after extreme lateral interbody fusion with percutaneous pedicle screws or traditional open posterior lumbar interbody fusion. BMC Musculoskelet Disord. 2017;18:415. doi: 10.1186/s12891-017-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242–50. doi: 10.1097/BSD.0b013e3181ecf995. [DOI] [PubMed] [Google Scholar]
- 17.Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine (Phila Pa 1976) 2010;35(26 Suppl):S312–21. doi: 10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35(26 Suppl):S322–30. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 19.Strom RG, Bae J, Mizutani J, Valone F, 3rd, Ames CP, Deviren V. Lateral interbody fusion combined with open posterior surgery for adult spinal deformity. J Neurosurg Spine. 2016;25:697–705. doi: 10.3171/2016.4.SPINE16157. [DOI] [PubMed] [Google Scholar]
- 20.Park HY, Ha KY, Kim YH, et al. Minimally invasive lateral lumbar interbody fusion for adult spinal deformity: clinical and radiological efficacy with minimum two years follow-up. Spine (Phila Pa 1976) 2018;43:E813–21. doi: 10.1097/BRS.0000000000002507. [DOI] [PubMed] [Google Scholar]
- 21.Theologis AA, Mundis GM, Jr, Nguyen S, et al. Utility of multilevel lateral interbody fusion of the thoracolumbar coronal curve apex in adult deformity surgery in combination with open posterior instrumentation and L5-S1 interbody fusion: a case-matched evaluation of 32 patients. J Neurosurg Spine. 2017;26:208–19. doi: 10.3171/2016.8.SPINE151543. [DOI] [PubMed] [Google Scholar]
- 22.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–48. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 23.Grubb SA, Lipscomb HJ, Coonrad RW. Degenerative adult onset scoliosis. Spine (Phila Pa 1976) 1988;13:241–5. doi: 10.1097/00007632-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Simmons ED. Surgical treatment of patients with lumbar spinal stenosis with associated scoliosis. Clin Orthop Relat Res. 2001;(384):45–53. doi: 10.1097/00003086-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Ponte A, Vero B, Sicicardi G. Surgical treatment of Scheuermann’s kyphosis. In: Winter R, editor. Progress in spinal pathology: kyphosis. Bologna: Aulo Gaggi; 1984. pp. 75–81. [Google Scholar]
- 26.Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6–9. [PubMed] [Google Scholar]
- 27.Yukawa Y, Matsumoto T, Kollor H, et al. Local sagittal alignment of the lumbar spine and range of motion in 627 asymptomatic subjects: age-related changes and sex-based differences. Asian Spine J. 2019;13:663–71. doi: 10.31616/asj.2018.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamato Y, Sato Y, Togawa D, et al. Differences in the geometrical spinal shape in the sagittal plane according to age and magnitude of pelvic incidence in healthy elderly individuals. J Orthop Sci. 2019;S0949-2658(19):30207–6. doi: 10.1016/j.jos.2019.07.005. [DOI] [PubMed] [Google Scholar]