Key Points
Question
What is the effect of the combination of ascorbic acid, corticosteroids, and thiamine on the trajectory of organ injury in septic shock?
Findings
In this randomized clinical trial that included 200 adults with septic shock, treatment for 4 days with a combination of parenteral ascorbic acid (1500 mg), hydrocortisone (50 mg), and thiamine (100 mg) vs placebo every 6 hours resulted in a change in the Sequential Organ Failure Assessment score of 4.7 in the intervention group vs 4.1 in the placebo group over 72 hours, a difference that was not statistically significant.
Meaning
This trial does not support the routine use of the combination of ascorbic acid, corticosteroids, and thiamine for organ protection in septic shock populations.
Abstract
Importance
The combination of ascorbic acid, corticosteroids, and thiamine has been identified as a potential therapy for septic shock.
Objective
To determine whether the combination of ascorbic acid, corticosteroids, and thiamine attenuates organ injury in patients with septic shock.
Design, Setting, and Participants
Randomized, blinded, multicenter clinical trial of ascorbic acid, corticosteroids, and thiamine vs placebo for adult patients with septic shock. Two hundred five patients were enrolled between February 9, 2018, and October 27, 2019, at 14 centers in the United States. Follow-up continued until November 26, 2019.
Interventions
Patients were randomly assigned to receive parenteral ascorbic acid (1500 mg), hydrocortisone (50 mg), and thiamine (100 mg) every 6 hours for 4 days (n = 103) or placebo in matching volumes at the same time points (n = 102).
Main Outcomes and Measures
The primary outcome was change in the Sequential Organ Failure Assessment (SOFA) score (range, 0-24; 0 = best) between enrollment and 72 hours. Key secondary outcomes included kidney failure and 30-day mortality. Patients who received at least 1 dose of study drug were included in analyses.
Results
Among 205 randomized patients (mean age, 68 [SD, 15] years; 90 [44%] women), 200 (98%) received at least 1 dose of study drug, completed the trial, and were included in the analyses (101 with intervention and 99 with placebo group). Overall, there was no statistically significant interaction between time and treatment group with regard to SOFA score over the 72 hours after enrollment (mean SOFA score change from 9.1 to 4.4 [−4.7] points with intervention vs 9.2 to 5.1 [−4.1] points with placebo; adjusted mean difference, −0.8; 95% CI, −1.7 to 0.2; P = .12 for interaction). There was no statistically significant difference in the incidence of kidney failure (31.7% with intervention vs 27.3% with placebo; adjusted risk difference, 0.03; 95% CI, −0.1 to 0.2; P = .58) or in 30-day mortality (34.7% vs 29.3%, respectively; hazard ratio, 1.3; 95% CI, 0.8-2.2; P = .26). The most common serious adverse events were hyperglycemia (12 patients with intervention and 7 patients with placebo), hypernatremia (11 and 7 patients, respectively), and new hospital-acquired infection (13 and 12 patients, respectively).
Conclusions and Relevance
In patients with septic shock, the combination of ascorbic acid, corticosteroids, and thiamine, compared with placebo, did not result in a statistically significant reduction in SOFA score during the first 72 hours after enrollment. These data do not support routine use of this combination therapy for patients with septic shock.
Trial Registration
ClinicalTrials.gov Identifier: NCT03389555
This randomized clinical trial compares the effect of combination hydrocortisone, ascorbic acid, and thiamine (the HAT or Marik protocol) vs placebo on SOFA score–measured organ injury at 72 hours in patients with septic shock.
Introduction
Sepsis is a common and frequently fatal condition, with recent estimates suggesting that nearly 20% of all deaths are sepsis related.1 Although one modeling study estimated that overall sepsis mortality improved between 1990 and 2017, sepsis survivors often experience residual organ injury.2 To date, therapies for sepsis management remain early antimicrobials, effective treatment of the infection source, and supportive care.
Ascorbic acid and thiamine deficiencies have been described in people with sepsis and are thought to result from a combination of reduced intake and increased metabolic demands.3,4,5 While ascorbic acid and thiamine supplementation during sepsis have been studied for decades, interest in these therapies has recently increased.6,7,8,9,10 Evidence regarding prescription of corticosteroids in septic shock is conflicting.11,12 Basic laboratory and uncontrolled clinical studies suggested that combining corticosteroids with ascorbic acid may have a synergistic effect,8,13 and one observational study found an association of ascorbic acid, corticosteroids, and thiamine coadministration with improved outcomes for people with sepsis.8,13 Recent randomized trials of ascorbic acid alone or in combination with corticosteroids and thiamine have had varied design and inconsistent results. Therefore, additional investigation is needed.9,14 Ascorbic acid has also been proposed as an adjunctive therapy for respiratory failure related to coronavirus disease 2019 (COVID-19).15
The Ascorbic Acid, Corticosteroids, and Thiamine in Septic Shock (ACTS) trial tested the hypothesis that the combination of ascorbic acid, corticosteroids, and thiamine, compared with placebo, would reduce the Sequential Organ Failure Assessment (SOFA) score from enrollment to 72 hours after enrollment in patients with septic shock.
Methods
Study Oversight
The trial protocol was approved by the institutional review boards at each participating medical center. Enrollment began February 9, 2018, and participant follow-up continued until November 26, 2019. The final study protocol, including the statistical analysis plan, was submitted for publication September 18, 2019, accepted November 22, 2019, and published December 17, 2019. All analyses in the published protocol and analysis plan were specified prior to completion of patient follow-up and study unblinding.16 The methodology publication16 serves as the protocol and statistical analysis plan and takes precedence over prior protocol versions (Supplement 1). Written informed consent was obtained from patients or their representatives. A data and safety monitoring board monitored the trial.
Design and Setting
This was a multicenter, randomized, blinded, placebo-controlled superiority trial comparing the combination of ascorbic acid, hydrocortisone, and thiamine with placebo in patients with septic shock. The study was conducted at 14 centers in the United States (eTable 1 in Supplement 2).
Study Population
Adult patients (aged ≥18 years) were eligible if they had a suspected or confirmed infection and were receiving a vasopressor because of sepsis. Patients were excluded if they were allergic to study drug components, had a clinical indication for any of the study drugs, had symptomatic kidney stones within the last year, had glucose-6-phosphate dehydrogenase deficiency or hemochromatosis, were receiving kidney replacement therapy (changed from stage 3b chronic kidney disease after the 19th enrolled patient because of difficulty ascertaining chronic kidney disease stage), were not expected to survive 24 hours, or were a member of a protected population (ie, pregnant, prisoner). Patients were enrolled within 24 hours once they were identified as meeting inclusion criteria.
Race and ethnicity were determined by the research team based on documented race/ethnicity in the electronic medical record and were categorized into fixed categories or an “other” category. Race/ethnicity information is required for clinical trials funded by the National Institutes of Health and therefore we collected this information to facilitate a more comprehensive description of the study population.
Randomization, Masking, and Intervention
Patients were randomized to receive intervention or placebo in a 1:1 ratio using random block sizes of 2 or 4, stratifying by site using a list created by an independent statistician. Site investigators, research staff, clinical staff, and patients remained blinded to group assignment for the duration of the study. Research pharmacists at each site held a site-specific randomization list and prepared study drugs. Study drug and placebo were delivered in light-protected bags.
Patients randomized to the intervention received ascorbic acid (1500 mg), hydrocortisone (50 mg), and thiamine (100 mg) every 6 hours for 4 days or until intensive care unit (ICU) discharge. Ascorbic acid and thiamine were mixed together in 100 mL of normal saline and administered intravenously over 45 to 60 minutes. At some sites, ascorbic acid and thiamine were administered as separate infusions. Hydrocortisone was administered intravenously as a push dose in 1 mL of saline over 1 to 2 minutes. Patients randomized to placebo received 0.9% sodium chloride in a matching volume (approximately 100 mL) using the same techniques at the same time points.
Sepsis Management
Investigators followed local sepsis management guidelines. The early administration of antibiotics, maintenance of a mean arterial pressure of at least 65 mm Hg with a combination of volume resuscitation and vasopressors, and early treatment of the source of infection were recommended. Details of sepsis management, including volume of intravenous fluid prior to study enrollment, were recorded in the case report form.
Outcomes
The primary outcome was change in the SOFA score between enrollment and 72-hour follow-up (eTable 2 in Supplement 2).17 The SOFA score ranges from 0 (best) to 24 (worst). Key secondary outcomes included kidney failure, defined as the development of Kidney Disease: Improving Global Outcomes (KDIGO) grade 3 or higher during the index ICU stay and all-cause mortality after the first 30 days after initial study drug administration. Additional secondary outcomes were ventilator-free days during the first 7 days, shock-free days during the first 7 days (with a maximum of 7 days [best] and a minimum of 0 days [worst]), days free of ICU stay during the first 28 days (with a maximum of 28 days [best] and a minimum of 0 days [worst]), all-cause mortality to ICU discharge, all-cause mortality to hospital discharge, posthospitalization disposition in survivors to hospital discharge, 72-hour change in individual SOFA score components, and delirium on day 3 (as determined by the Confusion Assessment Method for the ICU).18 For a complete list of outcome and adverse event definitions, see eTables 3 and 4 in Supplement 2.
Statistical Analysis
Estimated effect sizes were determined based on a prior observational study.8 A between-group difference of 2 points in the SOFA score at the 72-hour time point was anticipated (intervention group decrease of 4 [±2] points vs control group decrease of 6 [±4] points over 72 hours). With these estimates, an α = .05, a t test with unequal variance, and a sample size of 200, the trial had greater than 99% power to detect a statistically significant between-group difference. A 2-point difference in SOFA score was considered to be both the minimal clinically important difference (MCID) and a reasonable predicted effect.8,19 For the key secondary outcome of kidney failure, 200 patients were estimated to provide 94% power, assuming 30% of participants in the intervention group and 55% in the placebo group would develop kidney failure. For 30-day mortality, 182 patients were estimated to provide 80% power, assuming a mortality of 40% vs 20% in the control and intervention groups, respectively.
Patients who received at least 1 dose of study drug were analyzed according to randomization group. Descriptive statistics summarized the study population with continuous variables presented as means and standard deviations or medians and interquartile ranges (IQRs). Categorical data (counts with percentages) were compared using Fisher exact tests.
The primary outcome was analyzed using a linear mixed-effects model where the correlation of within-patient repeated SOFA score measures was accounted for via the use of an unstructured variance-covariance matrix and linear contrasts. Covariates included age, sex, treatment group, time (as a categorical variable defined as baseline, 24 hours, and 72 hours), and the interaction between treatment group and time. Study site was included as a random intercepts effect. The reference group was placebo, and baseline was the reference variable for time. The mean difference in SOFA score at each of the 3 time points was estimated using the above model. The effect estimate for the primary outcome was the mean between-group difference in SOFA score change between enrollment and 72 hours. All patients were included in the longitudinal model, even if only the baseline SOFA score was available. The original trial protocol (Supplement 1) specified a comparison of mean or median SOFA score change; however, this analysis plan was revised prior to completion of trial enrollment.16
Survival to 30 days was assessed using Kaplan-Meier analysis and a Cox proportional hazards model controlling for site. Data met the criterion for the proportionality assumption (P = .62), using Schoenfeld residuals. Continuous, nonlongitudinal outcomes were compared using linear regression and categorical outcomes were compared using logistic regression controlling for site. Distributions for ventilator-free days, shock-free days, and ICU-free days were highly skewed and were analyzed using quantile regression. Prespecified subgroup analyses included analyses above/below median baseline SOFA, above/below lactate level of 27.0 mg/dL (3 mmol/L) or higher,6 time from inclusion to study drug administration above/below 12 hours (time from inclusion determined on the basis of vasopressor start time while meeting other inclusion criteria), and predicted 30-day survival by the clinician who enrolled the participant (categorized as likely, uncertain, or unlikely). Subgroups were analyzed using the relevant interaction term and assessed for effect modification. Prespecified sensitivity analyses of the primary outcome were performed by (1) including only patients whose SOFA score at 72 hours was available and (2) assigning a 20% SOFA score increase for death prior to 72 hours. A sensitivity analysis was also performed in which kidney failure was defined as either kidney replacement therapy or death (while meeting KDIGO 3 criteria).
All statistical tests were 2-sided and an P < .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, results for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were conducted with Stata, version 15 (StataCorp).
Results
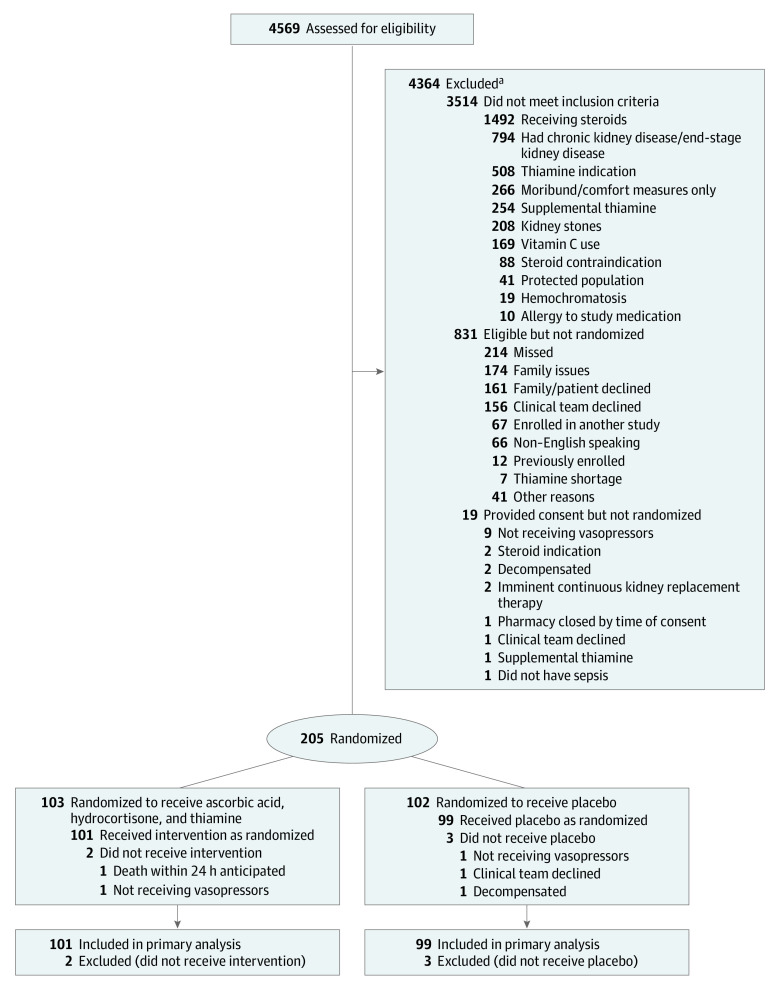
A total of 4569 patients met inclusion criteria, 831 were eligible, 224 consented, 205 were randomized, and 200 received at least 1 dose of study drug (see Figure 1). One hundred one patients (50.5%) were analyzed in the intervention group and 99 (49.5%) were analyzed in the placebo group. Baseline characteristics were generally well matched between the groups (Table 1). Seven (6.9%) and 14 (14.1%) patients received open-label corticosteroids after enrollment and prior to the 72-hour time point in the intervention and placebo groups, respectively. Less than 5% of patients received open-label ascorbic acid or thiamine in either group (eTable 5 in Supplement 2). Forty-seven (46.5%) and 42 (42.4%) patients in the intervention and placebo groups were discharged alive from the ICU within 96 hours of enrollment, respectively. The number of study drug doses administered per patient can be found in eFigure 1 in Supplement 2.
Figure 1. Flow of Patients Through the Ascorbic Acid, Corticosteroids, and Thiamine in Septic Shock (ACTS) Trial.
aPatients may have met more than 1 exclusion criterion.
Table 1. Baseline Cohort Characteristics.
| Characteristics | Intervention (n = 101) | Placebo (n = 99) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 68.9 (15.0) | 67.7 (13.9) |
| BMI, mean (SD) | 28.8 (10.1) [n = 100] | 27.9 (8.4) [n = 97] |
| Sex, No. (%) | ||
| Female | 44 (43.6) | 45 (45.5) |
| Male | 57 (56.4) | 54 (54.6) |
| Race, No. (%) | n = 91 | n = 93 |
| White | 68 (74.7) | 73 (78.5) |
| Black | 18 (19.7) | 16 (17.2) |
| Asian | 5 (5.5) | 3 (3.2) |
| ≥1 Race | 0 | 1 (1.1) |
| Hispanic, No./total (%) | 4/90 (4.4) | 4/96 (4.2) |
| Medical history, No. (%) | ||
| Malignancy | 26 (25.7) | 32 (32.3) |
| Coronary artery disease | 26 (25.7) | 26 (26.3) |
| Congestive heart failure | 14 (13.9) | 23 (23.2) |
| Liver disease | 11 (10.9) | 7 (7.1) |
| Chronic kidney disease stagea | ||
| 2 (Mild) | 1 (1.0) | 1 (1.0) |
| 3 (Moderate) | 4 (4.0) | 6 (6.1) |
| 4 (Severe) | 2 (2.0) | 3 (3.0) |
| Unknown | 3 (3.0) | 3 (3.0) |
| Clinical characteristics | ||
| Primary infectious source, No. (%) | n = 94 | n = 93 |
| Pneumonia | 31 (33.0) | 28 (30.1) |
| Intra-abdominal | 30 (31.9) | 23 (24.7) |
| Urinary tract infection | 20 (21.3) | 22 (23.7) |
| Otherb | 13 (13.8) | 20 (21.5) |
| Volume of intravenous fluids prior to study drug, median (IQR), mLc | 2000 (1062-3000) | 2000 (1125-3000) |
| Baseline cardiovascular component of total SOFA score, median (IQR)d | 4 (3-4) | 4 (3-4) |
| Time from vasopressor initiation to first study drug, median (IQR), h | 14.5 (8.1-19.1) | 13.0 (7.5-20.5) |
| Time from informed consent to first study drug, median (IQR), h | 2.2 (1.7-3.0) | 2.0 (1.5-2.7) |
| Mechanical ventilation, No. (%) | 48 (47.5) | 44 (44.4) |
| Acute respiratory distress syndrome, No. (%)e | 22 (21.8) | 18 (18.2) |
| Lactate level, median (IQR), mg/dL | 16.2 (12.6-25.2) | 16.2 (11.7-26.1) |
| 30-d Predicted survival, No. (%)f | ||
| High likelihood | 34 (33.7) | 38 (38.4) |
| Uncertain | 61 (60.4) | 54 (54.6) |
| Low likelihood | 6 (5.9) | 7 (7.1) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
SI conversion: To convert lactate to millimoles per liter, multiply by 0.111.
Chronic kidney disease stage 2 (mild): glomerular filtration rate = 60-89 mL/min; stage 3 (moderate): glomerular filtration rate = 30-59 mL/min; and stage 4 (severe): glomerular filtration rate <29 mL/min but not receiving kidney replacement therapy.
Other sources of infection in the intervention group: skin or soft tissue (6), central nervous system (2), endocarditis (1), biliary (1), bacteremia (1), prostatic abscess (1), prosthetic hip infection (1); in the placebo group: skin or soft tissue (8), vascular catheter (6), endocarditis (2), epidural abscess (2), dental abscess (1), biliary (1).
Volume of intravenous fluids received in the 12 hours preceding enrollment.
The cardiovascular component of the SOFA score ranges from 0 (best) to 4 (worst), with a score of 3 indicating a norepinephrine dose ≤1 µg/kg/min (or equivalent) and a score of 4 indicating a norepinephrine dose >1 µg/kg/min (or equivalent).
Defined by onset within 7 days, bilateral pulmonary infiltrates, and a ratio of arterial partial pressure of oxygen/fraction of inspired oxygen <300 on positive end-expiratory pressure of ≥5 mm Hg.
At time of enrollment, the physician enrolling the patient was asked to predict 30-day survival.
Primary Outcome and Key Secondary Outcomes
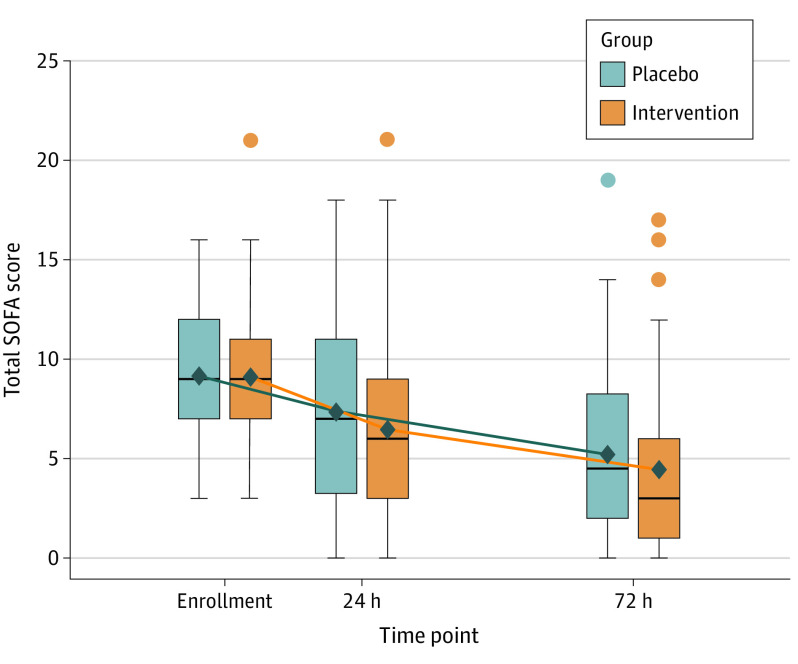
Prior to the 72-hour time point, 9 patients (9.1%) died in the placebo group and 10 patients (9.9%) died in the intervention group. One patient in each study group was discharged from the hospital alive prior to 72 hours, and 1 patient in the placebo group refused study-related blood draws after 24 hours. There was no statistically significant interaction between intervention group and time over 72 hours for the primary outcome of change in SOFA score (mean difference, −0.8; 95% CI, −1.7 to 0.2; P = .12) (Figure 2).
Figure 2. Longitudinal Plot of Mean Sequential Organ Failure Assessment (SOFA) Score Over Time.
Box tops and bottoms indicate interquartile ranges of the data; the bars inside the boxes indicate medians. Whiskers extend to the most extreme points within 1.5 interquartile ranges. Outliers are presented as dots colored by group. Means are presented as black diamonds and are joined by lines shaded by group.
Fifty-nine patients (29.5%) developed kidney failure during their index ICU stay. There was no statistically significant difference in kidney failure incidence between groups (31.7% in the intervention group vs 27.3% in the placebo group; adjusted risk difference, 0.03; 95% CI, −0.10 to 0.17; P = .58).
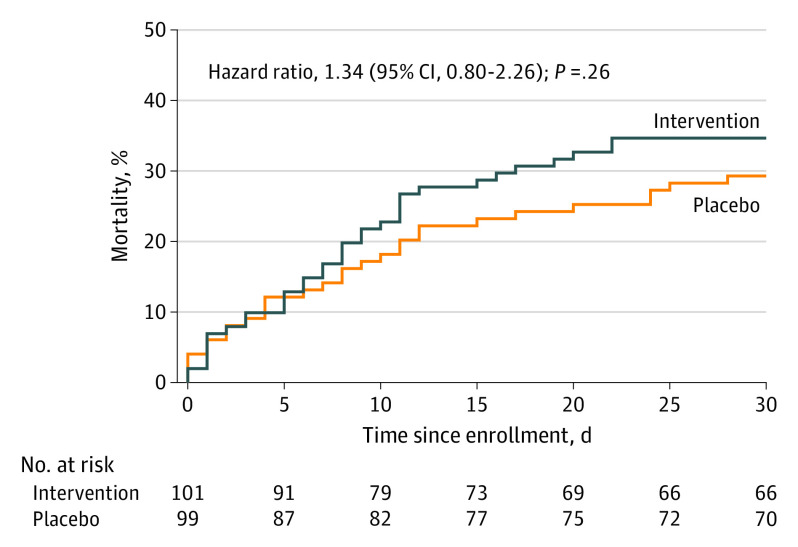
Sixty-four patients (32%) died within 30 days of enrollment. There was no statistically significant difference in 30-day mortality between the intervention and placebo groups (34.7% vs 29.3%; hazard ratio, 1.3; 95% CI, 0.8-2.2; P = .26) (Figure 3). Among the 51 patients (25.5%) who died prior to discharge, the most common reason for death was withdrawal of care by the clinical team, family, and/or patient because of an underlying (either preexisting or newly discovered) terminal illness or expected poor quality of life in the context of sepsis (64.7%) (eTable 6 in Supplement 2).
Figure 3. All-Cause Mortality From Enrollment to 30 Days After Enrollment.
Median observation time was 30 (interquartile range, 11-30) days in the intervention group and 30 (interquartile range, 20-30) days in the placebo group.
Sensitivity analyses yielded results similar to the primary analysis (eTable 7 in Supplement 2).
Additional Secondary Outcomes
The median number of ventilator-free days within the first 7 days after enrollment was 6 (IQR, 2-7) days in the intervention group vs 6 (IQR, 0-7) days in the placebo group (median difference, 0.0 days; 95% CI, −1.9 to 1.9 days; P > .99). The median number of shock-free days was higher in the intervention group compared with the placebo group (5 [IQR, 3-5] days vs 4 [IQR, 1-5] days; median difference, 1.0 days; 95% CI, 0.2-1.8 days; P < .01). Patients in the intervention group had a statistically significantly greater reduction in cardiovascular SOFA score during the first 72 hours (mean difference, −0.5; 95% CI, −0.9 to −0.1; P = .03 for interaction). There was no difference between the intervention and placebo groups in any other SOFA score component, including the liver component (mean difference, −0.1; 95% CI, −0.3 to 0.1; P = .22), the neurologic component (mean difference, −0.3; 95% CI, −0.6 to 0.1; P = .14), the kidney component (mean difference, 0.1; 95% CI, −0.2 to 0.4; P = .52), the respiratory component (mean difference, 0.0; 95% CI, −0.3 to 0.3; P = .84), or the coagulation component (mean difference, 0.0; 95% CI, −0.2 to 0.2; P = .92). See Table 2 for secondary outcomes and eTable 8 in Supplement 2 for SOFA elements.
Table 2. Primary and Secondary Outcomes.
| Outcomes | Intervention (n = 101) | Placebo (n = 99) | Effect estimate (95% CI) | P value |
|---|---|---|---|---|
| SOFA score over time (primary outcome measure), mean (SD) | ||||
| At enrollment | 9.1 (3.5) | 9.2 (3.2) | ||
| At 72 h | 4.4 (4.1) [n = 90] | 5.1 (4.3) [n = 88] | Adjusted mean difference: −0.8 (−1.7 to 0.2)a | .12 |
| Secondary outcomesb | ||||
| All-cause mortality over 30 d, No. (%) | 35 (34.7) | 29 (29.3) | Hazard ratio: 1.3 (0.8 to 2.2) | .26 |
| Kidney failure, No. (%) | 32 (31.7) | 27 (27.3) | Adjusted risk difference: 3% (−10% to 20%) | .58 |
| Ventilator-free days, median (IQR)c | 6 (2-7) | 6 (0-7) | Median difference: 0.0 (−1.9 to 1.9)d | >.99 |
| Shock-free days, median (IQR) | 5 (3-5) | 4 (1-5) | Median difference: 1.0 (0.2 to 1.8)d | .02 |
| Incidence of delirium, No./total (%) | 31/83 (37.4) | 35/76 (46.1) | Adjusted risk difference: −12% (−25% to 4%) | .16 |
| ICU-free days, median (IQR)e | 22 (3-25) | 21 (4-25) | Median difference: 1.0 (−3.0 to 6.0)d | .69 |
| All-cause mortality to ICU discharge, No. (%) | 23 (22.7) | 20 (20.2) | Adjusted risk difference: 2% (−10% to 10%) | .80 |
| All-cause mortality to hospital discharge, No. (%) | 28 (27.7) | 23 (23.2) | Adjusted risk difference: 3% (−10% to 20%) | .55 |
| Survivors discharged home, No./total (%) | 34/73 (46.6) | 35/76 (46.1) | Adjusted risk difference: −1.8% (−18% to 14%) | .82 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Adjusted mean difference of the difference in SOFA score change between enrollment and 72 hours. P value is for the interaction term.
Individual SOFA score outcomes over 72 hours can be found in eTable 8 in Supplement 2.
During the first 7 days after enrollment.
Median difference using quantile regression.
During the first 28 days after enrollment.
Subgroup Analyses
There was no statistically significant effect modification by time to enrollment (P = .65) or baseline lactate level (P = .37), although the number of patients in the high lactate subgroup was low (n = 45). In patients with a baseline SOFA score above the median, the mean difference in SOFA score change was −1.6 (95% CI, −2.8 to −0.3) points, favoring the intervention; however, there was no statistically significant effect modification by SOFA subgroup (P = .06). There was a significant effect modification by investigator-predicted 30-day survival (P = .046), with a greater effect seen among patients for whom their enrolling clinician thought survival was uncertain (eFigure 2 in Supplement 2).
Adverse Events
There were no unexpected serious adverse events related to the study drug. The most common serious adverse events were hyperglycemia (occurring in 12 patients in the intervention group and 7 patients in the placebo group), hypernatremia (occurring in 11 and 7 patients, respectively), and new hospital-acquired infection (occurring in 13 and 12 patients, , respectively) (see eTable 9 in Supplement 2 for complete details).
Discussion
In this multicenter randomized trial, the combination of ascorbic acid, corticosteroids, and thiamine, compared with placebo, did not result in a statistically significant difference in SOFA score change over 72 hours among adult patients with septic shock. There was no statistically significant difference between the 2 groups in the incidence of kidney failure or 30-day mortality. There were similar numbers of serious adverse events in each group.
Given the hypothesized organ-protective effect of the intervention, combined with the central focus of sepsis definitions on organ dysfunction, SOFA score trajectory was chosen as the primary outcome. The absence of a statistically significant difference between the intervention vs the control in SOFA score change over 72 hours contrasts with observational data, which suggested an association of this drug combination on measures of organ injury.8 The primary results of this trial are more consistent with those of 2 other recently published randomized trials of either ascorbic acid alone (CITRUS-ALI) or ascorbic acid in combination with hydrocortisone and thiamine (VITAMINS).9,14 In these prior trials, there was either no effect or only small effects of the intervention on SOFA scores.
There is no established MCID for change in SOFA score in the literature.20 In this trial, a 2-point difference in change in SOFA score was specified as the MCID, reflecting the Sepsis-3 clinical criteria for the definition of sepsis. The study that formed the basis of the Sepsis-3 clinical definition of sepsis found that an increase in SOFA score (from baseline) of 2 points or more was associated with an approximate 10% increase in mortality.19 Although prior analyses have concluded that trials are unlikely to identify a difference in SOFA score change of more than 1 point,21 a larger effect size was anticipated here on the basis of preliminary observational data suggesting that a large effect size was reasonable. It is possible, however, that a smaller difference in change in SOFA score is clinically important, and a smaller effect size is not excluded by the results of the present study.
There was no statistically significant difference in 30-day mortality between the groups. This differs from the results of the CITRUS-ALI study, which reported a 17% 28-day mortality benefit favoring the ascorbic acid group. Compared with the CITRUS-ALI trial, which enrolled patients with septic shock and acute respiratory distress syndrome, this trial did not require presence of acute respiratory distress syndrome for enrollment. In addition, the CITRUS-ALI trial used a different intervention that included a higher dose of ascorbic acid without concomitant thiamine or corticosteroids. Whether higher doses of ascorbic acid have added benefits is unclear, although in a nested cohort of the VITAMINS trial, the ascorbic acid level at 6 hours was comparable with that in the CITRUS-ALI trial despite a lower ascorbic acid dosing regimen.9,22
Patients in this trial were not selected on the basis of vitamin deficiency. This differs from a prior randomized trial of thiamine in septic shock, in which patients at increased risk of thiamine deficiency were included based on a serum lactate level greater than 27.0 mg/dL (3 mmol/L) after volume resuscitation (and exclusion of other causes of high lactate).6 Approximately 10% of enrolled patients in the current trial would have fit these criteria. In addition, some patients were excluded because clinicians were administering thiamine, potentially excluding patients more likely to respond to thiamine. Whether ascorbic acid and/or thiamine supplementation would have a greater effect in people deficient in these measures remains unknown.
The mean SOFA score at enrollment in this trial was 9.1, and 32% of patients died within 30 days. In contrast to the randomized trial of thiamine in septic shock, in which the median lactate level was 36.9 mg/dL (4.1 mmol/L), the median lactate level at enrollment in this trial was 16.2 mg/dL (1.8 mmol/L.)6 Lactate was measured at the time of first study drug administration in this trial and was more consistent with a measure of “persistently elevated” lactate as opposed to the highest lactate. Although there was no statistically significant effect modification for the primary outcome by median enrollment SOFA score or lactate level of 27.0 mg/dL (3 mmol/L) or higher, the confidence intervals of the stratified estimates include the possibility of a greater effect in patients with higher illness severity. There was also significant effect modification by investigator-predicted 30-day mortality, with a potentially beneficial effect of the intervention for patients considered to have uncertain survival outcome by their enrolling clinicians. These results are strictly hypothesis generating. Differential effects of interventions, with greater effects in patients with higher illness severity and the possibility of increased harm in those who are likely to survive regardless of the intervention, are a previously described phenomenon in critical care trials.23
Compared with the VITAMINS study, this trial did not include corticosteroids in the control group. Ultimately, 14 patients (14.1%) in the control group received open-label corticosteroids during the first 72 hours. As corticosteroids are known to reduce vasopressor requirements in septic shock,11,12 the improved hemodynamic parameters observed with the intervention in this trial may be related to corticosteroids, although a synergistic effect or effect from other interventions is possible. Given the number of comparisons made, results regarding days alive and vasopressor free and the cardiovascular component of the SOFA score should be considered hypothesis generating.
Limitations
This study has several limitations. First, ongoing or planned corticosteroid use was the most common exclusion criterion, perhaps eliminating a subset of patients more likely to benefit from corticosteroids. The protocol was written prior to publication of the ADRENAL and APROCCHSS studies, which demonstrated potential benefit of corticosteroids in some septic shock populations and may have led to increased corticosteroid prescription in the study period.11,12 Second, a large number of patients were screened but not randomized, which potentially reduces generalizability. Of those meeting all inclusion criteria and no exclusion criteria, approximately one-quarter were randomized. Data were not collected on the characteristics of these eligible participants who were not enrolled. Third, time from vasopressor initiation to study drug administration was 13.5 hours, which was similar to that of other clinical trials of pharmacologic interventions for sepsis.11,12,14 However, it is possible that a shorter time from vasopressor initiation to study drug administration may have resulted in improved outcomes. Fourth, some patients were discharged alive from the ICU within 96 hours of enrollment; thus, many patients did not receive a full 4 days of study drug. Fifth, the study did not have statistical power for subgroup analyses and cannot exclude the possibility that the intervention might have been more effective in certain subgroups. Sixth, the study did not have statistical power to detect small differences in mortality.
Conclusions
In patients with septic shock, the combination of ascorbic acid, corticosteroids, and thiamine, compared with placebo, did not result in a statistically significant reduction in SOFA scores during the first 72 hours after enrollment. These data do not support routine use of this combination therapy for patients with septic shock.
Trial Protocol and Statistical Analysis Plan
eTable 1. ACTS Trial Sites and Enrollment by Site
eTable 2. SOFA Score Definitions
eTable 3. Outcome Measure Definitions
eTable 4. Adverse Event Definitions
eTable 5. Open Label Medication Use
eTable 6. Reasons for Death
eTable 7. Sensitivity Analyses
eTable 8. SOFA Score Elements
eTable 9. Adverse Events
eFigure 1. Study Drug Doses Received
eFigure 2. Subgroup Analyses
Data Sharing Statement
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319(1):62-75. doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moskowitz A, Andersen LW, Huang DT, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care. 2018;22(1):283. doi: 10.1186/s13054-018-2217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marik PE, Hooper MH. Doctor—your septic patients have scurvy! Crit Care. 2018;22(1):23. doi: 10.1186/s13054-018-1950-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallat J, Lemyze M, Thevenin D. Do not forget to give thiamine to your septic shock patient! J Thorac Dis. 2016;8(6):1062-1066. doi: 10.21037/jtd.2016.04.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnino MW, Andersen LW, Chase M, et al. ; Center for Resuscitation Science Research Group . Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med. 2016;44(2):360-367. doi: 10.1097/CCM.0000000000001572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz A, Andersen LW, Cocchi MN, Karlsson M, Patel PV, Donnino MW. Thiamine as a renal protective agent in septic shock: a secondary analysis of a randomized, double-blind, placebo-controlled trial. Ann Am Thorac Soc. 2017;14(5):737-741. doi: 10.1513/AnnalsATS.201608-656BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229-1238. doi: 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 9.Fowler AA III, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261-1270. doi: 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler AA III, Syed AA, Knowlson S, et al. ; Medical Respiratory Intensive Care Unit Nursing . Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh B, Finfer S, Cohen J, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group . Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797-808. doi: 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 13.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954-962. doi: 10.1016/j.chest.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii T, Luethi N, Young PJ, et al. ; VITAMINS Trial Investigators . Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323(5):423-431. doi: 10.1001/jama.2019.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46(2):315-328. doi: 10.1007/s00134-020-05943-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz A, Yankama T, Andersen LW, Huang DT, Donnino MW, Grossestreuer AV; ACTS Clinical Trial Investigators . Ascorbic Acid, Corticosteroids and Thiamine in Sepsis (ACTS) protocol and statistical analysis plan: a prospective, multicentre, double-blind, randomised, placebo-controlled clinical trial. BMJ Open. 2019;9(12):e034406. doi: 10.1136/bmjopen-2019-034406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. doi: 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 19.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambden S, Laterre PF, Levy MM, Francois B. The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1):374. doi: 10.1186/s13054-019-2663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Grooth H-J, Geenen IL, Girbes AR, Vincent J-L, Parienti J-J, Oudemans-van Straaten HM. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21(1):38. doi: 10.1186/s13054-017-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson EP, Collie JT, Fujii T, et al. Pharmacokinetic data support 6-hourly dosing of intravenous vitamin C to critically ill patients with septic shock. Crit Care Resusc. 2019;21(4):236-242. [PubMed] [Google Scholar]
- 23.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045-1051. doi: 10.1164/rccm.201411-2125CP [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. ACTS Trial Sites and Enrollment by Site
eTable 2. SOFA Score Definitions
eTable 3. Outcome Measure Definitions
eTable 4. Adverse Event Definitions
eTable 5. Open Label Medication Use
eTable 6. Reasons for Death
eTable 7. Sensitivity Analyses
eTable 8. SOFA Score Elements
eTable 9. Adverse Events
eFigure 1. Study Drug Doses Received
eFigure 2. Subgroup Analyses
Data Sharing Statement