Key Points
Question
Among patients with diabetes and depression in India, does a 12-month collaborative care intervention that includes nonphysician care coordinators, decision support functions in electronic health records, and specialist case reviews improve depressive symptoms and measures of cardiometabolic health more than usual care at 24 months?
Findings
In this randomized clinical trial that included 404 patients at urban clinics in India with poorly controlled diabetes and depression, patients in the collaborative care intervention group, compared with the usual care group, were significantly more likely to achieve the composite outcome of at least a 50% reduction in the 20-item Symptom Checklist Depression Scale score and at least 1 of the following: reduction of at least 0.5 percentage points in hemoglobin A1c, reduction of at least 5 mm Hg in systolic blood pressure, or reduction of at least 10 mg/dL in low-density lipoprotein cholesterol at 24 months (71.6% vs 54.7%).
Meaning
Among patients with diabetes and depressive symptoms in urban India, a multicomponent collaborative care intervention resulted in statistically significantly greater improvements in a composite measure of depressive symptoms and cardiometabolic indices compared with usual care.
Abstract
Importance
Mental health comorbidities are increasing worldwide and worsen outcomes for people with diabetes, especially when care is fragmented.
Objective
To assess whether collaborative care vs usual care lowers depressive symptoms and improves cardiometabolic indices among adults with diabetes and depression.
Design, Setting, and Participants
Parallel, open-label, pragmatic randomized clinical trial conducted at 4 socioeconomically diverse clinics in India that recruited patients with type 2 diabetes; a Patient Health Questionnaire-9 score of at least 10 (range, 0-27); and hemoglobin A1c (HbA1c) of at least 8%, systolic blood pressure (SBP) of at least 140 mm Hg, or low-density lipoprotein (LDL) cholesterol of at least 130 mg/dL. The first patient was enrolled on March 9, 2015, and the last was enrolled on May 31, 2016; the final follow-up visit was July 14, 2018.
Interventions
Patients randomized to the intervention group (n = 196) received 12 months of self-management support from nonphysician care coordinators, decision support electronic health records facilitating physician treatment adjustments, and specialist case reviews; they were followed up for an additional 12 months without intervention. Patients in the control group (n = 208) received usual care over 24 months.
Main Outcomes and Measures
The primary outcome was the between-group difference in the percentage of patients at 24 months who had at least a 50% reduction in Symptom Checklist Depression Scale (SCL-20) scores (range, 0-4; higher scores indicate worse symptoms) and a reduction of at least 0.5 percentage points in HbA1c, 5 mm Hg in SBP, or 10 mg/dL in LDL cholesterol. Prespecified secondary outcomes were percentage of patients at 12 and 24 months who met treatment targets (HbA1c <7.0%, SBP <130 mm Hg, LDL cholesterol <100 mg/dL [<70 mg/dL if prior cardiovascular disease]) or had improvements in individual outcomes (≥50% reduction in SCL-20 score, ≥0.5-percentage point reduction in HbA1c, ≥5-mm Hg reduction in SBP, ≥10-mg/dL reduction in LDL cholesterol); percentage of patients who met all HbA1c, SBP, and LDL cholesterol targets; and mean reductions in SCL-20 score, Patient Health Questionnaire-9 score, HbA1c, SBP, and LDL cholesterol.
Results
Among 404 patients randomized (mean [SD] age, 53 [8.6] years; 165 [40.8%] men), 378 (93.5%) completed the trial. A significantly greater percentage of patients in the intervention group vs the usual care group met the primary outcome (71.6% vs 57.4%; risk difference, 16.9% [95% CI, 8.5%-25.2%]). Of 16 prespecified secondary outcomes, there were no statistically significant between-group differences in improvements in 10 outcomes at 12 months and in 13 outcomes at 24 months. Serious adverse events in the intervention and usual care groups included cardiovascular events or hospitalizations (4 [2.0%] vs 7 [3.4%]), stroke (0 vs 3 [1.4%]), death (2 [1.0%] vs 7 [3.4%]), and severe hypoglycemia (8 [4.1%] vs 0).
Conclusions and Relevance
Among patients with diabetes and depression in India, a 12-month collaborative care intervention, compared with usual care, resulted in statistically significant improvements in a composite measure of depressive symptoms and cardiometabolic indices at 24 months. Further research is needed to understand the generalizability of the findings to other low- and middle-income health care settings.
Trial Registration
ClinicalTrials.gov Identifier: NCT02022111
This randomized clinical trial compares the effect of a collaborative care model that integrates management of depression and enhanced diabetes care on depressive symptoms and HbA1c, SBP, and LDL cholesterol measures among individuals with depression and diabetes in India.
Introduction
Globally, suboptimal quality of health care accounts for 10% to 15% of mortality (5.7 million–8.4 million deaths) annually.1,2 Patients with multiple chronic conditions are at particular risk of poor quality of care because the need for multiple specialists increases care fragmentation and the likelihood of medical errors, poor outcomes, lower quality of life, and higher cost.3,4 Additional barriers to effective care for people with multiple chronic conditions include physical distances, financial costs, stigma, and shortages of health professionals. These barriers are pervasive, especially in low- and middle-income countries, and lead to avoidable poor outcomes for an increasing number of people with comorbid diseases.
Compared with the general population, people with chronic conditions, such as diabetes and heart disease, are twice as likely to experience mental health comorbidities, such as depression.5,6 Among patients with diabetes, comorbid depression is associated with poor self-management, worse glycemic control, and higher mortality.7,8 Depression is highly prevalent worldwide.9 Only 1 in 10 patients with depression receives adequate treatment,10 largely due to shortages of mental health professionals (psychiatrists, psychologists, therapists).11 To address the growing burdens of comorbid conditions globally, the World Health Organization has endorsed integrated care as feasible and efficient to increase access to mental health care and improve outcomes.12
The objective of this study was to develop and examine the effectiveness and sustainability of a collaborative care model that integrates management of depression and enhanced diabetes care in diabetes clinics in India. The collaborative care intervention was adapted from 2 evidence-based models13,14 and culturally tailored for the context. In these previous studies, continued exposure to the intervention was associated with sustained improvements. To assess whether collaborative care has sustained health effects without continued exposure, patients in the collaborative care group in this study received 12 months of the intervention and were reassessed after an additional 12 months without intervention.
Methods
Study Design
The Integrating Depression and Diabetes Treatment (INDEPENDENT) study was a multicenter, open-label, pragmatic clinical trial with randomization at the patient level, conducted at 4 urban diabetes clinics in India. The trial protocol and prespecified statistical analysis plan (Supplement 1) were published separately prior to study completion,15 and findings were reported following convention (eTable 1 in Supplement 2). Institutional ethics committees at each participating site and the coordinating centers (Madras Diabetes Research Foundation and Emory University) approved the study, and eligible patients gave written informed consent prior to enrollment.
Study Population
The study was conducted at 4 clinic sites (private clinics in Chennai, Bangalore, and Visakhapatnam, and a large public hospital outpatient clinic in Delhi). Patients were screened and enrolled from March 9, 2015, to May 31, 2016. The final 24-month study visit was completed on July 14, 2018.
Patients were eligible for inclusion if they were aged at least 35 years and had type 2 diabetes, moderate to severe depressive symptoms (9-item Patient Health Questionnaire [PHQ-9] score ≥10; range, 0-27; higher scores indicate more severe depressive symptoms), and at least 1 poorly controlled cardiometabolic parameter (hemoglobin A1c [HbA1c] ≥8%, systolic blood pressure (SBP) ≥140 mm Hg, or low-density lipoprotein [LDL] cholesterol ≥130 mg/dL). Site investigators reviewed clinic site records to identify patients with elevated HbA1c, SBP, and/or LDL cholesterol values and referred these patients to be screened for depressive symptoms using the PHQ-9. Patients with alcohol or substance use disorders, cognitive disorders, bipolar or psychotic disorders, type 1 diabetes, kidney failure, or cardiovascular disease (CVD) events in the past 12 months (myocardial infarction, unstable angina, or stroke) were excluded.
Study Randomization and Treatment
Randomization
Once informed consent was obtained, a blinded outcomes assessor conducted a detailed baseline assessment. Then, blinded study staff assigned patients to receive the collaborative care intervention or usual care using a password-protected web-based data management system (Interactive Web Response System). The system randomized patients in randomly generated blocks of 4, 6, 8, or 10 stratified by site, and randomization was communicated by the coordinating center to the site. Given the nature of the intervention, patients and their physicians could not be blinded to treatment randomization, but the outcomes assessor who conducted assessments at baseline and every 6 months thereafter remained blinded to treatment assignment for the duration of the study. Analysts were also blinded to treatment assignment.
Intervention Components
The collaborative care intervention was designed to improve depression and cardiometabolic indices and lower risks of microvascular and macrovascular complications of diabetes. Prior to finalizing the care model, the study team obtained and incorporated formative qualitative feedback regarding feasibility and acceptability of intervention components and training and educational materials from clinic site investigators, patients, and family members.16
The intervention used evidence- and team-based, patient-centered methods to overcome barriers and facilitate patient-level (eg, self-care), clinician-level (eg, measurement-based treatment to target), and system-level (eg, monitoring patient panels, outreach to patients with the most poorly controlled health) improvements. Each site had a team of a care coordinator and 2 consulting specialists (psychiatrist and diabetologist) who supplemented usual care by diabetes physicians.
To assist with patient-level self-management of diabetes and depression, the intervention involved nonphysician care coordinators who had backgrounds in allied health fields (eg, nutritional counseling, social work) but no prior training in mental health management. Care coordinators received training by the study team in behavioral activation and skills to support self-management: adherence to diet plans, exercise, medications, tobacco cessation, and follow-up visits with physicians. Care coordinators established regular (every 2-4 weeks) phone or in-person contact with patients; during these clinical contacts, care coordinators administered the PHQ-9 to monitor depressive symptoms, reviewed patients’ blood glucose and/or blood pressure logs, and provided counseling toward achieving individualized treatment goals.
To support diabetes physicians in their clinical decision-making and initiation and/or timely modification of evidence-based behavioral or pharmacotherapies for depression, glucose, blood pressure, and lipid management, the study team designed and incorporated a decision support electronic health record system into clinic workflows. This tool integrated patient characteristics, depressive symptom scores, and laboratory data over interim visits and provided evidence-based clinical prompts to physicians from built-in algorithms based on prevailing treatment guidelines.17 Prompts were adapted to prioritize medications that are standard care in India and lower-cost generic medications. If physicians made a clinical decision that was different from the prompt, they could reject the prompt and document their justification. Care coordinators also used the system to record interactions with patients and to track process and outcome measures, which also provided important input for team caseload review discussions.
To facilitate management of each clinic team’s population of patients, clinics held caseload review meetings every 2 to 4 weeks. Caseload reviews were attended by care coordinators and consulting specialists. This team reviewed data in the electronic health record and developed consensus recommendations for patients whose depression and/or cardiometabolic indices were poorly controlled. Recommendations included individualized outreach, treatment intensification, and/or behavioral activation to support patients in achieving individualized goals.
Prior to patient enrollment, teams at each site received a 3-day in-person training on the model and clinical workflows, systematic caseload review, and use of the decision support electronic health record system. Care coordinators received additional training18 focused on identification and management of suicide risk, motivational interviewing, and behavioral activation. After initial training, care coordinators had monthly coaching calls facilitated by a study team psychiatrist, psychologist, and nurse.
In the usual care group, study staff informed patients’ physicians about their depressive symptoms. The study investigators also provided site physicians with basic training in suicide risk assessment and management. Cardiometabolic management in the usual care group continued at physicians’ discretion without the use of the electronic health record system or prompts.
Data Collection and Follow-up
The total follow-up period for all patients was 24 months. Patients randomized to the intervention group received collaborative care for 12 months. The remaining 12 months involved passive follow-up without intervention to determine whether 12 months of exposure to the intervention had a sustained effect.
Patients in both groups attended study assessments at baseline (prior to randomization) and every 6 months thereafter with a blinded outcomes assessor. These data collection visits were paid for by the study. Because this was a pragmatic trial, costs of clinical care between study assessments for both groups, including visits with usual physicians, diagnostic tests, medications, or procedures, were borne by the patients (or clinics in the case of publicly funded settings). The intervention “dose” was measured based on tracking care coordinator and physician contacts with patients and the percentage of patients receiving antidepressant medications. For the usual care group, data regarding visits and treatments were retrospectively collected every 6 months via clinical chart review (paper and electronic).
At study assessments, outcome assessors administered the 20-item Symptoms Checklist Depression Scale (SCL-20; range, 0-4; higher scores indicate worse symptoms), a validated and sensitive measure of depression symptom severity,19 as well as questionnaires regarding anxiety (Generalized Anxiety Disorder 7-item scale); diet; exercise; medication adherence; health care use; and use of tobacco, alcohol (10-item Alcohol Use Disorders Identification Test), and other substances (10-item Drug Abuse Screening Test). The use of the SCL-20 for outcome measurements was intended to minimize potential test-retest bias given the repeated use of the PHQ-9 for clinical care. Assessors also used standardized protocols for measuring blood pressure (3 readings spaced 5 minutes apart in resting position using Omron T9P instruments) and collected blood samples for measurements of HbA1c and LDL cholesterol. The study used local laboratories to analyze blood samples; all laboratories were enrolled in an external quality assurance scheme (Bio-Rad Laboratories, Inc) with 2 SDs of coefficients of variation for metabolic markers, ranging from −0.51 to 0.66.
Throughout the study, patients were asked about the occurrence of serious adverse events, specifically hypoglycemia, microvascular and macrovascular diabetes complications, and hospitalizations. After classifying severity of the events and whether the events were related to the intervention, each adverse event was reported to ethics committees and the data and safety monitoring board.
Outcomes
The primary outcome was the between-group difference in the unadjusted percentage of patients who, at 24 months, had at least 50% improvement in SCL-20 scores and at least 1 of the following: at least 0.5-percentage point (ppt) reduction in HbA1c, at least 5-mm Hg reduction in SBP, or at least 10-mg/dL reduction in LDL cholesterol.
Prespecified secondary outcomes reported here include 12- and 24-month between-group differences in: the percentage of patients who met treatment targets (HbA1c <7.0%, SBP <130 mm Hg, LDL cholesterol <100 mg/dL [<70 mg/dL if history of CVD]) or had significant reductions in individual outcomes (≥50% reduction in SCL-20 score, ≥0.5-percentage point reduction in HbA1c, ≥ 5-mm Hg reduction in SBP, and ≥10-mg/dL reduction in LDL cholesterol); the percentage of patients who met HbA1c, SBP, and LDL cholesterol targets together; and mean changes in SCL-20 score, PHQ-9 score, HbA1c, SBP, and LDL cholesterol. Other prespecified secondary outcomes that will be reported separately included mean health utility, quality of life, and treatment satisfaction; mean health expenditures and within-trial cost utility; and whether the intervention had a similar beneficial effect on all 4 targets (SCL-20 score, HbA1c, SBP, and LDL cholesterol). Post hoc analyses examining overall improvements, achievement of the primary outcome at 12 months, PHQ-9 score less than 10, and within- and between-group mean changes over the 24 months were also completed.
Statistical Analysis
A sample size of 360 patients (180 per group) at 3 sites offered greater than 80% power (α = .05) to detect a 15% absolute risk difference (30% of patients in the collaborative care group vs 15% in the usual care group) in achieving the primary outcome. The effect size was estimated based on similar previous between-group differences in CVD risk factor improvements in collaborative care interventions13; the context of India, where documented achievement of care goals is lower than in high-income countries20,21; data from a quality improvement study in India14; and the longer follow-up without intervention. Because of concerns about meeting recruitment targets, an additional site was added in May 2015. To account for variation introduced by the additional site and provide greater robustness against type II error, the target sample size was revised to 400 after consultation with the data and safety monitoring board and subsequently amended in the protocol and methods publication.15
The patterns of missingness were analyzed, and missing measurements and patients lost to follow-up were imputed using a bootstrap expectation-maximization algorithm to construct 10 completed data sets for analysis.22 All results, unless noted otherwise, are derived from the imputed data, which were analyzed following convention.23
Patient characteristics by treatment assignment at baseline were described. For all analyses of intervention effects, data were analyzed according to patients’ randomization group. The risk differences (RDs) in achieving the primary outcome and prespecified secondary outcomes (improvements in individual depressive and cardiometabolic indices) between the intervention and usual care groups at 12 and 24 months accounted for treatment × time interaction and a site fixed effect, but did not adjust for baseline characteristics. The RDs were estimated using gaussian generalized estimating equations (GEEs) with identity link to account for correlation within patients over time (ie, linear probability models).24 Within- and between-group differences in means of continuous measures of SCL-20 score, PHQ-9 score, HbA1c, SBP, and LDL cholesterol over time were estimated using the same GEE approach.
Prespecified subgroup analyses were conducted to assess whether there was heterogeneity in effectiveness of the intervention, defined by the RD in the primary outcome at 24 months across age groups (35-49, 50-64, ≥65 years), sex (men, women), educational attainment (≤primary, secondary, ≥college), household income (Indian rupees per month: <10 000, 10 001-20 000, >20 000; mean conversion rate over 2015-2018 was 65 rupees to 1 US dollar), baseline SCL-20 score (≥1.3 vs <1.3), baseline HbA1c (≥8.0% vs <8.0%), baseline SBP (≥140 mm Hg vs <140 mm Hg), and baseline LDL cholesterol (≥130 mg/dL vs <130 mg/dL). For cardiometabolic indices, the eligibility criteria, which are also clinically meaningful thresholds, were used for this analysis. Because there is no clinically defined SCL-20 threshold for depression, the mean baseline SCL-20 score was used as the threshold. In addition to the prespecified subgroup analyses, clinically relevant post hoc analyses included examining whether there was variation in the primary outcome at 24 months by study site, care setting (public vs private), body mass index (<25, 25-29.9, ≥30), duration of diabetes (<8 years, ≥8 years; based on median duration of follow-up), insulin use, history of CVD, and history of microvascular disease. For each of the aforementioned subgroups, the primary analysis was repeated with the addition of the subgroup variable along with its interaction with treatment.24
Post hoc analyses included examining all primary and secondary outcomes over the whole study period (ie, using data from all study assessments) and estimating within- and between-group baseline to end-of-study differences in continuous outcomes with t tests and gaussian GEE models. Post hoc analyses also included examination of PHQ-9 score less than 10 to examine whether there was consistency with the SCL-20 depression measure trajectory.
Post hoc sensitivity analyses were conducted to assess the robustness of findings to alternative modeling approaches. First, analyses of the primary outcome were conducted, adjusted for age, sex, and baseline clinical characteristics. Second, RDs and associated standard errors were estimated using (1) GEE with site as a cluster variable (rather than fixed effect) and (2) linear mixed-effects models with nested random effects for patients within sites. Third, the influence of a single site with results that were dissimilar to other sites were examined by conducting analyses excluding this site. Fourth, PHQ-9 score less than 10 and meeting any of the HbA1c, SBP, or LDL cholesterol targets were examined, limited to those with uncontrolled levels (HbA1c ≥8.0%, SBP ≥140 mm Hg, LDL cholesterol ≥130 mg/dL) at baseline.
Analyses were conducted using R, version 3.6.1.25 Estimates were reported with 95% CIs using 2-sided statistical tests and a significance threshold of P < .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
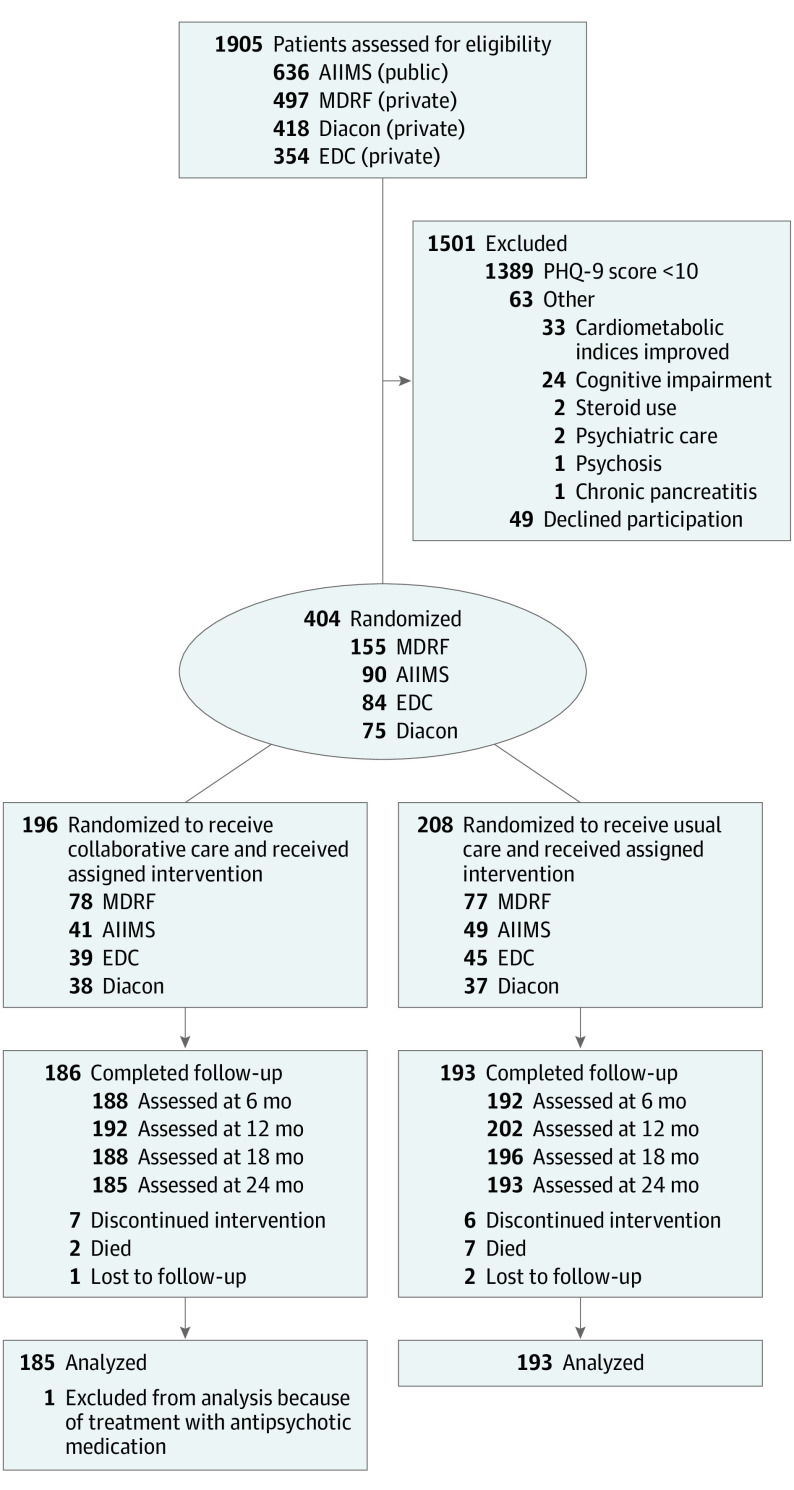
Of 2860 patients with type 2 diabetes who were approached, 1905 respondents were screened for eligibility; 453 patients were eligible for inclusion and 404 consented to enrollment and randomization (196 to collaborative care group and 208 to usual care; Figure 1). A total of 378 patients completed the 24-month study visit (185 [94.4%] in the collaborative care group and 193 [92.8%] in the usual care group); 18 (4.8%) patients were missing a key variable necessary for analysis.
Figure 1. Enrollment, Randomization, Follow-up, and Analysis of INDEPENDENT Study Patients.
Patient Health Questionnaire-9 (PHQ-9) screening was offered to patients identified as having at least 1 poorly controlled cardiometabolic parameter through chart review. AIIMS indicates All India Institute of Medical Sciences; EDC, Endocrine and Diabetes Center; Diacon, Diacon Diabetes Hospital; MDRF, Madras Diabetes Research Foundation.
At baseline, patients in the intervention and usual care groups had similar characteristics (Table 1). The mean (SD) age of the patients was 52.7 (8.6) years, 165 (40.8%) were men, 345 (85%) were married, 91 (22.5%) had more than a high school education, and 71 (17.6%) had health insurance. Patients had diabetes for a median (interquartile range) of 8.0 (3.9-14.2) years, 137 (33.9%) used insulin, 29 (7.2%) had preexisting CVD, and 216 (53.8%) had a previous microvascular complication. Among patients, the mean (SD) baseline SCL-20 score was 1.3 (0.5); PHQ-9 score, 13.2 (2.5); HbA1c, 9.1% (1.9%); blood pressure, 132/80 (16.3/10.1) mm Hg; and LDL cholesterol, 101.0 (37.8) mg/dL. The median (interquartile range) body mass index was 26.8 (23.9-29.7) among participants, and 6.7% were current smokers.
Table 1. Baseline Characteristics of Patients in a Study of the Effect of a Collaborative Care Model on Depressive Symptoms and Cardiometabolic Indices Among Patients With Depression and Diabetes in India.
| Characteristic | No. (%) | |
|---|---|---|
| Collaborative care group (n = 196) | Usual care group (n = 208) | |
| Sociodemographic characteristics | ||
| Age, mean (SD) | 52.1 (8.2) | 53.3 (8.9) |
| Sex | ||
| Women | 107 (54.6) | 132 (63.5) |
| Men | 89 (45.4) | 76 (36.5) |
| Married | 166 (84.7) | 179 (86.1) |
| Private or employer health insurance | 33 (16.8) | 38 (18.3) |
| Level of education, No. | 196 | 206 |
| No education | 19 (9.7) | 20 (9.7) |
| Primary or secondary | 134 (68.4) | 138 (67.0) |
| More than secondary | 43 (21.9) | 48 (23.3) |
| Occupation | ||
| Employed | ||
| Skilled | 62 (31.6) | 55 (26.4) |
| Unskilled | 29 (14.8) | 19 (9.1) |
| Homemaker | 91 (46.4) | 112 (53.8) |
| Retired | 12 (6.1) | 19 (9.1) |
| Unemployed | 2 (1.02) | 3 (1.4) |
| Household income, Indian rupeea | ||
| <3000 | 6 (3.1) | 4 (1.9) |
| 3000-10 000 | 49 (25.0) | 62 (29.8) |
| 10 001-20 000 | 57 (29.1) | 60 (28.8) |
| 20 001-30 000 | 36 (18.4) | 39 (18.8) |
| 30 001-40 000 | 12 (6.1) | 12 (5.8) |
| 40 001-50 000 | 13 (6.6) | 11 (5.5) |
| >50 000 | 23 (11.7) | 20 (9.6) |
| Clinical characteristics | ||
| Parental history of diabetes | 102 (69.4) | 103 (71.0) |
| Diabetes duration, median (IQR), y | 7.7 (3.3-13.7) | 8.5 (4.4;14.4) |
| Tobacco use | ||
| Never | 174 (88.8) | 190 (91.3) |
| Quit | 8 (4.1) | 5 (2.4) |
| Currently | 14 (7.1) | 13 (6.3) |
| Weight, median (IQR), kg | 67.5 (59.0-77.2) | 65.0 (58.9-72.7) |
| Waist circumference, median (IQR), cm | 95.0 (87.0-102.0) | 94.0 (87.0-101.0) |
| Body mass index, median (IQR) | 26.8 (24.2-29.8) | 26.8 (23.6-29.6) |
| Medication use | ||
| Oral hypoglycemic agent | 184 (93.9) | 191 (91.8) |
| Insulin | 66 (33.7) | 71 (34.1) |
| BP-lowering drug | 95 (48.5) | 105 (50.5) |
| Antidepressantb | 12 (6.1) | 7 (3.4) |
| Statin | 91 (46.4) | 114 (54.8) |
| Preexisting comorbidities | ||
| Neuropathy | 85 (44.7) | 83 (40.7) |
| Retinopathy | 54 (27.6) | 58 (27.9) |
| Heart disease | 9 (4.6) | 11 (5.3) |
| Depression | 5 (2.6) | 8 (3.9) |
| Stroke | 2 (1.0) | 7 (3.4) |
| Peripheral vascular disease | 0 | 5 (2.4) |
| Nephropathy | 1 (0.5) | 1 (0.5) |
| Cardiovascular and depression indices, mean (SD) | ||
| SCL-20 scorec | 1.3 (0.5) | 1.4 (0.5) |
| PHQ-9 scored | 13.0 (2.5) | 13.4 (2.5) |
| Fasting blood glucose, mg/dL | 183.0 (67.2) | 181.0 (77.1) |
| Hemoglobin A1c, % | 9.3 (2.0) | 9.0 (1.9) |
| Cholesterol, mean (SD), mg/dL | ||
| Total | 173 (44.1) | 175 (42.7) |
| Low-density lipoprotein | 101 (37.6) | 101 (38.1) |
| High-density lipoprotein | 40.1 (10.0) | 43.2 (12.6) |
| Triglycerides, median (IQR), mg/dL | 146.5 (107.5-199.5) | 140.5 (102.0-194.2) |
| BP, mean (SD), mm Hg | ||
| Systolic | 132 (15.4) | 133 (17.1) |
| Diastolic | 80.1 (10.5) | 80.3 (9.7) |
| Serum creatinine, median (IQR), mg/dL | 0.7 (0.6-0.9) | 0.7 (0.6-0.9) |
| Albumin-creatinine ratio, median (IQR) | 18.0 (6.6-31.2) | 17.4 (5.0-35.8) |
| Site | ||
| MDRF (private) | 78 (39.8) | 77 (37.0) |
| AIIMS (public) | 41 (20.9) | 49 (23.6) |
| EDC (private) | 39 (19.9) | 45 (21.6) |
| Diacon (private) | 38 (19.4) | 37 (17.8) |
Abbreviations: AIIMS, All India Institute of Medical Sciences; BP, blood pressure; Diacon, Diacon Diabetes Hospital; EDC, Endocrine and Diabetes Center; IQR, interquartile range; MDRF, Madras Diabetes Research Foundation; PHQ-9, Patient Health Questionnaire-9; SCL-20, Symptoms Checklist Depression Scale.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; to convert creatinine to µmol/L, multiply by 88.4; to convert cholesterol to mmol/L, multiply by 0.0259; and to convert triglycerides to mmol/L, multiply by 0.0113.
Mean conversion rate over 2015 to 2018 was 65 Indian rupee to 1 US dollar.
Use of lower doses of antidepressants for neuropathic symptoms.
Symptoms Checklist Depression Scale (SLC-20) score ranges from 0 (best) to 4 (worst).
Patient Health Questionnaire (PHQ-9) score ranges from 0 (best) to 27 (worst); score ≥10 indicates moderate to severe depressive symptoms.
During 12 months of active intervention, patients in the collaborative care group had 4.4 more recorded in-person clinic contacts and 2.9 more recorded phone contacts than patients in the usual care group. A higher percentage of patients in the collaborative care group, compared with the usual care group, received antidepressant medications (17.3% vs 5.6%).
Statistically significant differences in the percentage of patients with the composite primary outcome were noted at 24 months between the collaborative care vs usual care group (71.6% vs 54.7%; RD, 16.9% [95% CI, 8.5%-25.2%]) (Figure 2 and Table 2). Post hoc sensitivity analyses showed that these findings were robust in adjusted models (eTable 2 and eTable 3 in Supplement 2) and when applying alternative modeling approaches (eTable 4 in Supplement 2).
Figure 2. Primary and Secondary Outcomes in a Study of the Effect of a Collaborative Care Model on Depressive Symptoms and Cardiometabolic Indices Among Patients With Depression and Diabetes in India.
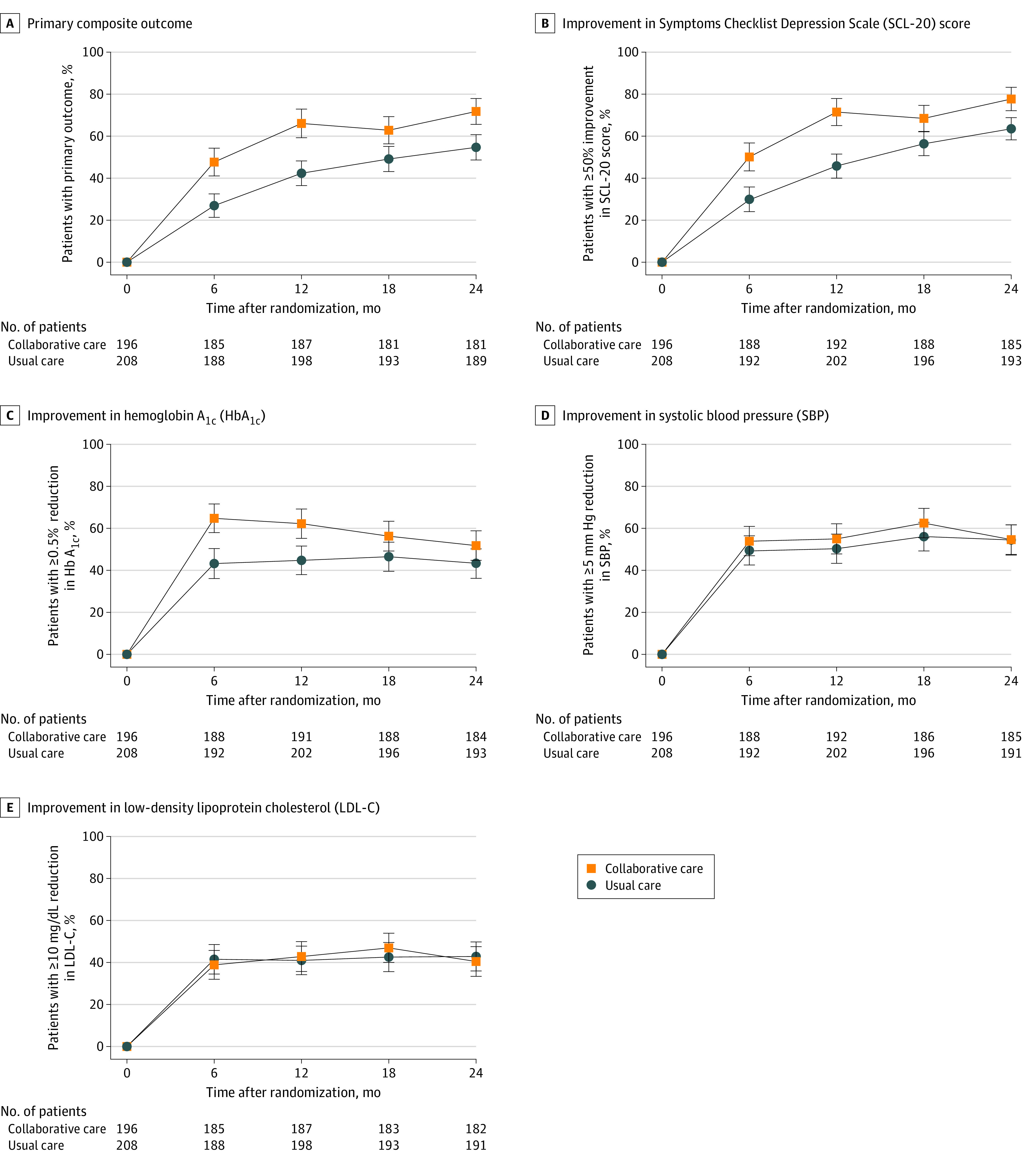
Primary outcome is the percentage of patients with ≥50% reduction in SCL-20 and at least 1 of the following: ≥0.5-percentage point reduction in HbA1c, ≥5-mm Hg reduction in SBP, or ≥10-mg/dL reduction in LDL-C. Markers indicate unadjusted percentages of patients by treatment group and error bars indicate the 95% CIs. Estimates were generated with gaussian models using an identity link and a generalized estimating equation approach to account for correlation of observations within patients over time. Model effects are treatment group, time, treatment × time interaction, and site.
Table 2. Outcomes in a Study of the Effect of a Collaborative Care Model on Depressive Symptoms and Cardiometabolic Indices Among Patients With Depression and Diabetes in Indiaa.
| Outcome | Collaborative care group | Usual care group | Risk difference (95% CI) | P valuec | ||
|---|---|---|---|---|---|---|
| At 24 months | Overallb | |||||
| Primary outcome, % | ||||||
| ≥50% Improvement in SCL-20 and ≥0.5-percentage point HbA1c reduction, ≥5-mm Hg SBP reduction, or ≥10-mg/dL LDL-C reductiond |
71.6 | 54.7 | 16.9 (8.5 to 25.2) | 18.7 (13.1 to 24.4) | <.001 | |
| Secondary outcomes, % | ||||||
| ≥50% improvement in SCL-20 scored | 77.7 | 63.6 | 14.1 (6.7 to 21.6) | 18.0 (12.4 to 23.7) | <.001 | |
| ≥0.5-percentage point reduction in HbA1c | 51.9 | 43.2 | 8.7 (−0.9 to 18.4) | 14.4 (7.1 to 21.7) | <.001 | |
| ≥5-mm Hg reduction in SBP | 54.8 | 54.5 | 0.3 (−9.6 to 10.2) | 4.0 (−3.6 to 11.1) | .28 | |
| ≥10-mg/dL reduction in LDL-C | 40.5 | 43.0 | −2.5 (−12.3 to 7.2) | 0.3 (−6.8 to 7.4) | .94 | |
| HbA1c <7% | 13.7 | 16.6 | −3.0 (−10.1 to 4.2) | 2.7 (−3.3 to 8.8) | .38 | |
| SBP <130 mm Hg | 63.7 | 63.4 | 0.3 (−9.1 to 9.7) | 0.6 (−5.9 to 7.1) | .85 | |
| LDL-C <100 mg/dL (<70 mg/dL with history of CVD) | 56.3 | 55.1 | 1.2 (−8.6 to 11.0) | 4.0 (−3.0 to 11.0) | .26 | |
| ≥0.5-percentage point reduction in HbA1c or HbA1c <7% | 55.1 | 50.4 | 4.7 (−5.0 to 14.5) | 12.1 (5.0 to 19.2) | <.001 | |
| ≥5-mm Hg reduction in SBP or SBP <130 mm Hg | 73.1 | 76.6 | −3.5 (−12.1 to 5.1) | −1.4 (−7.2 to 4.5) | .65 | |
| ≥10-mg/dL reduction in LDL or LDL-C <100 mg/dL (<70 with history of CVD) | 66.0 | 66.8 | −0.8 (−10.3 to 8.7) | 3.0 (−3.4 to 9.4) | .36 | |
| HbA1c <7%, SBP <130 mm Hg, and LDL-C <100 mg/dL (<70 with history of CVD) | 5.4 | 4.8 | 0.6 (−4.0 to 5.2) | 3.3 (−0.3 to 6.9) | .08 | |
| Mean difference (95% CI) | ||||||
| SCL-20 score, meand | 0.4 | 0.6 | −0.2 (−0.2 to −0.1) | −0.2 (−0.3 to −0.2) | <.001 | |
| PHQ-9 score, meane | 4.5 | 5.7 | −1.2 (−1.9 to −0.6) | −1.9 (−2.4 to −1.4) | <.001 | |
| HbA1c, mean, % | 8.7 | 8.6 | 0.0 (−0.3 to 0.4) | −0.2 (−0.4 to 0.1) | .12 | |
| SBP, mean, mm Hg | 122.4 | 123.2 | −0.8 (−4.6 to 2.9) | −1.6 (−4.2 to 1.0) | .22 | |
| LDL-C, mean, mg/dL | 95.9 | 94.0 | 2.0 (−5.6 to 9.6) | −0.6 (−5.9 to 4.7) | .82 | |
| Post hoc outcomes, % | Risk difference (95% CI) | |||||
| PHQ-9 score < 10e | 95.2 | 78.0 | 17.2 (11.2 to 23.2) | 19.1 (14.4 to 23.8) | <.001 | |
Abbreviations: CVD, cardiovascular disease; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Risk differences, percentages, and mean differences were estimated with gaussian models using an identity link and a generalized estimating equation approach to account for correlation of observations within patients over time. Model effects are treatment group, time, treatment × time interaction, and site.
Post hoc analysis.
P values represent statistical significance of overall between-group risk difference or mean difference.
Symptoms Checklist Depression Scale (SLC-20) score ranges from 0 (best) to 4 (worst).
Patient Health Questionnaire (PHQ-9) score ranges from 0 (best) to 27 (worst); score ≥10 indicates moderate to severe depressive symptoms.
Of 7 prespecified secondary outcomes examining single improvements or treatment target achievement at 12 months, statistically significantly greater improvements were observed in the collaborative care group compared with the usual care group for the percentage of patients who had at least a 50% reduction in SCL-20 scores (71.5% vs 45.8%; RD, 25.7% [95% CI, 17.0%-34.4%]) and reduction in HBA1c of at least 0.5 percentage points (62.2% vs 44.7%; RD, 17.5% [95% CI, 7.8%-27.2%]) (eTable 2 and eTable 5 in Supplement 2). At 24 months, statistically significant differences in SCL-20 score improvements persisted (77.7% in the collaborative care group vs 63.6% in the usual care group; RD, 14.1% [95% CI, 6.7%-21.6%]), but this was not observed for HbA1c. There were no statistically significant between-group differences in SBP or LDL cholesterol improvement at either time point. In post hoc analyses, a statistically significantly greater percentage of patients in the collaborative care group had PHQ-9 scores less than 10 at 12 months than in the usual care group (89.3% vs 65.5%; RD, 23.8% [95% CI, 16.2%-31.4%]), which persisted at 24 months (95.2% vs 78.0%; RD, 17.2% [95% CI, 11.2%-23.2%]).
Of 4 prespecified composite cardiometabolic secondary outcomes, the collaborative care group, compared with the usual care group, had a statistically significantly higher percentage of patients with a reduction in HBA1c of at least 0.5 percentage points or HbA1c less than 7.0% (69.4% vs 53.3%; RD, 16.1% [95% CI, 6.5%-25.7%]) and with HbA1c less than 7.0%, SBP less than 130 mm Hg, and LDL cholesterol less than 100 mg/dL (13.3% vs 4.7%; RD, 8.6% [95% CI, 3.0%-14.3%]) at 12 months, but not at 24 months (eTables 2 and 5 in Supplement 2).
For 5 prespecified continuous measures of depressive symptoms and cardiometabolic indices, compared with usual care, patients in the collaborative care group had greater reductions in depression scores at 12 months (SCL-20 score: mean difference, −0.3 [95% CI, −0.4 to −0.2]; PHQ-9 score: mean difference, −2.4 [95% CI, −3.2 to −1.6]) and 24 months (SCL-20 score: mean difference, −0.2 [95% CI, −0.2 to −0.1]; PHQ-9 score: mean difference, −1.2 [95% CI, −1.9 to −0.6]) (Table 2 and eFigure 1 in Supplement 2). In post hoc analyses, mean reductions in depression scores in patients in the collaborative care group were statistically significantly larger than for patients in the usual care group (eTable 3 and eTable 6 in Supplement 2). Between-group and within-group changes over time in SBP and LDL cholesterol showed no significant difference.
There were no significant differences in intervention effects on the primary outcome at 24 months by age, sex, education, income, public vs private clinics, body mass index, clinical history, baseline levels of indices, duration of diabetes, or insulin use (Figure 3). There was variation in the intervention effect on primary and secondary outcomes across sites, with 1 site exhibiting very large improvements in depression scores (eTable 7, eTable 8, and eFigure 2 in Supplement 2). Post hoc sensitivity analyses excluding this site showed overall effects were mildly attenuated, although the patterns and direction of findings remained consistent.
Figure 3. Primary Outcome at 24 Months by Socioeconomic and Clinical Characteristics in a Study of the Effect of a Collaborative Care Model on Depressive Symptoms and Cardiometabolic Indices Among Patients With Depression and Diabetes in India.
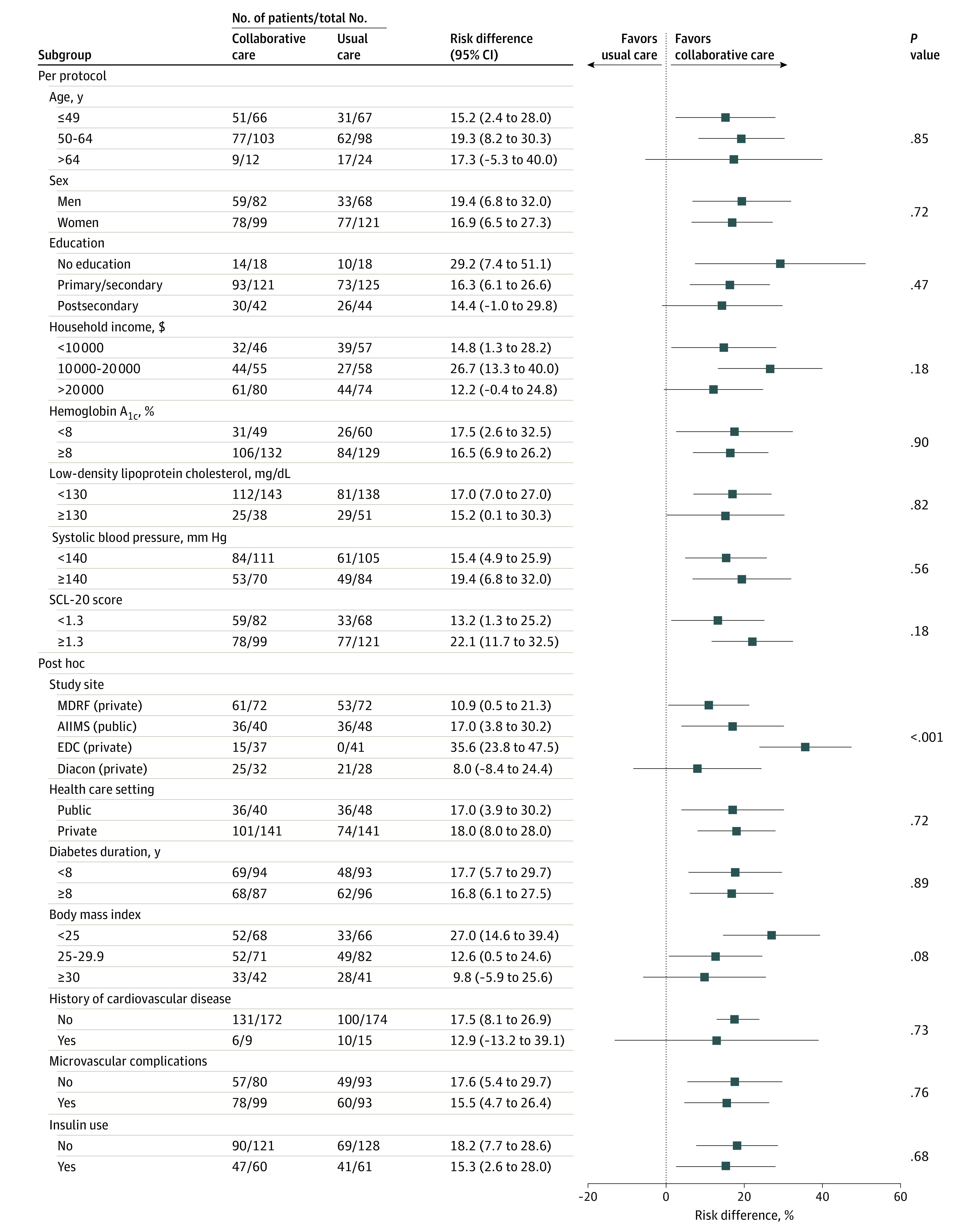
Risk differences were estimated with gaussian models using an identity link and a generalized estimating equation approach to account for correlation of observations within patients over time. Model effects are treatment group, time, treatment-by-time and treatment-by-subgroup interactions and site (except for subgroup analysis by site). AIIMS indicates All India Institute of Medical Sciences; EDC, Endocrine and Diabetes Center; Diacon, Diacon Diabetes Hospital; MDRF, Madras Diabetes Research Foundation; SCL-20, Symptoms Checklist Depression Scale.
Post hoc sensitivity analyses limited to patients with depressive symptoms and specific poorly controlled cardiometabolic indices were associated with 12-month statistically significantly larger improvements in the collaborative care group compared with the usual care group for mean HbA1c (mean difference, −0.5 percentage points [95% CI, −0.9 to −0.1]), SBP (mean difference, −6.4 mm Hg [95% CI, −11.0 to −1.9]), and LDL cholesterol (mean difference, −15.3 mg/dL [95% CI, −28.4 to −2.3]), but these did not persist at 24 months (eTable 9 in Supplement 2).
Incident severe adverse events in the collaborative care and usual care groups included CVD events or hospitalizations (4 [2.0%] vs 7 [3.4%]), stroke (0 vs 3 [1.4%]), deaths (2 [1.0%] vs 7 [3.4%]), and severe hypoglycemia (8 [4.1%] vs 0) (eTable 10 in Supplement 2).
Discussion
In this multicenter randomized clinical trial of patients with diabetes and depressive symptoms attending urban diabetes clinics in India, a multicomponent collaborative care intervention, compared with usual care, achieved clinically meaningful improvements in combined depression and cardiometabolic indices. Improvements were significantly larger during the active intervention from baseline to 12 months, with narrowing of between-group differences in the 12 months of follow-up without the intervention. These findings may have implications for sustainable management of comorbid diabetes and depression in diverse, low-resource, fragmented health care settings in which care is often inconsistent.
This study adds to the literature by finding short- and longer-term effectiveness of addressing physical-mental comorbidities in patients through collaborative care that targets multiple interacting barriers to care in India. Access to mental health care is exceedingly limited in India, with 0.3 psychiatrists, 0.8 mental health nurses, 0.1 social workers, and 0.1 psychologists per 100 000 population.26 Collaborative care increases access to effective mental health treatment, and these findings demonstrate the feasibility and acceptability of implementing this model in diverse diabetes care settings. The study was conducted in contexts in which, other than study-related assessment costs, patients were responsible for their treatment and follow-up costs and received no study-funded medications or incentives. Also, consistent with national data, 82.4% of study patients paid out of pocket for their health care.
This study also offers insights regarding differential sustainability of intervention effects on clinical parameters. Mean depressive symptom scores—both the SCL-20 and PHQ-9 scores—improved over the first 12 months of follow-up, with relatively moderate use of medications, and continued to improve over months 13 through 24 despite no intervention. This may reflect sustained effects of the intervention. Alternatively, these findings may be explained by the fluctuating nature of depressive symptoms27 or the subjective nature of measurement tools for depression, or they may represent social desirability bias. The consistency of sustained improvements in both depressive symptom measures minimizes concerns that patients learn the answers to these tests through repeated measurement over time.
In contrast, for cardiometabolic indices, the largest improvements and divergence between groups occurred in the 6- to 12-month active phase of the intervention, which is similar to what has been observed in similar studies,13,14 with gradual returns toward baseline over months 13 to 24. These findings suggest the need for more intensive patient self-management support and/or for ongoing use of decision support tools. This interpretation is supported by the CARRS trial,14 which demonstrated sustained cardiometabolic improvements with continued exposure to the active intervention, which included patient-focused (nonphysician care coordinators) and clinician-focused (decision support prompts in the electronic health record) quality improvement strategies. Meta-analyses of quality improvement studies have shown similar aggregated cardiometabolic improvements at 12 months (between-group differences of −0.37% for HbA1c, −3.1/1.6 mm Hg for blood pressure, and −3.9 mg/dL for LDL cholesterol)28 and that certain aspects of care (eg, workflow redesign, team-based care, electronic registries) can be leveraged to achieve sustained improvements of −0.28% in HbA1c, −2.3/1.1 mm Hg in blood pressure, and −5.4 mg/dL in LDL cholesterol beyond 12 months.29 Although short-lived, similar between-group differences in cardiometabolic improvements that dissipated after 12 months in the Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION; n = 3057)30 were still associated with lower cardiovascular and microvascular outcomes over 5 years.31,32
Although a number of studies have shown that comorbid depression is associated with worse control of cardiometabolic indices and higher risk of events and mortality,4,8,33,34,35 a corollary of these findings is that improvements in depressive symptoms alone do not improve cardiometabolic indices. Further analyses examining self-care behaviors by group and study phase may provide more nuanced data that inform how future practice change interventions might be designed. The observation that there were noteworthy incremental improvements in glycemia but not in blood pressure and cholesterol likely reflects the lower baseline levels for SBP and LDL cholesterol, because statistically significant reductions were noted over 12 months for those with elevated baseline SBP and LDL cholesterol levels. These findings also highlight that treat-to-target approaches have an excess risk of hypoglycemia, consistent with previous studies.
Few other studies have evaluated interventions to address comorbid cardiometabolic diseases and depression, and all of these studies, to our knowledge, have been based in high-income European countries or the US. In 14 primary care clinics in Washington State (n = 214), a collaborative care intervention in a study by Katon et al13 led to a 0.58% greater improvement in HbA1c, 0.40-point greater improvement in SCL-20 scores, 5.1-mm Hg greater reductions in SBP, and 6.9-mg/dL greater reductions in LDL cholesterol at 12 months. Compared with the study by Katon et al,13 the baseline profile of the 404 patients in this trial was slightly younger (mean age, 52.7 vs 56.8 years) and leaner (body mass index, 26.8 vs 36.7), with similar depressive symptom scores and somewhat higher HbA1c (9.1% vs 8.0%), lower LDL cholesterol levels (101 vs 107 mg/dL), and lower percentage of patients with prior history of CVD (7.2% vs 26.5%). The care model tested in this trial was also somewhat different in that the intervention was adapted to Indian settings, included allied health professionals as care coordinators (while the study by Katon et al13 used registered nurses), and included implementing a decision support electronic health record system that was new to most clinic sites.
Results of the current study may have implications in the context of the concurrent increase in life expectancy and of multiple morbidities, and their effects worldwide.3,4 First, because shortages of skilled mental health personnel11 are unlikely to be overcome in the near future, this study offers evidence that diabetes clinicians can be supported to manage depression effectively. This study included psychiatrists to train and support care coordinators and provide indirect consultation through caseload review. This distinguishes collaborative care interventions from other task-shifting interventions and has implications for scalability; models of delivery that are less reliant on implementation support need to be tested. Second, integrated care models that align with local clinic capacity, opportunities, and motivations for practice change36 have greater potential to strengthen care delivery worldwide and address a wide range of converging multiple morbidities. Third, these findings suggest a potential role for decision support functions in electronic medical records, especially in the context of increasing complexity and heterogeneity of patient profiles, speed of emerging evidence and guideline updates that are challenging for physicians to keep up with, and ongoing high demand for care; cumulatively, this will require more tailored management plans in shorter visit times. Fourth, to monitor whether quality improvement initiatives can reach population-scale improvements, as have been observed in the US,37,38 a parallel emphasis on regular reporting and surveillance of quality of care are needed in low- and middle-income countries.
The strengths of this study include the randomized trial design, diverse population and clinics, very low attrition, and standardized measures across sites. The study trained allied health professionals without any previous experience of mental health care to address mental health access gaps. The findings from this study are derived from a context in which only study visits were paid for by the research; all other follow-up, medication, and self-care costs were borne by patients. Additionally, because generic dichotomous targets (eg, HbA1c <7.0%) may motivate more aggressive care, which is not always appropriate (eg, higher risk of hypoglycemia) or patient-centered, this study examined clinically meaningful improvements from baseline for the main outcomes and reported target achievement as secondary outcomes also.
Limitations
This study has several limitations. First, the findings were relevant to adults with diabetes and moderate to severe depressive symptoms; the study excluded those with severe depressive symptoms, suicidality, or substance use disorders and referred them to specialists. Second, the study was conducted in urban diabetes clinics; further adaptation, dissemination, and testing are needed to assess whether these effects can be replicated and scaled in primary care, rural, or other low- and middle-income country settings. Third, this study could not disaggregate the effects of individual intervention components; that said, multicomponent interventions are strongly recommended to achieve sustainable quality improvement.29,39,40
Conclusions
Among patients with diabetes and depression in India, a 12-month collaborative care intervention, compared with usual care, resulted in statistically significant improvements in a composite measure of depressive symptoms and cardiometabolic disease indices at 24 months. Further research is needed to understand the generalizability of the findings to other low- and middle-income health care settings.
Trial protocol and statistical analysis plan
eResults
Data sharing statement
References
- 1.National Academies of Sciences Engineering and Medicine Crossing the Global Quality Chasm: Improving Health Care Worldwide. National Academies Press; 2018. [PubMed] [Google Scholar]
- 2.Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6(11):e1196-e1252. doi: 10.1016/S2214-109X(18)30386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci. 2016;71(2):205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arokiasamy P, Uttamacharya U, Jain K, et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the Study on Global Ageing and Adult Health (SAGE) reveal? BMC Med. 2015;13(1):178. doi: 10.1186/s12916-015-0402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24(6):1069-1078. doi: 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- 6.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383-2390. doi: 10.2337/dc08-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154-2160. doi: 10.2337/diacare.27.9.2154 [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MD, O’Connor P, Feeney P, et al. Depression predicts all-cause mortality: epidemiological evaluation from the ACCORD HRQL substudy. Diabetes Care. 2012;35(8):1708-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depression and Other Common Mental Disorders: Global Health Estimates World Health Organization ; 2017.
- 10.Patel V, Maj M, Flisher AJ, et al. Reducing the treatment gap for mental disorders: a WPA survey. World Psychiatry. 2010;9(3):169-176. doi: 10.1002/j.2051-5545.2010.tb00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel V, Xiao S, Chen H, et al. The magnitude of and health system responses to the mental health treatment gap in adults in India and China. Lancet. 2016;388(10063):3074-3084. doi: 10.1016/S0140-6736(16)00160-4 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization; Calouste Gulbenkian Foundation . Integrating the Response to Mental Disorders and Other Chronic Diseases in Health Care Systems World Health Organization ; 2014:6-50.
- 13.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611-2620. doi: 10.1056/NEJMoa1003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali MK, Singh K, Kondal D, et al. ; CARRS Trial Group . Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Ann Intern Med. 2016;165(6):399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalski AJ, Poongothai S, Chwastiak L, et al. The integrating depression and diabetes treatment (INDEPENDENT) study: design and methods to address mental healthcare gaps in India. Contemp Clin Trials. 2017;60:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao D, Lipira L, Kumar S, et al. Input of stakeholders on reducing depressive symptoms and improving diabetes outcomes in India: formative work for the INDEPENDENT study. Int J Noncommunicable Dis. 2016;1(2):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 18.University of Washington Education and training. AIMS Center: Advancing Integrated Mental Health Solutions website. Published 2020. Accessed February 10, 2020. https://aims.uw.edu/program-area/education-training
- 19.Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins Symptom Checklist (HSCL): a measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79-110. doi: 10.1159/000395070 [DOI] [PubMed] [Google Scholar]
- 20.Nagpal J, Bhartia A. Quality of diabetes care in the middle- and high-income group populace: the Delhi Diabetes Community (DEDICOM) survey. Diabetes Care. 2006;29(11):2341-2348. doi: 10.2337/dc06-0783 [DOI] [PubMed] [Google Scholar]
- 21.Menon VU, Guruprasad U, Sundaram KR, et al. Glycaemic status and prevalence of comorbid conditions among people with diabetes in Kerala. Natl Med J India. 2008;21(3):112-115. [PubMed] [Google Scholar]
- 22.Honaker J, King G, Blackwell M.. Amelia II: a program for missing data. J Statl Software. 2011;45(7). doi: 10.18637/jss.v045.i07 [DOI] [Google Scholar]
- 23.Marchenko YV, Eddings W A note on how to perform multiple-imputation diagnostics in Stata. Stata website. Published 2011. Accessed March 25, 2019. https://www.stata.com/users/ymarchenko/midiagnote.pdf
- 24.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed John Wiley & Sons; 2011. doi: 10.1002/9781119513469 [DOI] [Google Scholar]
- 25.A language and environment for statistical computing. R Foundation for Statistical Computing website. Last accessed April 2020. https://www.R-project.org/
- 26.Global Health Observatory data repository. World Health Organization website. Published 2016. Accessed February 1, 2020.https://apps.who.int/gho/data/node.main.MHHR?lang=en
- 27.Whiteford HA, Harris MG, McKeon G, et al. Estimating remission from untreated major depression: a systematic review and meta-analysis. Psychol Med. 2013;43(8):1569-1585. doi: 10.1017/S0033291712001717 [DOI] [PubMed] [Google Scholar]
- 28.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252-2261. doi: 10.1016/S0140-6736(12)60480-2 [DOI] [PubMed] [Google Scholar]
- 29.Lim LL, Lau ESH, Kong APS, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care. 2018;41(6):1312-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauritzen T, Griffin S, Borch-Johnsen K, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24(suppl 3):S6-S11. doi: 10.1038/sj.ijo.0801420 [DOI] [PubMed] [Google Scholar]
- 31.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156-167. doi: 10.1016/S0140-6736(11)60698-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandbæk A, Griffin SJ, Sharp SJ, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial. Diabetes Care. 2014;37(7):2015-2023. doi: 10.2337/dc13-1544 [DOI] [PubMed] [Google Scholar]
- 33.Heckbert SR, Rutter CM, Oliver M, et al. Depression in relation to long-term control of glycemia, blood pressure, and lipids in patients with diabetes. J Gen Intern Med. 2010;25(6):524-529. doi: 10.1007/s11606-010-1272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7(5):414-421. doi: 10.1370/afm.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934-942. [DOI] [PubMed] [Google Scholar]
- 36.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613-1624. [DOI] [PubMed] [Google Scholar]
- 38.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol. 2018;6(5):392-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eResults
Data sharing statement