Abstract
The high human labor demand involved in collecting paired medical imaging data severely impedes the application of deep learning methods to medical image processing tasks such as tumor segmentation. The situation is further worsened when collecting multi-modal image pairs. However, this issue can be resolved through the help of generative adversarial networks, which can be used to generate realistic images. In this work, we propose a novel framework, named TumorGAN, to generate image segmentation pairs based on unpaired adversarial training. To improve the quality of the generated images, we introduce a regional perceptual loss to enhance the performance of the discriminator. We also develop a regional loss to constrain the color of the imaged brain tissue. Finally, we verify the performance of TumorGAN on a public brain tumor data set, BraTS 2017. The experimental results demonstrate that the synthetic data pairs generated by our proposed method can practically improve tumor segmentation performance when applied to segmentation network training.
Keywords: medical image augmentation, generative adversarial network, brain tumor segmentation, image-to-image
1. Introduction
An accurate tumor segmentation model is pivotal for early tumor determination and radiotherapy arrangement [1]. Traditionally, tumor segmentation is performed by finding a mapping function between a real medical image (e.g., an MRI image) and a semantic label image of a real tumor, as depicted by medical professionals. With the rapid development of medical imaging equipment, substantial effort has been directed towards the research of segmentation tasks using multi-modal data pairs [2,3,4,5]. Generally, multi-modal data can lead to a better performance result, as compared to approaches based on a single modality, because more information about the tumor could be captured by different imaging methods [6]. Motivated by the success of deep learning, researchers soon applied deep neural networks to solve various medical imaging-related problems [7,8,9]. However, unlike classification, labeling medical images for segmentation is challenging, as it is time-consuming and requires medical specialists [10]. Labeling multi-modal medical images further increases the complexity of such a task. The lack of properly labeled tumor masks limits the potential of data-driven medical image segmentation such as those involving deep learning-based methods. Data augmentation (e.g., rotation, flipping) is one possible way to expand a data set with limited labeled samples. However, these methods are insufficient to represent the variations of shape, location, and pathology.
Recently, many researchers have used generative adversarial networks (GANs) for image synthesis and data augmentation. Although the earlier variants of GANs can only generate images from random noise [11], conditional GAN-based image-to-image translation models provide new solutions for pixel-wise image augmentation [12]. Many powerful GAN-based image-to-image variants have been proposed [13,14], which can generate realistic images by considering an input image and a given condition. In fact, some popular image-to-image translation frameworks such as Pix2pix [15] or CycleGAN [16] have already shown potential for pixel-wise image augmentation by converting an image only including semantic information to a realistic image. However, we still need to address two challenges before such methods can be applied to multi-modal medical image augmentation. The first challenge is a lack of source data, which means that we need to generate reasonable semantic labels first before feeding them into the image-to-image translation models. An incorrect lesion area may lead to an useless augmentation output. Furthermore, we need to guarantee the quality of the synthesized images without enough ground truth during the augmentation stage due to the absence of image pairs. Hence, to obtain a realistic medical image at the pixel level, adversarial training is necessary but requires further improvement.
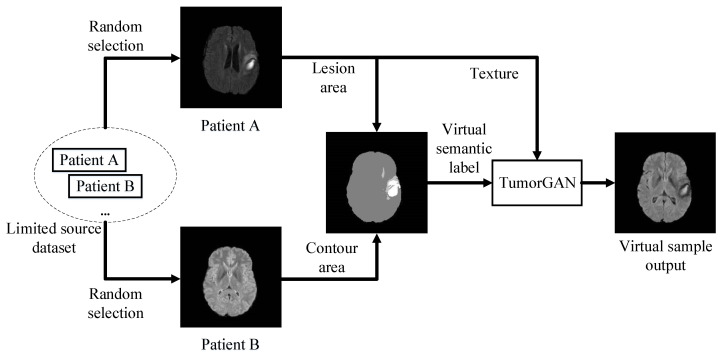
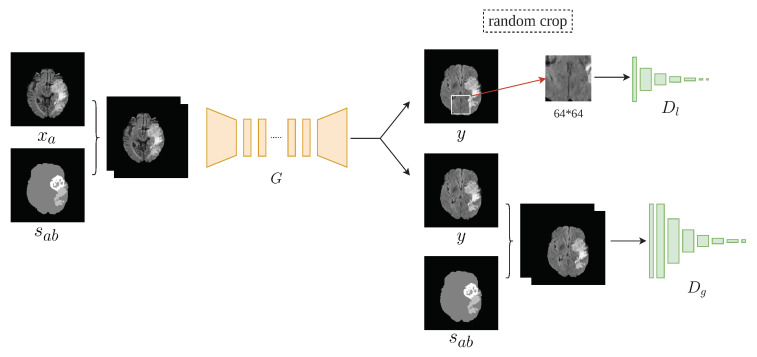
To generate realistic paired data with limited source data for medical image augmentation, we synthesize a pixel-wise semantic label image by combining the lesion area with the brain contour from two real medical images. Then, we feed the virtual semantic label image with texture image from patient A to generate the corresponding output, as displayed in Figure 1. By doing so, in an ideal case, we can obtain virtual samples from n patients for data augmentation. Furthermore, the validity of the synthetic semantic label images can be guaranteed, as both the contour and lesion area come from real samples. The synthetic semantic label image can also provide an attention region to help us build a regional perceptual loss, as well as a regional loss, in order to train the image-to-image translation model with a regional ground truth and improve the generalization performance. To further enhance the efficiency of adversarial learning, we include an additional local discriminator co-operating with the main discriminator.
Figure 1.
An overview of TumorGAN-based medical image augmentation.
In this work, we have the following contributions:
We propose an image-to-image translation framework called TumorGAN, which can synthesize virtual image pairs from n real data pairs for brain tumor segmentation. Our experimental results show that TumorGAN is able to augment brain tumor data sets and can improve the performance of tumor segmentation for both single-modality data and multi-modal data.
We design a region perceptual loss and an loss based on attention areas provided by the semantic labels to preserve the image details.
We included an extra local discriminator co-operating with the main discriminator, in order to increase the efficiency of the discriminator and help TumorGAN to generate medical image pairs with more realistic details.
2. Related Work
2.1. Brain Tumor Segmentation
Brain tumor segmentation is a challenging task, due to the structural complexity of brain tissue [17]. Many brain tumor segmentation approaches have been proposed. Researchers have earlier developed brain segmentation approaches based on contours [18], regions [19], statistics [20], and some traditional machine learning models [21]. As deep learning approaches have become more popular, more and more deep learning-based brain tumor segmentation methods have also been presented [22,23]. As an increasing number of multi-modal data sets, like BraTS challenge [24], have been released, several deep learning based multi-modal medical image segmentation methods [25,26] have also been proposed. As image-to-image translation frameworks have become popular for segmentation tasks, GAN-based image-to-image translation provides another solution for brain tumor segmentation. In this paper, we take advantage of a GAN-based image-to-image framework for multi-modal brain tumor processing. Differing from previous approaches, we aimed at applying the framework to the pre-processing stage for multi-modal brain tumor data augmentation. Our main aim is to use the augmented data to improve the robustness and performance of the segmentation model.
2.2. Generative Adversarial Network Based Medical Image Augmentation
Collecting and labeling medical images is a difficult task, due to the lack of experts and privacy concerns, thus limiting the application of supervised deep learning approaches [27]. Typically insufficient numbers of medical images requires the development of medical image augmentation methods. Earlier simple geometric deformation methods, including scaling, rotation, and flipping, have been employed to increase the variety of data sets [28]; however, such augmentation methods cannot represent the variations in the shape, location, and pathology of a brain tumor.
The success of generative adversarial networks (GANs) has provided a general solution for image augmentation [29]. A straightforward way of applying a GAN to medical image argumentation is to use the noise-to-image structure, which originates from the vanilla GAN and some variants [11,14,30], generating images from one-dimensional vectors [31]. However, these approaches cannot obtain a pixel-wise matching between two images. Another way is to take the advantage of the image-to-image translation framework for medical image augmentation. Earlier image-to-image translation models such as Pix2pix [15] need paired training data, which are expensive to obtain for medical images. CycleGAN, which can translate images from one domain to another with unpaired data [16], is more popular in solving medical image augmentation problems. Unpaired image-to-image translation models can even translate medical imaging across different modalities, such as MRI to CT translation [32,33,34]. Image-to-image frameworks have also been widely used for medical image quality enhancement or denoising [35,36]. Unlike the methods for medical image processing mentioned above, which attempt to build a mapping function between two modalities, our proposed method, TumorGAN, aims to build a mapping function between an edited semantic label image and a modality (flair, t1, t1ce, and t2).
3. Method
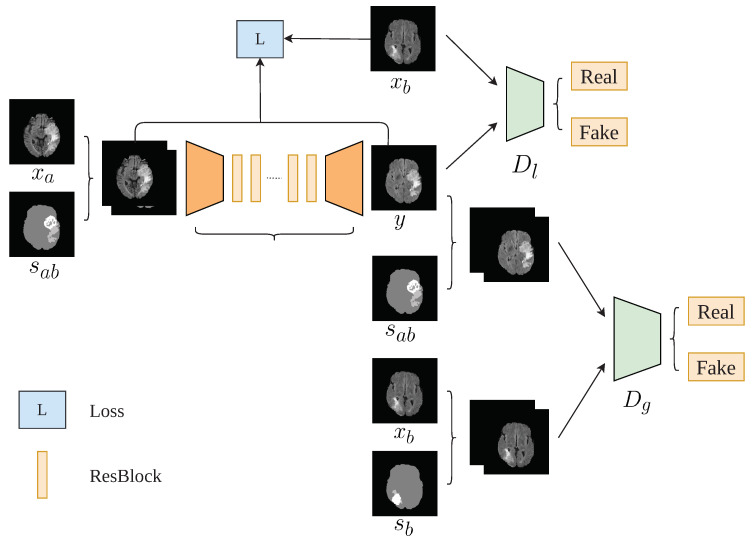
In the following, we introduce a brain data augmentation approach called tumorGAN, followed by a graphical description shown in Figure 2.
Figure 2.
TumorGAN architecture. We use one generator G and two discriminators and . The output of G is entered into the different discriminators in different ways to determine whether it is real or fake; is a semantic segmentation label corresponding to y and is a semantic label corresponding to .
3.1. Synthesis of Semantic Label Image
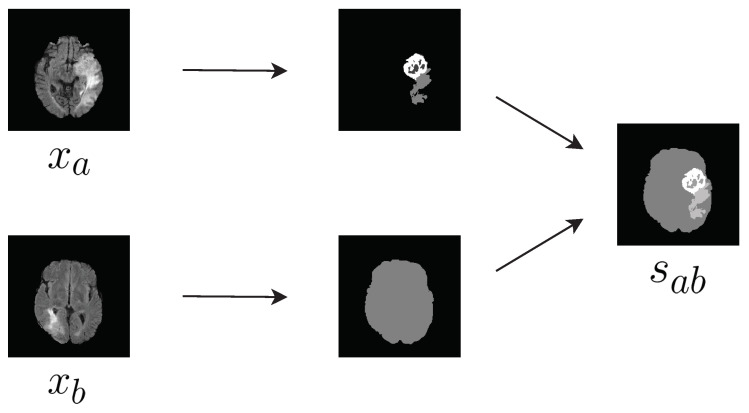
The contour and shape of brain tumor areas are complicated, due to the underlying pathology and anatomy. Thus, it would be difficult to synthesize a reasonable tumor image directly. Instead, we developed a way to design the tumor image from existing tumor shapes. Let x denote a brain image from a given imaging modality (i.e., FLAIR, T1, T2ce, and T2), and and denote a brain image and the corresponding semantic label for patient A, respectively. We can create a new virtual semantic label image by combining the tumor area from and the brain background from (Figure 3). In other words, our virtual semantic label image tries to mimic a case in which patient B has the same lesion as patient A.
Figure 3.
Composition of the label .
3.2. Architecture
Similar to most GAN implementations, TumorGAN includes two parts: a generator G and a pair of discriminators, and . We follow our previous work [37] to build the generator G and inherit the discriminator structure from Pix2pix [15] to form the global discriminator . As shown in Figure 2, the generator G is fed a segmentation label image concatenated with a brain image from a given modality (i.e., flair, t1, t1ce, or t2) to generate a synthetic image y. The segmentation label provide the outline information include brain background and tumor, and the brain image provide concrete tumor information.We can obtain a brain image from a slice of a patient A, and we synthesize a segmentation label image following the method described in Figure 3. Another brain image from patient B is used for adversarial and local perceptual loss calculation. To make the y fit the semantic label image , we designed a regional loss, as well as regional perceptual loss . Inspired by the local–global discriminator framework [38], we included an additional discriminator, , to improve the generation performance in terms of fine details. The input size of is a image cropped from y or . The flow chart of our method is shown in Figure 4.
Figure 4.
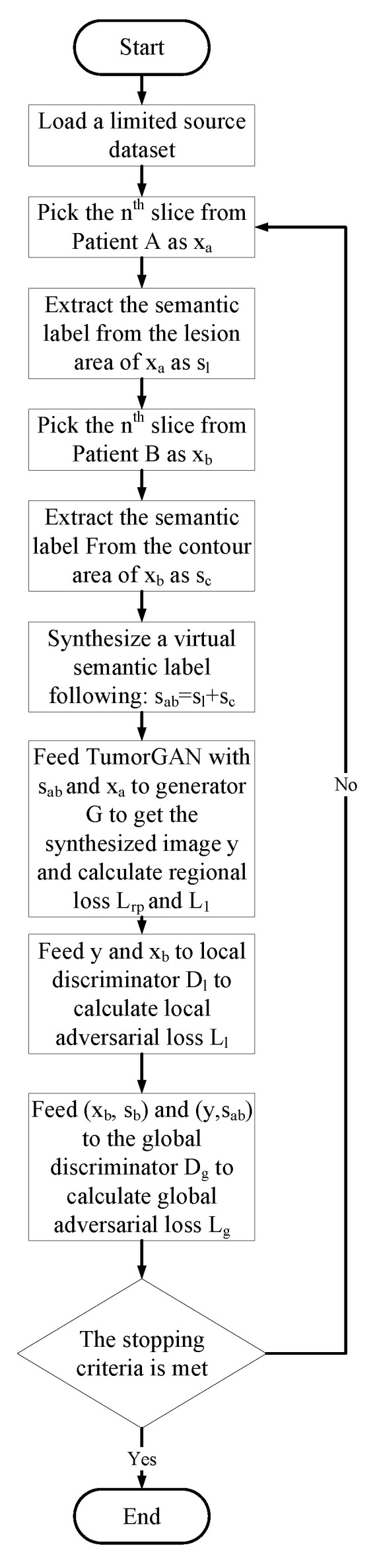
Flow chart of the proposed method.
3.3. Formulation
To generate a realistic brain image for each modality, we need to handle the generation task of brain tissue area and the tumor area simultaneously. To achieve this goal, we developed a regional perceptual loss from the perceptual loss [39], as follows:
| (1) |
| (2) |
| (3) |
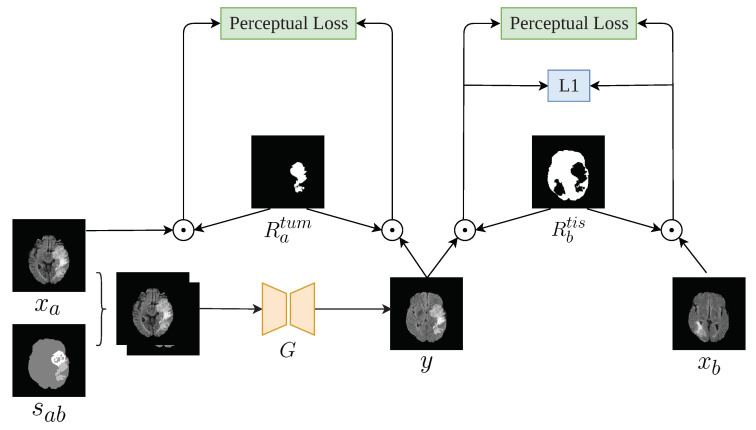
where and are the tissue region and the tumor region of the output image y, respectively; and are the feature maps obtained by the fourth convolutional layer before the third max-pooling layer in VGG-19 [40]. The parameters of are set as . The implementation details of the point multiplication operation can be found in Figure 5.
Figure 5.
Computation details of the perceptual loss and loss. is a mask for the x tumor; is a mask of the tissue, except for the and tumor sites.
As the healthy tissue area usually occupies a large area, as compared to the tumor lesion, we adopt a regional loss to improve the tissue texture:
| (4) |
Finally, an extra local discriminator was introduced, which co-operates with the vanilla discriminator (Figure 6) and encourages the generator to generate more realistic images. We designed our local–global adversarial loss function based on the least squares adversarial loss [41]:
| (5) |
| (6) |
| (7) |
where and are the adversarial losses of the global discriminator and the local discriminator, respectively; is the concatenation of the and the corresponded semantic label ; and is the randomly cropped region of y.
Figure 6.
Details of the generator and discriminator structures. is the global discriminator and is the local discriminator. The input of is a randomly cropped section of y.
The total loss function can be given as:
| (8) |
where , , and denote the objective weights; in our experiment, we used a ratio of 1:1000:1000 for these.
4. Experiment
To prove the efficiency of the proposed TumorGAN, we first applied TumorGAN on the BraTS 2017 data set to increase the sample number. Then, we validated the usefulness of the synthetic data by using them as part of the training data for several segmentation models, including the cascade model [42], U-Net [43] and deeplabv3 [44].
4.1. Implementation Details
The generator structure was derived from CycleGAN, which consists of nine residual blocks in the bottleneck. The local–global discriminators are similar, but with one less convolutional layer due to the reduced size of the input image. The detail of the structure are as follows:
Generator:;
Global discriminator:; and
Local discriminator:,
where CIRmFn in the generator means the Convolutional–InstanceNorm–ReLU layer with m filters, Res256 means a residual block with 256 filters, and the last layer of the generator uses Sigmoid as the activation function. CILRjFk in the discriminator means the Convolutional–InstanceNorm–LeakyReLU layer with j filters of .
4.2. Data Set Pre-Processing and Data Augmentation
The Multi-modal Brain Tumor Segmentation (BraTS) Challenge provides an annotated 3D MRI data set. In this work, the experiment was conducted on the BraTs 2017 data set, consisting of four MRI imaging modalities with different characteristics, including FLAIR, T1, T1CE, and T2. The BraTS 2017 data set provides 285 labeled patients, from which we used 226 patients (HGG(high-grade gliomas): 166, LGG(low-grade gliomas): 60) as a training set to train TumorGAN, as well as the segmentation network. The remaining cases (HGG: 44, LGG: 15) were used as the testing set, in order to evaluate the algorithm’s performance. We normalized all input images according to the following equation:
| (9) |
where and are the minimum and maximum pixel values of the input image, respectively.
The image size for a patient was 240 × 240 × 155. As the tumors were always located in the brain tissue, we took slices from 30 to 110 from each patient and resized them to 256 × 256. We used the pre-trained TumorGAN to generate synthetic brain images as well as semantic labels. The augmentation details are shown in Table 1. Theoretically, we can generate virtual samples with 226 real samples in the training data set. Considering the computing time issue, we generated 226 virtual samples—the same amount of samples—for the training data set, in order to support the semantic segmentation task.
Table 1.
Data split.
| Data Sets | All | HGG | LGG |
|---|---|---|---|
| Total | 285 | 210 | 75 |
| Train | 226 | 166 | 60 |
| Augmentation | 226 | 166 | 60 |
| Test | 59 | 44 | 15 |
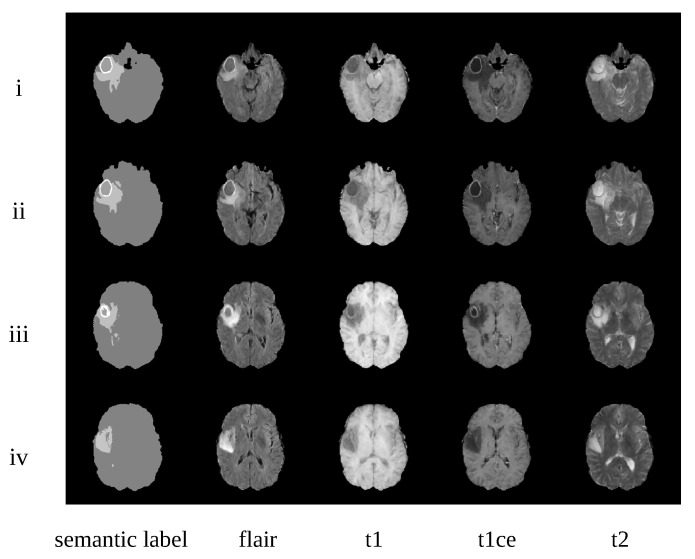
Some synthetic examples are shown in Figure 7. We can observe that the synthesized images are well-matched to the semantic labels. Furthermore, different modality brain images have different features. For example, the T1-CE (t1-weighted contrast-enhanced) modality can ensure that the tumor core is brighter, in order to distinguish the tumor core and edema regions. This can also been seen in our augmentation data.
Figure 7.
Examples of synthetic images from one patient. i–iv represent slices 52, 58, 66, and 73 from this patient, respectively. Images from the left to the right are the corresponding semantic labels and four synthetic modality images (i.e., flair, t1, t1ce, and t2).
4.3. Qualitative Evaluation
Ablation Study
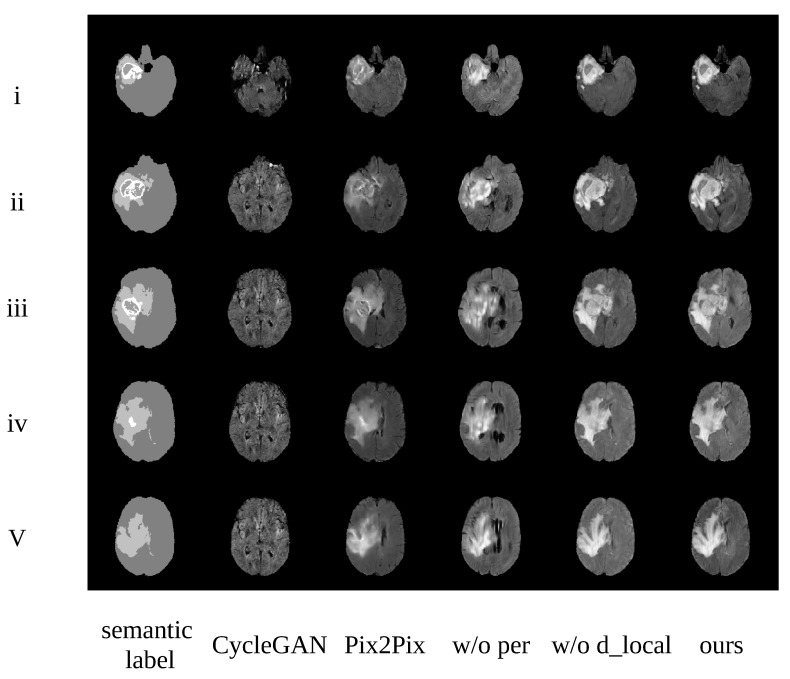
We designed an ablation study based on the flair modality, in order to demonstrate the efficiency of each component in our proposed TumorGAN, as shown in Figure 8. The i–v lines denoted the samples from slices 50, 60, 70, 80, and 90, respectively. The first column shows the semantic label; the last column includes the results obtained from TumorGAN; and the forth and the fifth columns contain synthetic images from TumorGAN without regional perceptual loss and local discriminator, respectively. It can be observed that, when we removed the regional perceptual loss, the synthetic image was lacking in detail and became blurred. Many artifacts appeared when we removed the local discriminator (see, e.g., Figure 8 w/o d_local).
Figure 8.
Examples generated from different methods. i–v represent slices 50, 60, 70, 80, and 90 from this patient, respectively. Images from the left to the right are the corresponding semantic label, CycleGAN, Pix2Pix, TumorGAN without regional perceptual loss, TumorGAN without the local discriminator, and TumorGAN.
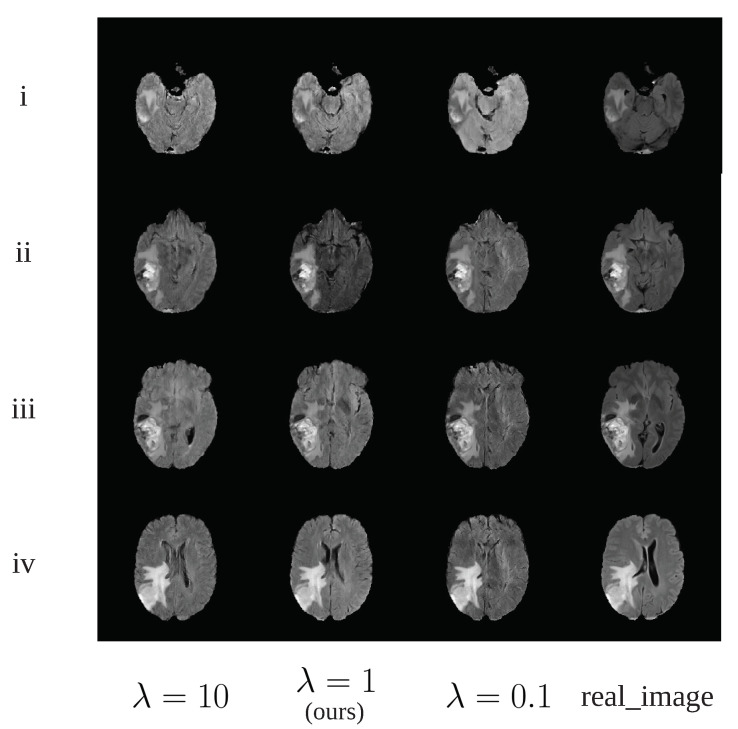
We used a traditional grid search method [45] to obtain the optimal values for , and in Equation (8). Take as an example, we searched a possible set for and finally choose for the rest of this study. Although we found that all choices of produce similar images, it was noticed provide better contrast in the generated tumor as shown in Figure 9ii. The procedure for selecting and is similar to that of . Since we treat , and as hyper parameters, identifying the optimal value of these hyper parameters requires re-train the network on the same training dataset many times. Hence, the computational burden is high and we cannot explore a large candidate set for these hyper parameters. It would be possible to obtain a better value for these parameters given enough computational resources.
Figure 9.
The synthetic image examples using different value.
Comparison with Baseline
TumorGAN obtained the best qualitative results, when compared to the baseline CycleGAN (see Figure 8). We used the same data (slices 30–110) as for TumorGAN in the 226 patients as the training data set to train CycleGAN. We created a semantic label image by combining the tumor area and the brain background from the same brain image; in this way, we could acquire the paired data to train the Pix2pix model. We also used the same data in CycleGAN.
To measure the quality of the generated images, we used the Fréchet inception distance (FID) [46] to evaluate the similarities between the real images and the generated samples. TumorGAN obtained FIDs that were favorable, as compared to the baselines (see Table 2). This shows that the proposed method can generate images that closely match the real data distribution. We can see that, compared with the score of CycleGAN (baseline), the FID score of our method was reduced by 50%.
Table 2.
FID (lower is better). TumorGAN generates diverse and real images that compare favorably to those of the state-of-the-art baselines.
| CycleGAN (Baseline) | Pix2Pix | w/o per | w/o d_lcoal | TumorGAN | |
|---|---|---|---|---|---|
| FID | 154.86 (0%) | 126.42 (18.36%) | 87.75 (43.34%) | 145.67 (5.93%) | 77.43 (50%) |
4.4. Tumor Segmentation Using Synthetic Data
Cascaded Net Wang et al. proposed a cascaded network and acquired the top rank in the BraTS 2018 challenge [42]. Cascaded Net segments the tumor following three stages: In the first stage, the Cascade Net locates and picks out the whole brain tumor area. In the second stage, it removes the useless surrounding tissue area and crops a square tumor region as the input to the next network, in order to segment the tumor core. In the third stage, the third network divides the tumor core into an enhanced region and a non-enhanced region. Finally, multi-view fusion is employed to combine the results from each stage. In our experiment, we only used the axial data for data synthesis. Due to the limited GPU memory, we changed the batch size to 3.
U-Net U-Net is a popular deep neural network for medical image segmentation [43]. U-Net has an encoder–decoder structure with several skip connections. Following the structure mentioned in [43], we used four times down-sampling and four times up-sampling to build the U-net in our experiment.
DeeplabV3 Deeplab and its variants have achieved a great of success in many common semantic segmentation tasks [47,48]. In this work, we used DeeplabV3 as another benchmark for the tumor image segmentation task. DeeplabV3 includes multiple dilated convolutional layers, which expand the field-of-view, and apply a pre-trained network. Furthermore, it augments the Atrous Spatial Pyramid Pooling module proposed previously. In this work, we used resnet50 as the backbone network to implement DeeplabV3.
All the three segmentation models were used for evaluation. The data split for each model is given in Table 1:
The dice score [49] was employed to evaluate the tumor segmentation performance on the testing set. The score is defined as follows:
| (10) |
where is the output and is the segmentation ground truth. The summation is voxel-wise and is a very small constant to prevent divsion by zero.
4.4.1. Training on Multi-Modal Dataset
The performance of the segmentation networks on multi-modal BraTS data included the original data and the augmented data, as shown in Table 3:
Table 3.
Dice score comparison of different segmentation networks trained on “Train” and “Train + TumorGAN Augmentation”. All networks were trained without usual data augmentation techniques (e.g., flip, crop, rotation, and so on). The best mean dice scores are highlighted bold.
| Networks | Whole | Core | en | Mean | |
|---|---|---|---|---|---|
| Cascaded Net | Without augmentation | 0.848 | 0.748 | 0.643 | 0.746 |
| With TumorGAN augmentation (ours) | 0.853 | 0.791 | 0.692 | 0.778 | |
| U-Net | Without augmentation | 0.783 | 0.672 | 0.609 | 0.687 |
| With TumorGAN augmentation (ours) | 0.806 | 0.704 | 0.611 | 0.706 | |
| Deeplab-v3 | Without augmentation | 0.820 | 0.700 | 0.571 | 0.697 |
| With TumorGAN augmentation (ours) | 0.831 | 0.762 | 0.584 | 0.725 | |
Table 3 shows that the dice score of all the three models with data augmentation outperformed the cases without augmentation. The average dice scores were improved by 2– for each of the segmentation models. All three models had a large improvement in tumor core segmentation. The performance of the whole-tumor and enhanced region segmentation tasks were also improved.
4.4.2. Training on Single Modality Data of U-Net
Table 4 shows the efficiency of TumorGAN-based augmentation in single modality data-based segmentation tasks. Without the support from other modalities, the scores provided in Table 4 were lower than that reported in Table 3. Based on the results of Table 3 and Table 4, we can conclude that the TumorGAN-based augmentation method can enhance the dice scores for most segmentation tasks, in the case of single modality inputs.
Table 4.
Dice score comparison of U-Net trained on single modality “Train”, “Train+Augmentation” and “Train + Pix2pix augmentation”. The best mean dice scores are highlighted bold
| Modality | Whole | Core | en | Mean | |
|---|---|---|---|---|---|
| flair | without augmentation | 0.754 | 0.513 | 0.286 | 0.518 |
| with pix2pix augmentation | 0.745 | 0.527 | 0.214 | 0.495 | |
| with TumorGAN augmentation | 0.765 | 0.522 | 0.289 | 0.525 | |
| t2 | without augmentation | 0.743 | 0.577 | 0.335 | 0.552 |
| with pix2pix augmentation | 0.729 | 0.593 | 0.220 | 0.514 | |
| with TumorGAN augmentation | 0.750 | 0.572 | 0.321 | 0.548 | |
| t1 | without augmentation | 0.628 | 0.422 | 0.199 | 0.416 |
| with pix2pix augmentation | 0.635 | 0.489 | 0.106 | 0.410 | |
| with TumorGAN augmentation | 0.628 | 0.467 | 0.235 | 0.443 | |
| t1ce | without augmentation | 0.597 | 0.534 | 0.570 | 0.567 |
| with pix2pix augmentation | 0.659 | 0.673 | 0.545 | 0.626 | |
| with TumorGAN augmentation | 0.671 | 0.681 | 0.589 | 0.647 | |
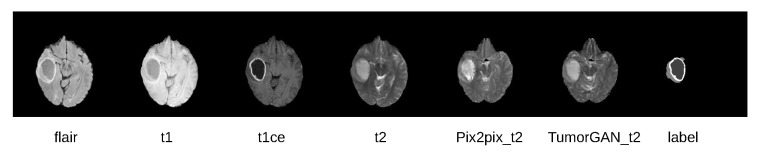
We evaluated the performance of various data augmentation methods on the brain tumor segmentation tasks with U-Net (See Table 4). The comparison analysis was done between our proposed TumorGAN and Pix2pix on four modalities (flair, t2, t1, and t1ce). In practical application, synthetic images are used to improve the segmentation accuracy. Obviously, most of the images generated by CycleGAN shown in Figure 8 do not have a clear tumor area. Thus, it would be meaningless to consider images generated by CycleGAN for comparison. It can be observed that augmenting dataset with both TumorGAN and Pix2pix generated images can enhance the segmentation performance in modalities including flair, t1 and t1ce. The performance obtained with TumorGAN was higher than that of Pix2pix, indicating that the images generated with the proposed TumorGAN can aid the segmentation task by providing more realistic tumor images as compared to Pix2pix. We also found that both TumorGAN and Pix2pix cannot improve the segmentation performance in t2. To investigate the cause regarding the performance degradation on t2, generated t2 images from both TumorGAN and Pix2pix and their ground truth segmentation label were shown in Figure 10. It can be observed that tumor regions including the “whole”, “core” and “en” can be clearly seen in flair, t1 and t1ce, and the boundary between “core” and “en” is also clear in these three modalities. However, the region of “en” can not be clearly visualized on the t2 images. Similarly, the synthesis t2 images from Pix2pix and TumorGAN also had a vague boundary between the “core” and “en”. Hence, the blurred boundary found on t2 images may cause performance to degrade when we use an image-to-image based augmentation method, as reflected on the poor segmentation performance on “en”.
Figure 10.
From left to right is the flair, t1, t1ce and t2 modality brain image from the real data. The pix2pix_t2 is the t2 modality image synthesized by the Pix2pix according to the same tumor. The TumorGAN_t2 is generated by TumorGAN. The last column is the segmentation label.
5. Conclusions and Future Work
In this paper, we proposed a novel GAN-based image-to-image framework called TumorGAN for brain tumor image augmentation. By combining the brain tissue area and the tumor area from two different patients, the proposed method can create virtual data pairs from the data of n patients. To further improve the generation performance, we introduced a regional perceptual loss and a regional loss with a local discriminator. Compared with other GAN-based image-to-image translation frameworks, our method can create high-quality image pairs from limited paired data. As proved by the experimental results, the synthesis image pairs from TumorGAN can practically help to improve tumor segmentation in both multi-modal and single-modality data sets.
In our work, we note that reasonable virtual semantic labels are the key to generating realistic synthesized samples and, so, we tried to obtain a reasonable tumor region from the existing samples, providing more possible combinations between tumors and healthy tissue. However, our method cannot provide an unseen tumor label, which restricts the diversity of the virtual semantic labels. Fortunately, multiple GAN-based studies have indicated that the generated shape or style can be controlled by latent codes [13,50], which may help us to control the generated tumor shape and increase the diversity of the virtual semantic labels. We leave this for a future work.
Author Contributions
Conceptualization, Z.Y. and H.Z.; Methodology, Q.L.; Software, Q.L.; Validation, Q.L. and Z.Y.; Formal Analysis, Q.L.; Investigation, Y.W.; Resources, Q.L.; Data Curation, Q.L. and Z.Y.; Writing—Original Draft Preparation, Q.L.; Writing—Review & Editing, Z.Y., Y.W. and H.Z.; Visualization, Q.L.; Supervision, Z.Y.; Project Administration, Z.Y. and Y.W.; Q.L. and Z.Y. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China under Grant Number 61701463; the Key Technology Research and Development Program of Shandong (Public welfare) under Grant Number 2019GHY112041; and Natural Science Basic Research Plan in Shaanxi Province of China (2019JQ-138).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Işın A., Direkoğlu C., Şah M. Review of MRI-based brain tumor image segmentation using deep learning methods. Procedia Comput. Sci. 2016;102:317–324. doi: 10.1016/j.procs.2016.09.407. [DOI] [Google Scholar]
- 2.Cordier N., Delingette H., Ayache N. A patch-based approach for the segmentation of pathologies: Application to glioma labelling. IEEE Trans. Med. Imaging. 2015;35:1066–1076. doi: 10.1109/TMI.2015.2508150. [DOI] [PubMed] [Google Scholar]
- 3.Menze B.H., Van Leemput K., Lashkari D., Riklin-Raviv T., Geremia E., Alberts E., Gruber P., Wegener S., Weber M.A., Székely G., et al. A generative probabilistic model and discriminative extensions for brain lesion segmentation—with application to tumor and stroke. IEEE Trans. Med Imaging. 2015;35:933–946. doi: 10.1109/TMI.2015.2502596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou T., Ruan S., Canu S. A review: Deep learning for medical image segmentation using multi-modality fusion. Array. 2019;3:100004. doi: 10.1016/j.array.2019.100004. [DOI] [Google Scholar]
- 5.Zhou C., Ding C., Wang X., Lu Z., Tao D. One-pass multi-task networks with cross-task guided attention for brain tumor segmentation. IEEE Trans. Image Process. 2020;29:4516–4529. doi: 10.1109/TIP.2020.2973510. [DOI] [PubMed] [Google Scholar]
- 6.Tseng K.L., Lin Y.L., Hsu W., Huang C.Y. Joint sequence learning and cross-modality convolution for 3d biomedical segmentation; Proceedings of the IEEE conference on Computer Vision and Pattern Recognition; Honolulu, HI, USA. 22–25 July 2017; pp. 6393–6400. [Google Scholar]
- 7.Havaei M., Davy A., Warde-Farley D., Biard A., Courville A., Bengio Y., Pal C., Jodoin P.M., Larochelle H. Brain tumor segmentation with deep neural networks. Med. Image Anal. 2017;35:18–31. doi: 10.1016/j.media.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Pereira S., Pinto A., Alves V., Silva C.A. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans. Med. Imaging. 2016;35:1240–1251. doi: 10.1109/TMI.2016.2538465. [DOI] [PubMed] [Google Scholar]
- 9.Fu M., Wu W., Hong X., Liu Q., Jiang J., Ou Y., Zhao Y., Gong X. Hierarchical combinatorial deep learning architecture for pancreas segmentation of medical computed tomography cancer images. BMC Syst. Biol. 2018;12:56. doi: 10.1186/s12918-018-0572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeiffer M., Funke I., Robu M.R., Bodenstedt S., Strenger L., Engelhardt S., Roß T., Clarkson M.J., Gurusamy K., Davidson B.R., et al. Generating large labeled data sets for laparoscopic image processing tasks using unpaired image-to-image translation; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Shenzhen, China. 13–17 October 2019. [Google Scholar]
- 11.Goodfellow I., Pouget-Abadie J., Mirza M., Xu B., Warde-Farley D., Ozair S., Courville A., Bengio Y. Advances in Neural Information Processing Systems. NIPS; Montreal, QC, Canada: 2014. Generative adversarial nets. [Google Scholar]
- 12.Mirza M., Osindero S. Conditional generative adversarial nets. arXiv. 20141411.1784 [Google Scholar]
- 13.Chen X., Duan Y., Houthooft R., Schulman J., Sutskever I., Abbeel P. Infogan: Interpretable representation learning by information maximizing generative adversarial nets; Proceedings of the 30th International Conference on Neural Information Processing Systems; Barcelona, Spain. 5–10 December 2016; pp. 2172–2180. [Google Scholar]
- 14.Odena A., Olah C., Shlens J. Conditional image synthesis with auxiliary classifier gans; Proceedings of the 34th International Conference on Machine Learning; Sydney, Australia. 6–11 August 2017; pp. 2642–2651. [Google Scholar]
- 15.Isola P., Zhu J.Y., Zhou T., Efros A.A. Image-to-image translation with conditional adversarial networks; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; Honolulu, HI, USA. 21–26 July 2017. [Google Scholar]
- 16.Zhu J.Y., Park T., Isola P., Efros A.A. Unpaired image-to-image translation using cycle-consistent adversarial networks; Proceedings of the IEEE International Conference on Computer Vision; Venice, Italy. 22–29 October 2017; pp. 2223–2232. [Google Scholar]
- 17.Sharma M.M. Brain tumor segmentation techniques: A survey. Brain. 2016;4:220–223. [Google Scholar]
- 18.Rajendran A., Dhanasekaran R. Fuzzy clustering and deformable model for tumor segmentation on MRI brain image: A combined approach. Procedia Eng. 2012;30:327–333. doi: 10.1016/j.proeng.2012.01.868. [DOI] [Google Scholar]
- 19.Zabir I., Paul S., Rayhan M.A., Sarker T., Fattah S.A., Shahnaz C. Automatic brain tumor detection and segmentation from multi-modal MRI images based on region growing and level set evolution; Proceedings of the 2015 IEEE International WIE Conference on Electrical and Computer Engineering (WIECON-ECE); Dhaka, Bangladesh. 19–20 December 2015; pp. 503–506. [Google Scholar]
- 20.Yousefi S., Azmi R., Zahedi M. Brain tissue segmentation in MR images based on a hybrid of MRF and social algorithms. Med. Image Anal. 2012;16:840–848. doi: 10.1016/j.media.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Benson C., Deepa V., Lajish V., Rajamani K. Brain tumor segmentation from MR brain images using improved fuzzy c-means clustering and watershed algorithm; Proceedings of the 2016 International Conference on Advances in Computing, Communications and Informatics (ICACCI); Jaipur, India. 21–24 September 2016; pp. 187–192. [Google Scholar]
- 22.Dvorak P., Menze B. Structured prediction with convolutional neural networks for multimodal brain tumor segmentation; In Proceeding of the Multimodal Brain Tumor Image Segmentation Challenge; Munich, Germany. 5–9 October 2015; pp. 13–24. [Google Scholar]
- 23.Colmeiro R.R., Verrastro C., Grosges T. International MICCAI Brainlesion Workshop. Springer; Cham, Switzerland: 2017. Multimodal brain tumor segmentation using 3D convolutional networks; pp. 226–240. [Google Scholar]
- 24.Menze B.H., Jakab A., Bauer S., Kalpathy-Cramer J., Farahani K., Kirby J., Burren Y., Porz N., Slotboom J., Wiest R., et al. The multimodal brain tumor image segmentation benchmark (BRATS) IEEE Trans. Med. Imaging. 2014;34:1993–2024. doi: 10.1109/TMI.2014.2377694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelhamer E., Long J., Darrell T. Fully convolutional networks for semantic segmentation. IEEE Ann. Hist. Comput. 2017;4:640–651. doi: 10.1109/TPAMI.2016.2572683. [DOI] [PubMed] [Google Scholar]
- 26.Ronneberger O., Fischer P., Brox T. International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer; Cham, UK: 2015. U-net: Convolutional networks for biomedical image segmentation. [Google Scholar]
- 27.Cunniff C.M., Byrne J.L., Hudgins L.M., Moeschler J.B., Olney A.H., Pauli R.M., Seaver L.H., Stevens C.A., Figone C. Informed consent for medical photographs. Genet. Med. Off. J. Am. Coll. Med. Genet. 2000;2:353–355. [Google Scholar]
- 28.Simard P.Y., Steinkraus D., Platt J.C. Best practices for convolutional neural networks applied to visual document analysis. Icdar. 2003;2:958. [Google Scholar]
- 29.Yi X., Walia E., Babyn P. Generative adversarial network in medical imaging: A review. Med. Image Anal. 2019;58:101552. doi: 10.1016/j.media.2019.101552. [DOI] [PubMed] [Google Scholar]
- 30.Radford A., Metz L., Chintala S. Unsupervised representation learning with deep convolutional generative adversarial networks. arXiv. 20151511.06434 [Google Scholar]
- 31.Frid-Adar M., Diamant I., Klang E., Amitai M., Goldberger J., Greenspan H. GAN-based synthetic medical image augmentation for increased CNN performance in liver lesion classification. Neurocomputing. 2018;321:321–331. doi: 10.1016/j.neucom.2018.09.013. [DOI] [Google Scholar]
- 32.Wolterink J.M., Dinkla A.M., Savenije M.H., Seevinck P.R., van den Berg C.A., Išgum I. Deep MR to CT synthesis using unpaired data; Proceedings of the International Workshop on Simulation and Synthesis in Medical Imaging; Quebec City, QC, Canada. 10–14 September 2017; pp. 14–23. [Google Scholar]
- 33.Hiasa Y., Otake Y., Takao M., Matsuoka T., Takashima K., Carass A., Prince J.L., Sugano N., Sato Y. Cross-modality image synthesis from unpaired data using CycleGAN; Proceedings of the 32nd International Conference on Neural Information Processing Systems; Granada, Spain. 16–20 September 2018; pp. 31–41. [Google Scholar]
- 34.Zhang Z., Yang L., Zheng Y. Translating and segmenting multimodal medical volumes with cycle-and shape-consistency generative adversarial network; Proceedings of the IEEE conference on computer vision and pattern Recognition; Salt Lake City, UT, USA. 18–22 June 2018; pp. 9242–9251. [Google Scholar]
- 35.Kang E., Koo H.J., Yang D.H., Seo J.B., Ye J.C. Cycle-consistent adversarial denoising network for multiphase coronary CT angiography. Med. Phys. 2019;46:550–562. doi: 10.1002/mp.13284. [DOI] [PubMed] [Google Scholar]
- 36.Yi X., Babyn P. Sharpness-aware low-dose CT denoising using conditional generative adversarial network. J. Digit. Imaging. 2018;31:655–669. doi: 10.1007/s10278-018-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Z., Wang C., Yu Z., Zheng H., Zheng B. Instance map based image synthesis with a denoising generative adversarial network. IEEE Access. 2018;6:33654–33665. doi: 10.1109/ACCESS.2018.2849108. [DOI] [Google Scholar]
- 38.Zheng Z., Wang C., Yu Z., Wang N., Zheng H., Zheng B. Unpaired photo-to-caricature translation on faces in the wild. Neurocomputing. 2019;355:71–81. doi: 10.1016/j.neucom.2019.04.032. [DOI] [Google Scholar]
- 39.Ledig C., Theis L., Huszár F., Caballero J., Cunningham A., Acosta A., Aitken A., Tejani A., Totz J., Wang Z., et al. Photo-realistic single image super-resolution using a generative adversarial network; Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition; Honolulu, HI, USA. 21–26 July 2017. [Google Scholar]
- 40.Simonyan K., Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv. 20141409.1556 [Google Scholar]
- 41.Mao X., Li Q., Xie H., Lau R.Y., Wang Z., Paul Smolley S. Least squares generative adversarial networks; Proceedings of the IEEE International Conference on Computer Vision 2017; Venice, Italy. 22–29 October 2017. [Google Scholar]
- 42.Wang G., Li W., Ourselin S., Vercauteren T. Automatic brain tumor segmentation using cascaded anisotropic convolutional neural networks; Proceedings of the International MICCAI Brainlesion Workshop; Quebec City, QC, Canada. 10–14 September 2017. [Google Scholar]
- 43.Brügger R., Baumgartner C.F., Konukoglu E. A Partially Reversible U-Net for Memory-Efficient Volumetric Image Segmentation; Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Shenzhen, China. 13–17 October 2019. [Google Scholar]
- 44.Chen L.C., Papandreou G., Schroff F., Adam H. Rethinking atrous convolution for semantic image segmentation. arXiv. 20171706.05587 [Google Scholar]
- 45.Bergstra J., Bengio Y. Random search for hyper-parameter optimization. J. Mach. Learn. Res. 2012;13:281–305. [Google Scholar]
- 46.Heusel M., Ramsauer H., Unterthiner T., Nessler B., Hochreiter S. GANs trained by a two time-scale update rule converge to a local Nash equilibrium; Proceedings of the 31st International Conference on Neural Information Processing Systems; Long Beach, CA, USA. 4–9 December 2017; pp. 6626–6637. [Google Scholar]
- 47.Chen L.C., Papandreou G., Kokkinos I., Murphy K., Yuille A.L. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected crfs. IEEE Trans. Pattern Anal. Mach. Intell. 2017;40:834–848. doi: 10.1109/TPAMI.2017.2699184. [DOI] [PubMed] [Google Scholar]
- 48.Chen L.C., Zhu Y., Papandreou G., Schroff F., Adam H. Encoder-decoder with atrous separable convolution for semantic image segmentation; Proceedings of the European Conference on Computer Vision; Munich, Germany. 8–14 September 2018. [Google Scholar]
- 49.Fidon L., Li W., Garcia-Peraza-Herrera L.C., Ekanayake J., Kitchen N., Ourselin S., Vercauteren T. Generalised wasserstein dice score for imbalanced multi-class segmentation using holistic convolutional networks; Proceedings of the International MICCAI Brainlesion Workshop; Quebec City, QC, Canada. 10–14 September 2017; pp. 64–76. [Google Scholar]
- 50.Karras T., Aila T., Laine S., Lehtinen J. Progressive growing of gans for improved quality, stability, and variation. arXiv. 20171710.10196 [Google Scholar]