Abstract
Objectives
Endoscopic pituitary surgery usually requires a collaboration between neurosurgeons and ENT surgeons to achieve optimal outcomes. However, neurosurgeons occasionally perform these procedures alone without an ENT surgeon. In this study, postoperative sinonasal quality of life and olfactory function were compared in patients who underwent endoscopic pituitary surgery performed by a single neurosurgeon or by a collaborative team of a neurosurgeon and an ENT surgeon.
Methods
A retrospective review of prospectively collected data was performed. Patients who underwent endoscopic pituitary surgery for pituitary adenoma from January 2015 to April 2018 were included. The study patients were divided into two groups; patients in group 1 underwent surgery performed by a single neurosurgeon, while patients in group 2 received surgery performed by a collaborative team of surgeons. Olfaction was assessed using a subjective Likert scale, the Cross-Cultural Smell Identification Test (CC-SIT), and the butanol threshold test (BTT). In addition, patients answered the Sino-nasal Outcome Test (SNOT-22) questionnaire regarding sinonasal quality of life before and 3 months after surgery.
Results
This study included 152 patients (46 patients in group 1 and 106 patients in group 2). Significant differences were not observed between the two groups regarding age, sex, tumor size, or operation time. Although subjective olfaction was not significantly different before and after surgery, group 2 showed significantly better objective olfactory function based on the CC-SIT (8.44±3.00 vs. 9.84±1.40; P=0.012) and BTT (4.67±0.84 vs. 5.02±0.33; P=0.022) scores at 3 months after surgery. The SNOT-22 scores were not statistically significantly different between the two groups (P>0.05).
Conclusion
In the present study, better olfactory outcomes were observed in patients who underwent surgery performed by a collaborative team of a neurosurgeon and an ENT surgeon. This result shows the need for collaboration between neurosurgeons and ENT surgeons in endoscopic pituitary surgery.
Keywords: Pituitary Neoplasms, Olfactory Perception, Sinonasal Outcome Test
INTRODUCTION
Endoscopic endonasal surgery is routinely performed to treat chronic sinusitis, and is also currently performed to treat sinonasal inflammatory disorders and extended malignant lesions [1,2]. Recently, neurosurgeons have applied endoscopic techniques through the transsphenoidal approach (TSA) to the sellar region because it provides improved visualization, a wider view inside the sella, and positive surgical outcomes [1]. However, several studies have reported that the endoscopic approach may disrupt normal anatomical structures to a greater extent than traditional approaches [3,4], indicating that collaboration with a otorhinology surgeon trained in endoscopic sinus surgery may be helpful for a successful approach and to avoid eventual complications. Therefore, endoscopic pituitary surgery requires a collaboration between neurosurgeons and ENT surgeons for optimal outcomes [5], particularly in patients with nasal anatomical variants and extensive skull base lesions [6].
However, patients with pituitary tumors usually visit the neurosurgery department; thus, in some centers, only neurosurgeons perform endoscopic pituitary surgery, including nasal approach procedures. With this in mind, we hypothesized that the surgical outcomes would be different when a neurosurgeon collaborated with an ENT surgeon. In several studies, sinonasal outcomes and quality of life have been compared between microscopic and endoscopic transsphenoidal surgical procedures for pituitary lesions [7,8], and no significant differences were found. However, the surgical outcomes in endoscopic pituitary surgery performed by neurosurgeons with a collaborative team have been compared in only a few studies [9,10].
In the present study, postoperative sinonasal quality of life and olfactory function were compared in patients who underwent endoscopic pituitary surgery by a neurosurgeon or by a collaborative team of a neurosurgeon and an ENT surgeon.
MATERIALS AND METHODS
A retrospective review of prospectively collected data was performed. This study protocol was approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC 201905-103-001) and the informed consents were waived.
Patients who underwent an endoscopic pituitary surgery for pituitary adenoma from January 2015 to April 2018 were included. All subjects underwent nasal endoscopy and computed tomography of the paranasal sinus before surgery, and patients who had abnormalities in their sinonasal spaces were excluded. Patients with a history of previous sinonasal or pituitary surgery were also excluded. We excluded patients used nasoseptal flap because the using the nasoseptal flap could cause more sinonasal morbidity that can cause bias. One hundred fifty-two patients were finally enrolled in this study (Fig. 1).
Fig. 1.
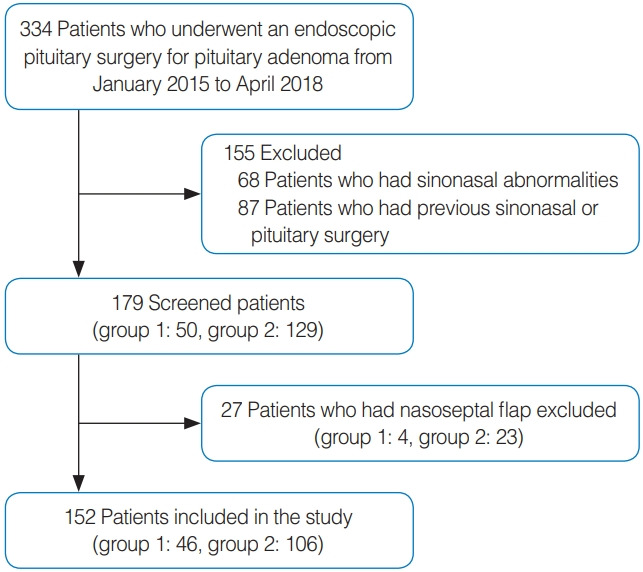
Flowchart of the study population. Group 1, single neurosurgeon group; Group 2, collaborative group.
The study patients were divided into two groups: group 1 (n=46) included patients who underwent surgery performed by a single neurosurgeon. The neurosurgeon was an experienced surgeon who performed 213 TSA surgeries by himself before this study. Group 2 (n=106) included patients who underwent surgery performed by a collaborative team consisting of a neurosurgeon and an ENT surgeon. All fundamental procedures, including postoperative management, were the same for the two groups. The entire procedure was performed with a 4-mm, 0°, 30°, or 45° endoscope. Surgery was performed using the endoscopic modified transseptal approach. The surgical procedure details were as follows. Before surgery, the nasal cavity was decongested with cottonoids soaked in a 1:10,000 epinephrine solution. Infiltration of the nasal septum with a lidocaine-containing epinephrine (1:100,000) solution was performed for vasoconstriction and hydrodissection. A hemitransfixion incision was made in the left nasal septum. Submucoperichondrial and submucoperiosteal dissection was performed toward the level of the rostrum sphenoidale. After posterior chondrotomy, the midportion of the bony septum was carefully removed to avoid damage to the superior 1 cm of the septum. The anterior wall and intersinus septum of the sphenoid sinus were then removed using a micro-Kerrison punch or high-speed drill. The endoscope was then inserted into the right nasal cavity. A small horizontal incision was made in the right nasal septal mucosa from the anterior end of the middle turbinate to 1 cm in front of the sphenoid natural ostium. The endoscope and suction device were introduced into the sphenoid sinus through this incision, and instruments such as a ring curette or dissector, were inserted through the left transseptal window, thus the surgeon could perform the binostril technique. After tumor resection using the binostril technique, the remnant septal bone was inserted between the septal mucosa and the hemitransfixion incision was sutured. Silastic sheets were placed in both nasal cavities for 1–2 weeks, followed by light nasal packing. We have used four hand and binostril surgery in both groups when resect tumors. In collaborative group, ENT surgeons approached to the sellar region by endonasal corridor and hold the scope and discuss with neurosurgeon in tumor resection time. But, in single neurosurgeon group, neurosurgical fellow hold the scope when performing tumor resection.
Olfactory function was subjectively evaluated using a Likert scale (0–100), and objectively with the cross-cultural smell identification test (CC-SIT; 0–12) and butanol threshold test (BTT). Preoperative anosmia patients were excluded, and for identifying olfactory function changes, differences from baseline to 3 months after surgery were calculated. Sinonasal quality of life was evaluated using the Sino-nasal Outcome Test (SNOT-22) questionnaire and subdomain scores analyzed. All tests were conducted before and 3 months after surgery. We tried to apply those metrics for every patients, but some patients missed test or questionnaire. We excluded missed patients and performed statistical processing. Number of processed patients is filled in parenthesis in each tables. After surgery, we examined patients with nasal endoscope in outpatient clinic, and septal perforation was identified as a nasal complication in the postoperative period. The cerebrospinal fluid (CSF) leakage was monitored during the intraoperative and postoperative periods. All statistical analyses were performed with IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA) using Mann-Whitney and chi-square tests. P-values ≤0.05 were considered statistically significant.
RESULTS
Among the 152 patients included in this study, group 1 consisted of 46 patients (26 men and 20 women) and group 2 consisted of 106 patients (51 men and 55 women) (Table 1). The mean age of the patients was 48.52±14.35 years in group 1 and 49.82±13.56 years in group 2. The operation time was 136.61±66.76 minutes in group 1 and 125.13±43.33 minutes in group 2. The mean size of the tumors was 22.09±7.70 mm in group 1 and 22.93±8.07 mm in group 2. CSF leakage was monitored during the intraoperative and postoperative periods. Nine of the 46 patients (19.6%) in group 1 had CSF leakage during the operation. In group 2, 15 of the 106 patients (14%) had CSF leakage during the operation. After surgery, CSF leakage did not occur in any patient in group 1, and in only one patient in group 2. Demographic characteristics and possible factors contributing to olfactory outcomes were not significantly different between the two groups.
Table 1.
Patients’ demographic characteristics
| Variable | NS (n=46) | NS+ENT (n=106) | P-value |
|---|---|---|---|
| Age (yr) | 48.52±14.35 | 49.82±13.56 | 0.595 |
| Sex (male:female) | 26:20 (56.5:43.5) | 51:55 (48:52) | 0.380 |
| Operation time (min) | 136.61±66.76 | 125.13±43.33 | 0.288 |
| Tumor size (mm) | 22.09±7.70 | 22.93±8.07 | 0.555 |
| Intraoperative CSF leakage | 9 (19.6) | 15 (14) | 0.469 |
| Postoperative CSF leakage | 0 | 1 (0.9) | 1.000 |
Values are presented as mean±standard deviation or number (%).
NS, single neurosurgeon; NS+ENT, neurosurgeon+ENT surgeon; CSF, cerebrospinal fluid.
Subjective olfactory function
Olfaction was subjectively assessed using a Likert scale. Subjective olfaction was evaluated before and after surgery at 1, 3, and 6 months. The mean preoperative score was 83.83 (standard deviation [SD], 20.33) for the patients in group 1 and 92.11 (SD, 11.11) for the patients in group 2. The mean postoperative score was 56.3 (SD, 33.93) for the patients in group 1 and 66.89 (SD, 26.08) for the patients in group 2 at 1 month after surgery. At 3 and 6 months after surgery, the mean postoperative scores were 70.42 (SD, 33.06) and 81.72 (SD, 21.74) in group 1, and 82.50 (SD, 28.71) and 89.21 (SD, 13.12) in group 2, respectively. Generally, patients’ scores in group 2 were better than those in group 1, but a statistically significant difference was not observed (Table 2). In addition, differences from baseline were calculated, and group 1 showed greater decreases at all intervals; however, statistically significant differences were not observed.
Table 2.
Subjective olfactory function
| Variable | NS | NS+ENT | P-value |
|---|---|---|---|
| Score | |||
| Pre | 83.83±20.33 (30) | 92.11±11.11 (102) | 0.054 |
| 1 mo | 56.38±33.93 (40) | 66.89±26.08 (84) | 0.088 |
| 3 mo | 70.42±33.06 (36) | 81.72±21.74 (90) | 0.064 |
| 6 mo | 82.50±28.71 (18) | 89.21±13.12 (38) | 0.484 |
| Difference | |||
| ∆1 mo | –35.40±35.06 (25) | –26.24±28.31 (81) | 0.185 |
| ∆3 mo | –19.56±32.29 (23) | –9.88±20.21 (88) | 0.182 |
| ∆6 mo | –10.77±27.30 (13) | –3.38±12.91 (37) | 0.363 |
Values are presented as mean±standard deviation. In the parenthesis, the number of examinees is filled in.
NS, single neurosurgeon; NS+ENT, neurosurgeon+ENT surgeon.
Objective olfactory function
Olfactory function was objectively evaluated using the CC-SIT and BTT. These tests were performed preoperatively and at 3 months after surgery. The preoperative CC-SIT scores of the patients in group 1 and group 2 were 9.12±2.60 and 10.03±1.35, respectively, which was not a statistically significant difference (Table 3). At 3 months after surgery, the CC-SIT scores of the patients in group 1 and group 2 were 8.44±3.00 and 9.84±1.40, respectively. The postoperative CC-SIT scores of the patients in group 2 was significantly higher than the scores of the patients in group 1 (P=0.012). Similarly, the preoperative BTT scores of the patients in both groups were not significantly different (4.91 ±0.66 vs. 5.18±0.23). However, the postoperative scores of the patients in group 2 were significantly higher than the scores of the patients in group 1 (4.67±0.84 vs. 5.02±0.33, P=0.022). When the differences from baseline were calculated, the change in CC-SIT scores from baseline to 3 months was significantly larger in the patients in group 1 (–1.55±2.80 vs. –0.227±1.39, P=0.027). The change in BTT scores was also larger in the patients in group 1, but a statistically significant difference was not seen (P=0.214).
Table 3.
Objective olfactory function
| Variable | NS | NS+ENT | P-value |
|---|---|---|---|
| Score | |||
| CC-SIT_pre | 9.12±2.60 (26) | 10.03±1.35 (104) | 0.93 |
| CC-SIT_3 mo | 8.44±3.00 (34) | 9.84±1.40 (90) | 0.012a) |
| BTT_Pre | 4.91±0.662 (26) | 5.18±0.23 (103) | 0.051 |
| BTT_3 mo | 4.67±0.84 (34) | 5.02±0.33 (89) | 0.022a) |
| Difference | |||
| ∆CC-SIT_3 mo | –1.55±2.80 (20) | –0.227±1.39 (88) | 0.027a) |
| ∆BTT_3 mo | –0.42±0.90 (20) | –0.16±0.28 (86) | 0.214 |
Values are presented as mean±standard deviation. In the parenthesis, the number of examinees is filled in.
NS, single neurosurgeon; NS+ENT, neurosurgeon+ENT surgeon; CC-SIT, Cross-Cultural Smell Identification Test; BTT, butanol threshold test.
P-values with statistical significance.
Sinonasal quality of life
Sinonasal quality of life was evaluated preoperatively and at postoperative 1, 3, and 6 months using the SNOT-22 questionnaire. No statistically significant difference in the total SNOT-22 scores between the two groups following surgery was observed (Table 4). Similarly, no statistically significant between-group difference was found in the SNOT-22 subdomain of rhinologic symptoms. Additionally, we compared the “sense of taste/smell” of the SNOT-22 in both groups. Group 2 showed better scores than group 1, albeit without a statistically significant difference. This result was similar to that of subjective olfactory function assessed using the Likert scale.
Table 4.
SNOT-22 scores
| Variable | NS | NS+ENT | P-value |
|---|---|---|---|
| Total score | |||
| Pre | 16.88±16.16 (26) | 16.08±12.75 (95) | 0.788 |
| 1 mo | 23.97±15.74 (39) | 19.58±12.39 (90) | 0.096 |
| 3 mo | 14.16±11.25 (37) | 18.11±13.23 (92) | 0.088 |
| 6 mo | 9.56±10.56 (25) | 13.79±16.14 (48) | 0.183 |
| Rhinologic symptom | |||
| Pre | 4.18±3.65 (27) | 3.80±3.74 (95) | 0.636 |
| 1 mo | 8.49±4.84 (39) | 7.12±3.89 (89) | 0.093 |
| 3 mo | 4.50±2.50 (34) | 5.34±3.71 (90) | 0.150 |
| 6 mo | 3.58±3.39 (19) | 4.47±4.97 (38) | 0.483 |
| Sense of taste/smell | |||
| Pre | 0.58±1.10 (26) | 0.35±0.81 (96) | 0.253 |
| 1 mo | 1.79±1.59 (39) | 1.35±1.29 (90) | 0.101 |
| 3 mo | 1.18±1.40 (34) | 0.92±1.12 (89) | 0.295 |
| 6 mo | 0.05±1.35 (19) | 0.67±0.98 (39) | 0.221 |
Values are presented as mean±standard deviation. In the parenthesis, the number of examinees is filled in.
SNOT-22, Sino-nasal Outcome Test; NS, single neurosurgeon; NS+ENT, neurosurgeon+ENT surgeon.
Postoperative endoscopic findings
After surgery, patients were examined using a nasal endoscope at the outpatient clinic to identify septal perforation as a sinonasal complication. Four patients in group 1 had septal perforation based on the postoperative endoscopic examination (4/46, 8.7%). In group 2, only one patient had septal perforation (1/106, 0.9%). That patient had severe septal deviation, so he underwent septoplasty concomitantly with endoscopic pituitary surgery. This might have affected the likelihood of septal perforation after surgery. The collaborative group showed a significantly lower septal perforation rate than the group treated by a single neurosurgeon (P=0.030).
DISCUSSION
In the present study, olfactory function and sinonasal outcomes were compared between patients who underwent surgery performed by a single neurosurgeon (group 1) and those who underwent surgery performed by a collaborative team of surgeons (group 2). We hypothesized that the patients in group 2 would have better postoperative olfactory function and sinonasal quality of life than the patients in group 1 because ENT surgeons have more experience with endoscopic endonasal surgery and managing nasal anatomical variations and extensive skull base lesions.
A few studies have compared the outcomes of endoscopic pituitary surgery between a single neurosurgeon and a collaborative team including ENT surgeons. Snyderman et al. [9] argued for the benefits of surgery performed by a multidisciplinary team in the management of sinonasal and ventral skull base malignancies, pointing out that the benefits include cross-fertilization of ideas, surgical innovation, and comprehensive patient care, and they emphasized the need for proper training for building a skull base team. Ismail et al. [10] reported a comparison between collaborative and single-surgeon approaches in endoscopic endonasal surgery on the sphenoid sinus. Due to the anatomical location, the endoscopic endonasal approach to the sphenoid sinus is a valuable procedure for both neurosurgeons and ENT surgeons; thus, the authors compared outcomes based on the approach to the sphenoid sinus. Although endonasal structural difficulties were more common in the collaborative group, the average time needed to reach to the sphenoid sinus and the incidence of intraoperative complications such as bleeding, a floppy middle turbinate, and septal complications were lower in the collaborative group. The results from that study underscore the necessity of collaboration in surgery involving an endoscopic endonasal approach. However, only the outcomes related to surgery were compared and the authors did not confirm postoperative sinonasal or olfactory function. To the best of our knowledge, the present study is the first in which sinonasal quality of life was compared between patients who underwent endoscopic pituitary surgery performed by a single neurosurgeon and those operated on by a collaborative team of surgeons. We also reviewed olfactory function and complications such as septal perforation in the two groups, and we obtained better outcomes in the group that underwent collaborative team surgery. We suggest that these results underscore the importance of ENT surgeons in endoscopic pituitary surgery, as performing endoscopic pituitary surgery in a collaborative team including ENT surgeons allows patients to benefit from the endoscopic endonasal approach, as well as other advantages including the preservation of olfactory function and sinonasal quality of life, reduced complications, and postoperative management.
In the present study, patients diagnosed with pituitary adenoma who did not undergo surgery using the nasoseptal flap technique for reconstruction were included. Other diagnoses such as craniopharyngioma, meningioma, and clival chordoma often require more extensive skull base surgery that could affect olfactory function or quality of life. The nasoseptal flap technique involves cutting the superior septal mucosa, which can result in disruption of the olfactory epithelium [11]. In many studies, the effects of the nasoseptal flap technique in patients have been examined, and a systematic review of 14 studies showed that nasoseptal flap elevation led to objective sinonasal function impairment [12]. After exclusion of these factors, the demographic characteristics of the patients in both groups were not significantly different. In the present study, the collaboration group showed a tendency for better subjective olfactory function during the postoperative period, as exhibited by better scores on a Likert scale; however, a statistically significant difference was not observed. The scores from baseline to the postoperative period were consistently lower in group 1, but without a statistically significant difference. Objective olfactory function was also significantly better in patients in group 2. Olfactory identification and threshold were evaluated using the CC-SIT and BTT, respectively. Both of these objective tests of olfaction showed significantly better postoperative results in group 2. We suggest that this difference may reflect distinctive characteristics of surgical technique between neurosurgeons and ENT surgeons. When the operation is carried out collaboratively, the approach to the sphenoid sinus and sellar region is managed by the ENT surgeon. The technique used for this approach includes skills of septoplasty, such as a hemitransfixion incision, septal flap elevation, and posterior chondrotomy. ENT surgeons are familiar with this technique, so the incidence of complications or olfactory dysfunction in these cases is minimal relative to when surgery is performed by a neurosurgeon. Although the postoperative change in subjective olfaction was not significantly different between the groups, the collaborative group showed a smaller decline in smell function than the group operated on by a single neurosurgeon. When we compared the “sense of taste/smell” subdomain of the SNOT-22 questionnaire, the collaborative group also showed better scores. As such, subjective olfactory function showed similar trends to those documented using objective olfactory function tests.
In a recent meta-analysis [13], no significant difference was found between preoperative and postoperative olfaction in patients who underwent endoscopic skull base surgery. However, an issue in that analysis was between-study heterogeneity in the surgical techniques and observation periods. Our study had a relatively short-term follow-up period, potentially raising the limitation of whether it analyzed a sufficiently long time interval for the restoration of olfactory function. However, our metrics of subjective and objective olfactory function gradually showed improvements over time. Furthermore, Puccinelli et al. [14] reported no significant change in patients’ University of Pennsylvania Smell Identification scores 1 year after undergoing surgery using the transnasal skull-base approach. However, their sample size was relatively small (n=22), and their follow-up period was longer than that of our study.
Sinonasal quality of life was evaluated using the SNOT-22 questionnaire, which was answered by patients preoperatively and postoperatively at 1, 3, and 6 months. The total SNOT-22 scores between groups were not significantly different. Furthermore, the SNOT-22 rhinologic symptom subdomain was also not significantly different between groups. We hypothesized the patients in group 2 would show better sinonasal quality of life, however, the results did not confirm that hypothesis. A reason for this may be that although the mucosa underwent more trauma during surgery performed only by a single neurosurgeon, the normal nasal mucosa (without inflammation) in TSA patients can normalize easily; therefore, sinonasal quality of life was not significantly different between the two groups.
The septal perforation rate was examined in the postoperative outpatient clinic as a sinonasal complication. When surgeons use the transseptal approach for TSA, septal perforation is a possible complication after surgery. In group 1, 8.7% of patients (4/46) showed postoperative septal perforation, while in group 2, only one patient (1/106, 0.9%) had septal perforation. ENT surgeons routinely perform septoplasty for patients with septal deviation, and are more familiar with the septal mucosal incision and septal flap elevation techniques than neurosurgeons. Therefore, septal complications occurred less frequently in patients in group 2. Septal perforation in endoscopic pituitary surgery has been analyzed in previous studies. The septal perforation rate was reported to range from 0% to 20% [15-17]. In our study, the septal perforation rate was 0.9% in the collaborative group, which is a comparable rate to those reported in previous studies. In the single surgeon group, the perforation rate was 8.7%, which is a somewhat higher rate than reported in former studies. If a nasoseptal flap is needed for reconstruction, the perforation rate could be higher. For instance, Soudry et al. [18] reported a septal perforation rate of 14.4% associated with the pedicled nasoseptal flap for skull base reconstruction.
The present study had several limitations. First, the two groups did not include a balanced number of patients. Generally, at our center, more patients undergo surgery performed by a collaborative team of surgeons than by a single neurosurgeon, which resulted in a different number of patients in each group. Second, the follow-up period was only 6 months. A longer follow-up period could have led to different results. However, it is the nature of our tertiary referral center that some patients may have to travel a great distance for care, rendering periodic follow-up after surgery very difficult. In addition, the number of olfactory tests or SNOT-22 questionnaires administered during the postoperative period was limited. However, differences were not observed between patients who were followed up after surgery and those who were not. Therefore, selection bias was likely minimal.
In the present study, sinonasal outcomes in endoscopic pituitary surgery were compared between operations performed by a single neurosurgeon (group 1) or by a collaborative team of surgeons (group 2). The patients in group 2 showed better postoperative olfactory function based on subjective and objective tests and had a lower septal perforation rate than the patients in group 1. However, sinonasal quality of life was not significantly different between the two groups. In conclusion, the results from the present study underscore the necessity of collaboration between neurosurgeons and ENT surgeons in endoscopic pituitary surgery to obtain better endoscopic outcomes and olfactory function.
HIGHLIGHTS
▪ Better olfactory identification outcomes were observed in patients who underwent surgery performed by a collaborative team of a neurosurgeon and an ENT surgeon.
▪ Collaboration between neurosurgeons and ENT surgeons in endoscopic pituitary surgery is necessary.
Footnotes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: SDH, DSK, DHN. Data curation: YN, JEC, KEL. Formal analysis: YGJ, HYK, SKC, SDH, YN. Methodology: SDH. Project administration: SDH, YN. Visualization: YN. Writing–original draft: YN. Writing–review & editing: YN, SDH.
REFERENCES
- 1.Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997 Jul;87(1):44–51. doi: 10.3171/jns.1997.87.1.0044. [DOI] [PubMed] [Google Scholar]
- 2.Stammberger H. Endoscopic endonasal surgery: concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngol Head Neck Surg. 1986;94(2):147–56. doi: 10.1177/019459988609400203. [DOI] [PubMed] [Google Scholar]
- 3.Jane JA, Jr, Han J, Prevedello DM, Jagannathan J, Dumont AS, Laws ER., Jr Perspectives on endoscopic transsphenoidal surgery. Neurosurg Focus. 2005 Dec;19(6):1–10. doi: 10.3171/foc.2005.19.6.3. [DOI] [PubMed] [Google Scholar]
- 4.D’Haens J, Van Rompaey K, Stadnik T, Haentjens P, Poppe K, Velkeniers B. Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol. 2009 Oct;72(4):336–40. doi: 10.1016/j.surneu.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Singh A, Wessell AP, Anand VK, Schwartz TH. Surgical anatomy and physiology for the skull base surgeon. Oper Tech Otolayngol Head Neck Surg. 2011 Sep;22(3):184–93. [Google Scholar]
- 6.van Lindert EJ, Ingels K, Mylanus E, Grotenhuis JA. Variations of endonasal anatomy: relevance for the endoscopic endonasal transsphenoidal approach. Acta Neurochir (Wien) 2010 Jun;152(6):1015–20. doi: 10.1007/s00701-010-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pledger CL, Elzoghby MA, Oldfield EH, Payne SC, Jane JA., Jr Prospective comparison of sinonasal outcomes after microscopic sublabial or endoscopic endonasal transsphenoidal surgery for nonfunctioning pituitary adenomas. J Neurosurg. 2016 Aug;125(2):323–33. doi: 10.3171/2015.6.JNS142695. [DOI] [PubMed] [Google Scholar]
- 8.Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL, et al. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: a prospective cohort study. J Neurosurg. 2015 Sep;123(3):799–807. doi: 10.3171/2014.10.JNS14921. [DOI] [PubMed] [Google Scholar]
- 9.Snyderman CH, Wang EW, Fernandez-Miranda JC, Gardner PA. The making of a skull base team and the value of multidisciplinary approach in the management of sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am. 2017 Apr;50(2):457–65. doi: 10.1016/j.otc.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Ismail M, Abdelaziz AA, Darwish M. A comparison between collaborative and single surgeon approach in endoscopic endonasal surgery to sphenoid sinus. Eur Arch Otorhinolaryngol. 2019 Apr;276(4):1095–100. doi: 10.1007/s00405-019-05305-y. [DOI] [PubMed] [Google Scholar]
- 11.Sowerby LJ, Gross M, Broad R, Wright ED. Olfactory and sinonasal outcomes in endoscopic transsphenoidal skull-base surgery. Int Forum Allergy Rhinol. 2013 Mar;3(3):217–20. doi: 10.1002/alr.21103. [DOI] [PubMed] [Google Scholar]
- 12.Greig SR, Cooper TJ, Sommer DD, Nair S, Wright ED. Objective sinonasal functional outcomes in endoscopic anterior skull-base surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016 Oct;6(10):1040–6. doi: 10.1002/alr.21760. [DOI] [PubMed] [Google Scholar]
- 13.Yin LX, Low CM, Puccinelli CL, O’Brien EK, Stokken JK, Van Abel KM, et al. Olfactory outcomes after endoscopic skull base surgery: asystematic review and meta-analysis. Laryngoscope. 2019 Sep;129(9):1998–2007. doi: 10.1002/lary.28003. [DOI] [PubMed] [Google Scholar]
- 14.Puccinelli CL, Yin LX, O’Brien EK, Van Gompel JJ, Choby GW, Van Abel KM, et al. Long-term olfaction outcomes in transnasal endoscopic skull-base surgery: a prospective cohort study comparing electrocautery and cold knife upper septal limb incision techniques. Int Forum Allergy Rhinol. 2019 May;9(5):493–500. doi: 10.1002/alr.22291. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Zhong C, Wang Y, Xu S, Guo Y, Dai C, et al. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a metaanalysis. World J Surg Oncol. 2014 Apr;12:94. doi: 10.1186/1477-7819-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeKlotz TR, Chia SH, Lu W, Makambi KH, Aulisi E, Deeb Z. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012 Mar;122(3):511–8. doi: 10.1002/lary.22479. [DOI] [PubMed] [Google Scholar]
- 17.Casler JD, Doolittle AM, Mair EA. Endoscopic surgery of the anterior skull base. Laryngoscope. 2005 Jan;115(1):16–24. doi: 10.1097/01.mlg.0000150681.68355.85. [DOI] [PubMed] [Google Scholar]
- 18.Soudry E, Psaltis AJ, Lee KH, Vaezafshar R, Nayak JV, Hwang PH. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015 Jan;125(1):80–5. doi: 10.1002/lary.24863. [DOI] [PubMed] [Google Scholar]


