Abstract
Ginsenosides are the major bioactive constituents of Panax ginseng, which have pharmacological effects. Although there are several reviews in regards to ginsenosides, new ginsenosides have been detected continually in recent years. This review updates the ginsenoside list from P. ginseng to 170 by the end of 2019, and aims to highlight the diversity of ginsenosides in multiple dimensions, including chemical structure, tissue spatial distribution, time, and isomeride. Protopanaxadiol, protopanaxatriol and C17 side-chain varied (C17SCV) manners are the major types of ginsenosides, and the constitute of ginsenosides varied significantly among different parts. Only 16 ginsenosides commonly exist in all parts of a ginseng plant. Protopanaxadiol-type ginsenoside is dominant in root, rhizome, leaf, stem, and fruit, whereas malonyl- and C17SCV-type ginsenosides occupy a greater proportion in the flower and flower bud compared with other parts. In respects of isomeride, there are 69 molecular formulas corresponding to 170 ginsenosides, and the median of isomers is 2. This is the first review on diversity of ginsenosides, providing information for reasonable utilization of whole ginseng plant, and the perspective on studying the physiological functions of ginsenoside for the ginseng plant itself is also proposed.
Keywords: ginsenoside, Panax ginseng, chemical structure, tissue spatial distribution
1. Introduction
Panax ginseng Meyer (P. ginseng), known as the king of all herbs, has been frequently used as traditional medicine and healthy food in China, Korea, and Japan. In 2012, P. ginseng was approved as a new food resource by Chinese government, and it has been widely used as the raw material of healthcare products [1]. Ginseng contains a large amount and number of ginsenosides. More than 289 saponins were reported from eleven different Panax species [2]. In addition, at least 123 ginsenosides have been identified in different P. ginseng species, and these include both naturally occurring compounds and those from steaming and biotransformation [3]. In addition, 112 saponins were reported from raw or processed ginseng, including hydrolysates, semisynthetic, and metabolites [4]. Ginsenosides are known to possess a lot of biological activities including regulatory effects on immunomodulation, protection functions in the central nervous and cardiovascular systems, anti-diabetic, anti-aging, anti-carcinogenic, anti-fatigue, anti-pyretic, anti-stress, boosting physical vitality, and promotion of DNA, RNA, and protein synthesis activities [5,6,7,8,9]. In addition, the biosynthesis of triterpenoid is an important factor of saponin diversity. Consequently, biosynthetic mechanisms for the backbone synthesis [4] and structural diversification and genes/enzymes involved in the biosynthesis [10] were reviewed in the cited references. Therefore, ginsenosides are recognized as the main bioactive components and a key index for quality evaluation of ginseng.
Due to the complexity of the ginsenosides and their structures, multi-platform analytical techniques are used in the detection of ginseng products, such as thin layer chromatography (TLC), high performance thin layer chromatography (HPTLC), gas chromatography (GC), high performance liquid chromatography (HPLC), ultra performance liquid chromatography (UPLC) [3,11,12]. However, these methods detect only small numbers of ginsenosides and lack in provision of structural information. Liquid chromatography coupled with tandem mass spectrometry can provide structural information with high sensitivity, specificity, and versatility in characterizing complex natural product samples. It has been successfully used as a powerful tool for ginsenoside analysis with high throughput [1]. In recent years, a number of novel ginsenosides have been detected in aerial parts of the ginseng plant using the HPLC-MS/MS method, such as stems, leaves, rhizomes, flowers, and flower buds, which enlarged the number of ginsenoside family members [13,14,15]. Several reviews have summarized the progress from a viewpoint of structural features, and conclude that ginsenosides are generally classified into four groups: protopanaxadiol type (PPD), protopanaxatriol type (PPT), C17 side-chain varied type (C17SCV), and oleanolic acid type (OA) [2,16,17,18]. However, spatial distribution of ginsenoside in different parts of P. ginseng is not yet summarized. This information will make better use of the whole ginseng plant and provide clues for studying the biological function of saponins. This review updates the ginsenoside list (from P. ginseng) to 170 by the end of 2019, and aims to highlight the diversity of ginsenosides in multiple dimensions, including chemical structure, tissue spatial distribution, time, and isomeride.
2. History of Saponins Isolated from P. ginseng
The history of ginsenoside isolation can be divided into three periods (before 1980 for Period I, 1980–2000 for Period II, after 2000 for Period III) based on the development of analytical techniques. The study on ginsenoside started in 1854. A ginsenoside-containing constituent was firstly isolated from American ginseng by American scholar Garriques [19], and subsequently, Japanese chemists reported panaquilon, panacon, panaxasapogenol, and ginsenin preliminarily separated from P. ginseng. For almost 100 years since the middle of the nineteenth century, it was difficult to obtain a pure ginsenoside due to the under development of separation techniques. In the early 1950s, with the development of separation technology and the invention of modern analytical instruments, such as GC, TLC, etc., the studies on the chemical ingredient of ginseng made remarkable progress. In 1963, for the first time, Shibata et al. reported the chemical property and structure of the panaxadiol separated from ginseng root [20]. In the 1970s, 17 ginsenosides were detected in ginseng, named as ginsenoside Ro, Ra, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, F1, F2, F3, Rb3, Rh, and 20-glucoginsenoside-Rf [21,22,23,24,25,26]. The second period began when the 13C NMR technique was introduced into the structure analysis of ginsenosides. By comparison of the measured 13C NMR spectroscopic data with known compounds, the accurate structure of new ginsenosides (G-Rh1, Rh2, Rh3, Rg4, Ra1, Ra2, Ra3, La, Rf2, Rs3, Ia, Ib, etc.) could be resolved from different parts of ginseng (root, steamed root, flower bud, stem, and leaf). In this period, more and more scientists focused on ginsenoside isolation, and most of ginsenosides were found in the aerial parts of ginseng [27,28,29,30,31,32,33,34,35,36]. The third period was defined by high-efficiency separation methods, as methods such as high-speed counter current chromatography (HSCCC), high performance centrifugal partition chromatography (HPCPC), and 2D NMR spectroscopic techniques were used for separating and identifying ginsenosides. The application of these powerful new techniques helps to identify the complex chemical structure, for instance, C17 side-chain variation and malonyl group. More than 50 new ginsenosides were isolated from 2000 to 2019, among which most of those possessed variations in the C17 side-chain, besides a part of malonyl ginsenosides [37,38,39,40,41].
3. Classification of Saponins Identified from P. ginseng
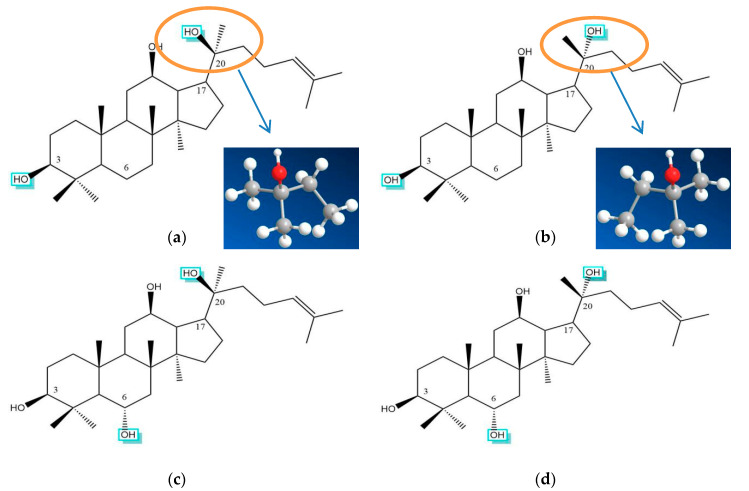
Although most ginsenosides have a rigid four-trans-ring steroid skeleton, they produce multiple pharmacological and biological effects that are different from one another due to minor variations on: (1) Type of sapogenins; (2) number, type, and site of glycosyl units; and (3) modification of C17 side-chains [11,42,43]. Therefore, the study of ginsenoside structure will help to elucidate the mechanism of multiple functions of ginsenosides. The reported ginsenosides are classified into protopanaxadiol type (PPD), protopanaxatriol type (PPT), oleanolic acid type (OA), and C17 side-chain varied (C17SCV) subtypes according to their determined sapogenin structures (Figure 1). The glycosyl components of saponin were mainly β-d-glucopyranosyl group, followed by α-l-rhamnopyranosyl group, a few binding α-l-arabinopyranosyl group and β-d-xylopyranosyl group, and the β-d-glucopyranosiduronyl group only appears in saponins with oleanolic acid-type (OA) sapogenin. In dammarane-type triterpenoid saponins, β-d-glucopyranosyl group (2→1)-β-d-glucopyranosyl oligosaccharide chains occur more frequently, and are mostly bound to C-3 of sapogenin to generate oxyglycoside; β-d-glucopyranosyl group (2→1)→α-l-rhamnopyranosyl group oligosaccharide chains are mostly bound to C-6 of sapogenin to form oxyglycoside. The tetracyclic parent nucleuses are relatively stable, whether they are PPT and/or PPD type. Moreover, the substituents that occur in the C17 side-chains often undergo oxidation, reduction, cyclization, and epimerization, contributing to diversity in chemical structure [12,16]. Table 1 displays the molecular formulas, molecular masses, and structural categories of 170 ginsenosides, isolated from different parts of P. ginseng. As a result, four ginsenosides are OA type, 59 ginsenosides are PPD type, 42 ginsenosides are PPT type, and 65 ginsenosides are C17CSV type. Among them, four PPD-type ginsenosides (Rb1, Rb2, Rc, Rd), three PPT-type ginsenosides (Re, Rf, Rg1), and one OA-type ginsenoside Ro (the structures are shown in Figure 2) are the most abundant in P. ginseng, and account for more than 70% of the total saponins [5].
Figure 1.
Structures of PPD, PPT, OA, and C17SCV sapogenins. The typical glycosylation sites for these sapogenins are marked in blue frame. (a) 20(S)-PPD: Protopanaxadiol type; (b) 20(R)-PPD: Protopanaxadiol type; (c) 20(S)-PPT: Protopanaxatriol type; (d) 20(R)-PPT: Protopanaxatriol type; (e) OA: Oleanolic acid type; (f) C17SCV: C17 side-chain variation type. R1 in C17SCV: -H, -OH, -OR. R2 in C17SCV: The variations in the C17 side-chain mainly comprise H2O-addition, hydroxylation, methoxylation, peroxidization, dehydration at C-20, carbonylation, dehydrogenation, cyclization, oxidation (at the double bond), and degradation. The stereochemistry of chiral centers are shown in (a) and (b).
Table 1.
The 170 ginsenosides isolated from P. ginseng.
| No. | Subtype | Saponins | Formula | Molecular Mass | Plant Part | Refs |
|---|---|---|---|---|---|---|
| 1 | OA 1 | Polyacetylene ginsenoside Ro | C65H100O21 | 1216.6757 | Root | [44] |
| 2 | OA | Ginsenoside Ro methyl ester | C49H78O19 | 970.5137 | Root(steamed) | [45] |
| 3 | OA | Calenduloside B | C48H78O18 | 942.5188 | Root | [46] |
| 4 | OA | Ginsenoside Ro | C49H80O18 | 956.5345 | Root, flower, fruit, leaf | [16,47] |
| 5 | PPD | Ginsenoside Ra1 | C58H98O26 | 1210.6346 | Root | [48] |
| 6 | PPD | Ginsenoside Ra2 | C58H98O26 | 1210.6346 | Root | [49] |
| 7 | PPD | Ginsenoside Ra3 | C59H100O27 | 1240.6452 | Root | [50] |
| 8 | PPD | Ginsenoside Rs1 | C55H92O23 | 1120.6029 | Root(steamed) | [51] |
| 9 | PPD | Ginsenoside Rs2 | C55H92O23 | 1120.6029 | Root(steamed) | [51] |
| 10 | PPD | Malonyl-ginsenoside Ra3 | C62H102O30 | 1326.6456 | Root(fresh) | [52] |
| 11 | PPD | Malonyl-notoginsenoside R4 | C62H102O30 | 1326.6456 | Root | [52] |
| 12 | PPD | Ginsenoside Ra4 | C62H102O27 | 1278.6608 | Root | [53] |
| 13 | PPD | Ginsenoside Ra5 | C60H99O27 | 1251.6373 | Root | [53] |
| 14 | PPD | Ginsenoside Ra6 | C58H96O24 | 1176.6292 | Root | [53] |
| 15 | PPD | Ginsenoside Ra7 | C57H93O23 | 1145.6108 | Root | [53] |
| 16 | PPD | Ginsenoside Ra8 | C57H94O23 | 1146.6186 | Root | [53] |
| 17 | PPD | Ginsenoside Ra9 | C57H94O23 | 1146.6186 | Root | [53] |
| 18 | PPD | 20(S)-ginsenoside Rg3 | C42H72O13 | 784.4973 | Root(steamed), fruit, leaf | [54] |
| 19 | PPD | Ginsenoside Rs3 | C44H74O14 | 826.5079 | Root(steamed) | [55] |
| 20 | PPD | Ginsenoside IV | C58H96O24 | 1176.6292 | Root | [47] |
| 21 | PPD | Ginsenoside V | C54H92O24 | 1124.5979 | Root | [47] |
| 22 | PPD | Gypenoside-V | C54H92O22 | 1092.6080 | Root | [46] |
| 23 | PPD | 20(R)-ginsenoside Rs3 | C44H74O14 | 826.5079 | Root(steamed) | [45] |
| 24 | PPD | Acetyl-ginsenoside Rd | C50H84O19 | 988.5607 | Root(mountain ginseng) | [56] |
| 25 | PPD | Ginsenoside F2 | C42H72O13 | 784.4973 | Root, fruit, leaf | [57] |
| 26 | PPD | Pseudoginsenoside Rc1 | C50H84O19 | 988.5607 | Fruit | [57] |
| 27 | PPD | Gypenoside XVII | C48H82O18 | 946.5501 | Fruit, leaf | [57] |
| 28 | PPD | Gypenoside IX | C47H80O17 | 916.5396 | Fruit, leaf | [57] |
| 29 | PPD | Quinquenoside L10 | C47H80O17 | 916.5396 | Fruit | [57] |
| 30 | PPD | 25-Hydroxyprotopanaxadiol | C30H54O4 | 478.4022 | Fruit | [58] |
| 31 | PPD | 20(S)-protopanaxadiol | C30H52O3 | 460.3916 | Fruit, leaf | [41,59] |
| 32 | PPD | 20(R)-protopanaxadiol | C30H52O3 | 460.3916 | Fruit | [59] |
| 33 | PPD | Notoginsenoside Fd | C47H80O17 | 916.5396 | Fruit | [60] |
| 34 | PPD | Ginsenoside Rd2 | C47H80O17 | 916.5396 | Leaf | [61] |
| 35 | PPD | 20(R)-ginsenoside Rg3 | C42H72O13 | 784.4973 | Root(steamed), fruit, leaf | [62,63] |
| 36 | PPD | 20(S)-ginsenoside Rh2 | C36H62O8 | 622.4445 | Root(steamed), fruit, leaf | [64] |
| 37 | PPD | 20(R)-ginsenoside Rh2 | C36H62O8 | 622.4445 | Fruit, leaf | [65] |
| 38 | PPD | Notoginsenoside Fe | C47H80O17 | 916.5396 | Fruit, leaf | [61] |
| 39 | PPD | Acetyl-ginsenoside Rb1 | C56H96O24 | 1152.6292 | Root(mountain ginseng), leaf | [56] |
| 40 | PPD | Acetyl-ginsenoside Rc | C55H92O23 | 1120.6029 | Root(mountain ginseng), leaf | [56] |
| 41 | PPD | Acetyl-ginsenoside Rb3 | C55H92O23 | 1120.6029 | Root(mountain ginseng), leaf | [56] |
| 42 | PPD | Ginsenoside compound O | C47H80O17 | 916.5396 | Root, fruit, leaf | [16,66] |
| 43 | PPD | Malonyl-ginsenoside Rb2 | C56H92O25 | 1164.5928 | Root, flower, fruit, leaf | [16] |
| 44 | PPD | Ginsenoside Mc | C41H70O12 | 754.4867 | Leaf | [16,66] |
| 45 | PPD | Ginsenoside compound Y | C41H70O12 | 754.4867 | Leaf | [16] |
| 46 | PPD | Ginsenoside compound K | C36H62O8 | 622.4445 | Root, fruit, leaf | [16] |
| 47 | PPD | Ginsenoside Rb1 | C54H92O23 | 1108.6029 | Root, flower, fruit, leaf | [16,67] |
| 48 | PPD | Malonyl-ginsenoside Rb1 | C57H94O25 | 1178.6084 | Root, flower, fruit, leaf | [16,67] |
| 49 | PPD | Ginsenoside Rc | C53H90O22 | 1078.5924 | Root, flower, fruit, leaf | [16,67] |
| 50 | PPD | Malonyl-ginsenoside Rc | C56H92O25 | 1164.5928 | Root, flower, fruit, leaf | [16,67] |
| 51 | PPD | Ginsenoside Rb2 | C53H90O22 | 1078.5924 | Root, flower, fruit, leaf | [16,67] |
| 52 | PPD | Ginsenoside Rb3 | C53H90O22 | 1078.5924 | Root, flower, fruit, leaf | [16,67] |
| 53 | PPD | Malonyl-ginsenoside Rb3 | C56H92O25 | 1164.5928 | Root, flower, leaf | [16,67] |
| 54 | PPD | Ginsenoside Rd | C48H82O18 | 946.5501 | Root, flower, fruit, leaf | [16,67] |
| 55 | PPD | Malonyl-ginsenoside Rd | C51H84O21 | 1032.5505 | Root, flower, fruit, leaf | [16,67] |
| 56 | PPD | Malonyl-floralginsenoside Rd2 | C51H84O21 | 1032.5505 | Flower | [68] |
| 57 | PPD | Malonyl-floralginsenoside Rd3 | C51H84O21 | 1032.5505 | Flower | [68] |
| 58 | PPD | Malonyl-floralginsenoside Rd4 | C51H84O21 | 1032.5505 | Flower | [68] |
| 59 | PPD | Malonyl-floralginsenoside Rd5 | C51H84O21 | 1032.5505 | Flower | [68] |
| 60 | PPD | Malonyl-floralginsenoside Rd6 | C54H87O24 | 1119.5587 | Flower | [68] |
| 61 | PPD | Malonyl-floralginsenoside Rc2 | C56H92O25 | 1164.5928 | Flower | [68] |
| 62 | PPD | Malonyl-floralginsenoside Rc3 | C56H92O25 | 1164.5928 | Flower | [68] |
| 63 | PPD | Malonyl-floralginsenoside Rc4 | C56H92O25 | 1164.5928 | Flower | [68] |
| 64 | PPT | 20(S)-ginsenoside Rg2 | C42H72O13 | 784.4973 | Root, fruit, leaf | [54,69] |
| 65 | PPT | Koryoginsenoside R1 | C46H76O15 | 868.5184 | Root | [36] |
| 66 | PPT | Ginsenoside Re6 | C46H76O15 | 868.5184 | Root | [70] |
| 67 | PPT | Ginsenoside Re2 | C48H82O19 | 962.5450 | Root | [70] |
| 68 | PPT | Ginsenoside Re3 | C48H82O19 | 962.5450 | Root | [70] |
| 69 | PPT | Ginsenoside Re4 | C47H80O18 | 932.5345 | Root | [70] |
| 70 | PPT | Notoginsenoside Rt | C44H74O15 | 842.5028 | Root | [46] |
| 71 | PPT | Majoroside F6 | C48H82O19 | 962.5450 | Root | [46] |
| 72 | PPT | Pseudoginsenoside Rt3 | C42H70O13 | 782.4816 | Root | [46] |
| 73 | PPT | Vinaginsenoside R15 | C42H72O15 | 816.4871 | Root | [46] |
| 74 | PPT | 20(R)-ginsenoside Rf | C42H72O14 | 800.4922 | Root | [45] |
| 75 | PPT | 20(R)-notoginsenoside R2 | C41H70O13 | 770.4816 | Root | [45] |
| 76 | PPT | Ginsenoside Ia | C42H72O14 | 800.4922 | Fruit | [71] |
| 77 | PPT | Chikusetsusaponin LM1 | C41H70O13 | 770.4816 | Fruit | [57] |
| 78 | PPT | 25-Hydroxyprotopanaxatriol | C30H54O5 | 494.3971 | Fruit | [58] |
| 79 | PPT | 20(S)-protopanaxatriol | C30H52O4 | 476.3866 | Fruit, leaf | [59] |
| 80 | PPT | 20(R)-protopanaxatriol | C30H52O4 | 476.3866 | Fruit | [59] |
| 81 | PPT | Notoginsenoside R3 | C48H82O19 | 962.5450 | Fruit | [60] |
| 82 | PPT | 20-glucoginsenoside Rf | C48H82O19 | 962.5450 | Root, flower, leaf | [16] |
| 83 | PPT | Saponin IIb | C36H62O9 | 638.4394 | Leaf | [72] |
| 84 | PPT | Saponin IIIc | C37H62O10 | 666.4343 | Leaf | [72] |
| 85 | PPT | 20(S)-ginsenoside Rh1 | C36H62O9 | 638.4394 | Leaf | [62] |
| 86 | PPT | 20(R)-ginsenoside Rh1 | C36H62O9 | 638.4394 | Root(steamed), leaf | [21] |
| 87 | PPT | Acetyl-ginsenoside Rg1 | C44H74O15 | 842.5028 | Root(mountain ginseng), leaf | [56] |
| 88 | PPT | Acetyl-ginsenoside Re | C50H84O19 | 988.5607 | Root(mountain ginseng), leaf | [56] |
| 89 | PPT | Notoginsenoside R2 | C41H70O13 | 770.4816 | Root, fruit, leaf | [16] |
| 90 | PPT | Notoginsenoside R1 | C47H80O18 | 932.5345 | Root, flower, fruit, leaf | [16,67] |
| 91 | PPT | Ginsenoside Rg1 | C42H72O14 | 800.4922 | Root, flower, fruit, leaf | [16,67] |
| 92 | PPT | Ginsenoside Re | C48H82O18 | 946.5501 | Root, flower, fruit, leaf | [16,67] |
| 93 | PPT | Malonyl-ginsenoside Rg1 | C45H74O17 | 886.4926 | Root, flower, leaf | [16,67] |
| 94 | PPT | Malonyl-ginsenoside Re | C51H84O21 | 1032.5505 | Root, flower, fruit, leaf | [16,67] |
| 95 | PPT | Ginsenoside Rf | C42H72O14 | 800.4922 | Root, flower, fruit, leaf | [16,67] |
| 96 | PPT | 20(R)-ginsenoside Rg2 | C42H72O13 | 784.4973 | Root(steamed), flower, fruit, leaf | [16,67] |
| 97 | PPT | Ginsenoside Rf3 | C41H70O13 | 770.4816 | Flower | [67] |
| 98 | PPT | Floralginsenoside M | C53H90O22 | 1078.5924 | Flower, leaf | [73] |
| 99 | PPT | Floralginsenoside N | C53H90O22 | 1078.5924 | Flower, leaf | [73] |
| 100 | PPT | Floralginsenoside P | C53H90O23 | 1094.5873 | Flower | [73] |
| 101 | PPT | Ginsenoside F1 | C36H62O9 | 638.4394 | Flower, fruit, leaf | [74] |
| 102 | PPT | Ginsenoside F3 | C41H70O13 | 770.4816 | Flower, fruit, leaf | [74] |
| 103 | PPT | Ginsenoside F5 | C41H70O13 | 770.4816 | Flower, fruit, leaf | [74] |
| 104 | PPT | Malonyl-floralginsenoside Re2 | C51H84O21 | 1032.5505 | Flower | [68] |
| 105 | PPT | Malonyl-floralginsenoside Re3 | C51H84O21 | 1032.5505 | Flower | [68] |
| 106 | C17SCV | Koryoginsenoside R2 | C54H92O24 | 1124.5979 | Root | [36] |
| 107 | C17SCV | Ginsenoside Re5 | C42H72O15 | 816.4871 | Root | [70] |
| 108 | C17SCV | Ginsenoside Rs4 | C44H72O13 | 808.4973 | Root(sun cured) | [75] |
| 109 | C17SCV | Dehydroprotopanaxadiol I | C30H50O2 | 442.3811 | Root(steamed) | [2] |
| 110 | C17SCV | Ginsenoside Rg5 | C42H70O12 | 766.4867 | Root(steamed) | [76] |
| 111 | C17SCV | Dehydroprotopanaxatriol I | C30H50O3 | 458.3760 | Root(steamed) | [2] |
| 112 | C17SCV | Ginsenoside Rs6 | C38H62O9 | 662.4394 | Root(sun cured) | [75] |
| 113 | C17SCV | Ginsenoside Rz1 | C42H70O12 | 766.4867 | Root(steamed) | [77] |
| 114 | C17SCV | Dehydroprotopanaxadiol II | C30H50O2 | 442.3811 | Root(steamed) | [2] |
| 115 | C17SCV | Ginsenoside Rs5 | C44H72O13 | 808.4973 | Root(sun cured) | [75] |
| 116 | C17SCV | Dehydroprotopanaxatriol II | C30H50O3 | 458.3760 | Root(steamed) | [2] |
| 117 | C17SCV | Ginsenoside Rg6 | C42H70O12 | 766.4867 | Root(steamed) | [78] |
| 118 | C17SCV | Ginsenoside Rk3 | C36H60O8 | 620.4288 | Root(steamed) | [76] |
| 119 | C17SCV | Ginsenoside Rs7 | C38H62O9 | 662.4394 | Root(sun cured) | [75] |
| 120 | C17SCV | Ginsenoside Rg9 | C42H70O13 | 782.4816 | Root(steamed) | [79] |
| 121 | C17SCV | 12-O-glucoginsenoside Rh4 | C42H70O13 | 782.4816 | Root(steamed) | [80] |
| 122 | C17SCV | Ginsenoside Rg10 | C42H69O13 | 781.4738 | Root(steamed) | [79] |
| 123 | C17SCV | Ginsenoside Rh10 | C36H62O8 | 622.4445 | Root(steamed) | [80] |
| 124 | C17SCV | Ginsenoside Rg11 | C42H70O14 | 798.4766 | Root(steamed) | [80] |
| 125 | C17SCV | Vinaginsenoside R8 | C48H82O19 | 962.5450 | Fruit | [57] |
| 126 | C17SCV | Ginsenoside Rh4 | C36H60O8 | 620.4288 | Root(steamed), fruit | [4,57] |
| 127 | C17SCV | Ginsenoside Rh5 | C36H60O9 | 636.4237 | Root(steamed), fruit | [4,57] |
| 128 | C17SCV | Isoginsenoside-Rh3 | C36H60O7 | 604.4339 | Fruit | [81] |
| 129 | C17SCV | Ginsenoside Rf2 | C42H72O14 | 800.4922 | Fruit | [82] |
| 130 | C17SCV | Ginsenoside Rk2 | C36H60O7 | 604.4339 | Root(steamed), fruit | [76,83] |
| 131 | C17SCV | Pseudoginsenoside RT5 | C36H62O10 | 654.4343 | Fruit | [83] |
| 132 | C17SCV | Ginsenoside Rh3 | C36H60O7 | 604.4339 | Root(steamed), fruit | [76,83] |
| 133 | C17SCV | Ginsenoside Rg4 | C42H70O12 | 766.4867 | Root, fruit | [16] |
| 134 | C17SCV | Ginsenoside F4 | C42H70O12 | 766.4867 | Root, fruit, leaf | [16] |
| 135 | C17SCV | Ginsenoside Rg7 | C36H60O9 | 636.4237 | Leaf | [39] |
| 136 | C17SCV | Ginsenoside Rh6 | C36H62O11 | 670.4292 | Fruit, leaf | [39] |
| 137 | C17SCV | Ginsenoside Ki | C36H62O10 | 654.4343 | Leaf | [39] |
| 138 | C17SCV | Ginsenoside Km | C36H62O10 | 654.4343 | Leaf | [84] |
| 139 | C17SCV | Ginsenoside Rh9 | C36H60O9 | 636.4237 | Leaf | [39] |
| 140 | C17SCV | 12,23-Epoxyginsenoside Rg1 | C42H70O14 | 798.4766 | Leaf | [85] |
| 141 | C17SCV | Ginsenoside Rh7 | C36H60O9 | 636.4237 | Leaf | [39] |
| 142 | C17SCV | Ginsenoside Rh8 | C36H60O9 | 636.4237 | Leaf | [39] |
| 143 | C17SCV | Hexanordammaran | C24H40O4 | 392.2927 | Leaf | [86] |
| 144 | C17SCV | Floralginsenoside A | C42H72O16 | 832.4820 | Flower | [87] |
| 145 | C17SCV | Ginsenoside La | C42H70O13 | 782.4816 | Leaf | [35] |
| 146 | C17SCV | Vinaginsenoside R4 | C48H82O19 | 962.5450 | Root, fruit, leaf | [16] |
| 147 | C17SCV | Ginsenoside Rk1 | C42H70O12 | 766.4867 | Root(steamed), fruit, leaf | [16] |
| 148 | C17SCV | Floralginsenoside H | C50H84O21 | 1020.5505 | Flower | [88] |
| 149 | C17SCV | Floralginsenoside Tc | C53H90O24 | 1110.5822 | Flower | [89] |
| 150 | C17SCV | Floralginsenoside Td | C53H90O24 | 1110.5822 | Flower | [84] |
| 151 | C17SCV | Ginsenoside I | C48H82O20 | 978.5400 | Flower | [90] |
| 152 | C17SCV | Ginsenoside II | C48H82O20 | 978.5400 | Flower | [90] |
| 153 | C17SCV | Floralginsenoside C | C41H70O15 | 802.4715 | Flower | [74] |
| 154 | C17SCV | Floralginsenoside J | C48H82O20 | 978.5400 | Flower | [88] |
| 155 | C17SCV | Floralginsenoside Ka | C36H62O11 | 670.4292 | Flower | [91] |
| 156 | C17SCV | Floralginsenoside La | C48H82O19 | 962.5450 | Flower | [88] |
| 157 | C17SCV | Floralginsenoside Lb | C48H82O19 | 962.5450 | Flower | [88] |
| 158 | C17SCV | Floralginsenoside Ta | C36H60O10 | 652.4187 | Flower | [89] |
| 159 | C17SCV | Floralginsenoside E | C42H72O15 | 816.4871 | Flower | [74] |
| 160 | C17SCV | Floralginsenoside F | C42H72O15 | 816.4871 | Flower | [74] |
| 161 | C17SCV | Floralginsenoside G | C50H84O21 | 1020.5505 | Flower | [88] |
| 162 | C17SCV | Floralginsenoside K | C48H82O21 | 994.5349 | Flower | [88] |
| 163 | C17SCV | Floralginsenoside O | C53H90O22 | 1078.5924 | Flower | [73] |
| 164 | C17SCV | Floralginsenoside B | C42H72O16 | 832.4820 | Flower | [74] |
| 165 | C17SCV | Floralginsenoside D | C41H70O15 | 802.4715 | Flower | [74] |
| 166 | C17SCV | Floralginsenoside I | C48H82O20 | 978.5400 | Flower | [88] |
| 167 | C17SCV | Floralginsenoside Kb | C45H76O19 | 920.4981 | Flower | [91] |
| 168 | C17SCV | Floralginsenoside Kc | C45H76O20 | 936.4930 | Flower | [91] |
| 169 | C17SCV | Floralginsenoside Tb | C35H62O11 | 658.4292 | Flower | [89] |
| 170 | C17SCV | Ginsenoside III | C48H80O19 | 960.5294 | Flower | [92] |
1 OA: Oleanolic acid; PPD: Protopanaxadiol; PPT: Protopanaxatriol; C17SCV: C17 side-chain varied.
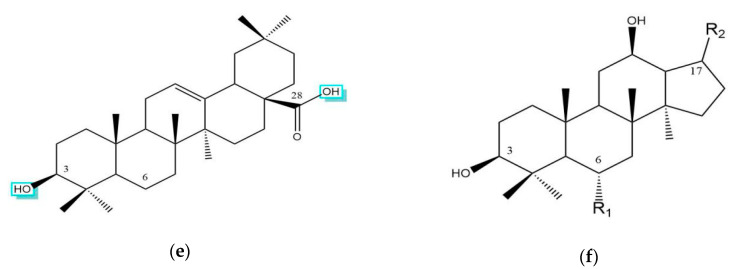
Figure 2.
Structures of eight high-abundance saponins in P. ginseng. (a) PPD-type ginsenoside Rb1; (b) PPD-type ginsenoside Rb2; (c) PPD-type ginsenoside Rc; (d) PPD-type ginsenoside Rd; (e) PPT-type ginsenoside Re; (f) PPT-type ginsenoside Rf; (g) PPT-type ginsenoside Rg1; (h) OA-type ginsenoside Ro.
4. Spatial Distribution of Ginsenosides in Different Parts
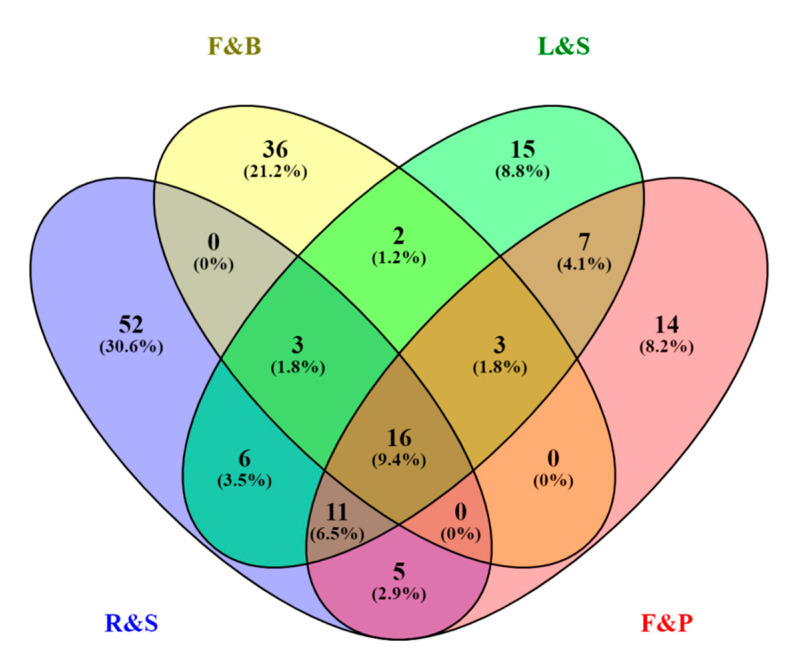
The Venn diagram (Figure 3) shows the number of ginsenosides commonly and separately shared by the following four groups: R&S (roots, rhizomes, and steamed roots), L&S (leaves and stems), F&P (fruits and fruit pedicels), and F&B (flowers and flower buds). Among them, the number of unique ginsenosides in group R&S, F&P, L&S, and F&B are 52, 15, 14, and 36, respectively, accounting for 30.6%, 8.8%, 8.2%, and 21.2% of the number of total ginsenosides, respectively. The result gives some explanation why ginseng root is designated as medicinal parts rather than the other parts. Sixteen ginsenosides are commonly existed in all tissues, and among them, there are nine PPD type (Rc, Rd, Rb2, Rb1, Rb3, m-ginsenoside Rb1, m-ginsenoside Rc, m-ginsenoside Rb2, m-ginsenoside Rd), six PPT type (Re, Rg1, Rf, 20(R)-ginsenoside Rg2, Notoginsenoside R1, m-ginsenoside Re), one OA type (Ro), and none of C17SCV type. Numbers of ginsenosides shared by R&S and F&P, F&P and L&S, L&S and F&B, R&S and F&B were 32 (18.8%), 37 (21.7%), 24 (14.1%), and 19(11.2%), respectively. In addition, 13 malonyl-ginsenosides were existing specifically in flowers and buds; however, none of them was observed in fruit. This implies that these malonyl-ginsenosides show not only spatial specificity, but also temporal specificity. Here in, we speculate that malonyl-ginsenosides may play a physiological role during tissue development.
Figure 3.
Venn diagram of ginsenosides according to different parts of P. ginseng. R&S: Roots, rhizomes, and steamed roots; L&S: Leaves and stems; F&P: Fruits and fruit pedicels; F&B: Flowers and flower buds.
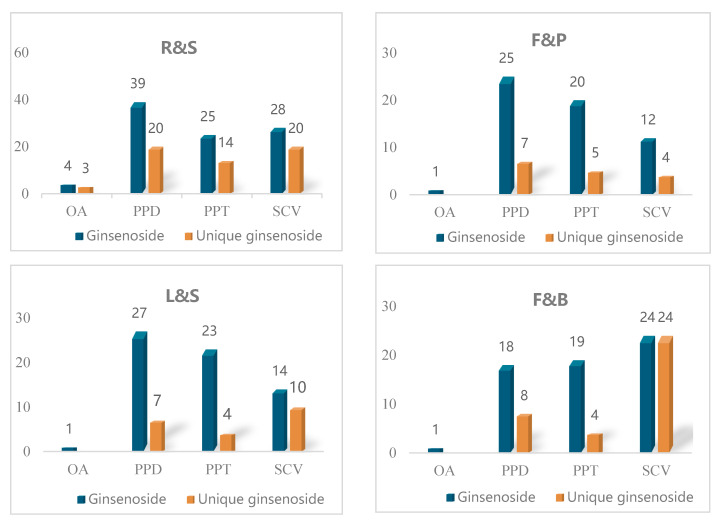
As indicated by Figure 4, the numbers of PPD-type ginsenosides (blue bar) are highest in R&S, F&P, and L&S, while the C17SCV-type ginsenoside is highest in F&B. Interestingly, C17SCV-type ginsenosides exhibit significant variation among different groups. Only nine C17SCV-type ginsenosides are shared by more than two groups, whereas the other 58 C17SCV-type ginsenosides are unique to a particular group. For the OA-type ginsenoside, three are specific to group R&S (Polyacetyleneginsenoside-Ro, Ginsenoside Ro methyl ester, Calenduloside-B) and one (Ginsenoside Ro) is commonly shared by all parts.
Figure 4.
Structural categories of ginsenosides in different parts of P. ginseng. R&S: Roots, rhizomes, and steamed roots; F&P: Fruits and fruit pedicels; L&S: Leaves and stems; F&B: Flowers and flower buds; OA: Oleanolic acid; PPD: Protopanaxadiol; PPT: Protopanaxatriol; C17SCV: C17 side-chain varied.
5. Isomers of Ginsenosides
The total 170 ginsenosides are divided into 69 molecular formula groups. Therefore, it is common that one molecular formula corresponds to several ginsenosides. (Table 2). The molecular formula with the largest number of isomers is C48H82O19 (molecular weight 962.5450), with a total of nine isomers; followed by C51H84O21 (molecular weight 1032.5505) with a total of eight isomers, and C41H70O13 (molecular weight 770.4816) with a total of seven isomers. The isomers median of 69 molecular formulas is 2, which means that one molecular formula corresponds to two isomers equally. Optical and position isomerism are the dominant types of ginsenoside isomers, whilst cis-trans isomerism and tautomerism are detected occasionally.
Table 2.
Isomers of 170 ginseng saponins.
| No. | Formula | Molecular Mass | No. of Isomers | No. | Formula | Molecular Mass | No. of Isomers |
|---|---|---|---|---|---|---|---|
| 1 | C24H40O4 | 392.2927 | 1 | 36 | C46H76O15 | 868.5184 | 2 |
| 2 | C30H50O2 | 442.3811 | 2 | 37 | C47H80O17 | 916.5396 | 6 |
| 3 | C30H50O3 | 458.3760 | 2 | 38 | C47H80O18 | 932.5345 | 2 |
| 4 | C30H52O3 | 460.3916 | 2 | 39 | C48H78O18 | 942.5188 | 1 |
| 5 | C30H52O4 | 476.3866 | 2 | 40 | C48H80O19 | 960.5294 | 1 |
| 6 | C30H54O4 | 478.4022 | 1 | 41 | C48H82O18 | 946.5501 | 3 |
| 7 | C30H54O5 | 494.3971 | 1 | 42 | C48H82O19 | 962.5450 | 9 |
| 8 | C35H62O11 | 658.4292 | 1 | 43 | C48H82O20 | 978.5400 | 4 |
| 9 | C36H60O10 | 652.4187 | 1 | 44 | C48H82O21 | 994.5349 | 1 |
| 10 | C36H60O7 | 604.4339 | 3 | 45 | C49H78O19 | 970.5137 | 1 |
| 11 | C36H60O8 | 620.4288 | 2 | 46 | C49H80O18 | 956.5345 | 1 |
| 12 | C36H60O9 | 636.4237 | 5 | 47 | C50H84O19 | 988.5607 | 3 |
| 13 | C36H62O10 | 654.4343 | 3 | 48 | C50H84O21 | 1020.5505 | 2 |
| 14 | C36H62O11 | 670.4292 | 2 | 49 | C51H84O21 | 1032.5505 | 8 |
| 15 | C36H62O8 | 622.4445 | 4 | 50 | C53H90O22 | 1078.5924 | 6 |
| 16 | C36H62O9 | 638.4394 | 4 | 51 | C53H90O23 | 1094.5873 | 1 |
| 17 | C37H62O10 | 666.4343 | 1 | 52 | C53H90O24 | 1110.5822 | 2 |
| 18 | C38H62O9 | 662.4394 | 2 | 53 | C54H87O24 | 1119.5587 | 1 |
| 19 | C41H70O12 | 754.4867 | 2 | 54 | C54H92O22 | 1092.6080 | 1 |
| 20 | C41H70O13 | 770.4816 | 7 | 55 | C54H92O23 | 1108.6029 | 1 |
| 21 | C41H70O15 | 802.4715 | 2 | 56 | C54H92O24 | 1124.5979 | 2 |
| 22 | C42H69O13 | 781.4738 | 1 | 57 | C55H92O23 | 1120.6029 | 4 |
| 23 | C42H70O12 | 766.4867 | 6 | 58 | C56H92O25 | 1164.5928 | 6 |
| 24 | C42H70O13 | 782.4816 | 4 | 59 | C56H96O24 | 1152.6292 | 1 |
| 25 | C42H70O14 | 798.4766 | 2 | 60 | C57H93O23 | 1145.6108 | 1 |
| 26 | C42H72O13 | 784.4973 | 5 | 61 | C57H94O23 | 1146.6186 | 2 |
| 27 | C42H72O14 | 800.4922 | 5 | 62 | C57H94O25 | 1178.6084 | 1 |
| 28 | C42H72O15 | 816.4871 | 4 | 63 | C58H96O24 | 1176.6292 | 2 |
| 29 | C42H72O16 | 832.4820 | 2 | 64 | C58H98O26 | 1210.6346 | 2 |
| 30 | C44H72O13 | 808.4973 | 2 | 65 | C59H100O27 | 1240.6452 | 1 |
| 31 | C44H74O14 | 826.5079 | 2 | 66 | C60H99O27 | 1251.6373 | 1 |
| 32 | C44H74O15 | 842.5028 | 2 | 67 | C62H102O27 | 1278.6608 | 1 |
| 33 | C45H74O17 | 886.4926 | 1 | 68 | C62H102O30 | 1326.6456 | 2 |
| 34 | C45H76O19 | 920.4981 | 1 | 69 | C65H100O21 | 1216.6757 | 1 |
| 35 | C45H76O20 | 936.4930 | 1 |
6. Mass Spectrometry-Based Metabolomics Analysis on P. ginseng
Recently, MS and its hyphenations with chromatographic separation techniques have emerged as an instrumental trend in ginsenoside analysis [93,94]. HPLC/MS can overcome the problems related to ginsenoside pre-analysis derivatization and the low abundance of molecular ions [95,96]. The use of on-line MS detection shows superior sensitivity and specificity compared with conventional UV and ELSD detection [97,98]. The sensitivity of MS detection can surpass 1000 times that of UV absorbance [99]. In addition, the possible matrix effects encountered with many Panax ginseng formulations may be compromised by MS [100]. Despite these advantages, MS remains costly for use in routine analysis. With the development of soft ionization techniques, HPLC/MS has been successfully applied for the qualitative and quantitative analyses of Panax ginseng [101]. Among the various mass spectrometry ionization techniques, electrospray mass spectrometry (ESI-MS) is the approach that is most commonly coupled with HPLC [15,102,103]. While ESI-MS suffers from matrix-induced ionization suppression difficulties [104], atmospheric pressure chemical ionization (APCI) can offer itself as one possible alternative [105]. Quadrupole time-of-flight mass spectrometry (QTOF-MS), a powerful tool for the identification of analytes, provides several advantages in structural analysis, such as a higher resolution and accuracy in mass measurements. Coupled with QTOF-MS, UPLC has been introduced for metabolite profiling and metabolomics purposes [99]. In recent years, orbitrap technology has achieved great breakthrough in resolution and scanning speed and realized the high-resolution detection of multi-stage mass spectrometry by combining the linear ion trap and quadrupole mass spectrometry, which can be widely applied in the development of new drugs [106].
According to the available literature, Wang et al. in 1999 [97] firstly identified ginsenosides by LC/MS/MS and differentiated P. ginseng and P. quinquefolius based on the ginsenoside Rg1/Rf and Rc/Rb2 ratios. A liquid chromatography-tandem mass spectrometry (LC/MS/MS) method was developed to distinguish Asian ginseng and North American ginseng. The method is based on the baseline chromatographic separation of two potential chemical markers: Rf and 24(R)-pseudo ginsenoside F11 [107]. Z X. et al. 2000 developed a similar LC/MS/MS method to determine ginsenoside in ginseng. Nine ginsenosides were determined, among which five of them were identified according to molecular weight [108]. In the late 1990s and early 2000s, the resolution of mass spectrometry was low and the number of identified ginsenosides was limited, which could be used for distinguishing Asian ginseng and American Ginseng, and identifying ginsenosides.
Chen et al. [109] established a chemical finger-print metabolomics approach using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS). The method was successfully used to authenticate and evaluate Panax Ginseng of various commercial grades. Using UPLC-QTOF-MS/MS, Zhang et al. evaluated the overall quality of commercially available white ginseng and red ginseng, and investigated their characteristic chemical composition indicators. Fifty-one major chromatographic peaks of white ginseng and red ginseng samples were separated within 24 min [110]. By means of UPLC-DAD-QTOF-MS/MS, Wang et al. conducted qualitative and quantitative analysis of ginsenosides of cultivated ginseng and mountain ginseng. A total of 131 ginsenosides were detected in cultivated ginseng and mountain ginseng, and all the components were completely separated within 10 min, among which contents of 19 typical ginsenoside were accurately quantified. This method has been validated for quality evaluation of ginseng and identification of cultivated ginseng and mountain ginseng [13]. Zhang et al. Quickly and comprehensively identified the ginsenosides using high-resolution time-of-flight mass spectrometry, electrospray dual-spray ion source, and negative ion mode. A total of 95 saponins in suncured ginseng were identified within 11 min, providing a feasible basis for the quality control of suncured ginseng [111]. With the emergence of high-resolution mass spectrometry and the development of high-throughput screening technologies, several time-saving methods were established for commercial ginseng product evaluation.
Since 2015, Orbitrap mass spectrometer had been applied in ginsenoside detection. In 2017, a total of 101 malonyl-ginsenosides were firstly systematic analyzed by hybrid LTQ-Orbitrap mass spectrometer after UHPLC separation, and ten potential malonyl-ginsenoside markers were discovered for the discrimination of P. ginseng, P. quinquefolius, and P. notoginseng [112]. Shi et al. established an untargeted profiling strategy on a linear ion-trap/Orbitrap mass spectrometer coupled to ultra-high performance liquid chromatography to analyze malonyl-ginsenosides in several Panax species. Finally, 178 malonyl-ginsenosides were characterized from roots, leaves, and flower buds of P. ginseng, P. quinquefolius, and P. notoginseng [113]. To investigate the variation of ginsenosides among different processed red ginseng, Zhong et al. tested steamed, vinegared and dried red ginseng samples by UPLC-Q-Orbitrap MS. In total, 32 ginsenosides were identified and ginsenosides m-Rb1, Rh1, F1, 20(R)-Rh1, Rg5, and Rs5 were only found in red ginseng processed by vinegar [114]. With the development of Orbitrap and multi-mass spectrometry techniques, ginsenosides with complex structures, such as malonyl and C17 side-chain variation, have been increasingly detected, and the types of ginsenosides have been greatly extended.
7. Conclusions
In this review, we summarized the existing studies related to saponin analysis of P. ginseng, and sorted out the information of structural characteristic, spatial distribution, and isomer of 170 ginsenosides. There are 16 common ginsenosides present in all parts of P. ginseng. In contrast, each part has unique ginsenosides, and ginsenosides in different parts show obvious structural diversity. It should be emphasized that ginseng aerial parts can regenerate every year, and there is a large amount of rare ginsenosides in stems, leaves, and flower buds. In light of previous research results of the rare ginsenoside bioactivity in red ginseng, it seems that the aerial parts of P. ginseng are highly worth developing and utilizing. A conclusion can also be drawn that C17SCV-type ginsenosides and malonyl-ginsenoside are rich in flowers and buds. Therefore, a hypothesis that ginsenosides have physiological roles in ginseng plant development is proposed. The rapid development of high-performance liquid chromatography and mass spectrometry techniques significantly raise the throughput and accuracy of ginsenoside determination.
In the future, (1) with the continuous advancement of detection and identification technology, the analysis method of ginsenosides will develop in the direction of being more sensitive, convenient, and environmentally-friendly, with high-throughput and high-precision. By leveraging these technologies, more monomer compounds will be separated and identified from ginseng, which will develop the knowledge of the diversity of chemical structure of ginsenosides. (2) It is necessary to conduct further research on spatial distribution of ginsenosides in different parts of ginseng, and multidisciplinary collaborations among genomics, proteomics, metabonomics, and transcriptomics could be used to study the physiological functions of ginsenosides. (3) With increasing separation of ginsenosides possessing a complex structure, such as malonyl and C17 side-chain variation, the pharmacological action and pharmacokinetics of these ginsenosides would be further studied to clarify the efficacy of ginseng.
Funding
This research was supported by the Youths of China (No. 31401606), the Project of the Jilin Province Department of Science and Technology, China (No. 20190201160JC), Central Public-interest Scientific Institution Basal Research Fund (No. 1610342019032 and No. 1610342020024), Jilin Province Development and Reform Commission (No. 2019C052-10), National Key Research and Development Project (No. 2017YFC1702101), and Technology Key Project of Jilin Province (No. 20180201006YY).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang H.P., Zhang Y.B., Yang X.W., Yang X.B., Xu W., Xu F., Cai S.Q., Wang Y.P., Xu Y.H., Zang L.X. High-performance liquid chromatography with diode array detector and electrospray ionization ion trap time-of-flight tandem mass spectrometry to evaluate ginseng roots and rhizomes from different regions. Molecules. 2016;21:603. doi: 10.3390/molecules21050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Qi L.W., Wang C.Z., Yuan C.S. ChemInform abstract: Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin B.H., Kwon S.W., Jeong Hill Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015;37:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Z.Y., Zeng J.Z., Wong A.S.T. Chemical structures and pharmacological profiles of ginseng saponins. Molecules. 2019;24:2443. doi: 10.3390/molecules24132443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim K.H., Cho J.Y., Kim B., Bae B.S., Kim J.H. Red ginseng (panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J. Med. Food. 2014;17:111. doi: 10.1089/jmf.2013.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung S.I., Kang M.Y., Lee S.C. In vitro and in vivo antioxidant activity of aged ginseng (panax ginseng) Prev. Nutr. Food Sci. 2016;21:24–30. doi: 10.3746/pnf.2016.21.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelova N., Kong H.W., Heijden R., Yang S.Y., Choi Y., Kim H., Wang M., Hankemeier T., van der Greef J., Xu G., et al. Recent methodology in the phytochemical analysis of ginseng. Phytochem. Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 9.Woo H.C., Shin B.K., Cho I., Koo H., Kim M., Han J. Anti-obesity Effect of Carbon Dioxide Supercritical Fluid Extracts of Panax Ginseng C. A. Meyer. J. Korean Soc. Appl. BI. 2011;54:738–743. doi: 10.1007/BF03253153. [DOI] [Google Scholar]
- 10.Kim Y.J., Zhang D.Z., Yang D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015;33:717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Lü J.M., Yao Q., Chen C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X.P., Lin Y., Hu Y., Liu C.X., Lan K., Jia W. Phytochemistry, Metabolism, and Metabolomics of Ginseng. Chin. Herb. Med. 2015;7:98–108. doi: 10.1016/S1674-6384(15)60026-0. [DOI] [Google Scholar]
- 13.Yang X.W. Triterpenoids in Panax ginseng. Mod. Chin. Med. 2016;18:7–15. doi: 10.13313/j.issn.1673-4890.2016.1.003. [DOI] [Google Scholar]
- 14.Wang H.P., Zhang Y.B., Yang X.W., Zhao D.Q., Wang Y.P. Rapid characterization of ginsenosides in the roots and rhizomes of panax ginseng by UPLC-DAD-QTOF-MS/MS and simultaneous determination of 19 ginsenosides by HPLC-ESI-MS. J. Ginseng Res. 2015;40:382–394. doi: 10.1016/j.jgr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao Q., Bai M., Xu J.D., Kong M., Zhu L.Y., Zhu H., Wang Q., Li S.L. Discrimination of leaves of Panax ginseng and P. quinquefolius by ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J. Pharm. Biomed. Anal. 2014;97C:129–140. doi: 10.1016/j.jpba.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.W., Choi B.R., Kim Y.C., Choi D., Lee Y.S., Kim G.S., Baek N.I., Kim S.Y., Lee D.Y. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. 2017;22:2147. doi: 10.3390/molecules22122147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu S.F., Zhang J.T. New achievements in ginseng research and its future prospects. Chin. J. Integr. Med. 2009;15:403–408. doi: 10.1007/s11655-009-0403-6. [DOI] [PubMed] [Google Scholar]
- 18.Jia L., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional chinese medicine. Curr. Med. Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garriques S.S. On panaquilon, a new vegetable substance. Am. J. Pharm. 1854;90:231–234. [Google Scholar]
- 20.Shibata S., Tanaka O., Sado M., Tsushima S. On genuine sapogenin of ginseng. Tetrahedron Lett. 1963;4:795–800. doi: 10.1016/S0040-4039(01)90718-X. [DOI] [Google Scholar]
- 21.Sanada S., Kondo N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng. I. structures of Ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem. Pharm. Bull. 1974;22:421–428. doi: 10.1248/cpb.22.421. [DOI] [Google Scholar]
- 22.Sanada S., Kondo N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng. II. Structures of ginsenoside Re, -Rf and -Rg2. Chem. Pharm. Bull. 1974;22:2407–2412. doi: 10.1248/cpb.22.2407. [DOI] [Google Scholar]
- 23.Nagai Y., Tanaka O., Shibata S. Chemical studies on the oriental plant drugs—XXIV: Structure of ginsenoside-Rg1, a neutral saponin of ginseng root. Tetrahedron. 1971;27:881–892. doi: 10.1016/S0040-4020(01)92488-3. [DOI] [Google Scholar]
- 24.Shibata Y., Nozaki T., Higashi T., Sanada S., Shoji J. Saponins of leaves of Panax ginseng C.A. Meyer. Chem. Pharm. Bull. 1976;24:2204–2209. [Google Scholar]
- 25.Yahara S. Sapinins of bads and flowers of Panax ginseng C. A. Meyer isolation of ginsenside Rd, Re, Rg1. Chem. Pharm. Bull. 1976;27:3212. doi: 10.1248/cpb.24.3212. [DOI] [Google Scholar]
- 26.Sanada S., Shoji J. Studies on the Saponins of ginseng III, Structures of ginsenoside-Rb3 and 20-Glucoginsenoside-Rf. Chem. Pharm. Bull. 1978;26:1694–1697. doi: 10.1248/cpb.26.1694. [DOI] [Google Scholar]
- 27.Cai P. Isolation and identification of ginsenosides in ginseng leaves of Jilin Province. Chinese Pharm. Bull. 1982;17:500–502. [Google Scholar]
- 28.Shao C.J., Xu J.D., Jiang X.K., Cheng G.R. Studies on the chemical constituents of flower-buds of Panax ginseng C.A. Meyer in Jilin(I) J. Jilin Univ. Med. Ed. 1984;4:21–24. [Google Scholar]
- 29.Shao C.J., Xu J.D. Chemical studies on the tetracyclic tirterpenic saponins in flower-buds of Panax ginseng C.A. Meyer. Chem. J. Chinese Unvi. 1984;5:20–23. [Google Scholar]
- 30.Xu S.X., Wang N.L., Shen M., Lu X.K. Chemical constituents of saponins of stems and leaves of Panax ginseng C.A. Meyer. Acta Bot. Sin. 1986;28:95–101. [Google Scholar]
- 31.Chen Y.J., Xu S.X., Ma Q.F. Study on the new minor new constituents of ginseng leaves. Acta Pharm. Sin. 1987;22:685–688. [Google Scholar]
- 32.Zhang S., Chen Y., Cui C., He G., Xu S., Pei Y., Yao X., Zhu T. A new minor saponin from the leaves of Panax ginseng C. A. Meyer. Acta Pharm. Sin. 1989;24:877–879. [PubMed] [Google Scholar]
- 33.Chen Y., Zhang S., Wang Z., Lu Y., Xu S., Yao X., Cui C., Tezuka Y., Kikuchi T., Ogihara Y. Isolation and elucidation of a new minor saponin from the leaves of Panax ginseng C.A. Meyer. Acta Pharm. Sin. 1990;25:379–381. [PubMed] [Google Scholar]
- 34.Kim D.S., Chang Y.J., Zedk U., Zhao P., Lin Y.Q., Yang C.R. Dammarane saponins from Panax ginseng. Phytochemistry. 1995;40:1493–1497. doi: 10.1016/0031-9422(95)00218-V. [DOI] [PubMed] [Google Scholar]
- 35.Dou D., Wen Y., Weng M., Pei Y., Chen Y. Studies on the Minor Saponins from Leaves of Panax ginseng C.A.Meyer. China J. Chin. Mat. Med. 1997;22:35–37. [PubMed] [Google Scholar]
- 36.Dou D.Q., Hou W.B., Chen Y.J. Studies of the Characteristic Constituents of Chinese Ginseng and American Ginseng. Planta Med. 1998;64:555–560. doi: 10.1055/s-2006-957526. [DOI] [Google Scholar]
- 37.Dou D.Q., Chen Y.J., Liang L.H., Pang F.G., Shimizu N., Takeda T. Six New Dammarane-type Triterpene Saponins from the Leaves of Panax ginseng. Chem. Pharm. Bull. 2001;49:442–446. doi: 10.1248/cpb.49.442. [DOI] [PubMed] [Google Scholar]
- 38.Dou D.Q., Ren J., Chen Y., Pei Y.P., Chen Y.J. Study on the chemical constituents of the roots of commercial ginseng. China J. Chin. Mater. Med. 2003;28:522–524. [PubMed] [Google Scholar]
- 39.Liu C.X., Xiao P.G. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-X. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa T., Oura H., Choi J.H., Bae J.W. Application of high-per-formance liquid chromatography to the isolation of ginsenosides from ginseng saponins; Proceedings of the Ginseng society Conference; Seoul, Korea. September 1980; pp. 207–215. [Google Scholar]
- 41.Shibata S., Tanaka O., Ando T., Sado M., Tsushima S., Ohsawa T. chemical studies on oriental plant drugs. XIV. Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem. Pharm. Bull. 1966;14:595–600. doi: 10.1248/cpb.14.595. [DOI] [PubMed] [Google Scholar]
- 42.Liu F.F., Zhang A.H., Lei F.J., Xiu Y.H., Zhang L.X. Inhibiting effects of total ginsenosides of ginseng stems and leaves against Fusarium solani and their antibacterial mechanism. J. Jilin Agric. Univ. 2018;40:85–91. doi: 10.13327/j.jjlau.2017.3633. (In Chinese) [DOI] [Google Scholar]
- 43.Zhang A.H., Tan S.Q., Zhao Y., Feng J.L., Zhang L.X. Effects of total ginsenosides on the feeding behavior and two enzymes activities of Mythimna separata (Walker) Larvae. Evid. Based Complementary Altern. Med. 2015;10:1–6. doi: 10.1155/2014/451828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H., Lu Z., Tan G.T., Qiu S., Farnsworth N.R., Pezzuto J.M., Fong H.H.S. Polyacetylene ginsenoside-Ro, a novel triterpene saponin from Panax ginseng. Tetrahedron Lett. 2002;43:973–977. doi: 10.1016/S0040-4039(01)02310-3. [DOI] [Google Scholar]
- 45.Zhou Q.L., Xu W., Yang X.W. Chemical constituents of Chinese red ginseng. China J. Chin. Mater. Med. 2016;41:233–249. doi: 10.4268/cjcmm20160214. [DOI] [PubMed] [Google Scholar]
- 46.Lee D.G., Lee J., Yang S., Kim K.T., Lee S. Identification of dammarane-type triterpenoid saponins from the root of Panax ginseng. Nat. Prod. Sci. 2015;21:111–121. [Google Scholar]
- 47.Yang W.Z., Ye M., Qiao X., Liu C.F., Miao W.J., Bo T., Tao H.Y., Guo D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal. Chim. Acta. 2012;739:56–66. doi: 10.1016/j.aca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Besso H., Kasai R., Saruwatari Y., Fuwa T., Tanaka O. Ginsenoside-Ra1 and ginsenoside-Ra2, new dammarane-saponins of ginseng roots. Chem. Pharm. Bull. 1982;30:2380–2385. doi: 10.1248/cpb.30.2380. [DOI] [Google Scholar]
- 49.Matsuura H., Kasai R., Tanaka O., Saruwatari Y., Kunihiro K., Fuwa T. Further studies on dammarane-saponins of ginseng roots. Chem. Pharm. Bull. 1984;32:1188–1192. doi: 10.1248/cpb.32.1188. [DOI] [Google Scholar]
- 50.Kasai R., Besso H., Tanaka O., Saruwatari Y., Fuwa T. Saponins of red ginseng. Chem. Pharm. Bull. 1983;31:2120–2125. doi: 10.1248/cpb.31.2120. [DOI] [Google Scholar]
- 51.Ruan C.C., Liu Z., Li X., Liu X., Wang L.J., Pan H.Y., Zheng Y.N., Sun G.Z., Zhang Y., Zhang L.X. Isolation and Characterization of a New Ginsenoside from the Fresh Root of Panax Ginseng. Molecules. 2010;15:2319–2325. doi: 10.3390/molecules15042319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun G.Z., Li X.G., Liu Z., Wang J.Y., Zheng Y.N., Yang X.W. Isolation and structure characterization of malonylnotoginsenoside-R4 from the root of Panax ginseng. Chem. J. Chin. Univ. 2007;28:1316–1318. [Google Scholar]
- 53.Zhu G.Y., Li Y.W., Hau D., Jiang Z.H., Yu Z.L., Fong W.F. Acylated Protopanaxadiol-Type Ginsenosides from the Root of Panax ginseng. Chem. Biodivers. 2011;8:1853–1863. doi: 10.1002/cbdv.201000196. [DOI] [PubMed] [Google Scholar]
- 54.Kaku T., Kawashima Y. Isolation and characterization of ginsenoside Rg2, 20R-prosapogenin, 20S-prosapogenin and D20 -prosapogenin. Chemical studies on saponins of Panax ginseng C.A. Meyer. Third report. Arzneimittelforschung. 1980;30:936–943. [PubMed] [Google Scholar]
- 55.Baek N.I., Kim J.M., Park J.H., Ryu J.H., Kim D.S., Lee Y.H., Park J.D., Kim S.I. Ginsenoside Rs3, a genuine dammarane-glycoside from Korean red ginseng. Arch. Pharm. Res. 1997;20:280–282. doi: 10.1007/BF02976158. [DOI] [PubMed] [Google Scholar]
- 56.Chen W., Balan P., Popovich D. Ginsenosides analysis of New Zealand grown forest Panax ginseng using LC-QTOF-MS/MS. J. Ginseng Res. 2020;44:552–562. doi: 10.1016/j.jgr.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao L., Li K., Li D., Gong X. Saponin Constituents from Fruits of Panax ginseng. Mod. Chin. Med. 2018;20:928–952. doi: 10.13313/j.issn.1673-4890.20180402004. [DOI] [Google Scholar]
- 58.Wang W., Rayburn E., Hill D., Wang H., Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother. Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 59.Xu M., Zhan Z.J., Zhang X.Y. Study on the chemical constituents of ginseng fruit. Zhong Cao Yao. 2007;38:667–669. [Google Scholar]
- 60.Lee M., Seo H., Singh D., Lee S.J., Lee C. Unraveling dynamic metabolomes underlying different maturation stages of berries harvested from Panax ginseng. J. Ginseng Res. 2019;2019. 44:413–423. doi: 10.1016/j.jgr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dou D.Q., Chen Y.J., Meng Z.Y., Wen Y., Pei Y.P., Xu S.X., Yao X.S., Kawai H., Fukushima H., Murkami Y. Two Minor Saponins from Leaves of Panax ginseng C. A. Meyer. J. Chin. Pharm. Sci. 1996;5:195–199. [Google Scholar]
- 62.Siddiqi M., Siddiqi M.Z., Sungeun A., Kang S., Kim Y.J., Natarajan S., Yang D.U., Yang D.C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang K., Ham J., Kim Y.J., Park J., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: Active components and action mechanism. J. Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S., Kim M.G., Ko S., Kim H.K., Leem K., Kim Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced by compound 48/80. J. Ginseng Res. 2014;38:89–96. doi: 10.1016/j.jgr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee C., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun M., Che Y., Liu Z. A tractable method for the preparation of the ginsenoside compounds O and Mc1. Anal. Methods. 2015;7:4757–4762. doi: 10.1039/C5AY00117J. [DOI] [Google Scholar]
- 67.Li F., Li Q., Wang J., Lv C., Song D., Liu P., Zhang D., Lu J. Chemical and bioactive comparison of flowers of Panax ginseng Meyer, Panax quinquefolius L. and Panax notoginseng Burk. J. Ginseng Res. 2016;41:487–495. doi: 10.1016/j.jgr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu S., Yang W.Z., Yao C., Shi X.J., Li J.Y., Lou Y., Duan Y.N., Wu W.Y., Guo D.A. Malonylginsenosides with potential antidiabetic activities from the flower buds of Panax ginseng. J. Nat. Prod. 2017;80:899–908. doi: 10.1021/acs.jnatprod.6b00789. [DOI] [PubMed] [Google Scholar]
- 69.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical Studies on Crude Drug Precession. I. On the Constituents of Ginseng Radix Rubra (1) Yakugaku Zasshi. 1983;103:612–622. doi: 10.1248/yakushi1947.103.6_612. [DOI] [PubMed] [Google Scholar]
- 70.Zhu G.Y., Li Y.W., Hau D., Jiang Z.H., Yu Z.L., Fong W.F. Protopanaxatriol-Type ginsenosides from the root of Panax ginseng. J. Agric. Food Chem. 2011;59:200–205. doi: 10.1021/jf1037932. [DOI] [PubMed] [Google Scholar]
- 71.Dou D.Q., Wen Y.E., Pei Y., Yao X.S., Chen Y., Kawai H., Fukushima H. Ginsenoside-Ia: A Novel Minor Saponin from the Leaves of Panax ginseng. Planta Med. 1996;62:179–181. doi: 10.1055/s-2006-957849. [DOI] [PubMed] [Google Scholar]
- 72.Ma H.Y., Gao H., Huang J., Sun B.H., Yang B. Three new triterpenoids from Panax ginseng exhibit cytotoxicity against human A549 and Hep-3B cell lines. J. Nat. Med. 2012;66:576–582. doi: 10.1007/s11418-012-0662-y. [DOI] [PubMed] [Google Scholar]
- 73.Yoshikawa M., Sugimoto S., Nakamura S., Sakumae H., Matsuda H. Medicinal Flowers. XVI. New Dammarane-Type Triterpene Tetraglycosides and Gastroprotective Principles from Flower Buds of Panax ginseng. Chem. Pharm. Bull. 2007;55:1034–1038. doi: 10.1248/cpb.55.1034. [DOI] [PubMed] [Google Scholar]
- 74.Yoshikawa M., Sugimoto S., Nakamura S., Matsuda H. Medicinal Flowers. XI. Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds of Panax ginseng. Chem. Pharm. Bull. 2007;55:571–576. doi: 10.1248/cpb.55.571. [DOI] [PubMed] [Google Scholar]
- 75.Park I., Han S., Kim J., Piao L., Kwon S., Kim N., Kang T., Park M., Park J. Four new acetylated ginsenosides from processed ginseng (sun ginseng) Arch. Pharm. Res. 2002;25:837–841. doi: 10.1007/BF02977001. [DOI] [PubMed] [Google Scholar]
- 76.Park I., Kim N., Han S., Kim J., Kwon S., Kim H.J., Park M., Park J. Three new dammarane glycosides from heat processed ginseng. Arch. Pharm. Res. 2002;25:428–432. doi: 10.1007/BF02976595. [DOI] [PubMed] [Google Scholar]
- 77.Lee S., Shon H., Choi C.-S., Tran Manh H., Min B., Bae K. Ginsenosides from Heat Processed Ginseng. Chem. Pharm. Bull. 2009;57:92–94. doi: 10.1248/cpb.57.92. [DOI] [PubMed] [Google Scholar]
- 78.Ryu J., Park J.-H., Eun J.-H., Jung J.-H., Sohn D. A dammarane glycoside from Korean red ginseng. Phytochemistry. 1997;44:931–933. doi: 10.1016/S0031-9422(96)00661-9. [DOI] [Google Scholar]
- 79.Lee S., Oh J., Na M. Updating chemical profiling of red ginseng via the elucidation of two geometric isomers of ginsenosides Rg9 and Rg10. Food Chem. 2013;141:3920–3924. doi: 10.1016/j.foodchem.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 80.Cho J.G., Lee D.Y., Shrestha S., Lee S.K., Kang H.M., Son S.H., Yang D.C., Baek N.I. Three New Ginsenosides from the Heat-Processed Roots of Panax ginseng. Chem. Nat. Compd. 2013;49:882–887. doi: 10.1007/s10600-013-0769-8. [DOI] [Google Scholar]
- 81.Wang J.Y., Li X.G., Zheng Y.N., Yang X. Isoginsenoside-Rh3, a new triterpenoid saponin from the fruits of Panax ginseng C. A. Mey. J. Asian Nat. Prod. Res. 2005;6:289–293. doi: 10.1080/10286020310001595980. [DOI] [PubMed] [Google Scholar]
- 82.Yu M., Zhao Y.Q. Chemical Study on triterpenoids in ginseng fruit. Zhong Cao Yao. 2004;35:1221–1223. [Google Scholar]
- 83.Han W., Lu S., Wen H., Xu L., Jin J., Tang S. Chemical constituents from fruit pedicels of Panax ginseng. Zhong Cao Yao. 2018;49:1751–1760. doi: 10.7501/j.issn.0253-2670.2018.08.003. [DOI] [Google Scholar]
- 84.Nguyen H., Song G., Kim J.A., Hyun J.H., Kang H.K., Kim Y.H. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia cells. Bioorganic Med. Chem. Lett. 2009;20:309–314. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 85.Wang L., Wu Z., Gao H., Huang J., Sun B., Wu L. A new compound with cytotoxic activities from the leaves of Panax ginseng C.A. Meyer. Chin. Chem. Lett. 2008;19:837–840. doi: 10.1016/j.cclet.2008.05.017. [DOI] [Google Scholar]
- 86.Wu L.J., Wang L.B., Gao H., Wu B., Song X.M., Tang Z.S. A new compound from the leaves of Panax ginseng. Fitoterapia. 2008;78:556–560. doi: 10.1016/j.fitote.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Lee D.Y., Lee J., Jeong Y.T., Byun G.H., Kim J.H. Melanogenesis inhibition activity of floralginsenoside A from panax ginseng berry. J. Ginseng Res. 2017;41:602–607. doi: 10.1016/j.jgr.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamura S., Sugimoto S., Matsuda H., Yoshikawa M. Structures of dammarane type triterpene triglycosides from the flower buds of Panax ginseng. Heterocycles. 2007;71:577–588. [Google Scholar]
- 89.Tung N.H., Song G.Y., Woo S.H., Hyun J.W., Koh Y.S., Kang H.K. Ginsenosides from the leaves and flower buds of panax ginseng and their pharmacological effects. Curr. Bioact. Compd. 2012;8:159–166. doi: 10.2174/157340712801784732. [DOI] [Google Scholar]
- 90.Qiu F., Ma Z., Xu S.X., Yao X.S., Che C.T., Chen Y.J. A Pair of 24-hydroperoxyl Epimeric Dammarane Saponins from Flower-buds of Panax Ginseng. J. Asian Nat. Prod. Res. 2001;3:235–240. doi: 10.1080/10286020108041396. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen H.T., Song G.Y., Nhiem N.X., Ding Y., Tai B.H., Jin L.G., Lim C.M., Hyun J.W., Park C.J., Kang H.K., et al. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J. Agric. Food chem. 2010;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- 92.Qiu F., Ma Z., Xu S., Yao X.S., Chen Y., Che Z. Studies on Dammarane-Type Saponins in the Flower-Buds of Panax ginseng C.A. Meyer. J. Asian Nat. Prod. Res. 1998;1:119–123. doi: 10.1080/10286029808039853. [DOI] [PubMed] [Google Scholar]
- 93.Fuzzati N. Analysis methods of ginsenosides. J. Chromatogr. B. 2005;812:119–133. doi: 10.1016/S1570-0232(04)00645-2. [DOI] [PubMed] [Google Scholar]
- 94.Baek S.H., Bae O.N., Park J. Recent Methodology in Ginseng Analysis. J. Ginseng Res. 2012;36:119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui J., Björkhem I., Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J. Chromatogr. B. 1997;689:349–355. doi: 10.1016/S0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- 96.Kim B.Y., Lee M., Cho K., Park J., Park M. Analysis of ginseng saponins by HPLC with photoreduction fluorescence detection. Arch. Pharmacol. Res. 1992;15:328–332. doi: 10.1007/BF02974107. [DOI] [Google Scholar]
- 97.Van Breemen R., Huang C.-R., Lu Z.-Z., Rimando A., Fong H., Fitzloff J. Electrospray Liquid Chromatography/Mass Spectrometry of Ginsenosides. Anal. Chem. 1995;67:3985–3989. doi: 10.1021/ac00117a026. [DOI] [Google Scholar]
- 98.Wang X., Sakuma T., Asafu-Adjaye E., Shiu G. Determination of ginsenosides in plant extracts from panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal. Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- 99.Chan D., But P.P.H., Cheng S.W., Kwok I.M.Y., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal. Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 100.Sloley B., Lin Y.C., Ridgway D., Semple H., Tam Y., Coutts R., Löbenberg R., Tam-Zaman N. A method for the analysis of ginsenosides, malonyl ginsenosides, and hydrolyzed ginsenosides using high-performance liquid chromatography with ultraviolet and positive mode electrospray ionization mass spectrometric detection. J. AOAC Int. 2006;89:16–21. doi: 10.1093/jaoac/89.1.16. [DOI] [PubMed] [Google Scholar]
- 101.Park H.W., In G., Kim J.H., Cho B.G., Han G.H., Chang I.M. Metabolomic approach for discrimination of processed ginseng genus (Panax ginseng and Panax quinquefolius) using UPLC-QTOF MS. J. Ginseng Res. 2014;38:59–65. doi: 10.1016/j.jgr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Popovich D., Kitts D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry. 2004;65:337–344. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 103.Sun B.S., Xu M.Y., Li Z., Wang Y.B., Sung C.K. UPLC-Q-TOF-MS/MS Analysis for steaming times-dependent profiling of steamed Panax quinquefolius and its ginsenosides transformations induced by repetitious steaming. J. Ginseng Res. 2012;36:277–290. doi: 10.5142/jgr.2012.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pascoe R., Foley J., Gusev A. Reduction in Matrix-Related Signal Suppression Effects in Electrospray Ionization Mass Spectrometry Using On-Line Two-Dimensional Liquid Chromatography. Anal. Chem. 2002;73:6014–6023. doi: 10.1021/ac0106694. [DOI] [PubMed] [Google Scholar]
- 105.Ma X., Xiao H., Liang X. Identification of Ginsenosides in Panax quinquefolium by LC-MS. Chromatographia. 2006;64:31–36. doi: 10.1365/s10337-006-0812-z. [DOI] [Google Scholar]
- 106.Xu W., Qiu X.-H., Zhang J., Zhu D.Y., Yang Y.M., Lu C.J. Analysis of saponins in Panax notoginseng by UPLC-LTQ-Orbitrap MS/MS. Acta Pharm. Sin. 2012;47:773–778. doi: 10.16438/j.0513-4870.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 107.Li W., Gu C., Zhang H., Awang D.V.C., Breemen R.B.V. Use of High-Performance Liquid Chromatography−Tandem Mass Spectrometry To Distinguish Panax ginseng C.A. Meyer (Asian Ginseng) and Panax quinquefolius L. (North American Ginseng) Anal. Chem. 2000;72:5417–5422. doi: 10.1021/ac000650l. [DOI] [PubMed] [Google Scholar]
- 108.Xü Z.X., Xiao H.B., Wang J.N., Liang X.M. Analysis of ginsenosides by high performance liquid chromatography/mass spectrometry/mass spectrometry (lc/ms/ms) Se Pu. 2000;18:521–524. [PubMed] [Google Scholar]
- 109.Chen Y., Zhao Z., Chen H., Qin M., Liang Z. Chemical Differentiation and Quality Evaluation of Commercial Asian and American Ginsengs based on a UHPLC–QTOF/MS/MS Metabolomics Approach. Phytochem. Anal. 2014;26:145–160. doi: 10.1002/pca.2546. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H.M., Li S.L., Zhang H., Wang Y., Zhao Z.L., Chen S.L., Xu H.X. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J. Pharm. Biomed. Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X.X., Wang H.P., Yang Y., Du M.B., Mao S., Chen C., Liu Y.X., Li S.J. Rapid analysis of ginsenosides in dried fresh Ginseng by ultra -performance liquid chromatography with quadrupole time-of-flight mass spectrometry. China Medical Herald. 2015;12:130–136. [Google Scholar]
- 112.Shi X.J., Yang W.Z., Qiu S., Yao C., Shen Y., Pan H.Q., Bi Q.R., Yang M., Wu W.Y., Guo D.A. An in-source multiple collision-neutral loss filtering based nontargeted metabolomics approach for the comprehensive analysis of malonyl-ginsenosides from Panax ginseng, P. quinquefolius, and P. notoginseng. Anal. Chim. Acta. 2016;952:59–70. doi: 10.1016/j.aca.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 113.Shi X., Yang W., Huang Y., Hou J., Qiu S., Yao C., Feng Z., Wei W., Wu W., Guo D. Direct screening of malonylginsenosides from nine Ginseng extracts by an untargeted profiling strategy incorporating in-source collision-induced dissociation, mass tag, and neutral loss scan on a hybrid linear ion-trap/Orbitrap mass spectrometer coupled to ultra-high performance liquid chromatography. J. Chromatogr. A. 2018;1571:213–222. doi: 10.1016/j.chroma.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 114.Zhong W., Dai Y.L., Li X.Y., Wang Y.B., Liu S.Y. Analysis of chemical differences in red ginseng by Uplc-Q-Orbitrap mass spectrometry. J. Chin. Mass Spectrom. Soc. 2015;36:529–536. doi: 10.7538/zpxb.youxian.2015.0030. [DOI] [Google Scholar]