To the Editor:
With the emergence of SARS‐CoV‐2, there are still many unknowns regarding the effects on pediatric patients with sickle cell disease (SCD). We report a case of a 6‐month‐old male child with history of hemoglobin SC (Hgb SC) disease who contracted SARS‐CoV‐2. He initially presented to the pediatric emergency department with 1 day of fever (101.4°F) and nasal congestion. The day prior to presentation he had increased fussiness and rhinorrhea. He had been breastfeeding well with a normal number of wet diapers but had one episode of nonbilious nonbloody emesis.
In the emergency department, he was febrile to 38.4°C with a pulse of 177 beats per minute, respiratory rate of 46 breaths per minute, automated blood pressure of 136/42 mm Hg, and oxygen saturation of 99% in room air. Further laboratory evaluation showed a WBC of 11.5 K/mcL with 64% neutrophils and a C‐reactive protein (CRP) of 0.7 mg/dL (Tables S1 and S2). Hgb was 9.0 g/dL with an mean corpuscular volume (MCV) of 62 fL (Table S1). A chest X‐ray (CXR) demonstrated perihilar streaky opacities consistent with a viral process (Figure 1). Nasopharyngeal SARS‐CoV‐2 testing by reverse transcriptase PCR (RT‐PCR) was positive.
FIGURE 1.
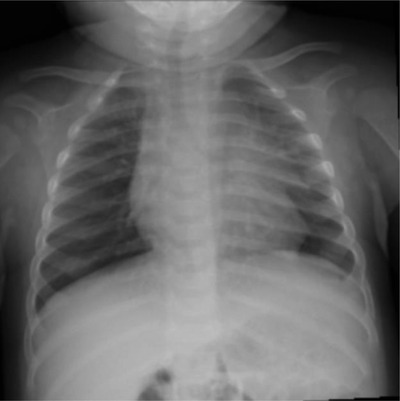
Chest X‐ray of the patient at presentation in emergency department
During his 2‐day inpatient hospitalization, the patient was placed in a negative pressure room on airborne precautions with continuous cardiac monitoring and pulse oximetry. The patient remained on room air without signs of hypoxia or cardiac instability throughout hospitalization. He received IV ceftriaxone 75 mg/kg/dose once daily for 48 hours. A blood culture obtained on admission did not have any growth after 5 days of incubation. Laboratory work during hospitalization showed an improvement in WBC with stable electrolytes (Tables S1 and S2). On admission, he had an elevated D‐dimer of 710 ng/mL, which decreased to 590 ng/mL on the day of discharge (Table S3). His laboratory data included ferritin 18.9 ng/mL, iron 37 mcg/dL, transferrin 311 mg/dL, total iron bonding capacity 444 mcg/dL, and iron saturation 8%; indicated a microcytic anemia due to iron deficiency. Throughout hospitalization, he remained medically stable.
More than half of all the children with SCD experience at least one episode of acute chest syndrome (ACS) in the first decade of life. 1 ACS is responsible for 25% of deaths in hospitalized SCD patients. 2 , 3 Current guidelines recommend that patients presenting with exam findings for ACS should be treated empirically and have a chest X‐ray. 4 However, radiological signs can be delayed compared to physical symptoms, so a normal X‐ray does not preclude the diagnosis of ACS if there is clinical suspicion. 4
Pediatric reports from Wuhan, China indicate that, of the RT‐PCR SARS‐CoV‐2 confirmed cases, 40.9% had moderate severity of illness, 2.5% had severe illness, and 0.4% were critically ill, which was defined as children who developed acute respiratory distress syndrome (ARDS). 5 Of the pediatric patients who became critically ill, the highest percentage (1.9%) was seen in patients aged <1‐year old. 5 The overall median age of onset in the Wuhan patient population was 7 years old with a 56.6% male predominance. 5 Cruz and Zeichner reported that 5% of symptomatic children have hypoxia, of which 0.6% progressed to ARDS. 6 Wu et al study of 201 adult patients from Wuhan, China with COVID‐19 showed that 95% had bilateral pulmonary infiltrates on CXR and 5% had unilateral infiltrates. 7 Patients who developed ARDS had higher temperatures and dyspnea prior to admission. 7 Nur et al reported two young adult patients with SCD and COVID‐19 who both developed vaso‐occlusive crisis (VOC) and ACS but had no flu‐like symptoms or findings characteristic of COVID‐19 on noncontrast chest computed tomography. 8 Another case report of a 21‐year‐old patient with HbS/β thalassemia with VOC received hydroxychloroquine, supplemental oxygen, and an exchange transfusion after which symptoms resolved. 9 The success of combining standard ACS therapies as well as tocilizumab, an antihuman IL‐6 receptor monoclonal antibody, is attributed to the abnormal high IL‐6 levels in SCD during VOC. 10 , 11 However, uncertainty remains regarding novel COVID‐19 treatments and their efficacy.
Reports of adult patients with COVID‐19 have shown a correlation between hypercoagulability, particularly higher D‐dimer, and increased mortality. 8 Data from Wuhan has shown that an increase in D‐dimer >1 mcg/mL was associated with fatal outcomes during hospitalization. 12 SARS‐CoV‐2 infection is thought to increase patient's hypercoagulability due to the proinflammatory cytokine response that can induce procoagulant factors causing thrombosis. 12 This raises concern for a higher risk of disseminated intravascular coagulation, which increases mortality rates. The higher risk of hyperviscosity in SCD patients indicates that close monitoring of D‐dimer levels might be beneficial. In our pediatric case, D‐dimer levels were elevated upon presentation, but improved prior to discharge. Further data analyses are needed to better correlate D‐dimer to disease severity.
To our knowledge, this is the first case report of a pediatric sickle cell patient with COVID‐19. The mild presentation and ultimate good outcome indicate that not all infants with comorbid conditions, including SCD, will develop severe outcomes following SARS‐CoV‐2 infection. It is, however, still critically important to monitor these patients carefully in a controlled setting due to the risk of ACS and other complications.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Supporting information
Supporting information
Supporting information
REFERENCES
- 1. Jain S, Bakshi N, Kristhnamurti L. Acute chest syndrome in children with sickle cell disease. Pediatr Allergy Immunol Pulmonol. 2017;30(4):191‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vinchinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood. 1997;89(5):1787‐1792. [PubMed] [Google Scholar]
- 3. Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84(2):643‐649. [PubMed] [Google Scholar]
- 4. Howard J, Hart N, Roberts‐Harewood M, Cummins M, Awogbade M, Davis B. Guideline on the management of acute chest syndrome in sickle cell disease. Br J Haematol. 2015;169(4):492‐505. [DOI] [PubMed] [Google Scholar]
- 5. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(5):e20200702. [DOI] [PubMed] [Google Scholar]
- 6. Cruz AT, Zeichner SL. COVID‐19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;145(5):e20200834. [DOI] [PubMed] [Google Scholar]
- 7. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biedmond BJ. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol. 2020. 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beerkens F, John M, Puliafito B, Corbett V, Edwards C, Tremblay D. COVID‐19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hemtol. 2020.. 10.1002/ajh.25809. [DOI] [PubMed] [Google Scholar]
- 10. De Luna G, Habibi A, Deux JF, et al. Rapid and severe COVID‐19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020.. 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020. 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information


