Since late 2019, a severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) viral outbreak has spread all over the world. The disease caused by SARS‐CoV‐2, named COVID‐19, has caused over 550 000 deaths and infected over 19 000 000 people worldwide (by Aug 7, 2020 1 ). Although individuals from all ages can be infected, the clinical prognosis is worse for patients harbouring one or more risk factors, including age, obesity, hypertension, diabetes, respiratory and/or cardiovascular disease, and cancer, or autoimmune diseases, among others. 2 , 3 , 4
Current models indicate that the surface unit (S1) of the spike (S) protein of SARS‐CoV‐2 binds to the angiotensin‐converting enzyme 2 (ACE2) as the entry receptor in host cells. SARS‐CoV‐2 uses then the host serine protease TMPRSS2 for S priming, to which fusion of viral and cellular membranes follows, allowing for viral entry into the cell and fast replication. 5 , 6 Although more information on the mechanisms of SARS‐CoV‐2 entry in different cell types is available on a daily basis, intra‐cellular modulation of viral replication is still under‐studied. Important intra‐cellular components for viral replication include mitochondria, which not only are involved in the production of cellular energy, regulation of redox and ionic fluxes, intermediate metabolism (including production of nucleotides) and regulation of cell death 7 , 8 but also have a critical role for the regulation of host innate immunity. 9 , 10
Interestingly, data obtained with SARS‐CoV, the aetiologic agent of the 2002‐2003 SARS outbreak, showed that SARS‐CoV‐encoded open reading frame‐9b (ORF‐9b) peptide localizes to mitochondria in A549 and HEK 293 cells and causes mitochondrial elongation, 11 by leading to ubiquitination and proteasomal degradation of dynamin‐like protein 1, a mitochondrial protein involved in mitochondrial fusion events. 12 Furthermore, the same paper demonstrated that ORF‐9b increased autophagy through ATG‐5‐mediated effects. Importantly, the viral peptide led to evasion of the host innate immunity by targeting the mitochondrial‐associated adaptor molecule MAVS (also known as ISP‐1/VISA/Cardiff) signalosome by usurping PCBP2 and the HECT domain E3 ligase AIP4. This chain of events leads to the degradation of MAVS, TRAF3 and TRAF 6, severely limiting the host capacity to trigger IFN responses. 11 The authors conclude that mitochondrial effects of the viral ORF‐9b peptide are a critical step in viral replication. This observation raises the interesting possibility that viral peptides from SARS‐CoV‐2 may alter mitochondrial functioning with selective advantages for viral proliferation. There is a gap in knowledge on how and why this may occur. It is likely that viral peptides may induce mitochondrial fusion to increase the respiratory activity of those organelles. Not only this remains to be determined, but also how this is selectively advantageous for viral replication is still not fully known (Figure 1).
Figure 1.
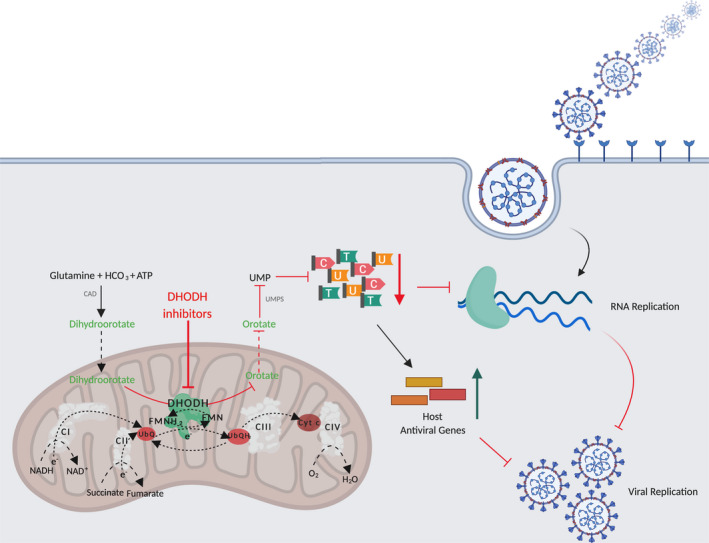
DHODH inhibitors prevent viral replication. Simplified depiction of de novo pyrimidine synthesis pathway and its role in viral replication. A series of enzymes catalyse the 6‐step pathway conversion of glutamine, bicarbonate (HCO3) and ATP into the final product, Uridine monophosphate (UMP), necessary for the production of pyrimidines. De novo pyrimidine synthesis is catalysed by the trifunctional CAD (carbamoyl phosphate synthase, aspartate carbamoyl transferase and dihydroorotase), DHODH and the bifunctional UMPS (orotate phosphoribosyltransferase and orotidine monophosphate decarboxylase). DHODH catalyses the fourth step, the oxidation of dihydroorotate to orotate, and is linked to the mitochondrial electron transport chain (ETC) via ubiquinone (UbQ) redox‐cycling. Inhibition of DHODH leads to pyrimidine nucleosides depletion that primarily affects RNA virus replication. The decrease of pyrimidine pools further triggers host antiviral genes expression and promotes innate immune responses. Both responses are a consequence of the reduction of pyrimidines and cause inhibition of virus replication and infection. Created with BioRender.com
Drugs targeting host metabolism may show advantage in comparison with virus‐targeted drugs, since they can be used against a wide spectrum of viruses and potentially overcome resistance. In the last decades, the host's pyrimidine biosynthesis pathway has become a potential target to fight viral, bacterial, parasitic and fungal infections. Several high‐throughput screenings for broad‐spectrum antivirals identified compounds which target de novo pyrimidine biosynthesis, in particular DHODH activity. These results highlighted the potential role of host DHODH targeting for antiviral activity. 13 , 14 , 15 , 16 Besides the salvage pathway, one particularly important cellular source of nucleotides for viral replication is the host de novo pyrimidine synthesis, which is catalysed by inner mitochondrial membrane protein Dihydroorotate Dehydrogenase (DHODH), in connection with the mitochondrial respiratory chain. DHODH converts dihydroorotate to orotate, which is channel to ultimately produce uridine monophosphate (UMP), the precursor for all pyrimidine nucleotides for RNA and DNA. 17 This enzyme is ubiquitously expressed in all tissues, including the liver and heart, 18 as well as lung, 19 linking mitochondrial respiration to pyrimidine synthesis. The link between OXPHOS and DHODH is maintained by CoQ redox‐cycling at mitochondrial complex III. 17 , 20
Two of the most well‐known DHODH inhibitors are brequinar and leflunomide. Brequinar competitively inhibits DHODH at the UbQ‐binding site, while leflunomide, which is metabolized to the active agent teriflunomide (A77 1726), promotes noncompetitively inhibition. None of the drugs interferes with the FMN‐binding site. 21 Leflunomide is well tolerated in humans, being clinically available since 1998 for a number of autoimmune, including treatment of psoriatic and rheumatoid arthritis, and virus‐related diseases. Teriflunomide has also been used for the treatment of multiple sclerosis. 22 Brequinar efficiently protects allograft and xenograft rejections following transplantation. 23
DHODH inhibitors were already demonstrated to have antiviral activity against positive and negative ‐sense RNA and DNA viruses, 13 , 14 namely foot‐and‐mouth disease virus, 24 Junín virus, 25 rotavirus 26 , and particularly against Ebola. 15 While the salvage pathway, that recycles products of DNA/RNA degradation and from nutrients, is usually sufficient to support nonrapidly proliferative cells, viruses ‘kidnap’ the de novo pyrimidine synthesis pathway of the infected‐host cells to sustain their rapid replication. Thus, targeting de novo pathway is an important approach, because a large nucleotide pool is more critical for pathogens replication, being less prone to cause toxicity. 22 A couple of studies suggests that the addition of DHODH inhibitors should occur at early stages of infection, since they interfere with viral RNA replication and act on early/middle stages of viral lifecycle. 13 , 24
Teriflunomide, the active open‐ring malononitrile metabolite of leflunomide, was indicated in the treatment of the single‐stranded foot‐and‐mouth disease virus. A concentration of 300 μM produced an antiviral activity and abolished viral mRNA levels, while the CC50 was set at 542.7 μM in IBRS‐2 cells. Moreover, a single 2 hours‐injection of teriflunomide concomitantly with lethal dose of foot‐and‐mouth disease virus exposure, increased survival rate in 25% at 96 hours post‐injection compared to nontreated controls. 24 A3, a small molecular weight pyrimidine biosynthesis inhibitor, was identified as an inhibitor of a broad spectrum of viruses, including 8 families of RNA, 2 of DNA and retroviruses, though with different effectiveness. Since addition of uracil or orotic acid reversed its antiviral effects, the possible mechanism of action is very likely to involve DHODH. 13 The inhibition of DHODH activity by A3 was found to be similar to the well‐known inhibitor teriflunomide, which further supported the idea of DHODH as the main target of A3. 27 The compound inhibited influenza virus replication in A549 cells, displaying an IC50 of 0.178 μM and a selective index of 1505. The selective index was even more representative in primary human tracheal‐bronchial epithelial cells, demonstrating a strong effect on viral replication. A3 inhibited virus polymerase function and RNA synthesis. 13 Other study also identified A3 as an antiviral drug against arenaviruses, further demonstrating its ability to hamper viral RNA replication and transcription. Moreover, the same study also suggested that A3 can induce mutations on the viral RNA that decrease the infectivity of the virus. 27 A high‐throughput screen for the detection of influenza A virus inhibitors revealed that FA‐613 has a high potential. Besides influenza A and B strains, FA‐613 showed antiviral activity against both SARS and MERS‐coronaviruses. However, its effects were not limited to the inhibition of RNA replication since FA‐613 also triggered host innate immunity. 14
This is clearly relevant as SARS‐CoV‐2 also impairs innate immune responses. 28 , 29 Thus, DHODH inhibitors could have antiviral capacity by inducing two different mechanism of action. If on one hand DHODH inhibition causes pyrimidine pool depletion and consequently interferes with replication of the viral genome, on the other hand it induces antiviral genes expression, namely interferon‐simulated genes, which stimulates innate immune responses. 14 , 15 , 16 Amino‐tetrahydrocarbazole‐containing compounds, as GSK983 and SW835, target DHODH activity and lead to activated ATM and IRF‐1 ‐dependent innate immune responses against Ebola virus. Thus, inhibition of de novo pyrimidine synthesis further increases the expression of interferon stimulated genes. Brequinar also showed similar effect. 15 Treatment with FA‐613, through the inhibition of DHODH, triggered the expression of the antiviral gene IFNB1, CXCL10 and ISG15. Survival of mice infected with H1N1 was prolonged by the treatment with FA‐613. 14 Another study used a cell‐based reporter screening to identify compounds that stimulate interferon‐inducible antiviral genes. The study found that DD264 enhances antiviral genes in human cells, which required the transcription factor IFR1. The authors further assumed that the antiviral activity of the pyrimidine biosynthesis inhibitor DD26 is rather caused through the activation of cellular defence responses. 16
Although FA‐613 lost its antiviral activity in interferon‐deficient Vero cell line, 14 A3 treatment did not show any induction of antiviral genes and exhibited antiviral properties in Vero cells. 13 This suggests that different DHODH‐targeting compounds can trigger different pathways, including through the activation of innate immune system. However, the mechanisms that mediate this response of the immune system is not known, and further studies are needed to understand if its activation is an alternative pathway or depends on pyrimidine synthesis inhibition.
Although in vitro studies have demonstrated exciting results, several DHODH inhibitors have failed in in vivo experiments or clinical trials, and this was usually associated with side effects, narrow therapeutic window and inconsistent pharmacokinetics. Leflunomide and teriflunomide are likely to have additional targets besides DHODH, promoting anti‐kinase effects or acting as aryl hydrocarbon antagonists. Another off‐target of leflunomide is probably CII, influencing mitochondrial ROS generation. 22 , 30 The compensation of pyrimidine pool through the salvage pathway was already demonstrated in vitro. In fact, addition of uridine abolished the beneficial effects achieved by several DHODH‐targeting drugs. 13 , 14 , 31 However, the circulating levels of uridine in mammalians (including humans), which are between 2 and 9 μM, 32 , 33 are usually, but not always, below the concentration levels used to reverse antiviral effects of different compounds in vitro. 16 , 26 , 34 , 35 Still, Wang et al showed that 5 μM of uridine partially restores viral replication of Dengue virus induced by NITD‐982. 35
The critical question focused in this paper is whether DHODH inhibitors can be used to treat COVID‐19. A paper by Xiong et al 36 demonstrated that DHODH inhibitors S312 and S416, with previously shown favourable drug‐like and pharmacokinetic profiles, 37 showed broad‐spectrum antiviral effects against various RNA viruses, including against SARS‐CoV‐2 in different cell models. In vivo effects were demonstrated against 4000 PFU of A/WSN/33 H1N1 and 600 PFU of A/SC/09 (H1N1), but not SARS‐CoV‐2 infection, although it is only a matter of time before more in vivo experiments are performed and published. Indeed, a phase I randomized clinical trial (NCT04425252) has just started to evaluate the effects of brequinar in hospitalized patients with COVID‐19 infection.
DHODH inhibitors as anti‐SARS‐coV‐2 therapies are gaining traction. Immunic, a US‐based company was awarded permission for a phase II clinical trial of IMU‐838, an oral selective immune modulator which blocks the metabolism of activated immune cells by inhibiting DHODH. 38 , 39 IMU‐838, also known as vidofludimus calcium, was initially proposed as a therapeutics for multiple sclerosis. 38 In this study, vidofludimus was determined to be 2.6 times more potent in inhibiting dehydroorotate oxidation by human DHODH compared to teriflunomide.
Leflunomide, teriflunomide and brequinar are usually well‐tolerated. Several animal studies and clinical trials have reported low‐grade adverse events. The main dose‐limiting side effect is usually associated with bone‐marrow suppression, including thrombocytopenia, leucopenia and anaemia, which is fully reversed upon treatment cessation. 30 , 40 , 41 It is also important to note that some of the side effects observed with these compounds could be triggered by off‐target effects instead of direct inhibition of host metabolism. 22
Potential strategies should then be developed to improve the therapeutic window of DHODH inhibitors in antiviral therapy, including compounds towards specific DHODH active sites. Targeting different pathways simultaneously, as the concomitantly use of presently used antiviral drugs with de novo pyrimidine biosynthesis inhibitors might also potentiate the benefits of antiviral treatments. For example, simultaneous in vitro addition of A3 and ribavirin, a ribosyl purine analog approved against a wide spectrum of virus, resulted in a more potent inhibition of viral activity than monotherapy. 27 An important approach to reduce cytotoxicity, maintaining antiviral activity against RNA viruses, such as SARS‐CoV‐2, is exogenous addition of deoxycytidine. 31 On the other hand, DHODH is located at the inner mitochondrial membrane. The use of mitochondrial‐targeted DHODH inhibitors, could further increase the biological activity and, at the same time, minimize the side effects.
RNA virus‐related infections are a public health issue responsible for millions of deaths worldwide every year. Virus‐specific prophylactic as well as antiviral treatments have been developed; however, they are not fully efficient and are useless against other RNA viruses. Each virus possesses its own replication machinery and is constantly mutating to evade from drugs that target viral proteins. Thus, targeting host pathways that are essential for their replication is an efficient therapeutic approach for a wide range of RNA viruses. Depletion of pyrimidine pool, achieved by DHODH inhibition, interferes with the transcription and replication of several viruses causing a halt in infection. Moreover, the stimulation of innate immune system, that induce elimination of the pathogen, is a more efficient strategy. In case of new outbreaks, as the one that we are experiencing nowadays, the repurposing of FDA approved drugs is crucial to reduce the time to find an efficient treatment. Furthermore, if molecules that target multiple viruses, instead of single virus‐directed drugs, are available in the market we would be more prepared for unforeseen pandemics.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
Work in the authors' laboratory is funded by FEDER funds through the Operational Programme Competitiveness Factors ‐ COMPETE and national funds by FCT ‐ Foundation for Science and Technology, grant UIDB/04539/2020.
REFERENCES
- 1. Johns Hopkins University & Medicine . COVID‐19 Map ‐ Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed August 7, 2020
- 2. Atzrodt CL, Maknojia I, McCarthy RDP, et al. A Guide to COVID‐19: a global pandemic caused by the novel coronavirus SARS‐CoV‐2. FEBS J. 2020. 10.1111/febs.15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang D, Comish P, Kang R. The hallmarks of COVID‐19 disease. PLoS Pathog. 2020;16(5):e1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clotman K, Twickler MB. Diabetes or endocrinopathy admitted in the COVID‐19 ward. Eur J Clin Invest. 2020;50(7):e13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581(7807):221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS‐CoV‐2 and discovery of its inhibitors. Nature. 2020;582(7811):289‐293. [DOI] [PubMed] [Google Scholar]
- 7. Branco AF, Ferreira A, Simões RF, et al. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46(3):285‐298. [DOI] [PubMed] [Google Scholar]
- 8. Scorrano L. Keeping mitochondria in shape: a matter of life and death. Eur J Clin Invest. 2013;43(8):886‐893. [DOI] [PubMed] [Google Scholar]
- 9. Wallings RL, Herrick MK, Tansey MG. Linking mitochondria to the immune response. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vilmen G, Glon D, Siracusano G, et al. BHRF1, a BCL2 viral homolog, disturbs mitochondrial dynamics and stimulates mitophagy to dampen type I IFN induction. Autophagy. 2020;1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi C‐S, Qi H‐Y, Boularan C, et al. SARS‐coronavirus open reading frame‐9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol. 2014;193(6):3080‐3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pernas L, Scorrano L. Mito‐morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505‐531. [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann H‐H, Kunz A, Simon VA, Palese P, Shaw ML. Broad‐spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci U S A. 2011;108(14):5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung NN, Lai KK, Dai J, et al. Broad‐spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J Gen Virol. 2017;98(5):946‐954. [DOI] [PubMed] [Google Scholar]
- 15. Luthra P, Naidoo J, Pietzsch CA, et al. Inhibiting pyrimidine biosynthesis impairs ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antiviral Res. 2018;158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucas‐Hourani M, Dauzonne D, Jorda P, et al. Inhibition of Pyrimidine Biosynthesis Pathway Suppresses Viral Growth through Innate Immunity. PLoS Pathog. 2013;9(10):e1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boukalova S, Hubackova S, Milosevic M, Ezrova Z, Neuzil J, Rohlena J. Dihydroorotate dehydrogenase in oxidative phosphorylation and cancer. Biochim Biophys acta Mol basis Dis. 2020;1866(6):165759. [DOI] [PubMed] [Google Scholar]
- 18. Löffler M, Jöckel J, Schuster G. Dihydroorotat‐ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol Cell Biochem. 1997;174(1/2):125‐129. [PubMed] [Google Scholar]
- 19. Namba T, Tanaka K‐I, Ito Y, et al. Induction of EMT‐like phenotypes by an active metabolite of leflunomide and its contribution to pulmonary fibrosis. Cell Death Differ. 2010;17(12):1882‐1895. [DOI] [PubMed] [Google Scholar]
- 20. Bajzikova M, Kovarova J, Coelho AR, et al. Reactivation of dihydroorotate dehydrogenase‐driven pyrimidine biosynthesis restores tumor growth of respiration‐deficient cancer cells. Cell Metab. 2019;29(2):399‐416.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu S, Neidhardt EA, Grossman TH, Ocain T, Clardy J. Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents. Structure. 2000;8(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 22. Munier‐Lehmann H, Vidalain P‐O, Tangy F, Janin YL. On dihydroorotate dehydrogenases and their inhibitors and uses. J Med Chem. 2013;56(8):3148‐3167. [DOI] [PubMed] [Google Scholar]
- 23. He T, Haapa‐Paananen S, Kaminskyy VO, et al. Inhibition of the mitochondrial pyrimidine biosynthesis enzyme dihydroorotate dehydrogenase by doxorubicin and brequinar sensitizes cancer cells to TRAIL‐induced apoptosis. Oncogene. 2014;33(27):3538‐3549. [DOI] [PubMed] [Google Scholar]
- 24. Mei‐jiao G, Shi‐fang LI, Yan‐yan C, et al. Antiviral Effects of Selected IMPDH and DHODH Inhibitors Against Foot and Mouth Disease Virus. Biomed Pharmacother. 2019;118:109305. [DOI] [PubMed] [Google Scholar]
- 25. Sepúlveda CS, García CC, Damonte EB. Antiviral activity of A771726, the active metabolite of leflunomide, against Junín virus. J Med Virol. 2018;90(5):819‐827. [DOI] [PubMed] [Google Scholar]
- 26. Chen S, Ding S, Yin Y, et al. Suppression of pyrimidine biosynthesis by targeting DHODH enzyme robustly inhibits rotavirus replication. Antiviral Res. 2019;167:35‐44. [DOI] [PubMed] [Google Scholar]
- 27. Ortiz‐Riano E, Ngo N, Devito S, et al. Inhibition of Arenavirus by A3, a Pyrimidine Biosynthesis Inhibitor. J Virol. 2014;88(2):878‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Golonka RM, Saha P, Yeoh BS, et al. Harnessing innate immunity to eliminate SARS‐CoV‐2 and ameliorate COVID‐19 disease. Physiol Genomics. 2020;52(5):217‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sykes DB, Kfoury YS, Mercier FE, et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell. 2016;167(1):171‐186.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deans RM, Morgens DW, Ökesli A, et al. Parallel shRNA and CRISPR‐Cas9 Screens Enable Antiviral Drug Target Identification. Nat Chem Biol. 2016;12(5):361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karle JM, Anderson LW, Erlichman C, Cysyk RL. Serum uridine levels in patients receiving N‐(phosphonacetyl)‐L‐aspartate. Cancer Res. 1980;40(8 Pt 1):2938‐2940. [PubMed] [Google Scholar]
- 33. Moyer JD, Oliver JT, Handschumacher RE. Salvage of circulating pyrimidine nucleosides in the rat. Cancer Res. 1981;41(8):3010‐3017. [PubMed] [Google Scholar]
- 34. Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1‐22. [DOI] [PubMed] [Google Scholar]
- 35. Wang Q‐Y, Bushell S, Qing M, et al. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J Virol. 2011;85(13):6548‐6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiong R, Zhang L, Li S, et al.Novel and potent inhibitors targeting DHODH are broad‐spectrum antivirals against RNA viruses including newly‐emerged coronavirus SARS‐CoV‐2. 10.1007/s13238-020-00768-w [DOI] [PMC free article] [PubMed]
- 37. Zhu J, Han L, Diao Y, et al. Design, synthesis, X‐ray crystallographic analysis, and biological evaluation of thiazole derivatives as potent and selective inhibitors of human dihydroorotate dehydrogenase. J Med Chem. 2015;58(3):1123‐1139. [DOI] [PubMed] [Google Scholar]
- 38. Muehler A, Peelen E, Kohlhof H, Gröppel M, Vitt D. Vidofludimus calcium, a next generation DHODH inhibitor for the Treatment of relapsing‐remitting multiple sclerosis. Mult Scler Relat Disord. 2020;43:102129. [DOI] [PubMed] [Google Scholar]
- 39. Muehler A, Kohlhof H, Groeppel M, Vitt D. Safety, Tolerability and Pharmacokinetics of Vidofludimus calcium (IMU‐838) After Single and Multiple Ascending Oral Doses in Healthy Male Subjects. Eur J Drug Metab Pharmacokinet. 2020. 10.1007/s13318-020-00623-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bork E, Vest S, Hansen HH. A phase I clinical and pharmacokinetic study of brequinar sodium, DUP 785 (NSC 368390), using a weekly and a biweekly schedule. Eur J Cancer Clin Oncol. 1989;25(10):1403‐1411. [DOI] [PubMed] [Google Scholar]
- 41. Read SW, DeGrezia M, Ciccone EJ, et al. The effect of leflunomide on cycling and activation of T‐cells in HIV‐1‐infected participants. PLoS One. 2010;5(8):e11937. [DOI] [PMC free article] [PubMed] [Google Scholar]


