Abstract
The ongoing coronavirus disease 2019 (COVID‐19) pandemic has led to a global public health emergency with the need to identify vulnerable populations who may benefit from increased screening and healthcare resources. Initial data suggest that overall, pregnancy is not a significant risk factor for severe coronavirus disease 2019 (COVID‐19). However, case series have suggested that maternal obesity is one of the most important comorbidities associated with more severe disease. In obese individuals, suppressors of cytokine signaling are upregulated and type I and III interferon responses are delayed and blunted leading to ineffective viral clearance. Obesity is also associated with changes in systemic immunity involving a wide range of immune cells and mechanisms that lead to low‐grade chronic inflammation, which can compromise antiviral immunity. Macrophage activation in adipose tissue can produce low levels of pro‐inflammatory cytokines (TNF‐α, IL‐1β, IL‐6). Further, adipocyte secretion of leptin is pro‐inflammatory and high circulating levels of leptin have been associated with mortality in patients with acute respiratory distress syndrome. The synergistic effects of obesity‐associated delays in immune control of COVID‐19 with mechanical stress of increased adipose tissue may contribute to a greater risk of pulmonary compromise in obese pregnant women. In this review, we bring together data regarding obesity as a key co‐morbidity for COVID‐19 in pregnancy with known changes in the antiviral immune response associated with obesity. We also describe how the global burden of obesity among reproductive age women has serious public health implications for COVID‐19.
Keywords: COVID‐19, maternal health, obesity, pneumonia, pregnancy
1. INTRODUCTION
The emerging coronavirus disease 2019 (COVID‐19) pandemic caused by infection with the novel betacoronavirus SARS‐CoV‐2 continues to challenge public health systems globally. Although the majority of patients with COVID‐19 have self‐limited disease consisting predominantly of mild respiratory symptoms, approximately 20%‐30% develop acute respiratory distress syndrome (ARDS). 1 , 2 , 3 , 4 The Centers for Disease Control have recently refined their risk categories for COVID‐19 to state that obesity was a major risk factor. 5 In pregnant women, severe COVID‐19 disease is also associated with obesity. 6 , 7 Over the last decade, non‐communicable metabolic diseases such as hypertension, diabetes, and obesity have increased in prevalence globally. 8 In the United States, more than one‐third of reproductive age women are considered to be obese (body mass index [BMI] ≥30 kg/m2). 9 At the same time, the immunology and pathophysiology associated with COVID‐19 in pregnancy and especially in the setting of other comorbidities such as obesity are poorly understood. This review is directed toward summarizing how obesity affects the severity of COVID‐19 clinical disease and negatively impacts the antiviral immune response.
2. OBESITY AS A RISK FACTOR FOR SEVERE COVID‐19
In non‐pregnant populations, obesity has been associated with severe COVID‐19 disease. A retrospective review of 770 patients with COVID‐19 from the two medical centers in New York found that obese patients were more likely to present with symptoms; obese patients also had a significantly increased risk of ICU admission or death (RR 1.58) even after adjusting for race, age, and troponin levels. 10 Another retrospective study from a third medical center in New York including 200 patients with COVID‐19 found that a BMI ≥35 kg/m2 was independently associated with higher in hospital mortality compared to a BMI of 25‐34 kg/m2 (adjusted odds ratio 3.78; 95% CI: 1.45‐9.83). Similarly, BMI ≥35 kg/m2 was a significant predictor for increasing oxygenation requirements and intubation. 11 An Italian retrospective study demonstrated similar findings with overweight or obese patients more often requiring ventilation and a higher level of care despite younger age than older patients with normal BMI. 12
Obese pregnant women are at increased risk for complications of viral infection from influenza, cytomegalovirus, and SARS‐CoV‐1 and related complications such as ARDS. 13 , 14 , 15 , 16 , 17 The increased risk of severe respiratory viral disease due to obesity and pregnancy was most striking with the H1N1 Pandemic in 2009. 17 In a study of hospitalized patients with a confirmed H1N1 influenza A viral infection in the United States, class III obesity was associated with hospitalization regardless of whether the patient had chronic medical conditions. 18 Immune changes in obesity have also been associated with increased susceptibility of viral infection including increased peak viral loads and delayed clearance in influenza. 19
Several case series and cohort studies have reported an increased severity of COVID‐19 in pregnancies complicated by elevated BMI and obesity (Table 1). An early report of two pregnant women with severe COVID‐19 necessitating ICU admission in the postpartum period was notable for a BMI of 38 and 47 kg/m2 in these cases. 20 In a cohort study of 46 pregnant women with COVID‐19 in Washington State, obesity emerged as a key co‐morbidity in women with severe COVID‐19 6 ; of five pregnant women with severe disease in which information to calculate the body mass index was available, four were overweight or obese prior to pregnancy. 6 In a study of pregnant women in Italy, it was reported that of 14 women with severe disease, the median BMI was 30 kg/m2, which was significantly elevated compared to women with mild disease (P = .02). 21 Another cohort study from 12 medical centers in the United States included 64 pregnant women hospitalized due to COVID‐19; of 64 women with severe or critical COVID‐19 disease, the average BMI was 33.5 kg/m2. 22 This study also demonstrated that critically ill pregnant women with COVID‐19 had a lower BMI than severely ill women, suggesting that while obesity may be a risk factor for severe disease, obese women may have lower mortality than lean women. Interestingly, the idea that obese women may have a greater disease severity, but lower mortality than lean women mirrors other studies from the critical care literature, which have coined this finding as the “obesity paradox”. 23 Maternal deaths have been linked with obesity, however. A case series from Iran including nine pregnant women with severe COVID‐19 disease of which seven died, three women had a BMI >30 kg/m2. 24 A case series of 124 maternal deaths from Brazil found that obesity (undefined) was significantly associated with mortality. 25 Further, several case reports or series from the United States and the United Kingdom have reported maternal deaths or severe maternal morbidity in women with obesity. 26 , 27 , 28 , 29 , 30 , 31 , 32 Finally, the largest series of pregnant women with COVID‐19 to date including 427 cases in the United Kingdom demonstrated that 34% of cases were obese compared to 23% of controls. 7
TABLE 1.
Studies on COVID‐19 in pregnancy with reports on obesity
| Study | Country | N | N with obesity | Maternal outcomes | Neonatal outcomes |
|---|---|---|---|---|---|
| Andrikopoulou et al 109 | USA (NY) | 158 | 80 (50.6%) | Similar rates of obesity for mild (52%) and severe (47%) COVID‐19 | Two PTB for maternal decompensation |
| Hantoushzadeh et al 24 | Iran | 9 | 3 (33%) | 1/7 (14%) maternal deaths in obese women | Three IUFD, two NND |
| Knight et al 7 | UK | 427 | 140 (34%) | 3 maternal deaths | 50/243 iatrogenic PTB, 29 (46%) due to COVID‐19. three IUFD, two NND |
| Lokken et al 6 | USA (WA) | 46 | 15 (35.7%) | 6 (15%) severe COVID‐19, 5/6 (80%) of severe cases in obese women | One PTB in an obese patient for respiratory compromise, one IUFD |
| Mendoza et al 110 | Spain | 42 | Mean BMI 26.1 in non‐severe COVID‐19 cases, 27.9 in severe COVID‐19 cases | 8/42 (19%) severe COVID‐19 | Three PTB due to respiratory compromise |
| Pierce‐Williams et al 22 | USA (PA, NY, NJ, OH) | 64 | Mean BMI 33.5 in severe COVID‐19 cases, BMI 29.7 in critical COVID‐19 cases | 44 (69%) severe, 20 (31%) critical COVID‐19 | 88% of women with critical disease had PTB |
| Savasi et al 21 | Italy | 77 | Mean BMI 22.8 in total study, Mean BMI 30 in cases with severe COVID‐19 | 14 (18%) severe COVID‐19 | 4/11 (36%) of severe COVID‐19 cases had PTB |
Abbreviations: BMI, body mass index; IUFD, intrauterine fetal demise; NJ, New Jersey; NND, neonatal demise; NY, New York; OH, Ohio; PA, Pennsylvania; PTB, preterm birth; UK, United Kingdom; USA, United States of America; WA, Washington State.
3. IMMUNOPATHOLOGY OF COVID‐19
Understanding the immunopathology of infection with this novel virus is rapidly evolving. SARS‐CoV‐2 shares 80% RNA sequence homology with SARS‐CoV‐1, allowing extrapolation of likely shared pathophysiology and immune response. 33 , 34 , 35 Both viruses enter the cell via angiotensin‐converting enzyme‐related carboxypeptidase 2 (ACE2) receptor, though the SARS‐CoV‐2 spike protein binds ACE2 with significantly higher affinity than SARS‐CoV‐1. 36 Healthy individuals have higher concentrations of ACE2 in lung tissues, specifically bronchial smooth muscle cells, alveolar epithelium, type II pneumocytes, and alveolar macrophages. 37 , 38 Extrapulmonary expression of ACE2 occurs in myocardial cells 38 ; enterocytes in the ileum and jejunum 39 ; and proximal tubular cells in the kidney, 38 oral mucosa, 40 and arterial and venous endothelium. 41 In contrast, the strongest evidence suggests negligible placental expression of ACE2 and TMPRSS2, a serine protease that acts as a canonical mediator of cell entry for SARS‐CoV‐2 in conjunction with ACE2. 42 Multiple cells, predominantly within the lung, but also within other target organs (eg, heart, kidney) express the canonical receptor for SARS‐CoV‐2 entry.
During the initial stage of most viral infections, the type I and type III interferon (IFN) response is the primary mechanism leading to viral clearance (Figure 1, right panel). Immune cells detect viral nucleic acids through pattern recognition receptors (PRRs), primarily endosomal receptors Toll‐like receptor (TLR)3/7/9 and cytosolic receptors melanoma differentiation‐associated protein‐5 (MDA‐5), and retinoic acid‐inducible gene‐I (RIG‐I), which leads to the activation of both type I IFN and inflammatory cytokine production. 43 , 44 Type I IFN upregulates hundreds of interferon‐stimulated genes, which activate antiviral signaling and provide positive feedback and amplification of inflammation. 45 SARS‐CoV‐1 has been demonstrated to use multiple mechanisms to evade this initial IFN response including ubiquitin degradation of MDA‐5 and RIG‐I, inhibition of downstream signaling molecules mitochondrial antiviral signaling protein (MAVS) and TNF‐receptor associated factor (TRAF)3/6, and blockage of phosphorylation of signal transduction and activation of transcription (STAT) family transcription factors. 46 , 47 , 48 In addition, ACE2 is itself an IFN‐induced gene. Activation of the normal antiviral response therefore leads to upregulation of the receptor for viral entry. 41 Early data from SARS‐CoV‐2 suggest that this virus is also able to modulate the IFN response. 49
FIGURE 1.
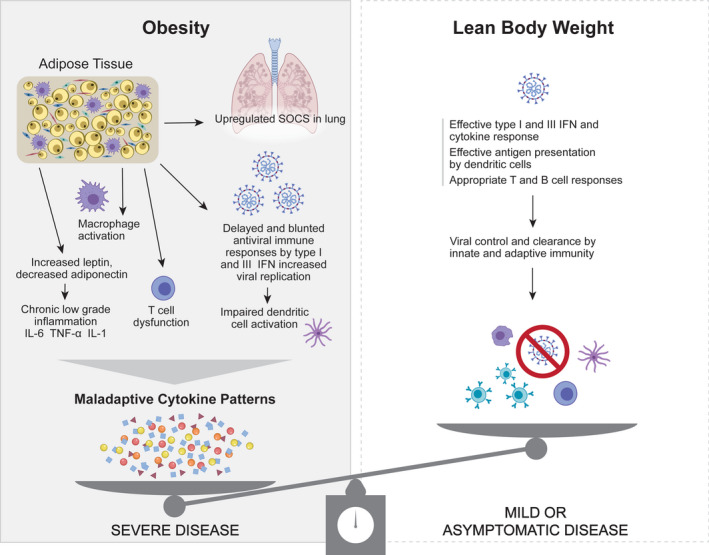
Potential mechanisms for increased COVID‐19 severity in pregnancy associated with obesity. In pregnant women with lean body weight (right panel), there is typically an effective type I and III IFN responses to viruses through antigen presentation by dendritic cells and coordinated innate and adaptive immune responses. In obese pregnant women (left panel), there is an inhibition of viral clearance through blunting of type I and III IFN responses, as well as inadequate antigen presentation by dendritic cells and T‐cell dysfunction. Obesity is also associated with chronic inflammation, M1 macrophage activation, altered adipokine production (eg, leptin, adiponectin) and upregulation of suppressors of cytokine gene signaling (SOCS), which can lead to excessive lung injury. The combination of defective viral clearance, increased inflammatory lung injury and altered lung mechanics in obese pregnant patients can synergize to increase the risk of severe or critical COVID‐19 disease
As the infection progresses, there is increasing viral‐induced cell death with release of additional viral particles as well as cellular components. In addition to infecting respiratory epithelial cells, 38 SARS‐CoV‐1 can also infect immune cells such as T cells and antigen‐presenting cells such as monocytes and dendritic cells. 50 These signals activate tissue macrophages that further amplify the inflammatory response by producing pro‐inflammatory cytokines (TNF‐α, IL‐1, IL‐6), which, in turn, lead to additional lung injury and immune cell recruitment. 51 , 52 Cytokines and chemokines result in the activation of adaptive immune T and B cells as well as recruitment of neutrophils and monocytes. Viral‐specific CD8 T cells are cytotoxic primarily to infected cells and serve to limit the release of additional viral particles, while neutrophils non‐specifically release reactive oxygen species and leukotrienes, which are directly toxic to pneumocytes and endothelial cells. Additionally, high levels of IFN and pro‐inflammatory cytokines also lead to cell death directly with and without viral infection through induction of apoptosis. Patients with severe COVID‐19 typically have high levels of systemic pro‐inflammatory cytokines, lymphopenia, and inflammatory lung infiltrates, which is consistent with a maladaptive patterns of cytokine production and inflammatory misfiring. 53 , 54 , 55 , 56 Elevated cytokines are also associated with multiple pathologic effects in the lung including endothelial apoptosis and vascular leaking, an ineffective antiviral response, diffuse alveolar damage, inflammatory cellular infiltrates, and intravascular thrombosis. 57 , 58 , 59
4. OBESITY‐INDUCED CHANGES TO IMMUNITY AND PHYSIOLOGY
Adipose tissue is an active endocrine and immune organ consisting primarily of adipocytes, but also multiple immune cell types, which represent the second most frequent type of cells in this tissue. 60 , 61 Macrophages are the most common immune cell type in adipose tissue and, in lean individuals, produce type 2 cytokines (IL‐4, IL‐10) and anti‐inflammatory molecules. 62 , 63 However, in obese individuals, activated macrophages in adipose tissue produce pro‐inflammatory cytokines TNF‐α, IL‐1β and IL‐6, which results in recruitment and activation of additional monocytes, as well as NKT cells and mast cells (Figure 1, left panel). Adaptive immune cells also play a role in obesity‐associated inflammation. Adipose tissue from lean individuals is composed primarily of CD4+ Th2 cells and regulatory T cells (Treg), which promote an anti‐inflammatory environment, while obese adipose tissue is enriched for CD4+ Th1 and Th17 cells as well as cytotoxic CD8+ T cells. 64 , 65 , 66 Changes in T‐cell polarization may be due to altered metabolite availability in obesity, which contributes to T‐cell differentiation and response to pulmonary infection. 67 , 68 In addition to changes in T‐helper cell phenotype, obesity is also associated with T‐cell dysfunction (Figure 1, left panel). Obesity results in increased production of memory T cells, and in a mouse model of viral infection, the memory T‐cell response to viral infection in obese animals resulted in increased pathogenesis rather than a protective response. 69 The chronic inflammation in obesity has also been associated with T‐cell exhaustion, which may be responsive to treatment with biologic therapies. 70 , 71
Adipose tissue and cytokine‐like hormone released from adipocytes, called adipokines, may directly and indirectly impair the pulmonary immune response (Figure 1, left panel). The adipocyte overflow hypothesis suggests that when an adipocyte can no longer hypertrophy to accommodate storage of new lipids, an “overflow” of fatty acids occurs into the body 72 , 73 ; lipids may then be recognized by innate immune pathogen recognition receptors at ectopic sites to stimulate a low‐grade inflammatory response. 74 Adipose tissues also release adipokines that can act as powerful regulators of the immune response. Leptin is a key adipokine and can regulate both innate and adaptive immunity to mediate a pro‐inflammatory immune response. 75 An inflammatory microenvironment can also downregulate production of adiponectin by adipocytes, which impairs the anti‐inflammatory response. Interestingly, high levels of leptin that is typical in obese individuals increase the risk of the severity of respiratory infections in both humans and mouse models. 76 High circulating leptin levels were associated with mortality in non‐pregnant adults hospitalized for acute respiratory distress syndrome due to pneumonia, even after adjusting for BMI. 76 The placental trophoblast and amnion also secrete leptin, which may further impair the pulmonary immune response in pregnant women. 77 Finally, adipose tissue is present in subcutaneous, visceral, and omental locations; the cellular and metabolic properties of each type of tissue are unique. Alterations in visceral adiposity have been associated more closely with adverse metabolic and health outcomes and immunologic dysfunction. 78 , 79
In addition to inducing immunologic dysfunction, excess adipose tissue also changes the mechanics and physiology of respiration. The increased metabolic requirements in obesity result in higher oxygen consumption and increased work of breathing. 80 Obesity also results in greater production of carbon dioxide, which leads to decreased respiratory drive. Mechanically, increased fat deposits within the abdominal cavity reduce the compliance of the respiratory system. 81 Increased abdominal adipose tissue mass leads to elevated abdominal pressure and lower lung volume by reducing expiratory reserve and functional residual capacity. 82 Obesity is also associated with airway narrowing which can lead to gas trapping. 83 The combination of decreased lung volumes, increased abdominal pressure, and narrowing of the airway leads to increased work of breathing with early fatigue of respiratory muscles.
5. IMMUNOLOGY IN PREGNANCY AND IMPACT OF OBESITY
Pregnancy provides both a physiologic challenge and a immunologic challenge for the maternal host during which it must balance providing access to nutrition, protection from infection, and tolerance of a genetically foreign fetus. To accommodate these functions, there is dynamic regulation of the maternal immune system, both systemically and at the maternal‐fetal interface during pregnancy. Pregnancy requires both pro‐inflammatory and tolerogenic immune responses at specific times during gestation. 84 , 85 , 86 During the early first trimester, a localized inflammatory response is necessary for embryonic implantation into the uterine decidua. 87 , 88 At the time of human parturition, a functional progesterone withdrawal in humans coupled with an inflammatory response direct the cascade of biological events that culminate in birth. 89 , 90 , 91 , 92 However, during the second and third trimesters, immune cells and cytokines promote a tolerogenic environment to accommodate the fetus and promote uterine quiescence. 93 , 94 Pathologic inflammation and inflammatory cytokine production (IL‐6, IL‐8, TNF‐α, IL‐1β) is associated with adverse pregnancy outcomes including abortion, fetal growth restriction, preterm birth, and preeclampsia. 95
Multiple immune adaptations during pregnancy result in alterations to antiviral immunity. First, syncytiotrophoblast cells that line the placental chorionic villous tree actively secrete type III IFN, which act as an immunologic and physical barrier to viral infection. 93 Systemic immune cellular and cytokine changes during pregnancy can favor either a pro‐inflammatory (IL‐1, IL‐6, IL‐12, IFN‐γ, TNF‐α) or tolerogenic (IL‐4, IL‐10, IL‐13) response depending on the time in gestation. 96 , 97 , 98 SARS‐CoV‐1 and influenza infections in pregnancy have been associated with an increased pro‐inflammatory response within the lungs, which resulted in decreased viral clearance and increase immune‐mediated lung injury. 99 , 100 Currently, data are insufficient to determine whether the systemic changes in pregnancy immunity play a role in enhancing COVID‐19 disease pathogenesis.
In overweight and obese pregnant women, immunologic and metabolic dysfunction likely contributes to the increased severity of COVID‐19 disease (Figure 1, left panel). The negative impact of obesity on the host response to respiratory viral pathogens may partially derive from an increased availability of glucose to the virus, changes to the adaptive immune system allowing propagation of viruses as well as a state of increased inflammation, inflammatory stresses, and poor wound healing. 101 Chronic inflammation and elevated adipokine leptin levels result in inhibition of the type I IFN antiviral response through upregulation of suppressor of cytokine signaling (SOCS) genes. 102 Obesity also results in higher baseline levels of inflammatory cytokines including IL‐6, TNF‐α, and IL‐1β 103 ; notably, serum IL‐6 levels are one of the strongest clinical correlates for severe COVID‐19 disease. 104 , 105 In addition, a change in CD4+ T‐cell polarization from Th2 and Treg cytokines (IL‐4, IL‐10, IL‐13) to a pro‐inflammatory Th1 and Th17 response observed in obese individuals is associated with production of pro‐inflammatory cytokines, such as TNF‐α, IL‐1, IL‐6, and IL‐17, which provides a potential mechanism for an earlier initiation of cytokine release and inflammatory misfiring in obese patients. The expression of ACE2 by adipocytes and immune cells also suggests the possibility that adipose tissue may represent a potential reservoir for viral infection and may lead to increased viral burden or persistence; however, no studies to date have demonstrated that adipocytes can be directly infected with SARS‐CoV‐2. These obesity‐driven alterations in the immune response likely contribute to the severity of COVID‐19 in obese pregnant women.
6. CONCLUSION
Maternal obesity has emerged as a key risk factor increasing susceptibility of pregnant women to severe COVID‐19 disease. This is likely the result of complex immunologic, metabolic, endocrine, and physiologic changes associated with obesity, which affect the immune response to viral infection. The increasing global burden of obesity may lead to more severe pregnancy morbidity and has the potential to regress decades of progress in global health and, by extension, to improvements in reproductive and pregnancy care worldwide (Figure 2). Analyses comparing obesity rates in over the last three decades show that obesity among pregnant women has increased drastically worldwide. 106 In 2017‐2018, obesity among women 20 years and older was 41.9% in the United States. 9 Currently, the United States also has the highest number of COVID‐19 infections worldwide. 107 In light of the global COVID‐19 pandemic, there has been a call for renewed prioritization of non‐communicable diseases such as obesity that increase susceptibility of women with SARS‐CoV‐2 infection to severe disease or mortality. 108 Carefully designed epidemiologic studies are required to assess the linkage between COVID‐19 disease severity, obesity, and associated socioeconomic factors. There is also an urgent need to focus research on how risk factors, like obesity, alter the immune response to SARS‐CoV‐2 and influence disease pathogenesis of COVID‐19 (Box 1). Finally, given global trends in the rise of obesity over the last 3 decades, urgent action is needed to address this critical health condition for global health. 106
FIGURE 2.
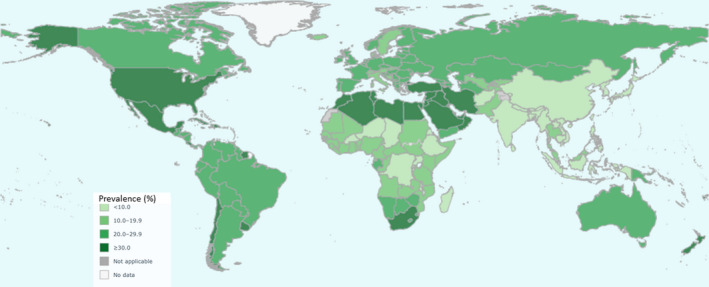
Global distribution of obesity among adult women. This global map demonstrates the geographic distribution of obesity (BMI >30) in adult women (>18 y old). The highest prevalence of obesity (>30%) is concentrated within the United States, Mexico, North Africa, South Africa, the Middle East and a few additional countries. Reprinted with permission: World Health Organization 2017 | Source: Global Health Observatory (http://www.who.int/gho/en/)
Box 1. Research questions.
What is the mechanism of increased risk for severe COVID‐19 disease in obese non‐pregnant and pregnant women?
Does the second and third trimester of pregnancy represent a time of increased risk for severe COVID‐19? If yes, how does gestational age modify the effect of obesity on COVID‐19 disease severity?
Is preterm birth more common in obese pregnant women with COVID‐19 due to concern for respiratory compromise?
Are certain therapies more effective for treatment of severe COVID‐19 in obese pregnant women compared to lean or non‐pregnant women?
Does increased surveillance for COVID‐19 in obese pregnant women improve health outcomes?
Can adipose tissue serve as a reservoir for SARS‐CoV‐2 viral infection through adipocyte ACE2 expression?
Are viral loads higher of SARS‐CoV‐2 in obese versus lean pregnant women? Are the kinetics of viral clearance different in obese versus lean pregnant women?
Can we design epidemiologic studies to further assess whether the risk of severe COVID‐19 infection in obese pregnancy is directly related to obesity itself or to associated socioeconomic factors?
ACKNOWLEDGMENTS
We would like to acknowledge Nicole Wothe, who provided administrative assistance with the manuscript submission. Jessie Brown provided graphical design assistance with the conceptual model figure. The authors report no conflict of interest. We note that Dr Alisa Kachikis has received honoraria for work on maternal immunization through Pfizer and GlaxoSmithKline, which are outside the scope of this manuscript. This work was supported by the National Institutes of Allergy and Infectious Diseases (grant numbers AI133976, AI145890, AI144938, and AI143265 to KAW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
McCartney SA, Kachikis A, Huebner EM, Walker CL, Chandrasekaran S, Adams Waldorf KM. Obesity as a contributor to immunopathology in pregnant and non‐pregnant adults with COVID‐19. Am J Reprod Immunol. 2020;84:e13320. 10.1111/aji.13320
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3,062 COVID‐19 patients: a meta‐analysis. J Med Virol. 2020;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coronavirus Disease 2019 (COVID‐19): people with certain medical conditions. 2020; https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html. Accessed July 29, 2020.
- 6. Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a SARS‐CoV‐2 infection in Washington State. Am J Obstet Gynecol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women hospitalised with confirmed SARS‐CoV‐2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS). medRxiv. 2020:2020.2005.2008.20089268.
- 8. Roura LC, Arulkumaran SS. Facing the noncommunicable disease (NCD) global epidemic–the battle of prevention starts in utero–the FIGO challenge. Best Pract Res Clin Obstet Gynaecol. 2015;29(1):5‐14. [DOI] [PubMed] [Google Scholar]
- 9. Hales CM, Carroll MD, Fryar CD, Ogden CL.Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. No. 360. In: February, 2020. [PubMed]
- 10. Hajifathalian K, Kumar S, Newberry C, et al. Obesity is associated with worse outcomes in COVID‐19: analysis of early data from New York City. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metabolism. 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Busetto L, Bettini S, Fabris R, et al. Obesity and COVID‐19: an Italian snapshot. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonmarin I, Belchior E, Bergounioux J, et al. Intensive care unit surveillance of influenza infection in France: the 2009/10 pandemic and the three subsequent seasons. Euro Surveill. 2015;20(46):1–10. [DOI] [PubMed] [Google Scholar]
- 14. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garg S, Jain S, Dawood FS, et al. Pneumonia among adults hospitalized with laboratory‐confirmed seasonal influenza virus infection‐United States, 2005–2008. BMC Infect Dis. 2015;15:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maruyama T, Fujisawa T, Suga S, et al. Outcomes and prognostic features of patients with influenza requiring hospitalization and receiving early antiviral therapy: a prospective Multicenter Cohort Study. Chest. 2016;149(2):526‐534. [DOI] [PubMed] [Google Scholar]
- 17. Poulakou G, Pérez M, Rello J. Severe acute respiratory infections in the postpandemic era of H1N1. Curr Opin Crit Care. 2012;18(5):441‐450. [DOI] [PubMed] [Google Scholar]
- 18. Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3):e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honce R, Schultz‐Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol. 2020;2:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savasi VM, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2020;136(2):252‐258. [DOI] [PubMed] [Google Scholar]
- 22. Pierce‐Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical COVID‐19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol. 2020:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Jong A, Chanques G, Jaber S. Mechanical ventilation in obese ICU patients: from intubation to extubation. Crit Care. 2017;21(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID‐19 disease. Am J Obstet Gynecol. 2020;223(1):109.e1‐109.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takemoto MLS, Menezes MO, Andreucci CB, et al. The tragedy of COVID‐19 in Brazil: 124 maternal deaths and counting. Int J Gynaecol Obstet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020;27:e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blauvelt CA, Chiu C, Donovan AL, et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2020;136(1):46–51. [DOI] [PubMed] [Google Scholar]
- 28. Hong L, Smith N, Keerthy M, et al. Severe COVID‐19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Rep Womens Health. 2020;27:e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly JC, Dombrowksi M, O'Neil‐Callahan M, Kernberg AS, Frolova AI, Stout MJ. False‐negative COVID‐19 testing: considerations in obstetrical care. Am J Obstet Gynecol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmed I, Azhar A, Eltaweel N, Tan BK. First Covid‐19 maternal mortality in the UK associated with thrombotic complications. Br J Haematol. 2020;190(1):e37‐e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallejo V, Ilagan JG. A postpartum death due to coronavirus disease 2019 (COVID‐19) in the United States. Obstet Gynecol. 2020;136(1):52–55. [DOI] [PubMed] [Google Scholar]
- 32. Cooke WR, Billett A, Gleeson S, et al. SARS‐CoV‐2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol. 2020;250:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: Immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 35. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14(2):185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016‐1035.e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pique‐Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS‐CoV‐2? bioRxiv. 2020:2020.2005.2018.101485. [DOI] [PMC free article] [PubMed]
- 43. Saito T, Gale M Jr. Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 44. Pestka S, Krause CD, Walter MR. Interferons, interferon‐like cytokines, and their receptors. Immunol Rev. 2004;202:8‐32. [DOI] [PubMed] [Google Scholar]
- 45. Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50(4):907‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kindler E, Thiel V, Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res. 2016;96:219‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu X, Pan J, Tao J, Guo D. SARS‐CoV nucleocapsid protein antagonizes IFN‐β response by targeting initial step of IFN‐β induction pathway, and its C‐terminal region is critical for the antagonism. Virus Genes. 2011;42(1):37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blanco‐Melo D, Nilsson‐Payant BE, Liu W‐C, et al. SARS‐CoV‐2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv. 2020:2020.2003.2024.004655.
- 50. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench‐to‐bedside review: damage‐associated molecular patterns in the onset of ventilator‐induced lung injury. Crit Care. 2011;15(6):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science (New York, NY). 2020;368(6490):473‐474. [DOI] [PubMed] [Google Scholar]
- 54. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vardhana SA, Wolchok JD. The many faces of the anti‐COVID immune response. J Exp Med. 2020;217(6);e20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lucas C, Wong P, Klein J, et al. Longitudinal immunological analyses reveal inflammatory misfiring in severe COVID‐19 patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 57. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Menter T, Haslbauer JD, Nienhold R, et al. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients With COVID‐19. Ann Intern Med. 2020. [DOI] [PubMed] [Google Scholar]
- 60. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low‐grade inflammation. J Endocrinol. 2014;222(3):R113‐R127. [DOI] [PubMed] [Google Scholar]
- 61. Maurizi G, Della Guardia L, Maurizi A, Poloni A. Adipocytes properties and crosstalk with immune system in obesity‐related inflammation. J Cell Physiol. 2018;233(1):88‐97. [DOI] [PubMed] [Google Scholar]
- 62. Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116(1):33‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415‐445. [DOI] [PubMed] [Google Scholar]
- 64. Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T‐cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145(2):366‐374.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Endo Y, Yokote K, Nakayama T. The obesity‐related pathology and Th17 cells. Cell Mol Life Sci. 2017;74(7):1231‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang M, Chen F, Wang J, Zeng Z, Yang Q, Shao S. Th17 and Treg lymphocytes in obesity and Type 2 diabetic patients. Clin Immunol. 2018;197:77‐85. [DOI] [PubMed] [Google Scholar]
- 67. Rao M, Dodoo E, Zumla A, Maeurer M. Immunometabolism and pulmonary infections: implications for protective immune responses and host‐directed therapies. Front Microbiol. 2019;10:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Q, Wu H. T Cells in adipose tissue: critical players in immunometabolism. Front Immunol. 2018;9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, Whitmire JK. Obesity expands a distinct population of T cells in adipose tissue and increases vulnerability to infection. Cell Rep. 2019;27(2):514‐524.e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saeidi A, Zandi K, Cheok YY, et al. T‐cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. 2018;9:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aguilar EG, Murphy WJ. Obesity induced T cell dysfunction and implications for cancer immunotherapy. Curr Opin Immunol. 2018;51:181‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14(9):398‐403. [DOI] [PubMed] [Google Scholar]
- 73. Vidal‐Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome. Endocrinol Nutr. 2013;60(Suppl 1):39‐43. [DOI] [PubMed] [Google Scholar]
- 74. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Francisco V, Pino J, Campos‐Cabaleiro V, et al. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ubags ND, Stapleton RD, Vernooy JH, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1(8):e82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta‐derived hormone in humans. Nat Med. 1997;3(9):1029‐1033. [DOI] [PubMed] [Google Scholar]
- 78. Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629‐639. [DOI] [PubMed] [Google Scholar]
- 79. Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129(10):3978‐3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160(3):883‐886. [DOI] [PubMed] [Google Scholar]
- 81. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12(9):755‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated‐paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144‐151. [DOI] [PubMed] [Google Scholar]
- 83. Hedenstierna G, Santesson J, Norlander O. Airway closure and distribution of inspired gas in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20(4):334‐342. [DOI] [PubMed] [Google Scholar]
- 84. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17(8):469‐482. [DOI] [PubMed] [Google Scholar]
- 85. Chavan AR, Griffith OW, Wagner GP. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr Opin Genet Dev. 2017;47:24‐32. [DOI] [PubMed] [Google Scholar]
- 86. Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol. 2019;14:185‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72(2):141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hadley EE, Richardson LS, Torloni MR, Menon R. Gestational tissue inflammatory biomarkers at term labor: a systematic review of literature. Am J Reprod Immunol. 2018;79(2):e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154‐167. [DOI] [PubMed] [Google Scholar]
- 91. Sivarajasingam SP, Imami N, Johnson MR. Myometrial cytokines and their role in the onset of labour. J Endocrinol. 2016;231(3):R101‐R119. [DOI] [PubMed] [Google Scholar]
- 92. Stanfield Z, Amini P, Wang J, et al. Interplay of transcriptional signaling by progesterone, cyclic AMP, and inflammation in myometrial cells: implications for the control of human parturition. Mol Hum Reprod. 2019;25(7):408‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal‐fetal interface. Sci Immunol. 2019;4(31):eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Erlebacher A. Immunology of the maternal‐fetal interface. Annu Rev Immunol. 2013;31:387‐411. [DOI] [PubMed] [Google Scholar]
- 95. Nadeau‐Vallée M, Obari D, Palacios J, et al. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152(6):R277‐R292. [DOI] [PubMed] [Google Scholar]
- 96. Aghaeepour N, Ganio EA, McIlwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2(15):eaan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res. 2012;54(1‐3):254‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601‐610. [DOI] [PubMed] [Google Scholar]
- 99. Raj RS, Bonney EA, Phillippe M. Influenza, immune system, and pregnancy. Reprod Sci. 2014;21(12):1434‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kalil AC, Thomas PG. Influenza virus‐related critical illness: pathophysiology and epidemiology. Crit Care. 2019;23(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tian Y, Jennings J, Gong Y, Sang Y. Viral infections and interferons in the development of obesity. Biomolecules. 2019;9(11):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. NCD Risk Factor Collaboration (NCD‐RisC) . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. World Health Organization . Coronavirus Disease (COVID‐19) Situation Report ‐ 153. June 21, 2020.
- 108. Kapur A, Hod M. Maternal health and non‐communicable disease prevention: an investment case for the post COVID‐19 world and need for better health economic data. Int J Gynaecol Obstet. 2020;150(2):151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Andrikopoulou M, Madden N, Wen T, et al. Symptoms and critical illness among obstetric patients with coronavirus disease 2019 (COVID‐19) infection. Obstet Gynecol. 2020;136(2):291‐299. [DOI] [PubMed] [Google Scholar]
- 110. Mendoza M, Garcia‐Ruiz I, Maiz N, et al. Pre‐eclampsia‐like syndrome induced by severe COVID‐19: a prospective observational study. BJOG. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


