Abstract
Background
In late December 2019 and on 1st January 2020, the coronavirus (COVID‐19) infecting humans was first identified in Wuhan, Hubei Province, China. Later cases have also been confirmed worldwide. Coronaviruses are RNA viruses that are phenotypically and genotypically diverse. Globally, as of 6th April 2020, laboratory confirmed cases of COVID‐19 reported to the World Health Organisation (WHO) amounted to 1 211 214, including 67 666 deaths.
Aim
In the current study, we performed a literature review on coronavirus outbreak to summarise details about the pathogenesis, epidemiology, diagnosis and the management strategies for the disease control.
Pathogenesis
Coronaviruses are tremendously precise and mature only in differentiated respiratory epithelial cells, as seen in both organ cultures as well as human volunteers. This virus will cause the antiviral T‐cell response to be erratic, owing to the T‐cell apoptosis activation, triggering the immune system to collapse.
Transmission
The understanding of the transmission of COVID‐19 risk is incomplete. The transmission mainly occurs through the respiratory droplets once an infected person sneezes, like the spread of flu and other respiratory infectious agents.
Clinical presentation
Presentations of COVID‐19 includes fever, cough, shortness of breath, malaise and respiratory distress.
Treatment
There have been no approved vaccines available for COVID‐19 until today. The Ministry of Science and Technology in the People’s Republic of China declared three potential antiviral medicines suitable for treating COVID‐19. Those three medicines are, namely, favilavir, chloroquine phosphate and remdesivir. Hydroxychloroquine combined with azithromycin enhances the reduction of the viral load in COVID‐19 patients.
Conclusion
The corona virus transmits quicker than its two predecessors the MERS‐CoV and SARS‐CoV, but has reduced casualty. The global effects of this latest pandemic are still unclear. Nevertheless, considering that so far no vaccine has been available; preventive approaches are the best way to fight against the virus.
1. INTRODUCTION
According to WHO, in December 2019, several pneumonia cases of unknown aetiology were identified in the city of Wuhan in central China. Towards the end of December 2019, patients presenting with viral pneumonia because of an unknown microbial agent were reported in Wuhan, China. A novel coronavirus was subsequently identified as the causative pathogen, provisionally named 2019 novel coronavirus (2019‐nCoV). On February 11th 2020, WHO announced the rapidly spreading coronavirus disease as COVID‐19. As of 26th January 2020, more than 2000 cases of COVID‐19 infection have been confirmed, most of which involved people living in or visiting Wuhan, and human‐to‐human transmission was confirmed. 1
The initial infected individuals mostly were linked to exposures to a seafood market in Wuhan. 2 In 2020, the Chinese authorities reported 2835 confirmed cases in mainland China, including 81 deaths. Additionally, 19 confirmed cases were identified in Hong Kong, Macao and Taiwan, and 39 imported cases were identified in Thailand, Japan, South Korea, United States, Vietnam, Singapore, Nepal, France, Australia and Canada. The pathogen was soon identified as COVID‐19, which is closely related to severe acute respiratory syndrome CoV (SARS‐CoV). 3
The Chinese authorities officially announced a novel coronavirus, 2019‐n CoV, as the causative agent. 4 , 5 Coronaviruses (CoV) are a family of viruses called Coronaviridae. The subfamily Coronavirinae has three genera, alphacoronavirus, betacoronavirus and gammacoronavirus. The subfamily Torovirinae has two genera, torovirus and bafinivirus. CoV can lead to a range of conditions as mild as the common cold, fever and cough and as severe as pneumonia, respiratory distress kidney failure or even death. 6 These viruses are zoonotic, that is, they are transmitted between animals and humans. A couple of coronaviruses were previously identified: MERS‐CoV, which causes Middle East respiratory syndrome and was transmitted from dromedary camels to humans, and SARS‐CoV, which causes severe acute respiratory syndrome and was transmitted from civet cats to humans. 7 , 8 COVID‐19 is believed to have been transmitted zoonotically, in a wet market in Wuhan where game animals and meat were sold. 9
However, common human coronaviruses including types 229E, NL63, OC43 and HKU1 cause mild to moderate upper respiratory tract symptoms, sore throat, runny nose and cough. Other symptoms include fever, headache and general feeling of being unwell. More severe conditions affecting the lower respiratory tract, such as pneumonia and bronchitis, are more common in people with cardiopulmonary illnesses, immune compromised patients, infants and older adults. These viruses are transmitted from infected humans to others through air by coughing or sneezing or close personal contact such as touching or shaking hands. 10
The outbreak of COVID‐19 has resulted in a total of 1 211 214 confirmed cases in the world, distributed in 81 countries, and, of these cases, 67 666 cases died. Of the total cases, the United States of America has the largest number, with 307 318 cases, followed by 130 759 cases in Spain, 128 948 cases in Italy, 95 391 cases in Germany, 83 005 cases in China, 69 607 cases in France, 58 226 cases in the Islamic Republic of Iran and 47 810 cases in the United Kingdom. 11 However, the corona virus' potential path is uncertain. An overview of this new corona virus is given in this article. Despite the rapidly developing awareness about this virus, readers are encouraged to refresh themselves periodically.
In the current research, we performed a detailed review that was widely available to summarise pathogenesis and ongoing epidemic, epidemiological facts, diagnosis, disease control approaches and methods of prevention.
2. METHODOLOGY
The literature search was performed using the following electronic databases: EMBASE, PubMed and Google Scholar. Hand searching of the reference lists of the retrieved studies was performed to identify further relevant publications. Terms and keywords used to conduct the literature search included the following: “COVID‐19,” “Prevention,” “Pneumonia outbreak” and “Coronavirus.” Alternative search terms were “2019‐nCoV,” “novel coronaviruses” and “supportive care.” The filters were set to search for studies related to humans and published in English. Attempts were made to identify all literature related to COVID‐19. Thus, no time limit was set for the search. Screening of the titles and abstracts of the retrieved studies was conducted to assess relevance. Studies included in the current literature review involved those covering the following important aspects: aetiology, pathogenesis, mode of transmission, clinical diagnosis, special attention to sensitive populations, clinical management and treatment; and early supportive therapy and monitoring. Studies that do not cover any of the above‐mentioned items were excluded. Data were extracted only from the full‐text articles, WHO interim guidelines and also from the Centres for Disease Control and Prevention (CDC).
3. AETIOLOGY
The aetiologic source responsible for the cluster of pneumonia cases in Wuhan was clearly identified as a novel betacoronavirus (same family as SARS‐CoV and MERS‐CoV) through next‐generation sequencing (NGS) from cultured virus or from several pneumonia patients’ samples. Electron microscopic imaging shows a virus with a crown morphology, which gives it the name coronavirus. Genetic amplification assays were established as a result of sequence information and used by laboratories linked with the China Centre for Disease Control (CCDC) to identify numerous cases in the future. 12
4. VIROLOGY
Coronaviruses, a family of viruses within the nidoviruses superfamily, are further classified according to their genera, alpha‐, beta‐, gamma‐ and deltacoronaviruses (α‐, β‐, γ‐ and δ‐). Among those, alpha and beta species are capable of contaminating only mammals, whereas the other two genera can infect birds and could also infect mammals. 13 , 14 Two of these genera belong to human coronaviruses (HCoVs): α‐coronaviruses, which comprise human coronavirus 229E (hcov229E) and human coronavirus NL63 (hcovNL63), and β‐coronaviruses, which are human coronavirus HKU1, human coronavirus OC43, MERS‐COV (known as Middle East respiratory syndrome coronavirus) and SARS‐CoV (referred to as severe acute respiratory syndrome coronavirus). 15
The severe acute respiratory syndrome CoV‐2 (SARS‐CoV‐2) is now named novel COVID‐19 (coronavirus disease 2019). 16 Genome sequencing and phylogenetic research revealed that the COVID‐19‐causing coronavirus is a beta‐coronavirus that belongs to the same subtypes as SARS virus, but still exists in a variant group. The receptor‐binding gene region appears to be very similar to that of the SARS‐CoV and it is believed that the same receptor would be used for cell entry. 17
4.1. Virion structure and its genome
Coronaviruses are structurally enveloped, belonging to the positive‐strand RNA viruses category that has the largest known genomes of RNA. The structures of the coronavirus are more spherical in shape, but their structure has the potential to modify their morphology in response to environmental conditions, being pleomorphic. The capsular membrane which represents the outer envelope usually has glycoprotein projection and covers the nucleus, comprising a matrix protein containing a positive‐strand RNA. Since the structure possesses 5'‐capped and 3'‐polyadenylated ends, it remains identical to the cellular mRNAs. 18 The structure is comprised of hemagglutinin esterase (HE) (present only in some beta‐coronaviruses), spike (S), small membrane (E), membrane (M) and nucleocapsid (N), as shown (Figure 1). The envelope containing glycoprotein is responsible for attachment to the host cell, which possesses the primary anti‐genic epitopes mainly those recognised by neutralising antibodies. The spike S‐protein being in a spike form is subjected to a structural rearrangement process so that fusing the outer membrane of the virus with the host‐cell membrane becomes easier. 19 , 20 Recent SARS‐CoV work has also shown that the membrane exopeptidase ACE enzyme (angiotensin‐converting enzyme) functions as a COVID‐19 receptor to enter the human cell. 21
FIGURE 1.
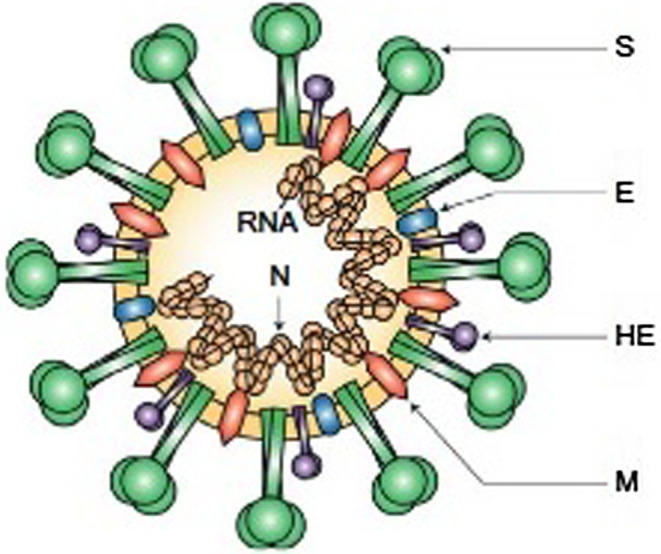
Virion structure and its genome
4.2. Viral replication
Usually replication of coronavirus occurs within the cytoplasm and is closely associated with endoplasmic reticulum and other cellular membrane organelles. Human coronaviruses are thought to invade cells, primarily through different receptors. For 229E and OC43, amino peptidase‐N (AP‐N) and a sialic acid containing receptor, respectively, were known to function in this role. After the virus enters the host cell and uncoating process occurs, the genome is transcribed, and then, translated. A characteristic feature of replication is that all mRNAs form an enclosed group of typical 3′ ends; only the special portions of the 5′ ends are translated. In total, about 7 mRNAs are produced. The shortest mRNA codes and the others can express the synthesis of another genome segment for nucleoprotein. At the cell membrane, these proteins are collected and genomic RNA is initiated as a mature particle type by burgeoning from internal cell membranes. 22 , 23
5. PATHOGENESIS
Coronaviruses are tremendously precise and mature in most of the airway epithelial cells as observed through both in vivo and in vitro experiments. There is an enhanced nasal secretion observed along with local oedema because of the damage of the host cell, which further stimulates the synthesis of inflammatory mediators. In addition, these reactions can induce sneezing, difficulty breathing by causing airway inhibition and elevate mucosal temperature. These viruses, when released, chiefly affect the lower respiratory tract, with the signs and symptoms existing clinically. Also, the virus further affects the intestinal lymphocytes, renal cells, liver cells and T‐lymphocytes. Furthermore, the virus induces T‐cell apoptosis, causing the reaction of the T‐cell to be erratic, resulting in the immune system’s complete collapse. 24 , 25
5.1. Mode of transmission
In fact it was accepted that the original transmission originated from a seafood market, which had a tradition of selling live animals, where the majority of the patients had either worked or visited, although up to now the understanding of the COVID‐19 transmission risk remains incomplete. 16 In addition, while the newer patients had no exposure to the market and still got the virus from the humans present there, there is an increase in the outbreak of this virus through human‐to‐human transmission, with the fact that it has become widespread around the globe. This confirms the fact similar to the previous epidemics, including SARS and MERS, that this coronavirus exhibited potential human‐to‐human transmission, as it was recently declared a pandemic by WHO. 26
Respiratory droplets are the major carrier for coronavirus transmission. Such droplets can either stay in the nose or mouth or enter the lungs via the inhaled air. Currently, it is known that COVID‐19’s transmission from one person to another also occurs through touching either an infected surface or even an object. With the current scant awareness of the transmission systems however, airborne safety measures with a high‐risk procedure have been proposed in many countries. Transmission levels, or the rates from one person to another, reported differ by both location and interaction with involvement in infection control. It is stated that even asymptomatic individuals or those individuals in their incubation period can act as carrier of SARS‐CoV2. 27 , 28 With the data and evidence provided by the CDC, the usual incubation period is probably 3 to 7 days, sometimes being prolonged up to even 2 weeks, and the typical symptom occurrence from incubation period to infection takes an average of 12.5 days. 29
6. CLINICAL DIAGNOSIS
The symptoms of COVID‐19 remain very similar to those of the other respiratory epidemics in the past, which include SARS and MERS, but here the range of symptoms includes mild rhinitis to septic shock. Some intestinal disturbances were reported with the other epidemics, but COVID‐19 was devoid of such symptoms. When examined, unilateral or bilateral involvement compatible with viral pneumonia is observed in the patients, and bilateral multiple lobular and sub‐segmental consolidation areas were observed in patients hospitalised in the intensive care unit. Comorbid patients showed a more severe clinical course than predicted from previous epidemics. Diagnosis of COVID‐19 includes the complete history of travel and touch, with laboratory testing. It is more preferable to choose serological screening, which can help to analyse even the asymptomatic infections; several serological tests are in progress for SARS‐CoV‐2. 14 , 30
6.1. Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases
The assessment of the patients with COVID‐19 should be based on the clinical features and also epidemiological factors. The screening protocols must be prepared and followed per the native context. 31 Collecting and testing of specimen samples from the suspected individual is considered to be one of the main principles for controlling and managing the outbreak of the disease in a country. The suspected cases must be screened thoroughly in order to detect the virus with the help of nucleic acid amplification tests such as reverse transcription polymerase chain reaction (RT‐PCR). If a country or a particular region does not have the facility to test the specimens, the specimens of the suspected individual should be sent to the nearest reference laboratories per the list provided by WHO. 32
It is also recommended that the suspected patients be tested for the other respiratory pathogens by performing the routine laboratory investigation per the local guidelines, mainly to differentiate from other viruses that include influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus, rhinovirus, human metapneumovirus and SARS coronavirus. It is advisable to distinguish COVID‐19 from other pneumonias such as mycoplasma pneumonia, chlamydia pneumonia and bacterial pneumonia. 33 Several published pieces of literature based on the novel coronavirus reported in China declared that stool and blood samples can also collected from the suspected persons in order to detect the virus. However, respiratory samples show better viability in identifying the virus, in comparison with the other specimens. 34 , 35 , 36
6.2. Nucleic acid amplification tests (NAAT) for COVID‐19 virus
The gold standard method of confirming the suspected cases of COVID‐19 is carried out by detecting the unique sequences of virus RNA through reverse transcription polymerase chain reaction (RT‐PCR) along with nucleic acid sequencing if needed. The various genes of virus identified so far include N, E, S (N: nucleocapsid protein, E: envelope protein gene, S: spike protein gene) and RdRP genes (RNA‐dependent RNA polymerase gene). 32
6.3. Serological testing
Serological surveys are also considered to be one of the most effective ones in facilitating outbreak investigation and it also helps us to derive a retrospective assessment of the disease by estimating the attack rate. 32 According to the recent literature, paired serum samples can also help clinicians to diagnose COVID‐19 in case of false negative results in NAAT essays. 37 The literature also declared that the commercial and non‐commercial serological tests are under consideration in order to support the practising clinicians by assisting them in diagnosis. Similarly, there are studies published on COVID‐19 which are comprised of the serological data on clinical samples. 38 , 39
6.4. Viral sequencing
Apart from confirming the presence of virus in the specimens, viral sequencing is also quite useful in monitoring the viral genomic mutations, which plays a very significant role in influencing the performance of the medical countermeasures inclusive of the diagnostic test. Genomic sequencing of the virus can also help further in developing several studies related to molecular epidemiology. 32
6.5. Specimen collection and storage
A Nasopharyngeal and oropharyngeal swab should be collected using Dacron or polyester flocked swabs. It should be transported to the laboratory at a temperature of 4°C and stored in the laboratory between 4 and −70°C on the basis of the number of days and, in order to increase the viral load, both nasopharyngeal and oropharyngeal swabs should be placed in the same tube. Bronchoalveolar lavage and nasopharyngeal aspirate should be collected in a sterile container and transported similarly to the laboratory by maintain a temperature of 4°C.
Sputum samples, especially from the lower respiratory tract, should be collected with the help of a sterile container and stored, whereas tissue from a biopsy or autopsy should be collected using a sterile container along with saline. However, both should be stored in the laboratory at a temperature that ranges between 4 and −70°C. Whole blood for detecting the antigen, particularly in the first week of illness, should be collected in a collecting tube and stored in the laboratory between 4 and −70°C. Urine samples must also be collected using a sterile container and stored in the laboratory at a temperature that ranges between 4 and −70°C. 32
7. PREGNANCY
Currently, there is a paucity of knowledge and data related to the consequences of COVID‐19 during pregnancy. 40 , 41 , 42 However, pregnant women seem to have a high risk of developing severe infection and complications during the recent 2019‐nCoV outbreak. 41 , 42 , 43 This speculation was based on previous available scientific reports on coronaviruses during pregnancy (SARS‐CoV and MERS‐CoV) as well as the limited number of COVID‐19 cases. 41 , 42 , 43 Analysing the clinical features and outcomes of 10 newborns (including two sets of twins) in China, whose mothers are confirmed cases of COVID‐19, revealed that perinatal infection with 2019‐nCoV may lead to adverse outcomes for the neonates, for example, premature labour, respiratory distress, thrombocytopenia with abnormal liver function and even death. 44 It is still unclear whether or not the COVID‐19 infection can be transmitted during pregnancy to the foetus through the transplacental route. 42 A recent case series report, which assessed intrauterine vertical transmission of COVID‐19 infection in nine infants born to infected mothers, found that none of the infants tested positive for the virus. 45 Likewise, there was no evidence of intrauterine infection caused by vertical transmission in the SARS and MERS epidemics. 43
The CDC asserts that infants born to mothers with confirmed COVID‐19 are considered persons under investigation (PUI) and should be temporarily separated from the mother and isolated. 46
7.1. Breastfeeding and infant care
The data available to date is limited and cannot confirm whether or not COVID‐19 can be transmitted through breast milk. 40 Assessing the presence of COVID‐19 in breast milk samples from six patients showed negative result. 45 The CDC points out that in case of a confirmed or suspected COVID‐19 infection, the decision of whether or how to start or continue breastfeeding should be made by the mother in collaboration with the family and healthcare practitioners. 47 Careful precautions need to be taken by the mother to prevent transmitting the disease to her infant through respiratory droplets during breastfeeding. This includes wearing a facemask and practising hand hygiene before feeding the baby. In addition, it is advisable that breast pumps are cleaned properly after each use and, if possible, a healthy individual is available to feed the expressed breast milk to the infant. 42
7.2. Children and elderly population
On the basis of the available reports, COVID‐19 among children accounted for 1‐5% of the confirmed cases, and this population does not seem to be at higher risk for the disease than adults. There is no difference in the COVID‐19 symptoms between adults and children. However, the available evidence indicated that children diagnosed with COVID‐19 have milder symptoms than the adults, with a low mortality rate. 48 , 49 On the contrary, older people who are above the age of 65 years are at higher risk for a severe course of disease. In the United Stated, approximately 31‐59% of those with confirmed COVID‐19 between the ages of 65 and 84 years old required hospitalisation, 11‐31% of them required admission to the intensive care unit, and 4‐11% died. 50
8. PREVENTION
The WHO and other agencies such as the CDC have published protective measures to mitigate the spread of COVID‐19. This involves frequent hand washing with handwash containing 60% of alcohol and soap for at least 20 seconds. Another important measure is avoiding close contact with sick people and keeping a social distance of 1 metre always to everyone who is coughing and sneezing. Not touching the nose, eyes and mouth was also suggested. While coughing or sneezing, covering the mouth and nose with a cloth/tissue or the bent elbow is advised. Staying at home is recommended for those who are sick, and wearing a facial mask is advised when going out among people. Furthermore, it is recommended to clean and sterilise frequently touched surfaces such as phones and doorknobs on a daily basis. 51 , 52 Staying at home as much as possible is advisable for those who are at higher risk for severe illness, to minimise the risk of exposure to COVID‐19 during outbreaks. 53
9. VACCINES
The strange coronavirus outbreak in the Chinese city of Wuhan, now termed COVID‐19, and its rapid transmission, threatens people around the world. Because of its pandemic nature, the National Institutes of Health (NIH) and pharmaceutical companies are involved in the development of COVID‐19 vaccines. Xu Nanping, China’s vice‐minister of science and technology, announced that the first vaccine is expected to be ready for clinical trials in China at the end of April 2020. 54 There is no approved vaccine and treatment for COVID‐19 infections.
Vaccine development is sponsored and supported by the Biomedical Advanced Research and Development Authority (BARDA), a component of the Office of the Assistant Secretary for Preparedness and Response (ASPR). Sanofi will use its egg‐free, recombinant DNA technology to produce an exact genetic match to proteins of the virus. 55
10. RECOMBINANT SUBUNIT VACCINE
Clover Biopharmaceuticals is producing a recombinant subunit vaccine based on the trimeric S‐protein of COVID‐19. 55 The oral recombinant vaccine is being expanded by Vaxart in tablet formulation, using its proprietary oral vaccine platform.
11. CLINICAL MANAGEMENT AND TREATMENT
In severe COVID‐19 cases, treatment should be given to support vital organ functions. People who think they may have been exposed to COVID‐19 should contact their healthcare provider immediately. Healthcare personnel should care for patients in an Airborne Infection Isolation Room (AIIR). Precautions must be taken by the healthcare professional, such as contact precautions and airborne precautions with eye protection. 56
Individuals with a mild clinical presentation may not require primary hospitalisation. Close monitoring is needed for the persons infected with COVID‐19. Elderly patients and those with prevailing chronic medical conditions such as lung disease, heart failure, cancer, cerebrovascular disease, renal disease, diabetes, liver disease and immunocompromising conditions and pregnancy are risk factors for developing severe illness. Management includes implementation of prevention and control measures and supportive therapy to manage the complications, together with advanced organ support. 57
Corticosteroids must be avoided unless specified for chronic obstructive pulmonary disease exacerbation or septic shock, as it is likely to prolong viral replication as detected in MERS‐CoV patients. 58
12. EARLY SUPPORTIVE THERAPY AND MONITORING
Management of patients with suspected or documented COVID‐19 consists of ensuring appropriate infection control and supportive care. WHO and the CDC posted clinical guidance for COVID‐19. 59
Immediate therapy of add‐on oxygen must be started for patients with severe acute respiratory infection (SARI) and respiratory distress, shock or hypoxaemia. Patients with SARI can be given conservative fluid therapy only when there is no evidence of shock. Empiric antimicrobial therapy must be started to manage SARI. For patients with sepsis, antimicrobials must be administered within 1 hour of initial assessments. The WHO and CDC recommend that glucocorticoids not be used in patients with COVID‐19 pneumonia except where there are other indications (exacerbation of chronic obstructive pulmonary disease). 59
Patients’ clinical deterioration is closely observed with SARI; however, rapidly progressive respiratory failure and sepsis require immediate supportive care interventions comprising quick use of neuromuscular blockade and sedatives, hemodynamic management, nutritional support, maintenance of blood glucose levels, prompt assessment and treatment of nosocomial pneumonia, and prophylaxis against deep venous thrombosis (DVT) and gastrointestinal (GI) bleeding. 60 Generally, such patients give way to their primary illness to secondary complications like sepsis or multiorgan system failure. 48
13. CONVALESCENT PLASMA THERAPY
Guo Yanhong, an official with the National Health Commission (NHC), stated that convalescent plasma therapy is a significant method for treating severe COVID‐19 patients. Among the COVID‐19 patients currently receiving convalescent plasma therapy in the virus‐hit Wuhan, one has been discharged from hospital, as reported by Chinese science authorities on Monday, 17th February 2020 in Beijing. The first dose of convalescent plasma from a COVID‐19 patient was collected on 1st and 9th February 2020 from a severely ill patient who was given treatment at a hospital in Jiangxia District in Wuhan. The presence of the virus in patients is minimised by the antibodies in the convalescent plasma. Guiqiang stated that donating plasma may cause minimal harm to the donor and that there is nothing to be worried about. Plasma donors must be cured patients and discharged from hospital. Only plasma is used, whereas red blood cells (RBC), white blood cells (WBC) and blood platelets are transfused back into the donor's body. Wang alleged that donor’s plasma will totally improve to its initial state after one or 2 weeks from the day of plasma donation of around 200 to 300 millilitres. 61
14. ANTIVIRAL THERAPY
COVID‐19 is an infectious disease caused by SARS‐CoV‐2, which is also termed the novel coronavirus and is diligently associated with the SARS virus. The Ministry of Science and Technology from the People’s Republic of China declared three potential antiviral medicines suitable for treating COVID‐19. Those three medicines are, namely, Favilavir, chloroquine phosphate and remdesivir. A clinical trial was conducted to test the efficacy of those three drugs, and the results proved that out of the three medicines above only Favilavir is effective in treating the patients with novel coronavirus. The remaining two drugs were effective in treating malaria. 62
Likewise a study carried out in the United States by the National Institute of Health proved that remdesivir is effective in treating the Middle East respiratory syndrome coronavirus (MERS‐CoV), which is also a type of coronavirus that was transmitted from monkeys. The drug remdesivir was also used in the United States for treating the patients with COVID‐19. There has been a proposal to use the combination of protease inhibitors lopinavir‐ritonavir for treating the patients affected by COVID‐19. 62
It is also evident that remdesivir was effective in treating the patients who were infected with Ebola virus. Per this evidence, China has already started testing the efficacy of remdesivir in treating the patients with COVID‐19, especially in Wuhan, where the outbreak occurred. Chloroquine, which is an existing drug which is currently used in treating malaria cases, was given to more than 100 patients who were affected with novel coronavirus to test its efficacy. 62
A multicentric study was conducted in China to test the effectiveness of remdesivir in treating the patients with COVID‐19. Thus, the results of the clinical trial proved that remdesivir has a considerably acceptable level of efficacy for treating the patients with COVID‐19. Therefore, the National Health Commission of the People's Republic of China decided to include remdesivir in the Guidelines for the Prevention, Diagnosis and Treatment of Pneumonia Caused by COVID‐19. 62
Chloroquine and hydroxychloroquine are existing anti‐malaria drugs also given to more than 30 patients infected with COVID‐19 in Guangdong province and Hunan province to test their effectiveness and efficacy. Thus, the results of the clinical trial showed that the patients who were given chloroquine had a significant reduction in their body temperature. The clinical trial also showed better recovery among the patients who were given chloroquine and hydroxy chloroquine. 63 , 64 , 65 Hydroxychloroquine treatment is significantly associated with viral load reduction as well as disappearance in COVID‐19 patients. Further, the outcome is reinforced by azithromycin. The role of lopinavir and ritonavir in the treatment of COVID‐19 is uncertain. A potential benefit was suggested by preclinical data, but additional data has failed to confirm it. Tocilizumab is an immunomodulating agent used as adjunct therapy in some protocols based on a theoretical mechanism and limited preliminary data. 66
15. HOME CARE
Home management may be appropriate for patients with mild infection who can be adequately isolated in the outpatient setting. Management of such patients should focus on prevention of transmission to others, and monitoring for clinical deterioration, which should prompt hospitalisation. Interim recommendations on home management of patients with COVID‐19 can be found on the WHO and CDC websites. 67
16. CONCLUSION
The corona virus (COVID‐19) spreads at an alarming rate all over the world. The outbreak of the virus has confronted the world's economic, medical and public health infrastructure. Elderly and immunocompromised patients also are susceptible to the virus's mortal impacts. Currently, there is no documented cure for the virus and no vaccine has been created, although some treatment protocols have been promising. Therefore, the virus can be controlled with the appropriate prevention strategies. Also, attempts have to be made to formulate systematic strategies to prevent such future zoonotic outbreaks.
Disclosure
The authors declare no conflict of interest.
Almaghaslah D, Kandasamy G, Almanasef M, Vasudevan R, Chandramohan S. Review on the coronavirus disease (COVID‐19) pandemic: Its outbreak and current status. Int J Clin Pract. 2020;74:e13637. 10.1111/ijcp.13637
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
References
- 1. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:564–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. 10.1016/S0140-6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Backer J, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019‐nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25:2000062. 10.2807/1560-7917.ES.2020.25.5.2000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desselberger U. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. In: van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB, eds. International Union of Microbiological Societies. San Diego: Virus Research; 2002:1162. [Google Scholar]
- 6. AlNajjar N, Attar L, Farahat F, et al. Psychobehavioural responses to the 2014 Middle East respiratory syndrome‐novel corona virus (MERS CoV) among adults in two shopping malls in Jeddah, western Saudi Arabia. Eastern Mediterranean Health Journal. 2016;22:817–823. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Li JJ, Xie X, et al. Game consumption and the 2019 novel coronavirus. Lancet Infect Dis. 2020;20:275–276. 10.1016/S1473-3099(20)30063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . https://www.cdc.gov/coronavirus/2019‐ccov/types.html. Accessed March 5, 2020.
- 9. World Health Organization . Coronavirus disease (COVID‐19) situation reports. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed March 5, 2020.
- 10. World Health Organization . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim guidance. https://www.who.int/publications/i/item/10665‐331501. Accessed January 17, 2020.
- 11. World Health Organization . Coronavirus disease (COVID‐19) pandemic. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019.
- 12. Gautret P, Charrel R, Belhouchat K, et al. Lack of nasal carriage of novel corona virus (HCoV‐EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect. 2013;19:E315–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enjuanes L, Zuñiga S, Castano‐Rodriguez C, et al. Molecular basis of coronavirus virulence and vaccine development. Adv Virus Res. 2016;96:245–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization . Novel coronavirus situation report—2. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200122‐sitrep‐2‐2019‐ncov.pdf. Accessed February 12, 2020.
- 17. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fehr AR, Athmer J, Channappanavar R, et al. The nsp3 macrodomain promotes virulence in mice with coronavirus‐induced encephalitis. J Virol. 2015;89:1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luk HKH, Li X, Fung J, et al. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol. 2019;71:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ViralZone. Coronavirinae . https://viralzone.expasy.org/785. Accessed February 25, 2019.
- 21. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kin N, Miszczak F, Lin W, et al. Genomic analysis of 15 human coronaviruses OC43 (HCoV‐OC43s) circulating in France from 2001 to 2013 reveals a high intra‐specific diversity with new recombinant genotypes. Viruses. 2015;7:2358–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu H, Zhou J, Ho‐Yin Wong B, et al. Productive replication of Middle East respiratory syndrome corona virus in monocyte‐derived dendritic cells modulates innate immune response. Virology. 2014;454:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lambeir A‐M, Durinx C, Scharpé S, et al. Dipeptidyl‐peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. [DOI] [PubMed] [Google Scholar]
- 26. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahin AR, Erdogan A, Agaoglu PM, et al. 2019 novel coronavirus (COVID‐19) outbreak: a review of the current literature. EJMO. 2020;4:1–7. [Google Scholar]
- 28. Hui DS, Azhar EI, Madani TA, et al. Novel coronavirus outbreak in Wuhan. China. Int J Infect Dis. 2019;2020:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization . Global Surveillance for human infection with coronavirus disease (COVID‐2019). Interim Guidance. https://www.who.int/publications/i/item/global‐surveillance‐for‐human‐infection‐with‐novel‐coronavirus‐(2019‐ncov) Accessed March 20, 2020.
- 30. World Health Organization . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. Interim Guidance, March 2, 2020. This work is available under the CC BY‐NC‐SA 3.0 IGO licence.
- 31. Shen K, Yang Y, Wang T, et al. Novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. 2020;16:223–231. 10.1007/s12519-020-00343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu K, Cai H, Shen Y, et al. Management of corona virus disease‐19 (COVID‐19): the Zhejiang experience. Jour Zhejiang Univ. 2020;49. 10.3785/j.issn.1008-9292.2020.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang W, Du R‐H, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Chen C, Zhu S, et al. Isolation of 2019‐nCoV from a stool specimen of a laboratory‐confirmed case of the coronavirus disease 2019 (COVID‐19). China CCDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer B, Drosten C, Müller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;19:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai SL, Wang JY, Zhou YQ, et al. Analysis of the first family epidemic situation of new coronavirus pneumonia in Gansu Province. Chin J Prev Med. 2020;54:E005. [DOI] [PubMed] [Google Scholar]
- 37. Xiao SY, Wu Y, Liu H, et al. Evolving status of the 2019 novel coronavirus infections: proposal of conventional serologic assays for disease diagnostics and infection monitoring. J Med Virol. 2020;92:464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization . Coronavirus disease (COVID‐19) advice for the public. https://www.who.int/emergencies/disease/novel‐coronavirus‐2019/advice‐for‐public. Accessed March 16, 2020.
- 39. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19): how to protect yourself. https://www.cdc.gov/coronavirus/2019‐ncov/prepare/prevention.html. Accessed March 16, 2020.
- 40. Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID‐19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. 10.1016/j.ajog.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Favre G, Pomar L, Musso D, et al. 2019‐nCoV epidemic: what about pregnancies? Lancet. 2020;395:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. The American College of Obstetricians and Gynaecologists . Practice advisory: novel coronavirus 2019 (COVID‐19). https://www.acog.org/Clinical‐Guidance‐and‐Publications/Practice‐Advisories/Practice‐Advisory‐Novel‐Coronavirus2019?Is‐MobileSet=false. Accessed March 12, 2020.
- 43. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) Coronavirus 2019‐nCoV infecting pregnant women: lessons from SARS, MERS and other human coronavirus infections. Viruses. 2020;12:E194. 10.3390/v12020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention . Interim considerations for infection prevention and control of coronavirus disease 2019 (COVID‐19) in inpatient obstetric healthcare settings. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/inpatient‐obstetric‐healthcare‐guidance.html. Accessed March 14, 2020.
- 47. Centers for Disease Control and Prevention . Pregnancy and breastfeeding: information about coronavirus disease 2019. https://www/cdc.gov/coronavirus/2019‐ncov/prepare/pregnancy‐breastfeeding.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019‐ncov%2Fsoecific‐grous%2Fpregnancy‐faq.html. Accessed March 13, 2020.
- 48. Duddu P.Coronavirus treatment: vaccines/drugs in the pipeline for COVID‐19. https://www.clinicaltrialsarena.com/analysis/coronavirus‐mers‐cov‐drugs/. Accessed 24 February 24, 2020
- 49. HHS . HHS engages Sanofi’s recombinant technology for 2019 novel coronavirus vaccine. https://www.hhs.gov/about/news/2020/02/18/hhs‐engages‐sanofis‐recombinant‐technology‐for‐2019‐novel‐coronavirus‐vaccine.html. Accessed February 18, 2020.
- 50. Centers for Disease Control and Prevention . Interim infection prevention and control recommendations for patients with confirmed coronavirus disease 2019 (COVID‐19) or persons under investigation for COVID‐19 in healthcare settings. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/infection‐control‐recommendations.html. Accessed February 21, 2020.
- 51. Centers for Disease Control and Prevention . Interim clinical guidance for management of patients with confirmed 2019 novel coronavirus (2019‐nCoV) infection. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html. Accessed May 20, 2020.
- 52. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473–475. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention . Interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for coronavirus disease 2019 (COVID‐19); 2020. https://www.cdc.gov/coronavirus/2019‐nCoV/lab/guidelines‐clinical‐specimens.html. Accessed February 28, 2020.
- 54. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395:473–475. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siegel MD, Siemieniuk R, Parsons PE, Guyatt G.Acute respiratory distress syndrome: supportive care and oxygenation in adults. https://www.uptodate.com/contents/acute‐respiratory‐distress‐syndrome‐supportive‐care‐and‐oxygenation‐in‐adults?topicRef=126981&source=see_link. Accessed February 29, 2020.
- 56. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected. Interim guidance. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19. Accessed January 28, 2020
- 57. Schmidt GA, Mandel J, Parsons PE, Sexton DJ, Hockberger RS.Evaluation and management of suspected sepsis and septic shock in adults. https://www.uptodate.com/contents/evaluation‐and‐management‐of‐suspected‐sepsis‐and‐septic‐shock‐in‐adults. Accessed February 11, 2020.
- 58. World Health Organization . Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts; February 4, 2020. https://www.who.int/publications‐detail/home‐care‐for‐patients‐with‐suspected‐novel‐coronavirus‐(ncov)‐infection‐presenting‐with‐mild‐symptoms‐and‐management‐of‐contacts. Accessed February 14, 2020.
- 59. Centers for Disease Control and Prevention . Interim guidance for implementing home care of people not requiring hospitalization for 2019 novel coronavirus (2019‐nCoV). January 31, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/guidance‐home‐care.html. Accessed February 4, 2020.
- 60. Pharmaceutical Technology . China approves first anti‐viral drug against coronavirus COVID‐19. https://www.pharmaceutical‐technology.com/news/china‐approves‐favilavir‐covid‐19/. Accessed February 18, 2020.
- 61. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and treatment coronavirus (COVID‐19). March 8, 2020. [PubMed]
- 62. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Centers of Disease Control and Prevention. Children and coronavirus disease 2019 (COVID‐19) . https.//www.cdc.gov/coronavirus/2019‐ncov/prepare/chilgren.html.Published2020. Accessed March 24, 2020.
- 65. Smith T, Bushek J, Prosser T. COVID‐19 drug therapy. Clinical Drug Information/Clinical Solutions. Amsterdam, The Netherlands: Elsevier; 2020. [Google Scholar]
- 66. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Centers of Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19): older adults. 2020. https://www.cdc.gov/coronavirus/2019‐ncov/specific‐groups/high‐risk‐complications/older‐adults.html. Accessed March 24, 2020.


