To the Editor,
Coronavirus disease 2019 (COVID‐19) pandemic has become one of the most challenging episodes in the history of modern public health, with particular emphasis in high‐risk population. 1 However, the evidence regarding their response to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the agent responsible for COVID‐19 is scant. 2 Herein, we present the clinical and therapeutic course of a SARS‐CoV‐2 infection in a patient with multivisceral transplant and a recent tuberculosis infection.
A 31‐year‐old male that on March 2003 was diagnosed with volvulus and required an extensive intestinal resection. On June 2006, he received an isolated intestinal graft. At the age of 29, he was diagnosed with chronic rejection, and 4 months later (July 2018), he received a multivisceral transplant. On December 2018, patient was diagnosed with disseminated tuberculosis and quadruple antibiotic therapy was initiated. During 2019, he required occasional hospital admissions to manage the mutifactorial respiratory problems.
Patient's history of SARS‐CoV‐2 infection is summarized in Figure 1. On March 9 (day −12), patient went to the outpatient clinic with mild fever, cough, runny nose, and diarrhea. Notable laboratory results are shown in Table 1. Pharyngeal adenovirus infection was diagnosed, with C‐reactive‐protein (CRP) and white blood cells (WBC) above normal range. On March 13, abdominal pain got worse and blood in stool appeared; therefore, he was admitted for hospital monitoring. Vital signs were between normal ranges during the whole hospitalization period. RT‐PCR testing was negative for both adenovirus (pharyngeal smear and peripheral blood) and SARS‐CoV‐2 (nasopharyngeal swab). As seen in the previous laboratory test, WBC, platelets, and CRP values were above normal range.
Figure 1.
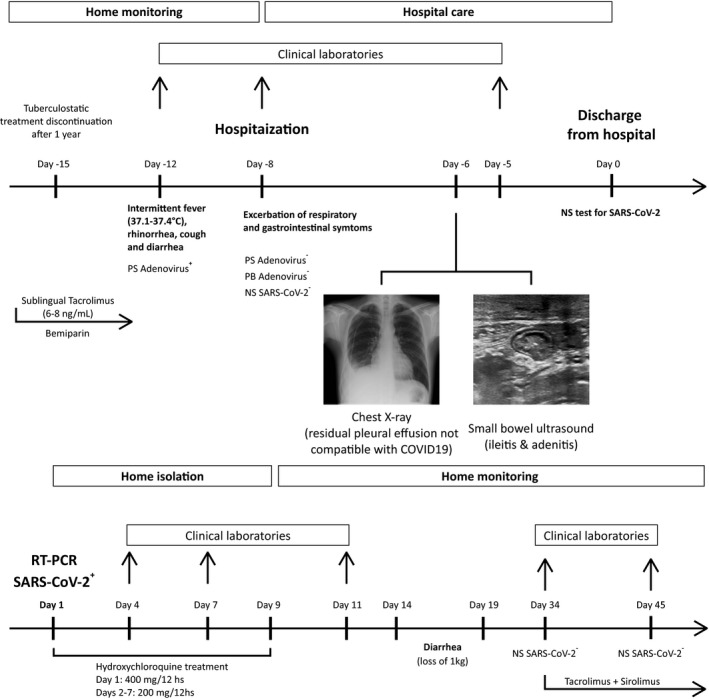
COVID‐19 episode timeline. Notable events from 12 days before COVID‐19 diagnosis and until 45 days after that moment are shown. Pharmacological treatments, patient symptoms, days on which laboratory samples were taken, imaging findings, and time of hospitalization are depicted.). Abbreviations: PS, pharyngeal smear; PB peripheral blood; NS, nasopharyngeal swab; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; RT‐PCR, reverse transcription polymerase chain reaction
Table 1.
Clinical laboratory results
| Variable | SARS‐CoV‐2‐ | SARS‐CoV‐2+ | SARS‐CoV‐2‐ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | Day −12 | Day −8 | Day −5 | Day 1 | Day 4 | Day 7 | Day 11 | Day 34 | Day 45 | |
| SARS‐CoV‐2 | (−) | (+) | (−) | (−) | ||||||
| Adenovirus | (+) | (−) | ||||||||
| Virus/Bacteria/Parasites | (−) | (−) | ||||||||
| Albumin (g/dL) | 2.9‐5.2 | 4.3 | 4.4 | 4 | 4.5 | 4.8 | ||||
| CRP (mg/mL) | 0‐5 | 35.1 | 23.2 | 9,6 | 5.8 | 5.8 | 6.6 | 7.2 | 19.5 | |
| AST (U/L) | <40 | 56 | 44 | 43 | 42 | 46 | ||||
| ALT (U/L) | <35 | 75 | 64 | 67 | 80 | 94 | ||||
| Ferritin (ng/mL) | 22‐322 | 24 | 248 | 169 | 157 | |||||
| eGFR (mL/min/1.73m2) | >75 | >90 | >90 | 85 | >90 | >90 | >90 | 88 | ||
| BUN (mg/dL) | 11‐49 | 57 | 38 | 35 | 62 | 80 | ||||
| SCr (mg/dL) | 0.7‐1‐3 | 0.98 | 0.85 | 1.01 | 1.03 | 1.07 | 1.11 | |||
| LDH (U/L) | 100‐190 | 253 | 213 | 232 | 269 | |||||
| Prothrombin time (s) | 11.9 | 12.7 | 12.5 | 11.9 | 11.9 | 11.5 | ||||
| Derived fibrinogen (mg/dL) | 150‐450 | 397 | 352 | 319 | 344 | 321 | 253 | |||
| Cephalin time (s) | 28.9 | 24.8 | 25.4 | 27.9 | 27.7 | 28.4 | ||||
| D‐dimer (ng/mL) | 0‐500 | 2114 | 1803 | 1844 | 1440 | 2150 | ||||
| WBC (x103/uL) | 3.9‐10.2 | 11.36 | 11.41 | 10.41 | 12.41 | 11.05 | 12.38 | 9.42 | 9.0 | |
| Neutrophils (x103/uL) | 1.5‐7.70 | 5.67 | 6.02 | 5.3 | 5.6 | 4.7 | 5 | 4.08 | 4.19 | |
| Lymphocytes (x103/uL) | 1.1‐4.5 | 1.91 | 1.71 | 1.47 | 2.54 | 2.19 | 2.85 | 2.33 | 1.82 | |
| Monocytes (x103/uL) | 0.10‐0.9 | 0.98 | 1 | 1.08 | 1.22 | 0.97 | 1.31 | 0.96 | 0.72 | |
| Eosinophils (x103/uL) | 0.02‐0.65 | 2.48 | 2.37 | 2.29 | 2.68 | 2.88 | 2.86 | 1.8 | 2.12 | |
| Platelets (x103/uL) | 150‐370 | 451 | 460 | 486 | 477 | 500 | 480 | 393 | 297 | |
| Total IgG (mg/dL) | 725‐1900 | 1890 | ||||||||
| IgG SARS‐CoV‐2 | (−) | |||||||||
| Total IgM (mg/dL) | 45‐280 | 52 | ||||||||
| IgM SARS‐CoV‐2 | (−) | |||||||||
| C3 (mg/dL) | 75‐135 | 131 | ||||||||
| C4 (mg/dL) | 14‐60 | 27.80 | ||||||||
| CD4+ SARS‐CoV‐2 | (−) | |||||||||
| CD8+ SARS‐CoV‐2 | (−) | |||||||||
| Tacrolimus (ng/mL) | 6‐8 | 4.3 | 13.9 | 9.3 | 3.2 | 7.2 | 7.6 | 7.5 | 8.6 | |
| Sirolimus (ng/mL) | 6‐8 | 10.3 | ||||||||
Values unbold were above reference range. Flow cytometry experiments: lymphocytes were gated according to their forward and side characteristics. CD4 and CD8 cells were previously defined as CD3+CD45RA‐7AAD‐.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C‐reactive protein; eGFP, estimated glomerular filtration rate (based on CKD‐EPI creatinine); LDH, lactate dehydrogenase; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; Scr, serum creatinine; WBC, white blood cells.
On day −6, thorax‐Rx showed residual right pleural effusion, similar to previous study (March 2019). No signs compatible with COVID‐19 lung infection were observed. Considering that patient still presented moderate abdominal pain and diarrhea, an abdominal ultrasound test was performed, revealing ileitis and adenitis.
Considering COVID‐19 epidemiological environment and patient health status on March 24 (Day 0), a nasopharyngeal sample was taken and patient was discharged from hospital. Nevertheless, SARS‐CoV‐2 RT‐PCR came back positive (Day 1). Hydroxychloroquine treatment was empirically started at that moment, and home monitoring with consulting every 2‐3 days was recommended. Along home isolation period, no respiratory symptoms were reported. Laboratory test results of days 4, 7, and 11 showed WBC above normal range but normal lymphocytes count. Although elevated compared to reference values, CRP was notably lower than before COVID‐19 diagnosis.
Platelet count and D‐dimer values were also above normal range during days 4‐11. Nonetheless, derived fibrinogen, prothrombin, and cephalin time remain between normal range during that time lapse. It is important to emphasize that patient was already receiving anticoagulant prophylaxis with bemiparin due to chronic central vein thrombosis and because he was carrier of a central catheter. Renal function presented elevated levels of blood urea nitrogen (BUN) and lactate dehydrogenase (LDH) during the COVID‐19 episode. However, estimated glomerular filtration rate and serum creatinine were in normal range. Both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were already above normal values during the days −12/‐5 and remain elevated at Day 11. Ferritin levels were between normal range, but 10 times higher compare to values of Day −12. At days 34 and 45, patient had two negative SARS‐CoV‐2 RT‐PCR. Interestingly, we could not detect specific IgG/IgM antibodies against SARS‐CoV‐2 in serum after recovery (Anti‐S glycoprotein ELISA determination) nor virus specific CD4+/CD8+ T cells (IFN‐γ production after in vitro stimulation with SARS‐CoV‐2 peptides‐flow cytometry determination). Patient was under tacrolimus administration along this episode, and despite physiological variations, blood levels remain between the desired range of 6‐8 ng/mL (Table 1).
In this multivisceral transplant recipient with SARS‐CoV‐2 infection, gastrointestinal manifestations could be related to the altered immunological status of the graft and should be taken into consideration in further cases. 3 Whether the favorable outcome was due to patient low risk because of his age, hydroxychloroquine, anticoagulant, or immunosuppressant treatment (or a combination of all those factors) cannot be concluded. 4 , 5 However, central role of SARS‐CoV‐2 early detection and treatment cannot be ruled out. Of note, the immunosuppressive regime used was higher than in other solid organ transplants and was not suspended nor diminished during COVID‐19 episode. 6 This fact could influence the patient T and B unresponsiveness against the virus; nevertheless, he achieved infection resolution without severe respiratory symptoms. More experience needs to be accumulated in order to conclude whether transplanted patients represent or not a group of risk for SARS‐CoV‐2 infection and determine the best way to handle immunosuppression treatment.
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.
AUTHOR CONTRIBUTIONS
Papa‐Gobbi, Rodrigo; Talayero, Paloma; Stringa, Pablo; and Rumbo, Martín involved in manuscript preparation. Papa‐Gobbi, Rodrigo involved in figures preparation. Pascual‐Miguel, Bárbara involved in flow cytometry analysis of SARS‐CoV‐2‐specific T cells. Papa‐Gobbi, Rodrigo; Alcolea‐Sánchez, Alida; and González‐Sacristan, Rocío involved in clinical data collection. Bueno, Alba; Serradilla, Javier; Andrés, Ane; and López‐Santamaría, Manuel involved in surgical data collection. Ramos‐Boluda, Esther and Hernández‐Oliveros, Francisco involved in study supervision.
ACKNOWLEDGEMENTS
The authors thank NUPA (Spanish Association of Help to Children and Adults with intestinal failure, parenteral nutrition, and multivisceral transplant) for its support, and Roberts J. for the critical revision of clinical laboratory results.
Papa-Gobbi R, Bueno A, Serradilla J, et al. Novel coronavirus (SARS-CoV-2) infection in a patient with multivisceral transplant. Transpl Infect Dis. 2021;23:e13430. 10.1111/tid.13430
Esther Ramos‐Boluda and Francisco Hernández‐Oliveros share senior authorship.
REFERENCES
- 1. Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn C, Amer H, Anglicheau D, et al. Global Transplantation COVID Report March 2020. Transplantation. 2020;104(10):1974–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian Y, Rong L, Nian W, et al. Review article: gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, Cron RQ, Hartwell J, et al. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2(6):e358‐e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez AV, Roman YM, Pasupuleti V, et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID‐19: A living systematic review. Ann Intern Med. 2020. 10.7326/M20-2496 [DOI] [PubMed] [Google Scholar]
- 6. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]


