Abstract
The severe acute respiratory syndrome coronavirus 2 pandemic has drastically altered all facets of clinical care and research. Clinical research in hepatology has had a rich tradition in several domains, including the discovery and therapeutic development for diseases such as hepatitis B and C and studying the natural history of many forms of chronic liver disease. National Institutes of Health, foundation, and industry funding have provided important opportunities to advance the academic careers of young investigators while they strived to make contributions to the field. Instantaneously, however, all nonessential research activities were halted when the pandemic started, forcing those involved in clinical research to rethink their research strategy, including a shift to coronavirus disease 2019 research while endeavoring to maintain their preexisting agenda. Strategies to maintain the integrity of ongoing studies, including patient follow‐up, safety assessments, and continuation of investigational products, have included a shift to telemedicine, remote safety laboratory monitoring, and shipping of investigational products to study subjects. As a revamp of research is being planned, unique issues that face the research community include maintenance of infrastructure, funding, completion of studies in the predetermined time frame, and the need to reprogram career path timelines. Real‐world databases, biomarker and long‐term follow up studies, and research involving special groups (children, the homeless, and other marginalized populations) are likely to face unique challenges. The implementation of telemedicine has been dramatically accelerated and will serve as a backbone for the future of clinical research. As we move forward, innovation in clinical trial design will be essential for conducting optimized clinical research.
Abbreviations
- BA
biliary atresia
- COVID‐19
coronavirus disease 2019
- EHR
electronic health record
- FDA
U.S. Food and Drug Administration
- MVP
Million Veterans Program
- NASH
nonalcoholic steatohepatitis
- NIH
National Institutes of Health
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VA
Veterans Administration
The impact of coronavirus disease 2019 (COVID‐19) on clinical research has been felt variably across the world, though there are several unique considerations in the United States. Clinical research in every domain of medicine during the early phases of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic came to a halt and has been largely relegated to a nonessential activity. Appropriately, the shift has been toward COVID‐19‐related research, including an accelerated strategy for developing and validating diagnostic tests, vaccines, and therapeutics. As such, nonessential research, including National Institutes of Health (NIH)‐ and pharmaceutical industry‐sponsored clinical trials, longitudinal biorepositories, and real‐world research studies, have faced significant challenges.
Clinical research in hepatology has a rich and long tradition of developing therapies through rigorously conducted trials for conditions such as chronic hepatitis B and C and now nonalcoholic steatohepatitis (NASH).( 1 , 2 , 3 ) Historically, materials from biorepositories have also led to important observations; serum collected after an outbreak of icteric hepatitis in army personnel in 1942 linked to contaminated yellow fever vaccine allowed for subsequent recognition that this outbreak was attributable to hepatitis B.( 4 ) Long‐term trials that allow for description of disease natural history, such as the HALT‐C (Hepatitis C Antiviral Long‐Term Treatment against Cirrhosis) study, have made essential observations that continue to inform clinical practice.( 5 ) Real‐life studies have provided an opportunity to address therapeutic outcomes in challenging populations and to compare standard‐of‐care therapeutic approaches.( 6 ) Furthermore, real‐world data have provided much needed information on patient characteristics and care outside of the clinical trial environment where patients are carefully selected and have generated postmarketing safety and clinical effectiveness data postapproval of hepatitis C therapy, allowing for the critical evaluation by the U.S. Food and Drug Administration (FDA).( 7 ) In addition, the Veterans Administration (VA) research over decades has made sentinel observations relevant to clinical practice while providing an enormous biorepository through the Million Veterans Program (MVP).( 8 , 9 , 10 )
Hepatology clinical research was halted when the pandemic took hold, and uncertainty in the timing and pace of reentry has created additional challenges. Perhaps more important, what the long‐term postpandemic era of “the new normal” state will hold is unknown. In the immediate future, prospects for maintenance of current research funding and infrastructure are uncertain as the community struggles with maintaining safe practice procedures to minimize the risk of transmission of COVID‐19. Clinical care revenue and foundation/association endowments, which have been vital to support unfunded and investigator‐initiated research, have also been considerably impacted. Thus, as investigators, it is important that we recognize these challenges, position ourselves for anticipated changes, and become innovative in order to achieve our research mission. These challenges have also created important opportunities and have already led to accelerated integration of telehealth into research practices and expanded the potential of real‐world datasets to rapidly disseminate knowledge as the pandemic evolved. Continued growth will require partnering with several stakeholders, including investigators, patients, information technology, industry, and national and international partners such as the FDA, European Medicines Agency, and NIH. This document endeavors to provide insights into the current status of clinical research in several domains of hepatology (Fig. 1), as well as its challenges and the direction it might be heading in, during and following the COVID‐19 pandemic (Table 1).
Fig. 1.
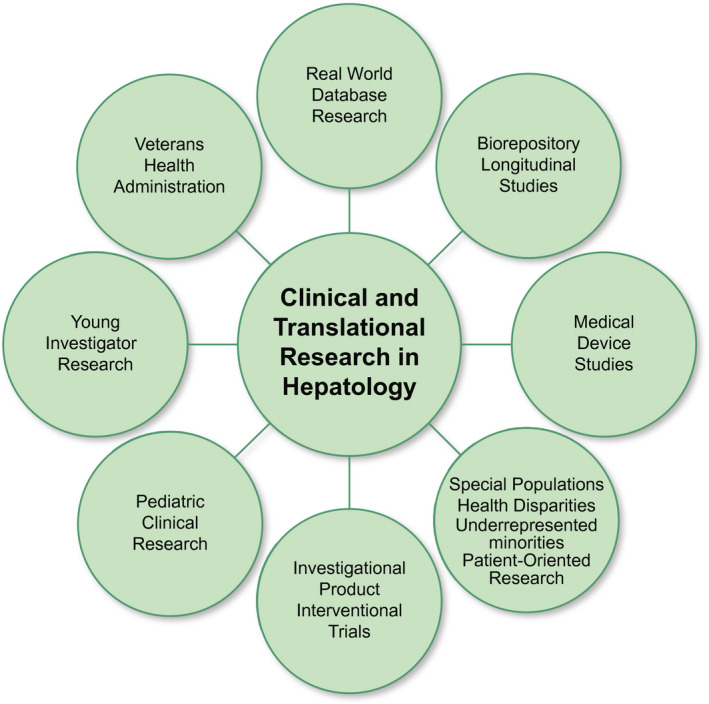
Domains of clinical and translational research in hepatology.
Table 1.
Domains of Clinical Research and the Changes, Challenges, and Opportunities
| Domain of Research | Initial Changes in COVID‐19 Pandemic | Considerations in Re‐entry | Long‐Term Challenges | Innovative Strategies and Opportunities |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: IP, investigational product; PRO, patient‐reported outcome; IT, information technology.
Investigational Product, Clinical, and Device Trials
Sponsored Clinical Trials
As SARS‐CoV‐2 made its way to the United States, research centers within and outside academic institutions quickly moved to close down nonessential activities, including face‐to‐face study visits and cessation of enrollment in new clinical trials. Investigators were required to determine which research visits were essential for safety purposes; clinical research infrastructure shifted to focus on supporting COVID‐19‐related research. For many, the loss of clinical research support staff and facilities made conducting even the essential safety visits challenging. Existing clinical trials had to adjust expectations—shifting to telemedicine visits, less reliance on laboratory or other invasive monitoring, and less collection of biospecimens for ancillary studies. The hard truth is that many active clinical trials have missed collection of critical study data that, in turn, may influence the successful and timely completion and interpretation of studies. Because of exceptional circumstances, protocol deviations/violations will likely be more frequent, and adverse events may also be more common, but causality assessment attributable to COVID‐19 as a confounder can be challenging. It is very likely that extension of data collection post‐COVID‐19 to capture critical safety and efficacy data may be needed. Both from the standpoint of regulatory agencies and the journals publishing study results, consideration of the impact of protocol deviations on the study outcomes and conclusions will be necessary.
As institutions reopen clinical activities and clinical research, the trajectory for clinical trials faces uncertainties. First, research staff, ancillary study services, and study participants need to be assured of a safe environment to conduct research. This will be stepwise and variable in pace across the United States. The focus will be initially on resuming work on clinical trials already underway, and any new study would need a COVID‐19 contingency plan. Going forward, clinical trials should consider provisions for telemedicine visits, remote monitoring, use of services for remote collection of biospecimens, and plans for direct delivery of investigational product to participants. Second, the COVID‐19 experience has served to highlight what is essential versus what is aspirational with regard to data and biospecimen collection. Clinical trials may need to focus more on the essential and less on the aspirational for the near future. Third, the research community needs to consider the impact of COVID‐19 on patients’ willingness to participate in clinical trials( 11 ) with efforts made to assure participants that their safety has been and remains a primary focus. Indeed, COVID‐19 may provide an opportunity to highlight the role of clinical research in health care advances. The importance of well‐designed clinical trials as a means of informing best practices—even under the threat of a pandemic—can be highlighted.
The long‐term impact of COVID‐19 on clinical trials is difficult to quantify, but is likely to be measured in years, even though the initial shutdown was only for a few months. This reflects the time needed to reboot the clinical research infrastructure, have investigators refocus on non‐COVID‐19 activities, and to reinvigorate participants to engage in clinical research. Furthermore, new financial challenges for institutions may reduce protected time for clinician researchers and/or an institution’s capacity to support investigator‐initiated clinical trials, as well as potentially limiting the space for the conduct of trials. Yet, there are clearly many positives that have emerged from the COVID‐19 experience that improve our capacity to conduct clinical trials, including, as detailed below, how to use technology to facilitate research activities, including teleconsenting and videoconferencing of investigator meetings.
Potential Methodological Changes in Clinical Trial Data Analysis
The disruption to clinical trial procedures by COVID‐19 creates analytical challenges given that some planned data will have not been collected. Both the goals of the trial as well as the analysis plan may therefore need to be revised. In conducting statistical analyses and model building, results should be more cautiously interpreted if based on a more‐limited data set, and sensitivity analyses are needed to determine the robustness of the results in the setting of protocol deviations.( 12 ) Imputation methods will also need to be considered to account for missing data.( 13 ) To avoid heavy reliance on complicated statistical methods as an adjustment for incomplete data, these challenges motivate us to develop more‐flexible, non‐fixed‐sample designs that are capable of extending the trial based on current cumulated data while still keeping the statistical plan rigorous. Adaptive designs are highly desirable for future clinical trials: such designs allow us to update the initial design parameters at the interim stages and re‐estimate the sample size based on the data observed in the course of the trial to ensure adequate power. Multiple adaptive designs are available with applicability across all phases of clinical investigation.( 14 )
Clinical Research at the VA
The Veterans Health Administration is the largest and oldest integrated health care system in the United States and is unique in that it has its own federally funded research program through the VA office of Research and Development that supports health research nationwide. The health system serves over 9 million veterans and research in the system has contributed to changes to clinical practice over decades.( 15 ) VA clinical research not only consists of clinical trials (most of them multicenter), but also of health‐services–related research that, rather than randomizing subjects, randomizes participating VA sites to implementation (or not) of different strategies to improve the care of specific groups of veterans or to improve performance/quality of care. An important part of VA research is observational analyses of large databases containing clinical information on all veterans enrolled in VA care, the most important of which is the VA Corporate Data Warehouse. As mentioned above, the MVP is a national research program to learn how genes, lifestyle, and military exposures affect health and illness.
The VA Office of Research and Development (ORD) ceased all in‐person recruitment and enrollment in clinical trials as of March 16, 2020, and measures similar to non‐VA clinical trial sites were implemented (e.g., virtual visits and enrollment). Despite this, there will be a delay in the completion and completeness of these studies, and it is foreseen that many of them will require extension with supplementary funds. On the other hand, database research at the VA has expanded in this period to include studies investigating the effect of COVID‐19 and its treatment on veterans. The MVP continued recruitment and enrollment through an online survey assessing veterans’ experiences with COVID‐19, including how the pandemic affects their physical and mental health. In addition, many requests for applications have been put forward from the VA ORD to support efforts related to better understanding, preventing, diagnosing, and treating COVID‐19.
Device‐Related Research
Device trials represent ~10% of all clinical trials—over 34,000 are currently registered on ClinicalTrials.gov.( 16 ) Device trials have several unique characteristics that make them especially vulnerable to disruption by the COVID‐19 pandemic. Therapeutic or diagnostic device trials may require an FDA‐issued investigational device exemption, are rarely “placebo‐controlled,” and may compare the investigational device to an existing technology. Traditionally, they have relatively small sample sizes and involve fewer study visits. Furthermore, they may require specialized training or unique expertise.
Depending upon the complexity of the device, device trials and device companies may be less able to cope with trial shutdown because of disruption in supply chain, limited resources, limited capital, and inability to replace key personnel. In contrast to pharmaceutical trials, device trials frequently involve a single critical intervention (e.g., surgical implantation, diagnostic assessment); if this critical intervention is delayed, the impact on the trial is substantial. If key personnel at the clinical research center who have been trained in administration of the device are furloughed or laid off, training of new personnel may be required, prolonging the time to restarting the device trial. With the decline in numbers of coordinators and investigators able to assess subjects and conduct device trials, sites are left to prioritize studies. As a result, recruitment, enrollment, execution, and completion of a device trial may suffer disproportionately.
FDA Perspective and NIH‐Funded Research
The FDA( 17 ) and NIH( 18 ) have each posted guidance documents for the conduct of clinical trials during the COVID‐19 pandemic. These statements acknowledge that because of the pandemic, for ongoing trials, protocol deviations may be necessary and will depend on many factors, including the intervention involved, the patient population, and environmental circumstances. The overarching principle is that patient safety is of utmost importance and should be used to guide decisions impacting the trial, including recruitment, continuation decisions, patient monitoring, delayed assessments, and investigational product dispensing. Implementation of alternative visit formats, including virtual, phone, or remote contact, should be considered, provided the safety of the subject can be assured with these approaches. Furthermore, there will need to be increasing flexibility for testing, assuming that the safety of the patient can be assured. Protocol modifications that reduce immediate danger or protect the well‐being of the research participants may be implemented before institutional review board (IRB) approval, but must be subsequently reported.
Even beyond the pandemic, it appears nearly certain that the complexion of clinical research will undergo lasting change. From a regulatory perspective, flexibility by the FDA toward the acceptance of virtual visits and remote safety monitoring, including laboratory testing at satellite facilities or even home phlebotomy, will almost certainly need to occur to maintain the viability and attractiveness of clinical trials. This is particularly true in the face of what will be a challenging environment for patient recruitment. Furthermore, the development of surrogate noninvasive end points for trials takes on greater urgency, given the even greater reluctance of prospective research subjects to agree to invasive procedures performed at the tertiary care center. For noninterventional studies, the challenges may be daunting, and measures to allow remote or virtual study visits and remote or home sample collection may be essential to permit their continuation.
In light of the uncertainty pertaining to the COVID‐19 crisis, there have been various measures to accommodate research expenditures during this pandemic. In response to the inevitable impact of COVID‐19 social distancing measures, the NIH has implemented guidance for applicants and recipients of funding to ensure that research funding measures are sustained.( 19 ) The current pandemic raises concern of potential biases in grant selection because of the impact of the pandemic on feasibility, including patient recruitment. However, guidance has been issued by the NIH urging reviewers not to factor in COVID‐19‐related concerns into the scoring of proposals.( 20 )
Real‐World Database Research
Real‐world data are generally defined as information acquired outside of controlled clinical trials and can encompass a vast array of sources, including medical and pharmacy claims, electronic health records (EHRs), wearable devices, social media, and observational registries, among others.( 21 ) The COVID‐19 pandemic has provided immediate opportunities to enlighten the medical community about the clinical course of this illness, whereby most of the earlier reports were descriptive real‐world studies of COVID‐19 patients hospitalized in hotspot regions of China, Italy, and New York.( 22 , 23 , 24 ) These real‐world datasets were critical to developing an early, real‐time framework for understanding the natural history, risk factors, and best practices for this novel disease, which also helped to inform the design of clinical trials of innovative therapeutics.
The pandemic has also generated many challenges to ongoing real‐world research in other disease states. Observational studies that rely on in‐person medical encounters have been impacted by the restrictions placed on nonemergent medical care at both academic and community outpatient practices. Fewer patient visits translated immediately into less real‐world EHR data and biospecimens being collected and analyzed. Real‐world studies were deprioritized when principal investigators were called upon to assist with the overwhelming number of critically ill patients with COVID‐19 and research site staff were working from home. Site startup and activation throughout the United States, and especially in Europe, was delayed because of the uncertainty created by the pandemic. Even data from wearable devices and social media were likely impacted by global lockdowns that mandated modification of real‐world activities and led to anxiety, decreased physical activity, change in eating habits, and other negative effects.
Despite these initial difficulties during the height of the pandemic, real‐world studies are extremely well positioned to adapt to what appears to be the new reality of medical care around the globe. Real‐world studies are ideally suited to acquire robust information on safety and effectiveness of drugs to treat COVID‐19 under Emergency Use Authorization designations or to fulfill postmarketing requirements when the rapidity of approval is paramount, but additional safety data are warranted. Indeed, the immediacy of the need for information to help guide management of this aggressive disease resulted in a flood of manuscripts derived from real‐world data sources, including many describing the effectiveness of therapy for unapproved drugs to treat COVID‐19.( 25 , 26 , 27 , 28 ) Two studies of hydroxychloroquine were retracted by high‐impact medical journals after irregularities were identified, raising questions about the balance between data integrity and the review process and the urgency for disseminating important research that can impact patient outcomes.( 29 , 30 ) Despite the desire and need to publish key observations that might be relevant to critical health care interventions with potential patient benefit, it remains important to conduct the research as guided by the principles of good clinical practice.
The unprecedented ramp‐up of telehealth will increase a patient’s access and comfort level with these platforms and facilitate the adoption of virtual study visits and procedures, such as electronic consent (eConsent) and completion of patient‐reported outcome measures. Furthermore, observational studies that utilize data from the EHR can maintain continuity in the remote working environments that will be in place for the foreseeable future; research coordinators can access medical records and complete case‐report forms from distant locations without compromising the safety of themselves or study participants.
Biorepository and Biomarker Research
Cohort studies designed to contribute to biorepositories or for the purposes of biomarker and microbiome analyses have been profoundly impacted by the COVID‐19 pandemic. Although many studies of potentially beneficial investigational products have continued to a limited capacity, nonessential clinical research activities were virtually completely halted. Importantly, as ramp‐up plans are put into place, often these studies remain deprioritized, jeopardizing the interpretability of the analyses if there are long periods between collections and/or if clinical events are missed. As a result of this disruption, prospective cohort studies specifically designed to identify or validate biomarkers of disease progression or clinical events could be severely impacted unless remote procedures have been effectively implemented.
There has been a temporary shift in focus in some institutions to biobanking of specimens to study COVID‐19 itself. Although this may represent a unique opportunity for novel discovery, including among patients with chronic liver disease and liver transplant recipients, the collection, processing, and storage of these samples has created unique challenges. Although the virus has been detected in blood samples in only limited numbers of confirmed positive patients, fecal and salivary samples being collected for microbiome analyses may have significantly higher quantities of detectable virus.( 31 , 32 ) Thus, these specimens, unless inactivated, are currently recommended to be processed in biosafety level 3 facilities,( 33 ) very few of which are available at most institutions. As biorepositories eventually increase their activities, because some patients with SARS‐CoV‐2 infection or viral shedding are asymptomatic, additional consideration may have to be given to the safety of handling specimens more broadly.
Given the minimal risk associated with most biomarker and biorepository studies, remote consenting even once the pandemic is over may be an enduring model. In addition, collection and shipping of specimens from home( 34 , 35 , 36 ) in conjunction with remote ascertainment of applicable clinical symptoms or outcomes could be an efficient model for some of these studies going forward. This pandemic has led to a swift acceleration in home‐based biospecimen sampling, which is even being utilized in the NIH‐sponsored population‐based SARS‐CoV‐2 serosurvey.( 37 ) These remote procedures will be crucial to ensuring that biorepositories and biomarker studies can fulfill their potential.
Clinical Research in Special Populations
Pediatric Clinical Research
Pediatric liver research has faced unique challenges during this COVID‐19 pandemic. Most impacted have been clinical research studies focused on diseases with precise developmental windows for diagnosis and treatment, namely biliary atresia (BA), the most common indication for liver transplantation,( 38 ) and other inherited disorders of cholestasis. Studies of BA done under the umbrella of The Childhood Liver Disease Research Network (ChiLDReN), an NIH‐funded consortium and biorepository,( 39 ) have been largely halted during this pandemic, with no new sample collection. The failure to capture new cases of BA will certainly extend the timeline of progress. More important, the diagnosis of BA may be delayed, given that parents have been fearful of bringing their infants to their pediatrician’s office during this critical window for BA diagnosis between hospital discharge at birth and 2‐month vaccinations.
As in adult liver research, pediatric clinical studies have been subject to inconsistent and conflicting regulatory guidelines from local hospitals and universities to state to federal government. There have been multiple conversations with sponsors and institutions to find a “blended current practice” of telemedicine, laboratories, and in‐person study visits. Intervention studies are often limited in children, but this pandemic risks delayed opening or abandonment of new intervention studies in pediatric NASH and inherited forms of pediatric cholestasis.
Given that many pediatric liver diseases fall under the category of rare disease, we may see the biggest impact of this pandemic and unstable economic times on funding of pediatric liver research. Freestanding children’s hospitals perhaps have been hit the hardest financially given that they drastically cut patient flow to prepare for a possible COVID‐19 surge and potential co‐optation of pediatric spaces for adult COVID‐19 patients. Unfortunately, most centers were left with empty hospitals and financial consequences, which will certainly impact decisions on new hires, pilot research grants, and bridge funding for clinician‐scientists. In addition, much of pediatric liver research relies on philanthropy and foundation support. As many nonprofits see a drastic decrease in donations with unprecedented financial challenges and cancellation of all fundraising events, this will certainly impact pediatric liver research funding.
The pediatric liver community is a small one in comparison to its adult counterparts. Professional networking, especially for trainees and junior faculty, has been affected with cancellation of many scientific meetings. The pandemic may impact the future directions of pediatric liver research with regard to pediatric nonalcoholic fatty liver disease, acute liver failure, and developmental outcomes research. The emergence of COVID‐19 pediatric multisystem inflammatory syndrome reveals similarities with secondary hemophagocytic lymphohistiocytosis and forms of pediatric acute liver failure presentations,( 40 , 41 ) which may prompt future investigation into overlapping and key regulatory immune pathways in children. Last, with social distancing, closure of schools and recreational activities, and less in‐person access to developmental therapies, we may see disproportionate changes in developmental outcomes of children with inherited or acquired liver disease.( 42 )
On a positive note, the COVID‐19 pandemic has strengthened an already tight‐knit pediatric hepatology community. The Society of Pediatric Liver Transplantation–The Transplantation Society and North American Society for Pediatric Gastroenterology, Hepatology & Nutrition have put forth a joint national registry for COVID‐19 in children with chronic liver disease and post–liver transplantation.( 43 ) This pandemic has propelled the pediatric liver community to discuss innovative ways in which to safely allow children and their families back into the clinical arena with necessary rethinking of clinical and research workspaces and behaviors with the goal of maintaining forward progress in pediatric liver research.
Health Disparities and Research in Marginalized Populations
Research involving marginalized populations, such as the homeless, has similarly been impacted by the COVID‐19 pandemic. Health disparities in association with COVID‐19 have been highlighted in the media and clinical literature,( 44 ) primarily in association with race and ethnicity. It is imperative to anticipate that marginalized groups, for example those experiencing homelessness or who inject drugs, will also experience health inequalities during and in the aftermath of the pandemic. Some basic principles are guiding this re‐entry planning, which hopefully will ensure ongoing and meaningful engagement with these populations: (1) be nimble, flexible, continue to look for alternate ways to perform activities; (2) ensure access to protective and sanitation equipment to prevent disease, through blood‐borne and sexually transmitted infections, apart from SARS‐CoV‐2. Syringe service programs should remain “essential”; (3) continue to promote antistigma as an essential value to minimize inequalities and social harms; and (4) engage with our participants to find out their priorities and ability to engage in research, recognizing that marginalized populations may have difficulty with access and operational aspects of technology tools.
Research teams have always had to implement “outside‐the‐box” ideas for working with marginalized populations, and now it is even more important than ever to be flexible and cognizant of the community. A significant area of research with marginalized populations includes practice‐based implementation research; for example, assessing models to enhance access to and success with care. The first challenge of this domain of research will be the effects of clinical care changes. Given that patients are seen less frequently and in‐person visits are limited, researchers may need to reduce recruitment and enrollment expectations when working within clinical practice. Research innovations include reducing the burden of data collection to what is essential to answer focused research questions while environments must remain “enabling,” allowing for physical distancing and health interventions to work. Throughout the country, clinical practice with persons experiencing homelessness or injecting drugs has rapidly changed: outside testing facilities, curbside services, telehealth visits, extended prescriptions and “take‐homes,” and rapid and home induction of medication for opioid use disorder are just some examples of the transformation that clinicians are reporting. These structural changes, mostly aimed at ensuring safe and effective clinical care with the lowest possible risk burden on patients and providers, would have taken years to study, test, and implement. There will be new opportunities to assess the impact of these changes.
It remains essential to promote stigma reduction and avoid further marginalization and unintended social consequences during this time of rapid change. Whereas telemedicine may be challenging because of lack of knowledge or access to technology, eConsenting by phone or video may need to take more time with some marginalized populations. Unique language and lexicon changes apply to these populations, and perhaps should be revisited with patients during the course of the research study to ensure they comprehend the process and procedures they are being asked to participate in. Remuneration for research participants is likely to shift from in‐person payments to mail, or transfers, and transportation arrangements may need to be made. Long‐term challenges of reaching and including marginalized and special populations in research should incorporate lessons and some “best practices” that clinicians have been developing during the acute phase of the pandemic. In addition, investigators must commit to increased representation of marginalized populations and under‐represented minorities in clinical research through partnering with health care delivery organizations that serve these communities. There have been several reasons for lack of inclusiveness of minorities in clinical research that include, but are not limited to, factors such as less access to academic medical centers, lack of transportation, lack of insurance, distrust of the medical system, and clinical research. As we move forward, it is imperative that we make studies more research “friendly” by decreasing study‐related burdens through remote assessment and care; however, it remains possible that increased use of technology might have the opposite effect in marginalized populations. It is of utmost importance to have culturally sensitive approaches to enrollment; in this regard, enlisting trusted community leaders can help. Extending clinical research from academic centers to community clinics with dense minority populations may be of added value. Finally, seeking input and ideas from our participants, those experiencing homelessness or who inject drugs, on the how, where, and why of our research to prevent and treat disease and improve health, are important.
Considerations for Early‐ and Mid‐Career Investigators
The unprecedented changes in research attributed to COVID‐19 have disproportionately affected early‐ and mid‐career investigators. In addition to disruption of research activities, in many centers, early‐ and mid‐career clinical investigators and physician scientists have been deployed to clinical settings interrupting research efforts. For those investigators with caregiver responsibilities, COVID‐19 has placed additional strain given the increasing scarcity of available childcare. These additional strains have likely disproportionately affected the academic productivity of women compared to men given the unequal burden of domestic work and childcare shouldered by women.( 45 )Early‐ and mid‐career investigators may be particularly vulnerable to the research setbacks created by this period of decreased productivity. For those with career development awards, this is a crucial but limited period of training and mentored research meant to propel them toward independent funding. In response to these challenges, many universities have enacted policies extending probationary periods for tenure or promotions for faculty on research tracks. In addition, the NIH has enacted more flexible policies for research delayed or negatively impacted by COVID‐19 by extending budget periods for approved projects and providing for urgent competitive revisions and administrative supplements.
As we enter the next phase of the COVID‐19 pandemic, clinical and research duties are likely to wax and wane, resulting in intermittent and increased clinical duties with decreased patient recruitment and cohort enrollment. For early‐career clinical investigators relying on prospective enrollment in clinical trials or biospecimen collection to quickly analyze for preliminary data for larger awards, there will be a need to think outside the box and develop new strategies for patient recruitment, follow‐up, and data collection. Furthermore, they can channel their energy toward alternative research endeavors in order to continue to demonstrate academic productivity while some aspects of their research programs remain on hold (Table 2).
Table 2.
Alternative Research and Scholarly Areas of Engagement
|
The Patient Perspective on Clinical Research
With the biggest barriers being lack of trust or misconception, ongoing communication and reassurance during the COVID‐19 pandemic is essential for continued and successful work in clinical research. Many patients are open to testing investigational products or treatments and become saddened when a trial ends abruptly at any phase. Most are heavily invested in their personal health and hopeful about potential outcomes. Other motives that may contribute to patients’ feelings of letdown during the pandemic might include curtailment of financial compensation, travel to see a medical expert, or the humanitarian aspect of helping patients.
Telemedicine could be thought of as a reassuring aspect of clinical trials and a boost to retention and new recruitment. Apart from an investigational product being delivered to homes, a patient self‐monitoring with a phone app, detecting body temperature or taking high‐tech images of jaundice, would be most welcome by research participants. Although there will always be a need for laboratory tests and physical presence in clinics, access through smartphones can be user‐friendly and potentially have a higher retention rate.( 46 ) In the United States, a patient survey assured that patients trust telemedicine during clinical trials.( 47 ) The FDA has launched the MyStudies app, linked with Google Cloud, and sent out new guidelines. Telemedicine use in clinical trials also has the potential to mitigate inequalities through decentralization, electronic consent, and increased awareness of studies, thereby reducing barriers to entry for minorities, as experienced by blacks.( 48 ) By embracing changing technologies, and partnering with research teams and clinicians, all patients can experience the benefits of clinical research for new therapies during and beyond the era of COVID‐19.
Innovative Strategies
Despite the challenges, the pandemic has uncovered many opportunities. For clinical research to remain viable in the short term, all stakeholders must embrace new ways of conducting almost every aspect of clinical trials (Fig. 2). Strategies such as social media and targeted online ads and online prescreening questionnaires must replace their face‐to‐face counterparts. We need to establish clinical trial processes that are safe and acceptable during the pandemic. eConsents can do more than facilitate consent from remote locations; the process can be interactive, which may facilitate retention of the information, and can be used to evaluate the subject’s comprehension of the information presented.( 49 )
Fig. 2.
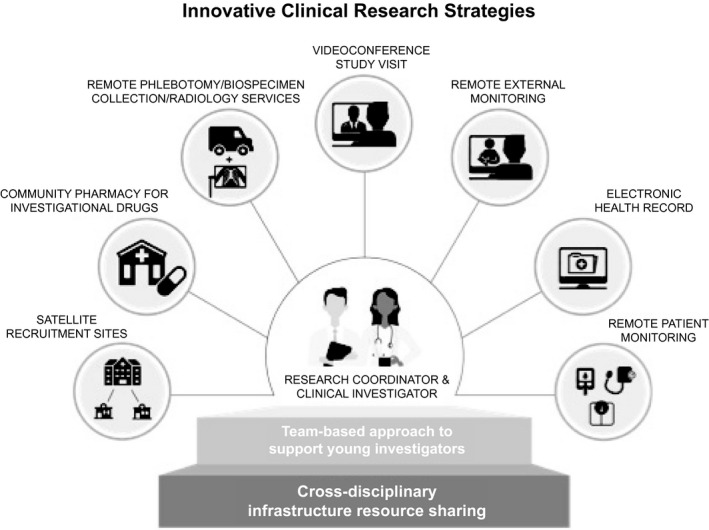
Innovative clinical research strategies.
Steps are being taken to facilitate innovative strategies in clinical trial enrollment, and much of the technology to enable remote patient recruitment, enrollment, investigational drug administration, and study measurement already exists. Telehealth in combination with mobile health (mHealth), defined as medical and public health practice supported by mobile devices, such as mobile phones, patient‐monitoring devices, personal digital assistants, and other wireless devices, can bring biomedical research to the participant’s home.( 50 ) These tools can improve convenience, reduce study visits, facilitate online surveys and assessments, increase retention and participation, deliver reminders, enable real‐time remote tracking, and enhance participant safety with real‐time alerts.( 51 ) There are successful examples of entirely Web‐based remote clinical trials in which participants are recruited by the Web, screened for eligibility using online questionnaires, receive study medications shipped directly to their home, and have laboratory testing in their community.( 52 ) Mobile technologies and Web‐based clinical trial design can recruit large numbers of participants in a short time span with minimal costs.( 53 ) Similarly, such technology may address long‐standing barriers to clinical research participation, such as the recruitment of subjects from rural and low‐income populations.( 54 )
With regard to study parameter measurements, a variety of validated toolkits supported by the NIH( 55 ) are available to enable virtual (online, tablet, etc.) assessment of health‐related quality of life, cognitive, and physical symptoms. Apart from remote specimen collection through mobile phlebotomy units, microsampling techniques that allow for participants to collect their own blood, though not yet available for prime time, are being developed.( 56 ) Partnerships can be established with retail community‐based pharmacies to provide investigational drugs. For studies that require in‐person visits, such as radiological imaging, study procedures can be altered to maximize the impact of this visit by performing additional assessments (e.g., phlebotomy, questionnaires) in the same visit. This will require a nimble research team and careful coordination. Additionally, sponsors can partner with ridesharing services such as Uber and Lyft to help overcome transportation barriers.( 57 ) Even more important, clinical trial designs that limit the need to travel and its associated costs can substantially improve subject participation during COVID‐19 and improve participation for typically under‐represented populations after COVID‐19.( 58 ) Finally, study monitoring may transition away from inefficient and expensive on‐site source document verification to remote online monitoring of EHRs.( 59 ) We must be mindful that prioritizing mHealth and other technologies can have the undesired effect of exacerbating existing disparities in clinical research, just as the over‐reliance on telemedicine can potentially widen disparities in clinical care.( 60 )
An effort to reduce the complexity, regulatory burden, and cost of clinical trials is already long overdue,( 61 ) and investments in clinical trial redesign will improve efficiency, feasibility, and cost.( 62 ) Removing geographical barriers to clinical trial participation will allow a broader range of participants and improve heterogeneity and generalizability.( 58 , 63 ) Innovative strategies are critical to permit clinical research to proceed during the COVID‐19 pandemic, but will also have a long‐lasting positive impact on the conduct of clinical trials for the foreseeable future.
Conclusions and Future Directions
Every facet of our lives has been changed by COVID‐19, and clinical research is no different. As COVID‐19 broadly started to hit the United States in March 2020, universities announced policies to shut down research that had to be implemented within 2‐3 days. The pandemic has caused varying degrees of interruptions in several domains of clinical research both within and outside of the United States (Table 3). Although many researchers quickly worked out solutions with sponsors, IRBs, investigational drug services, and in some instances home care services and local laboratories to enable participants in clinical trials to receive study medications and to have some clinical and laboratory monitoring, protocol deviations were inevitable. Fortunately, the FDA recognized these challenges and quickly issued guidance allowing protocol modifications to prioritize patient safety; IRBs promoted and broadened teleconsenting and expedited approval of protocol modifications, and the NIH reassured researchers that grant funding periods will be extended to enable research to be completed. Although these measures softened the blow, COVID‐19 will have a long‐term impact on clinical research and the career of clinical investigators. Completion of clinical trials will be delayed and missing data might affect conclusions of clinical trials as well as observational studies if data at critical time points are missing. Early‐ and mid‐career investigators dependent on preliminary data for grant funding and publication of completed studies for promotion will be most affected not only because of the pause on clinical research, but also deployment to other clinical services and for women and men with young children the added responsibilities of home schooling and childcare. For many investigators, decreased activities meant decreased research‐related revenue, necessitating layoff or reduction in effort of staff, making subsequent recovery more difficult.
Table 3.
The Global Perspective on Clinical Research in Hepatology*
| Domain of Clinical Research | Europe and United Kingdom | Asia | South America | |
|---|---|---|---|---|
| Clinical trials (industry and NIH/government agencies) | Initial changes |
|
|
|
| Re‐entry and long‐term strategy |
|
|
|
|
| Real‐world data | Initial changes |
|
|
|
| Re‐entry and long‐term strategy |
|
|
|
|
| Biorepository, natural history, and biomarker studies | Initial changes |
|
|
|
| Re‐entry and long‐term strategy |
|
|
|
|
Based on valuable contributions from professors Stefan Zeuzem and Jonel Trebicka (Germany), Graham Foster (United Kingdom), Pietro Lampertico (Italy), Fabien Zoulim (France), Grace Wong (Hong Kong), Jia‐Horng Kao (Taiwan), and Dr. Manuel Mendizabal (Argentina).
Abbreviations: IT, information technology; HCC, hepatocellular carcinoma.
As states begin to reopen and hospitals are ramping up clinical services, reopening of clinical research lags behind driven by concerns over unnecessary exposure of staff and participants. However, as with all major crises, innovations adopted to provide clinical service and to maintain clinical research during the COVID‐19 pandemic can be leveraged to improve efficiency of clinical research in the future (Fig. 3). Foremost is the adoption of telemedicine, eConsents, and remote platforms for patient monitoring and data collection. Both in clinical care and in clinical research, restrictions during the COVID‐19 pandemic forced an evaluation of what is essential and what is aspirational. These lessons will lead to leaner study designs, lower costs, and reduced burden to participants, without sacrificing science and quality of the study. Physicians and researchers have also learned how to utilize technology to evaluate patients remotely and to document findings electronically. However, access to technology is not uniform, and we must make sure that new studies include representative populations regardless of access to and comfort with technology. Furthermore, a team effort and a shared resource platform is likely to reduce costs and sustain research and also provide young investigators the necessary and critical support to foster their research. This is evident by the rapidly evolving collaborative research environment, including the NIH initiative of public‐private and multiindustry partnership, permitting rapid launch of rigorously designed large clinical trials, head‐to‐head comparison of new therapeutics and vaccines, as well as early investigations into combinations of investigational therapies.
Fig. 3.
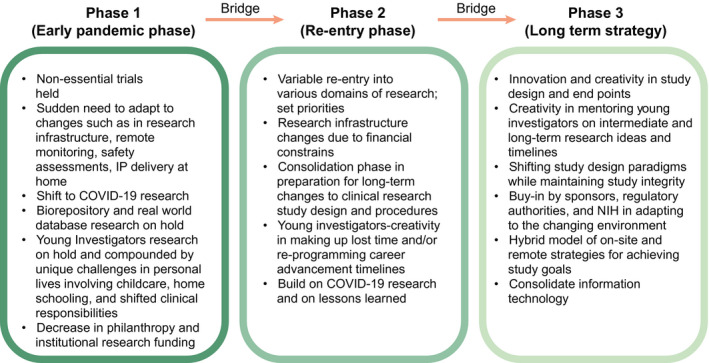
Overview on the current status, re‐entry phase, and potential future strategies and expectations in clinical research.
The COVID‐19 pandemic created havoc on our lives and on clinical research, but if we all embrace it as a disruptive innovation, we can come out on the other side of the curve with more robust and efficient study designs that will accelerate rather than hinder progress.
Author Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: E.V., R.T.C., K.R.R.; Drafting the article or revising it critically for important intellectual content: E.V., M.S., J.C., K.C., O.F., K.H., K.P., R.L., M.L., G.T.E., M.W.F., G.G.T., N.T., A.S.L., R.T.C., K.R.R.; Final approval of the version to be published: E.V., M.S., J.C., K.C., O.F., K.H., K.P., R.L., M.L., G.T.E., M.W.F., G.G.T., N.T., A.S.L., R.T.C., K.R.R.
Acknowledgment
AASLD COVID‐19 Clinical Oversight Subcommittee (COS):
Oren K. Fix, M.D., M.Sc., F.A.A.S.L.D. (co‐chair)
Swedish Medical Center, Seattle, WA
Elizabeth C. Verna, M.D., M.S. (co‐chair)
Columbia University, New York, NY
Kimberly A. Brown, M.D., F.A.A.S.L.D.
Henry Ford Health System, Detroit, MI
Jaime Chu, M.D.
Icahn School of Medicine at Mt Sinai, New York, NY
Bilal Hameed, M.D.
University of California, San Francisco, CA
Laura M. Kulik, M.D.
Northwestern Medicine, Chicago, IL
Ryan M. Kwok, M.D.
Uniformed Services University, Bethesda, M.D.
Brendan M. McGuire, M.D.
University of Alabama, Birmingham, AL
Daniel S. Pratt, M.D., F.A.A.S.L.D.
Massachusetts General Hospital, Boston, MA
Jennifer C. Price, M.D., Ph.D.
University of California, San Francisco, CA
Nancy S. Reau, M.D., F.A.A.S.L.D.
Rush University, Chicago, IL
Mark W. Russo, M.D., M.P.H., F.A.A.S.L.D.
Carolinas Medical Center, Charlotte, NC
Michael L. Schilsky, M.D., F.A.A.S.L.D.
Yale University, New Haven, CT
Norah A. Terrault, M.D., M.P.H., F.A.A.S.L.D.
Keck School of Medicine of USC, Los Angeles, CA
Andrew Reynolds (patient advocate)
The writing group acknowledges and thanks Professors Stefan Zeuzem and Jonel Trebicka (Germany), Graham Foster (United Kingdom), Pietro Lampertico (Italy), Fabien Zoulim (France), Grace Wong (Hong Kong), Jia‐Horng Kao (Taiwan), and Dr. Manuel Mendizabal (Argentina) for providing valuable perspective on the status of clinical research in their countries. Also acknowledged are Anita Kalluri and Vanessa Weir (helped in collating the document), Thelmelis Abreu (illustrations), and Katherine Wagner, M.I.P.H. (co‐contributor to part on marginalized population).
Potential conflict of interest: Dr. Verna received grants from Salix. Dr. Loomba consults for, advises for, and received grants from Boehringer Ingelheim, Bristol‐Myers Squibb, Cirius, Eli Lilly, Galmed, Gilead, Intercept, Janssen, Merck, NGM, Pfizer, Prometheus, and Siemens. He consults for and advises for Anylam/Regeneron, Arrowhead, AstraZeneca, Bird Rock, Celgene, CohBar, Conatus, Gemphire, Glympse, GNI, GRI, Inipharm, Ionis, Metacrine, Novartis, Novo Nordisk, Promethera, Sanofi, and Viking. He received grants from Allergan, Galectin, GE, Grail, Madrigal, NuSirt, and pH Pharma. Dr. Everson is employed by and owns stock in HepQuant. Dr. Fried consults for and owns stock in TARGET. He received grants from AbbVie and Gilead. Dr. Terrault consults for Intercept and EXIGO. She received grants from Gilead, Roche, and Genentech. Dr. Chung received grants from AbbVie, Gilead, Merck, Bristol‐Myers Squibb, Boehringer Ingelheim, Janssen, Roche, Kaleido, and Synlogic. Dr. Reddy advises for and received grants from Mallinckrodt and Gilead. He received grants from Grifols, Bristol‐Myers Squibb, Intercept, Exact Sciences, NASH‐TARGET, and HCC‐TARGET. He is on the data security monitoring board for Novartis.
R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (R01DK106419, 1R01DK121378, R01 DK124318, and P30DK120515), and DOD PRCRP (CA170674P2). G.T.E. is an equity member and CEO of HepQuant LLC. M.W.F. receives fees and is a stockholder in TARGET PharmaSolutions. N.T.: research grant support (to Institution) from Gilead and Roche/Genentech. RTC: research grant support (to Institution) from AbbVie, Gilead, Merck, BMS, Janssen, Boehringer Ingelheim, Roche, Kaleido, and Synlogic. K.R.R.: advisory board: Mallinckrodt and Gilead; research support (to Institution) from Mallinckrodt, Grifols, Gilead, Merck, BMS, Intercept, Exact Sciences, NASH‐TARGET, and HCC‐TARGET.
References
- 1. Falade‐Nwulia O, Suarez‐Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct‐acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166:637‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zoulim F, Lebossé F, Levrero M. Current treatments for chronic hepatitis B virus infections. Curr Opin Virol 2016;18:109‐116. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2019;394:2184‐2196. [DOI] [PubMed] [Google Scholar]
- 4. Seeff LB, Beebe GW, Hoofnagle JH, Norman JE, Buskell‐Bales Z, Waggoner JG, et al. A serologic follow‐up of the 1942 epidemic of post‐vaccination hepatitis in the United States Army. N Engl J Med 1987;316:965‐970. [DOI] [PubMed] [Google Scholar]
- 5. Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low‐dose peginterferon. N Engl J Med 2008;359:2429‐2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evon DM, Sarkar S, Amador J, Lok AS, Sterling RK, Stewart PW, et al. Patient‐reported symptoms during and after direct‐acting antiviral therapies for chronic hepatitis C: the PROP UP study. J Hepatol 2019;71:486‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mishra P, Florian J, Peter J, Vainorius M, Fried MW, Nelson DR, et al. Public‐private partnership: targeting real‐world data for hepatitis C direct‐acting antivirals. Gastroenterology 2017;153:626‐631. [DOI] [PubMed] [Google Scholar]
- 8. Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology 1993;17:564‐576. [DOI] [PubMed] [Google Scholar]
- 9. Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, et al. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo‐controlled clinical trial of patient survival. Gastroenterology 2005;128:882‐890. [DOI] [PubMed] [Google Scholar]
- 10. Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, et al. Million veteran program: a mega‐biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214‐223. [DOI] [PubMed] [Google Scholar]
- 11. Padala PR, Jendro AM, Gauss CH, Orr LC, Dean KT, Wilson KB, et al. Participant and caregiver perspectives on clinical research during Covid‐19 pandemic. J Am Geriatr Soc 2020;68:E14‐E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssen KJ, Donders ART, Harrell FE, Jr., Vergouwe Y, Chen Q, Grobbee DE, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010;63:721‐727. [DOI] [PubMed] [Google Scholar]
- 14. Yin G. Clinical trial design: Bayesian and frequentist adaptive methods. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 15. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system‐wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health 2017;38:467‐487. [DOI] [PubMed] [Google Scholar]
- 16. U.S. National Library of Medicine . Trend, Charts, and Maps. September 2, 2020. https://clinicaltrials.gov/ct2/resources/trends#RegisteredStudiesOverTime. Accessed September 2, 2020.
- 17. Food and Drug Administration . FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID‐19 Public Health Emergency. July 2, 2020. https://www.fda.gov/media/136238/download. Accessed September 2, 2020.
- 18. National Institutes of Health . Guidance for NIH‐funded Clinical Trials and Human Subjects Studies Affected by COVID‐19. March 16, 2020. https://grants.nih.gov/grants/guide/notice‐files/NOT‐OD‐20‐087.html. Accessed September 2, 2020.
- 19. Mehta HB, Ehrhardt S, Moore TJ, Segal JB, Alexander GC. Characteristics of registered clinical trials assessing treatments for COVID‐19: a cross‐sectional analysis. BMJ Open 2020;10:e039978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amero S. Temporary, emergency situations due to COVID‐19 and application scores received during peer review. Extramural Nexus. 2020. https://nexus.od.nih.gov/all/2020/04/21sz/temporary‐emergency‐situations‐due‐to‐covid‐19‐and‐application‐scores‐received‐during‐peer‐review/. Accessed August 19, 2020. [Google Scholar]
- 21. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real‐world evidence—what is it and what can it tell us? N Engl J Med 2016;375:2293‐2297. [DOI] [PubMed] [Google Scholar]
- 22. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019—COVID‐NET, 14 states, March 1‐30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabio C, Antonella C, Patrizia RQ, Francesco C, Annalisa R, Laura G, et al. Early predictors of clinical outcomes of COVID‐19 outbreak in Milan, Italy. Clin Immunol 2020;217:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med 2020;382:2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, et al. Compassionate remdesivir treatment of severe Covid‐19 pneumonia in intensive care unit (ICU) and non‐ICU patients: clinical outcome and differences in post‐treatment hospitalisation status. Pharmacol Res 2020;158:104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim AHJ, Sparks JA, Liew JW, Putman MS, Berenbaum F, Duarte‐Garcia A, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID‐19. Ann Intern Med 2020;172:819‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehra MR, Desai SS, Ruschitzka F, Patel AN. Retraction—hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐19: a multinational registry analysis. Lancet 2020;395:1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med 2020;382:2582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA 2020;323:1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina DD, Genoni A, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect 2020;81:e45‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases . Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID‐19). August 11, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/lab/lab‐biosafety‐guidelines.html. Accessed September 2, 2020.
- 34. Abrahamson M, Hooker E, Ajami NJ, Petrosino JF, Orwoll ES. Successful collection of stool samples for microbiome analyses from a large community‐based population of elderly men. Contemp Clin Trials Commun 2017;7:158‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hughes SR, Chapleau RR. Comparing DNA quantity and quality using saliva collection following food and beverage consumption. BMC Res Notes 2019;12:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrett JR. Do‐it‐yourself biospecimens: the benefits of home collection. Environ Health Perspect 2004;112:A51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Institutes of Health . NIH begins study to quantify undetected cases of coronavirus infection. April 10, 2020. https://www.nih.gov/news‐events/news‐releases/nih‐begins‐study‐quantify‐undetected‐cases‐coronavirus‐infection. Accessed September 2, 2020.
- 38. Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. Biliary atresia: clinical and research challenges for the twenty‐first century. Hepatology 2018;68:1163‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. The Childhood Liver Disease Research Network (ChiLDReN) . https://childrennetwork.org. Accessed September 2, 2020.
- 40. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration UK. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alonso EM, Horslen SP, Behrens EM, Doo E. Pediatric acute liver failure of undetermined cause: a research workshop. Hepatology 2017;65:1026‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Squires JE, Ng VL, Hawthorne K, Henn LL, Sorensen LG, Fredericks EM, et al. Neurodevelopmental outcomes in preschool and school aged children with biliary atresia and their native liver. J Pediatr Gastroenterol Nutr 2020;70:79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Society of Pediatric Liver Transplantation . COVID‐19 Post Liver Transplantation Data Collection Registry for Pediatric Patients (0‐21 years). https://tts.org/initiatives/split‐covid‐19‐post‐liver‐transplantation‐data‐collection‐registry. Accessed September 2, 2020.
- 44. Chowkwanyun M, Reed AL, Jr . Racial health disparities and Covid‐19—caution and context. N Engl J Med 2020;383:201‐203. [DOI] [PubMed] [Google Scholar]
- 45. Alessandra M. The pandemic and the female academic. April 17, 2020. https://www.nature.com/articles/d41586‐020‐01135‐9AMn. Accessed September 2, 2020.
- 46. Lucy F. Moving remote: the post‐pandemic clinical trial. April 3, 2020. https://social.eyeforpharma.com/clinical/moving‐remote‐post‐pandemic‐clinical‐trial. Accessed September 2, 2020.
- 47. Nicola D. Telemedicine: The future of clinical trials? December 13, 2019. https://social.eyeforpharma.com/clinical/telemedicine‐future‐clinical‐trials. Accessed September 2, 2020.
- 48. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723‐750. [DOI] [PubMed] [Google Scholar]
- 49. U.S. Department of Health and Human Services Office for Human Research Protections, Food and Drug Administration Center for Drug Evaluation and Research .Use of electronic informed consent: questions and answers. Published December 2016. https://www.fda.gov/media/116850/download. Accessed June 2020.
- 50. World Health Organization . mHealth: new horizons for health through mobile technologies. 2011. http://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed June 2020.
- 51. Kakkar AK, Sarma P, Medhi B. mHealth technologies in clinical trials: opportunities and challenges. Indian J Pharmacol 2018;50:105‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web‐based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials 2014;38:190‐197. [DOI] [PubMed] [Google Scholar]
- 53. Anguera JA, Jordan JT, Castaneda D, Gazzaley A, Arean PA. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innov 2016;2:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borno HT, Zhang L, Siegel A, Chang E, Ryan CJ. At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. Oncologist 2018;23:1242‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. HealthMeasures . www.healthmeasures.net. Accessed June, 2020.
- 56. Lei BUW, Prow TW. A review of microsampling techniques and their social impact. Biomed Microdevices 2019;21:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gould B.Lyft and Uber address transportation challenges for clinical trial patients. https://www.mdconnectinc.com/medical‐marketing‐insights/lyft‐and‐uber‐address‐transportation‐challenges. Accessed June, 2020.
- 58. Seidler EM, Keshaviah A, Brown C, Wood E, Granick L, Kimball AB. Geographic distribution of clinical trials may lead to inequities in access. Clin Investig 2014;4:373‐380. [Google Scholar]
- 59. Uren SC, Kirkman MB, Dalton BS, Zalcberg JR. Reducing clinical trial monitoring resource allocation and costs through remote access to electronic medical records. J Oncol Pract 2013;9:e13‐e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nouri S, Khoong EC, Lyles CR, Karliner L. Addressing equity in telemedicine for chronic disease management during the Covid‐19 pandemic. NEJM Catalyst Innovations in Care Delivery 2020;1. [Google Scholar]
- 61. Duley L, Antman K, Arena J, Avezum A, Blumenthal M, Bosch J, et al. Specific barriers to the conduct of randomized trials. Clin Trials 2008;5:40‐48. [DOI] [PubMed] [Google Scholar]
- 62. Borno HT, Small EJ. Does the COVID‐19 outbreak identify a broader need for an urgent transformation of cancer clinical trials research? Contemp Clin Trials 2020;92:105997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. King TE, Jr. Racial disparities in clinical trials. Mass Medical Soc 2002;346:1400‐1402. [DOI] [PubMed] [Google Scholar]


