Summary
SARS‐CoV2 is a novel coronavirus; the seventh of its species to infect humans. The spread of this virus emerged in Wuhan, China in late December, 2019. Since then, this virus has spread to more than 200 countries and has caused a worldwide pandemic. Being a new species of coronaviruses, any cure or vaccines for this virus has not yet been obtained. A large amount of scientific studies and clinical trials are being carried out across the world to find a potential vaccine for this virus. Current work reports a review of potential drugs and vaccines that may be effective against this virus. Different scientific therapies that may potentially be effective against the SARS‐CoV2 virus are also reviewed. The mechanisms of various drugs, their efficiency in various clinical trials and their side effects are also studied.
Keywords: affinity, coronavirus, FDA approved drug, infection, vaccines
Abbreviations
- ACE
Angiotensin Converting Enzyme
- ADE
Antibody Dependent Enhancement
- CDC
Centres for Disease Control and Prevention
- CQ
Chloroquine
- DCGI
Drug Controller General of India
- DNA
Deoxyribonucleic Acid
- HCoV
Human Coronavirus
- HCQ
Hydroxychloroquine
- HIV
Human Immunodeficiency Virus
- ICMR
Indian Council of Medical Research
- MERS‐CoV
Middle East Respiratory Syndrome‐Coronavirus
- NIH
National Institute of Health
- RAS
Renin‐Angiotensin System
- RNA
Ribonucleic Acid
- SARS‐CoV
Severe Acute Respiratory Syndrome‐Coronavirus
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome‐Coronavirus‐2
- WHO
World Health Organization
1. INTRODUCTION
Human coronaviruses were first discovered in the 1960's. The name “Corona” is a Latin word meaning “crown,” chosen because of the appearance of a crown around the virus particles when viewed under a two dimensional transmission electron microscope. The earliest coronaviruses 1 , 2 discovered were an infectious bronchitis virus in chickens and two in humans (229E and OC43). Other members in this family were later identified: SARS‐CoV in 2003, HCoV NL63 in 2004, HCoV HKU1 in 2005, MERS‐CoV in 2012 and SARS‐CoV‐2 (previously named 2019‐nCoV) in 2019. Most of these viruses cause respiratory tract infections. The first known severe illness caused by a coronavirus was with the Severe Acute Respiratory Syndrome (SARS) epidemic 3 , 4 which started in China in 2003. This pathogen was found to be an animal virus; transmitted to humans from civets. A second coronavirus epidemic 5 was caused by the Middle East Respiratory Syndrome (MERS) in 2012 in Saudi Arabia. This virus was transmitted to humans from camels.
FIGURE 1.
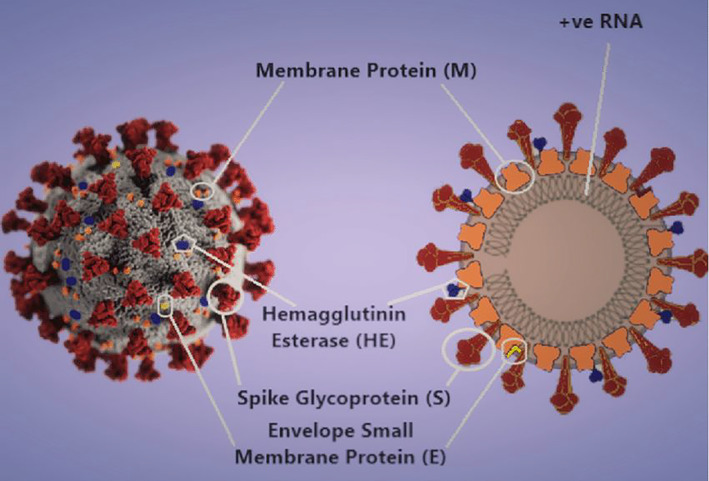
3‐D model 6 and schematic diagram of the SARS‐CoV‐2 virion. Image component modified from CDC Public Health Image Library (ID 23312: Alissa Eckert and Dan Higgins)
SARS‐CoV‐2 is the seventh coronavirus to infect humans. The first cases emerged in Wuhan, China, in late 2019, and then spread worldwide. This Wuhan strain 7 has been identified as a new strain of Beta coronaviruses from group 2B (Figure 1). Genome 8 of this virus has been found to be over 80% similar to the SARS‐CoV virus. This virus also has a 96% similarity to a bat coronavirus and hence it is suspected that it most likely originated from bats. It is transmitted between humans by contact via respiratory droplets or fluids ejected while coughing or sneezing. Secondary infections also occur when there is a contact with inanimate surfaces contaminated with the virus.
2. SCOPE OF WORK
Given the lack of approved drugs for the SARS‐CoV‐2 virus, it is essential to evaluate pre‐existing drugs for activity. In principle, a molecule can act as an anti‐viral drug if it inhibits some stage of the virus replication cycle, without being too toxic to the body cells. The possible modes of action of anti‐viral agents would include being able to‐.
Inactivate extracellular virus particles.
Prevent viral attachment and/or entry.
Prevent replication of the viral genome.
Prevent synthesis of specific viral protein(s).
Prevent assembly or release of new infectious virions.
Different anti‐viral drugs perform one or more actions from the above mentioned, so as to stop the spread of viral infection. We have showed a general mechanism of the process of viral replication in human cells and have also indicated at which stages different types of anti‐viral drugs perform, along with their primary functions (Figure 2).
FIGURE 2.
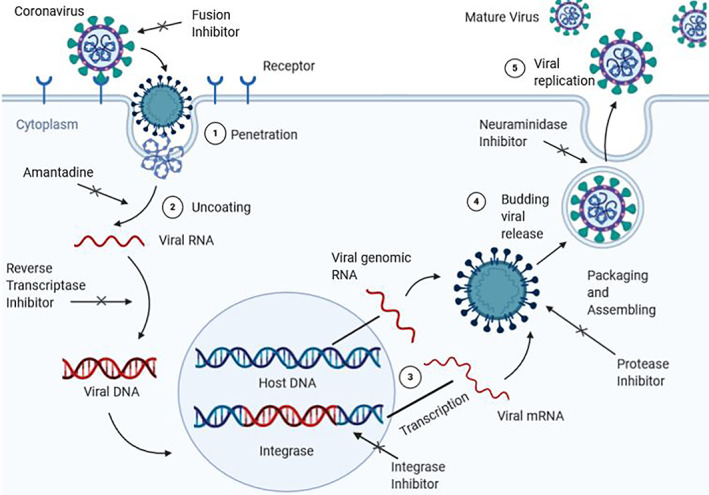
A general mechanism of viral replication in host cells and functions of inhibitors at various stages during the process. The virus first binds with the receptor of the host. A drug which is designed to be a fusion inhibitor will function at this stage by preventing the virus from binding with the receptor. Then, the virus will penetrate into the cell cytoplasm of the host. It undergoes uncoating at this stage. An amantadine inhibitor targets this stage and prevents uncoating. The viral RNA then transforms into a viral DNA. A reverse transciptase inhibitor works at this stage and prevents the viral DNA from being formed. The viral DNA then brings changes in the amino acid sequence of the host DNA and inserts itself in the sequencing. An integrase inhibitor prevents the virus from changing this sequence. With this, we get viral genomic RNA and viral mRNA. A protease inhibitor functions at this stage and prevents viral replication by selectively binding to viral proteases (eg, HIV‐1 protease) and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particle. The neuraminidase inhibitor targets this stage and prevents the virus from replicating, hence, preventing its reproduction by budding from the host cell. After this stage, the mature virus replicates itself and viral infection occurs. Image created with https://biorender.com/
This SARS‐CoV‐2 has now become a pandemic affecting over 5.4 million people and claiming 0.34 million lives worldwide, as of 24 May 2020. A graphical representation of the percentage of cases in some selected countries is shown, which helps us in getting a clear overview of the situation of the pandemic in countries across the world (Figure 3).
FIGURE 3.
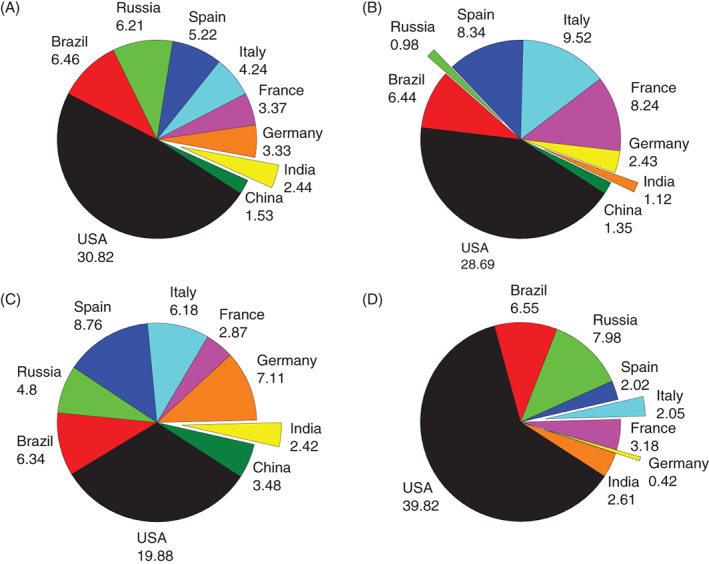
A, Percentage values of total number 9 of coronavirus cases recorded in respective countries. B, Percentage values of total number of coronavirus deaths 9 recorded in respective countries. C, Percentage values of total number of recovered coronavirus cases 9 recorded in respective countries. D, Percentage values of total number of active 9 coronavirus cases recorded in respective countries (as of 24 May 2020)
3. RECENT SCIENTIFIC WORK
On 20th March, WHO 10 announced a large global trial called SOLIDARITY to check for various therapeutic drugs that may possibly help with the cure for Covid‐19. For these trials, possibly involving thousands of patients across the world, scientists have suggested various existing compounds for testing; however, WHO is primarily focusing on what may be the four most promising drugs‐ remdesivir, chloroquine and hydroxychloroquine, ritonavir and lopinavir and this mixture with interferon‐beta. To get robust results, WHO 10 remarks that several thousands of patients will have to be recruited all across the world. A global data safety monitoring board will closely inspect results repeatedly and conclude whether any of these drugs have proven effect (or not, in which case trials for that drug may be dropped) or more drugs need to be added to the trial.
3.1. Remdesivir
Remdesivir 10 , 11 is an adenosine analogue originally tested against Ebola and some related viruses during the Ebola outbreak in the Democratic Republic of Congo. However this drug did not show promising results back then. This compound shuts down viral replication by inhibiting a key viral enzyme‐ RNA dependent RNA polymerase. It works against a broad spectrum of RNA viruses like SARS and MERS and functions post viral entry in a living cell. In 2017, researchers 12 at the University of North Carolina showed in test tube and animal studies that this drug can inhibit coronaviruses causing SARS and MERS. This drug 13 is not specifically designed to target SARS‐CoV‐2. As of 22nd March, two patients 10 in the United States of America were administered remdesivir and both of them showed improved conditions. Such individual‐case evidences do not prove that this drug is efficient and safe for use; yet scientists do believe this drug has the best potential in clinical trials owing to the fact that even high doses of this compound does not cause toxicities.
According to the study results 14 , 15 prematurely posted by the WHO on it's website by accident, the Chinese clinical trial on this drug was unsuccessful. The trial included 237 patients out of which 158 were administered remdesivir and the remaining 79 were given placebo. After a month of observations, results showed that 13.9% of the patients administered remdesivir died whereas the patients given placebo had a mortality rate of 12.8%. This drug was proven to be ineffective in curing Covid‐19 patients in this trial. However, in the first clinical trial to evaluate an experimental drug in the United States by NIH, 16 this drug was proven to be effective. This trial was a randomized controlled trial involving 1063 patients. Out of these, one group of patients was administered remdesivir and the other group received placebo. According to the preliminary results, patients receiving remdesivir showed 31% faster recovery rates than the other group. The median recovery time for patients receiving remdesivir was 11 days, while that for the patients given placebo was 15 days. Results also showed improved survival rates. The remdesivir group had a mortality rate of 8% while the placebo group had a rate of 11.6%.
Another trial 17 , 18 on this drug was performed, which included 61 patients. Out of these, the data of 8 patients could not be analyzed and the results from analysis of data of the remaining 53 patients were published. These patients were administered remdesivir for 10 days and a median follow up of 18 days showed that 68% patients had improvements in oxygen support class. 47% patients were discharged and 13% died. The mortality rates in patients receiving invasive ventilation were found to be 18% while the rates in patients not receiving invasive ventilation was 5%. This trial reported reduced mortality rates and also clinical improvements in 68% of the patients by the use of remdesivir.
3.2. Chloroquine and hydroxychloroquine
Chloroquine 11 , 13 is an anti‐malarial and autoimmune disease drug and has been in use for the past 70 years. It appears to act by preventing viruses from binding to human cells. As these drugs are weak bases, they work by decreasing the acidity in endosomes. They prevent viral infection by increasing the endosornal pH required by the virus to enter the cell and also by interfering with the receptors of the virus. Chloroquine phosphate is widely available, but has side effects, including headaches, diarrhea, rashes, itching and muscle problems like muscle pain and weakness. In cell culture, chloroquine shows activity against the SARS‐CoV‐2 virus, but the dosage requirements are usually high which may lead to serious toxicities if administered to humans. In rare cases, this drug affects the heart muscle which may lead to heart failure. Cases 19 , 20 of chloroquine poisoning have been reported in Nigeria. In the USA, 21 a man and his wife reportedly fell critically ill after self‐medication with chloroquine phosphate derived from a fish tank cleaner where it is used as an additive. The man later died and his wife was placed in critical care.
Hydroxychloroquine also has activity 19 against SARS‐CoV‐2 infection. Hydroxychloroquine sulfate 19 is a derivative of chloroquine phosphate, synthesized in 1946. It is less toxic than chloroquine in animals. This drug is widely used for treatment of diseases like rheumatoid arthritis. Currently, at least 7 clinical trials are being carried out in China to test the effectiveness of this drug against SARS‐CoV‐2. Researchers in France 12 have treated 20 Covid‐19 patients with hydroxychloroquine and observed that this drug reduced viral load in nasal swabs significantly. However, this study was not a randomized controlled trial and hence did not report clinical outcomes such as deaths. They 22 have also reported positive outcomes by combining hydroxychloroquine with the antibiotic azithromycin for treatment of Covid‐19 patients. However scientists have found many inconsistencies 23 in their results and have many conflicts of interests. Only rigorous randomized clinical trials can determine if either of these drugs is a viable therapeutic option.
3.3. Ritonavir/Lopinavir
The ritonavir and lopinavir combination 10 was originally developed in 2000 to treat HIV infections. Lopinavir was developed to inhibit the HIV protease‐ an enzyme that cleaves long protein chains into peptides during assembly of new viruses. Lopinavir is rapidly broken down by proteases in the human body and hence, it is administered with low dosages of ritonavir which makes lopinavir persist in the human body longer.
Cytochrome P450 3A4 are a superfamily 24 , 25 of heme containing monooxygenase enzymes that act as a catalyst during the transfer of oxygen atom from molecular oxygen into a large number of biological substrates. Ritonavir is a HIV inhibitor and also a cytochrome P450 3A4 inhibitor. This enzyme is a major human hepatic drug metabolizing enzyme. Ritonavir inhibits this enzyme very strongly and hence, small doses of ritonavir are administered along with lopinavir for increasing their plasma concentrations; thus increasing their lifetime in the human host.
This combination can inhibit coronavirus proteases as well. This has been tested in SARS and MERS patients, but the results are inconclusive. In Wuhan, China, the first trial 10 , 26 of this drug was not largely encouraging as no significant changes in patient conditions were recorded. This randomized, controlled, open lab trial included 199 patients out of which 99 patients received two pills of ritonavir/lopinavir twice a day along with standard care and the remaining 100 patients received standard care alone. At the end of this trial, results showed no significant difference between the two groups. Mortality at 28 days was found to be same in both the groups. However, doctors commented that all trial patients were critically ill (with more than one fifth of them dying) and the drugs may have been given too late. If administered at earlier stages of viral infection, these drugs may be more effective. Though these drugs are usually safe, while curing critically ill patients, they may interact with other drugs administered to them and could possibly cause significant liver damage.
3.4. Ritonavir/lopinavir and interferon‐beta
Interferon‐beta 10 is a molecule involved in regulating body inflammation and has been proven effective 27 in marmosets infected with MERS. A combination of ritonavir, lopinavir and interferon‐beta, is being tested 28 in MERS patients in Saudi Arabia. Recruitment for this trial started in November 2016 and is still underway; the reason for this slow process is the decline in number of patients infected with MERS in Saudi Arabia. This trial is a recursive, 2 stage, group sequential, randomized controlled trial. As of 22nd March, clinical trials of this combination drug have yet to be carried out on Covid‐19 patients.
3.5. Favipiravir
This is designed to target RNA viruses including influenza viruses, SARS‐CoV and MERS‐CoV by inhibiting RNA‐dependent RNA polymerase. Favipiravir was used in clinical trials 13 , 29 by Chinese scientists in Wuhan and Shenzhen in 340 patients. In this trial, the drug appeared to shorten the course of the disease. The group administered favipiravir cleared the disease in a median of 4 days, whereas the group not treated with this drug cleared the disease in a median of 11 days. Along with this, lung conditions improved in nearly 91% cases treated with this drug compared to the 62% in cases not treated with it.
3.6. Convalescent plasma
Another potential therapy 13 against SARS‐CoV‐2 involves recovering plasma from the blood of recovered Covid‐19 patients and infusing it into infected patients. Such therapies have been in use since as far back as 1918 and have also been used 30 during the SARS, 31 MERS and H1N1 influenza 32 pandemics. In 2003, during the SARS epidemic, 80 patients were treated 31 with convalescent plasma in a hospital in Hong Kong. Patients given this treatment before day 14 of illness showed higher discharge rates of 58.3% as compared to the rate of 15.6% in patients given this treatment after day 14 of illness. The former group also showed lower mortality rates compared to the latter. From a prospective, cohort study 32 conducted by scientists in Hong Kong in 2009‐10, convalescent plasma proved to be effective in the treatment of severe H1N1 2009 infection. In this study, 93 patients were recruited, out of which 20 received this treatment. Results showed that mortality rates in the group receiving treatment were nearly 20%‐ which was far less than the rate of 54.8% in the group not receiving this treatment. Results also showed reduced respiratory tract viral load and serum cytokine response in the patients undergoing this treatment.
Chinese scientists have reported 33 some levels of success with this therapy in a trial. They administered 245 Covid‐19 patients convalescent plasma, in February, and 91 of them showed improvement in symptoms. On 24th March, in the United States, this therapy has been allowed 34 for severely ill patients and New York doctors are also ready to conduct various clinical trials for the testing of this therapy. Along with China and USA, South Korea, UK and recently India, have also started trials 35 , 36 , 37 , 38 on this therapy.
On 17 April 2020, the Drug Controller General of India (DGCI) approved initiation 39 of trials for this therapy and nearly 100 institutes 38 , 40 have shown interest in its studies. This phase II, open label, randomized controlled trial 36 will contain 452 patients out of which half shall be given plasma therapy and the other half shall not. This trial is aimed at assessing the safety and efficiency of this treatment in patients at moderate stages of the disease. States like Kerala, Gujarat and Punjab have already begun to use this therapy for treating COVID‐19 patients. The protocols for administering this therapy are being framed by DGCI and large scale trials are being carried out by Indian Council of Medical Research (ICMR).
However, there are also some risks 41 involved with this. During blood transfusion, there is a possibility of accidental infections being passed to the patient. This therapy may not work for some patients and can instead enhance the infection. Antibody Dependent Enhancement (ADE) is a phenomenon in which the virus binds with an antibody and increases virulence in the body. There are many theories on how ADE occurs and scientists believe there may be more than one mechanism of action. One mechanism includes the case when some immune cells do not have usual receptors through which viruses bind and enter the cell; but instead have Fc receptors. Such receptors bind with one end of the antibody and the virus then binds to the binding site at the other end. In this manner, the virus gains entry in the cell through the antibodies. Hence, presence of antibodies does not suppress the viral infection, but rather enhances it. The ability of an antibody to neutralize a virion depends on the antibody concentration and on the strength of antibody‐antigen interaction. When the strength of such interactions is less than a threshold value, ADE occurs.
Also, administering antibodies may suppress the patient's natural immune system leaving them at risk of re‐infection. Presently, we face a lot of unknowns in this therapy. We do not know the exact dosage of antibodies needed to be administered, we do not know at which stage of infection this therapy should be given so that results are most efficient and we also do not know the age‐group of patients this will this benefit most. Doctors also remark that the patients who have been previously treated using this therapy and shown improved conditions had also received antiviral drugs and hence, it is hard to precisely determine the effect of this therapy. As sample sizes in convalescent plasma trials are small, we need to carry out more vigorous studies and trials to know the exact efficiency of this therapy.
3.7. Other therapy
Wu 42 has suggested a mechanism for a potential therapy for SARS‐CoV‐2 induced lung injury which is based on the idea of balancing the renin‐angiotensin system (RAS). Here, the activation of the RAS cascade involves renin. Renin cleaves angiotensinogen to generate a decapeptide hormone (Ang I), which is then converted to an octapeptide hormone (Ang II) by angiotensin‐converting enzyme (ACE). It binds to its receptors (AT1 is angiotensin II type I and AT2 is angiotensin II type II) which results in vasoconstriction and promotion of the release of aldosterone. ACE2 is a homologue 43 of ACE and plays a pivotal role of a counter‐regulatory enzyme, for balancing responses initiated from ACE. Furthermore, ACE2 hydrolyses Ang I and Ang II to generate Ang‐(1–9) and Ang‐(1–7). Ang‐(1–7) binds to the MAS receptor for antagonizing Ang II‐mediated actions. SARS‐CoV uses ACE2 as the entry receptor and we make an assumption that SARS‐CoV‐2 functions by the same mechanism. In mouse models 44 of SARS‐CoV, ACE2 has been observed to be reduced by viral replication and viral spike protein, but not ACE. Inhibition of ACE2 expression or down‐regulation of surface ACE2 by these coronaviruses may disrupt function balances between ACE/ACE2 and Ang II/Ang‐(1–7), leading to lung injuries. From this, we can conclude that by compensation of ACE2 and maintenance of balance between ACE/ACE2, we may be able to stop severe lung injuries occurring due to SARS‐CoV‐2. At present, this approach yet awaits clinical trials to determine its efficiency. 45
4. CONCLUSIONS
Such drugs and therapies are being studied and experimented on intensively for determining a potential cure for the SARS‐CoV‐2. At this stage, clinical trials have had both positive outcomes in some cases while inconclusive outcomes in others. With only an increased number of clinical trials, can we determine more accurately the efficiencies of these drugs. Meanwhile, over 90 vaccines are under development at various research institutes, universities and companies across the world, through a variety of different approaches. Usually, during the preparation of any vaccine, the developmental and clinical trial phase takes a long time. However, researchers are accelerating their efforts and hope that a viable vaccine for the SARS‐CoV‐2 could be prepared and distributed within 18 months.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGEMENTS
S.K.G. would like to thank Science and Engineering Research Board (SERB) and Department of Science and Technology (DST), New Delhi, India for the financial support. P.N.G. is thankful to the Department of Science and Technology (DST), India for the support under DST‐FIST and the University Grants Commission, India for the support under DRS‐SAP.
Shah N, Davariya V, Gupta SK, Gajjar P, Parmar J, D'Cruz L. Review: An insight into coronaviruses: Challenges, security and scope. Rev Med Virol. 2020;30:e2138. 10.1002/rmv.2138
Funding information University Grants Commission, India; Department of Science and Technology (DST), New Delhi, India; Science and Engineering Research Board (SERB)
Contributor Information
Sanjeev K. Gupta, Email: sanjeev.gupta@sxca.edu.in.
Pankaj Gajjar, Email: pngajjar@gujaratuniversity.ac.in.
REFERENCES
- 1. Science Daily . COVID‐19 coronavirus epidemic has a natural origin. 2020. https://www.sciencedaily.com/releases/2020/03/200317175442.htm. Accessed May 26, 2020.
- 2. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11):S223‐S227. 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 3. Published by Centers for Disease Control and Prevention . CDC SARS Response Timeline. 2020. https://www.cdc.gov/about/history/sars/timeline.htm. Accessed May 26, 2020.
- 4. Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319‐1325. 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Middle East respiratory syndrome coronavirus (MERS‐CoV). 2019. https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov). Accessed May 26, 2020.
- 6. Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID‐19). Cureus. 2020;12(4). 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26:450‐452. 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91‐98. 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Real time data pertaining to number of coronavirus cases. https://www.worldometers.info/coronavirus/. Accessed May 26, 2020.
- 10. Science Magazine . WHO launches global megatrial of the four most promising coronavirus treatments. 2020. https://www.sciencemag.org/news/2020/03/who‐launches‐global‐megatrial‐four‐most‐promising‐coronavirus‐treatments. Accessed May 26, 2020.
- 11. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheahan TP, Sims AC, Graham RL, et al. Broad‐spectrum antiviral GS‐5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CNET . Coronavirus treatments: Remdesivir, hydroxychloroquine and vaccines for COVID‐19. 2020. https://www.cnet.com/how-to/coronavirus-treatments-hydroxychloroquine-vaccines-and-drugs-for-covid-19/. Accessed May 26, 2020.
- 14. STAT . New data on Gilead's remdesivir, released by accident, show no benefit for coronavirus patients. Company still sees reason for hope. 2020. https://www.statnews.com/2020/04/23/data‐on‐gileads‐remdesivir‐released‐by‐accident‐show‐no‐benefit‐for‐coronavirus‐patients/. Accessed May 26, 2020.
- 15. BBC News . Hopes dashed as coronavirus drug remdesivir ‘fails first trial’. https://www.bbc.com/news/world-52406261. 2020. access date 26 May 2020.
- 16. National Institutes of Health . NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID‐19. 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed May 26, 2020.
- 17. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020;382:2327‐2336. 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: immunology and treatment options. Clin Immunol. 2020;215:108448. 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:16. 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CNN . Nigeria records chloroquine poisoning after Trump endorses it for coronavirus treatment. 2020. https://edition.cnn.com/2020/03/23/africa/chloroquine-trump-nigeria-intl/index.html. Accessed May 26, 2020.
- 21. Banner Health . Banner Health experts warn against self‐medicating to prevent or treat COVID‐19. 2020. http://bannerhealth.mediaroom.com/chloroquinephosphate. Accessed May 26, 2020.
- 22. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949. 10.1016/j.ijantimicag.2020.105949 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Science Integrity Digest . Thoughts on the Gautret et al. paper about Hydroxychloroquine and Azithromycin treatment of COVID‐19 infections. 2020. https://scienceintegritydigest.com/2020/03/24/thoughts-on-the-gautret-et-al-paper-about-hydroxychloroquine-and-azithromycin-treatment-of-covid-19-infections/. Accessed May 26, 2020.
- 24. Rock BM, Hengel SM, Rock DA, Wienkers LC, Kunze KL. Characterization of ritonavir‐mediated inactivation of cytochrome P450 3A4. Mol Pharmacol. 2014;86(6):665‐674. 10.1124/mol.114.094862. [DOI] [PubMed] [Google Scholar]
- 25. Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci USA. 2010;107(43):18422‐18427. 10.1073/pnas.1010693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan J, Yao Y, Yeung M, et al. Treatment with lopinavir/ritonavir or interferon‐β1b improves outcome of MERS‐CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904‐1913. 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arabi YM, Asiri AY, Assiri AM, et al; and the Saudi Critical Care Trials group. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon‐β1b (MIRACLE trial): statistical analysis plan for a recursive two‐stage group sequential randomized controlled trial. Trials. 2020;21:8. 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Guardian . Japanese flu drug ‘clearly effective’ in treating coronavirus, says China. 2020. https://www.theguardian.com/world/2020/mar/18/japanese-flu-drug-clearly-effective-in-treating-coronavirus-says-china. Accessed May 26, 2020.
- 30. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(1):44‐46. 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hung IFN, To KKW, Lee C‐K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza a (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xinhua Net . China puts 245 COVID‐19 patients on convalescent plasma therapy. 2020. www.xinhuanet.com/english/2020-02/28/c_138828177.htm. Accessed May 26, 2020.
- 34. U.S. Food and Drug Administration . Recommendations for Investigational COVID‐19 Convalescent Plasma. 2020. https://www.fda.gov/vaccines‐blood‐biologics/investigational‐new‐drug‐ind‐or‐device‐exemption‐ide‐process‐cber/recommendations‐investigational‐covid‐19‐convalescent‐plasma. Accessed May 26, 2020.
- 35. LiveMint . Plasma therapy: Do we finally have treatment for coronavirus disease? 2020. https://www.livemint.com/news/india/plasma‐therapy‐do‐we‐finally‐have‐a‐treatment‐for‐coronavirus‐disease‐11587454621132.html. Accessed May 26, 2020.
- 36. The Science . Convalescent plasma's success against COVID‐19 continues in new study. 2020. https://science.thewire.in/the‐sciences/convalescent‐plasmas‐success‐against‐covid‐19‐continues‐in‐new‐study/. Accessed May 26, 2020.
- 37. Forbes . Hundreds of U.S. hospitals join convalescent plasma study to treat coronavirus. 2020. https://www.forbes.com/sites/brucejapsen/2020/04/24/hundreds‐of‐us‐hospitals‐join‐convalescent‐plasma‐study‐to‐treat‐coronavirus/#22ba09717dd6. Accessed May 26, 2020.
- 38. NHS Blood and Transplant . Could you donate plasma to help treat coronavirus (COVID‐19) patients? n.d. https://www.nhsbt.nhs.uk/how-you-can-help/convalescent-plasma-clinical-trial/. Accessed May 26, 2020.
- 39. Hindu BusinessLine . DCGI clears clinical trial of plasma therapy in Covid‐19 patients. https://www.thehindubusinessline.com/news/national/drug‐controller‐general‐of‐india‐approves‐clinical‐trial‐of‐convalescent‐plasma‐therapy‐in‐covid‐19‐patients/article31375934.ece. Accessed May 26, 2020.
- 40. Business Today . Coronavirus cure: India to start plasma therapy; Kerala first off the block. 2020. https://www.businesstoday.in/latest/trends/coronavirus‐cure‐india‐to‐start‐plasma‐therapy‐kerala‐first‐off‐the‐block/story/401108.html. Accessed May 26, 2020.
- 41. India Today . What is Plasma Therapy: A possible treatment for coronavirus? 2020. https://www.indiatoday.in/science/story/what‐is‐convalescent‐plasma‐therapy‐possible‐treatment‐coronavirus‐covid‐19‐1669050‐2020‐04‐20. Accessed May 26, 2020.
- 42. Wu Y. Compensation of ACE2 function for possible clinical management of 2019‐nCoV‐induced acute lung injury. Virol Sin. 2020. 10.1007/s12250-020-00205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. CAS . Targeting ACE2 – Closing COVID‐19's cellular doorway. 2020. https://www.cas.org/blog/ace2‐covid‐19‐target?utm_source=hootsuite&utm_medium=facebook&utm_term=cas&utm_content=cca8d5e3‐964a‐4820‐b71c‐8f6ae406d9d2&utm_campaign=COVID%20Outreach. Accessed May 26, 2020.
- 44. Kuba K, Imai Y, Penninger JM. Angiotensin‐converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271‐276. 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580:576‐577. 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]


