Abstract
Background
To systematically review clinical and biochemical characteristics associated with the severity of the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐related disease (COVID‐19).
Materials and methods
Systematic review of observational studies from PubMed, ISI Web of Science, SCOPUS and Cochrane databases including people affected by COVID‐19 and reporting data according to the severity of the disease. Data were combined with odds ratio (OR) and metanalysed. Severe COVID‐19 was defined by acute respiratory distress syndrome, intensive care unit admission and death.
Results
We included 12 studies with 2794 patients, of whom 596 (21.33%) had severe disease. A slightly higher age was found in severe vs non‐severe disease. We found that prevalent cerebrovascular disease (odds ratio [OR] 3.66, 95% confidence interval [CI] 1.73‐7.72), chronic obstructive pulmonary disease (OR: 2.39, 95% CI 1.10‐5.19), prevalent cardiovascular disease (OR: 2.84, 95% CI 1.59‐5.10), diabetes (OR: 2.78, 95% CI 2.09‐3.72), hypertension (OR: 2.24, 95% CI 1.63‐3.08), smoking (OR: 1.54, 95% CI 1.07‐2.22) and male sex (OR: 1.22, 95% CI 1.01‐1.49) were associated with severe disease. Furthermore, increased procalcitonin (OR: 8.21, 95% CI 4.48‐15.07), increased D‐Dimer (OR: 5.67, 95% CI 1.45‐22.16) and thrombocytopenia (OR: 3.61, 95% CI 2.62‐4.97) predicted severe infection.
Conclusion
Characteristics associated with the severity of SARS‐CoV‐2 infection may allow an early identification and management of patients with poor outcomes.
Keywords: d‐dimer, infection, procalcitonin, SARS‐CoV‐2, severity, sex, thrombocytopenia
1. INTRODUCTION
The novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has become a serious public health emergency in Eastern Countries as well as in Europe and United States, causing the new pandemic with increasingly numbers of infected people and deaths. The clinical presentation of SARS‐CoV‐2‐related disease (COVID‐19) is highly variable, ranging from asymptomatic/pauci‐symptomatic patients to acute respiratory distress syndrome (ARDS) and sepsis, with patients needing admission to intensive care unit (ICU) and mechanical ventilation. 1 Thus, SARS‐CoV‐2 infection primarily affects the lungs, but may cause a systemic involvement leading to sepsis and multi organ failure. 2
For this reason, the management of COVID‐19‐infected patients may differ, ranging from patients potentially being cured at home to those transferred to hospital for more intensive treatment. Of note, even in patients admitted to the hospital, there may be a lag phase between illness onset and disease complications, 3 , 4 , 5 and it is still unclear whether it is possible to early identify patients who will need mechanical ventilation or ICU admission. This issue is of clinical relevance as early identification of COVID‐19‐infected patients at risk of experiencing more severe complications may prompt to treatment diversification. As clinical and laboratory characteristics associated with severe SARS‐CoV‐2 infection are not completely clarified, we performed a systematic review and meta‐analysis of the data so far reported in SARS‐CoV‐2‐infected patients to identify clinical and laboratory variables associated with a high‐risk ARDS, ICU or poor survival.
2. METHODS
The following variables were included in the analysis: age, male sex, prevalent cerebrovascular disease, chronic obstructive pulmonary disease (COPD), prevalent cardiovascular disease, diabetes, hypertension, cancer, smoking, increased procalcitonin, increased D‐Dimer, thrombocytopenia, prothrombin time (PT) and activated partial thromboplastin time (aPTT). Definition of comorbidities was based on medical record review.
Increased D‐Dimer was defined ≥0.5 mg/L (µg/mL) by Guan et al. and >1 µg/mL by Tang et al and Zhou et al Increased procalcitonin was defined as ≥0.5 ng/mL in all studies but Wang et al that used a lower cut‐off (≥0.05 ng/mL). Thrombocytopenia was generally defined as platelet count <100 × 109/L, except for Guan et al (<150 × 109/L) and Wu et al (<125 × 109/L).
2.1. Eligibility criteria
Types of studies: Clinical studies in patients with SARS‐CoV‐2 infection that reported comorbidities and laboratory analysis of patients distinguished in severe vs non‐severe infection. A SARS‐CoV‐2 infection was considered as severe if patients presented with one of the following characteristics (a) ARDS (vs non‐ARDS), (b) admission to ICU (vs non‐ICU) and (c) non‐survivors (vs survivors). No publication date or publication status restrictions were imposed. Only publications written in English language with full‐text available were included in the metanalysis.
2.2. Information sources
The studies were identified by searching electronic databases. This search was applied to PubMed, ISI Web of Science, SCOPUS and Cochrane database. The last search was run on 28 May 2020. Reference lists of all studies included in the present metanalysis were screened for potential additional eligible studies.
2.3. Search
Two investigators (DP and LL) independently searched in the electronic databases combining the following text terms and MeSH terms: "COVID‐19"[All Fields] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "2019‐nCoV"[All Fields] OR "SARS‐CoV‐2"[All Fields]. PRISMA flow diagram is reported in the Figure S1.
2.4. Study selection
Two authors (LL, DP) independently reviewed titles and abstracts generated by search. Studies were excluded if the title and/or abstract showed that the papers did not meet the selection criteria of our meta‐analysis. For potentially eligible studies or if the relevance of an article could not be excluded with certitude, we procured the full text. Disagreements were resolved by discussion between LL and DP; if no agreement reached, a third author (PP) decided. Studies not including a control group and animal studies were excluded. Case reports, editorials, commentaries, letters, review articles and guidelines were also excluded from the analysis.
Reporting of the study conforms to broad EQUATOR guidelines. 6
2.5. Statistical
The outcome for each study was set as the odds ratio (OR) and was computed based on reported counts of severe patients, for each risk factor. A continuity correction of 0.5 was also used. The standard error of the log‐hazard ratio was also computed directly based on these counts, which were available for all studies, and used to compute 95% confidence intervals. For continuous variables, the outcome was set as the proportion of severe patients in the study, and the variable was summarized through the overall median. The I2‐statistic was quantified to measure heterogeneity. For categorical predictors, the DerSimonian and Laird random‐effects model was used when an I2 >25% was observed, fixed‐effects methods otherwise. Forest plots summarized the results. For continuous predictors, a similar criterion was used to compare random‐ and fixed‐effects meta‐regressions. Bubble plots were used to summarize the results. All analyses were done using the R (R Development Core Team) software version 3.5.1. A P‐value <.05 was considered statistically significant.
3. RESULTS
We included 12 studies with 2794 patients with SARS‐CoV‐2 infection. The weighted median age of this population was 50 years (Table 1). The mean percentage of men was 54.6%, ranging from 48.5% (Yang et al) to 73.2% (Huang et al). The most frequent comorbidity was represented by hypertension (19.31%), followed by diabetes (10.09%) and prevalent cardiovascular disease (8.85%) (Table 2). Furthermore, 13.7% of patients were smokers (Table 2).
Table 1.
Demographic characteristics of patients included in the metanalysis
| Study name | Country | Study design | Total patients | Severe patients | Non severe patients | Age (median) | Age (median) severe | Age (median) non‐severe |
|---|---|---|---|---|---|---|---|---|
| Guan W 17 | China | R | 1099 | 173 | 926 | 47 | 52 | 45 |
| Huang C 18 | China | P | 41 | 13 | 28 | 49 | 49 | 49 |
| Wang D 4 | China | R | 138 | 36 | 102 | 56 | 66 | 51 |
| Zhou F 3 | China | R | 191 | 54 | 137 | 56 | 69 | 52 |
| Yang X 5 | China | P | 52 | 32 | 20 | 52 | 65 | 52 |
| Han H 19 | China | R | 94 | 45 | 49 | — | — | — |
| Tang N 20 | China | R | 183 | 21 | 162 | 54 | 64 | 52 |
| Wu C 21 | China | R | 201 | 84 | 117 | 51 | 59 | 48 |
| Xiong Z 22 | China | R | 421 | 59 | 362 | 52 | 56 | 51 |
| Sun Y 23 | China | R | 63 | 19 | 44 | 47 | 60 | 42 |
| Yang Q 24 | China | R | 136 | 33 | 103 | 56 | 64 | 53 |
| Israelsen SB 25 | Netherland | R | 175 | 27 | 148 | 71 | 68 | 73 |
| Total | 2794 | 596 | 2198 | 50.1 a | 54.5 a | 48.8 a |
Abbreviations: P, prospective; R, retrospective.
Calculated weighted mean.
Table 2.
Prevalence and odds ratio of comorbidities in severe and non‐severe patients with SARS‐CoV‐2 infection
| Total (n = 2794) | Severe (n = 596) | Non‐severe (n = 2198) | Odds ratio (95% confidence interval) | |
|---|---|---|---|---|
| Clinical | ||||
| Prevalent cerebrovascular disease (%) | 2.06 | 6.01 | 1.19 | 3.66 (1.73‐7.72) |
| Prevalent cardiovascular disease (%) | 8.85 | 13.94 | 7.40 | 2.84 (1.59‐5.10) |
| Chronic obstructive pulmonary disease (%) | 2.65 | 4.82 | 2.15 | 2.39 (1.10‐5.19) |
| Diabetes (%) | 10.09 | 19.06 | 7.70 | 2.78 (2.09‐3.72) |
| Arterial hypertension (%) | 19.31 | 30.72 | 16.42 | 2.24 (1.63‐3.08) |
| Cancer (%) | 1.11 | 2.00 | 1.37 | 1.62 (0.78‐3.35) |
| Smoking (%) | 13.74 | 15.38 | 13.34 | 1.54 (1.07‐2.22) |
| Male sex (%) | 54.55 | 57.21 | 53.82 | 1.22 (1.01‐1.49) |
| Laboratory | ||||
| Procalcitonin (increased, %) a | 6.88 | 21.38 | 3.52 | 8.21 (4.48‐15.07) |
| D‐Dimer (increased, %) a | 22.54 | 52.42 | 18.20 | 5.67 (1.45‐22.16) |
| Thrombocytopenia (%) a | 21.40 | 33.04 | 16.64 | 3.61 (2.62‐4.97) |
See Section 2 for definitions.
3.1. Severe vs non‐severe
Overall, 596 (21.33%) patients had severe and 2198 non‐severe infection. Thus, the criteria for severity definition were ARDS in 240, ICU admission in 76, non‐survivors in 107 and a composite of ARDS/ICU/death in 173.
Patients with severe disease had a slightly higher age compared with non‐severe ones (Table 1).
In the severe group, 38.1% were women and 61.9% were men. Among men, the proportion of those presenting with severe disease was 22.4% (range 15.7%‐60.0%). A lower percentage of severity was found in women, which was 17.9% (range 5.9%‐64.7%).
A metanalysis of the OR of each variable (Figures 1 and 2) showed that the strongest factors associated with a severe SARS‐CoV‐2 infection were in decreasing order, and prevalent cerebrovascular disease (OR: 3.66), chronic obstructive pulmonary disease (OR: 2.76), prevalent cardiovascular disease (OR: 2.58), diabetes (OR: 2.31), hypertension (OR: 1.93), smoking (OR: 1.67), cancer (OR: 1.62) and male sex (OR: 1.25) were associated with severe disease (Table 2).
Figure 1.
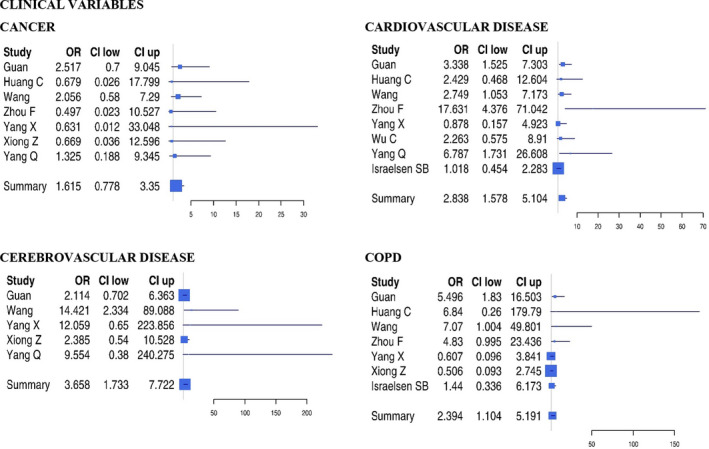
Forest plots of odds ratio of clinical variables for severe SARS‐CoV‐2 infection
Figure 2.
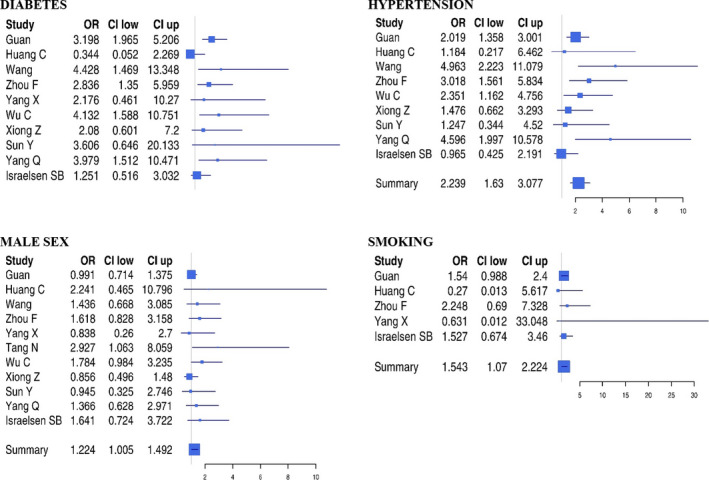
Forest plots of odds ratio of clinical variables for severe SARS‐CoV‐2 infection
For the laboratory variables, the study by Tang et al was not used, as the proportion of patients with increased laboratory values was reported only for non‐survivor patients, so that it was not possible to calculate the relative odds ratio. Among laboratory variables (Figure 3), increased procalcitonin (OR: 8.22), increased D‐Dimer (OR: 5.67) and thrombocytopenia (OR: 3.61) predicted severe infection.
Figure 3.
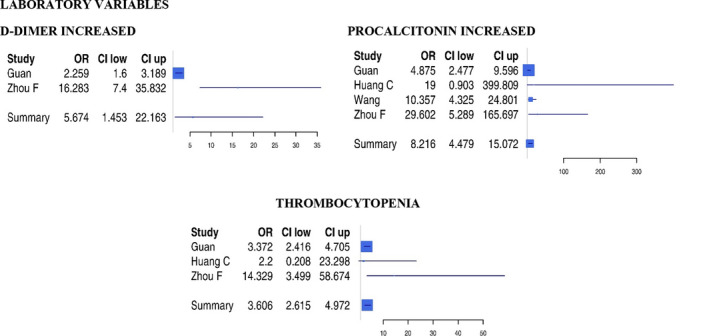
Forest plots of odds ratio of biochemical variables for severe SARS‐CoV‐2 infection
4. DISCUSSION
Our pooled analysis on a large sample of patients with SARS‐CoV‐2 infection showed that the strongest factors associated with severe COVID‐19 were cerebrovascular disease, COPD, prevalent cardiovascular disease, diabetes, increased procalcitonin, increased D‐Dimer and thrombocytopenia. The prevalence of cardiovascular risk factors reported in our analysis is similar to that reported in another recent review including >50 000 patients on this topic. 7
Prior cardiovascular disease and coexistence of risk factors such as diabetes were more associated with poor outcomes suggesting that atherosclerosis and its complications predispose to worse outcomes. Such association may be explained by the fact that cardiac damage is a frequent complication of SARS‐CoV‐2 as shown in patients needing ICU in whom troponin elevation may be detected up to approximately 40% of patients. 8 Thus, a recent analysis showed that patients with SARS‐CoV‐2 infection admitted with elevated troponin levels had worse outcomes compared to those with normal levels. 9
The non‐significant difference of age between severe and non‐severe patients is related to the relatively young age of patients included in the studies. A quality analysis however showed that patients with severe/complicated SARS‐CoV‐2 infection had a mean higher age (7 years) compared to those with non‐severe disease. Studies including elderly and very elderly patients are needed to better investigate this association.
Our results also show that male sex is associated with a more severe SARS‐CoV‐2 infection. This finding may be explained by several factors, including hormonal levels of steroids such as 17β‐estradiol and progesterone, which may play a role as immunomodulators in the host response to the viral infection. 10 In addition, there is evidence that some X‐linked genes, such as ACE2 which is involved in the SARS‐CoV‐2 infection pathogenesis by allowing SARS‐CoV‐2 entering into the cells, 11 and Y‐linked genes, such as SRY and SOX9 which codify for proteins involved in the immune response during viral infections, may explain sex‐based difference. 12
Among the laboratory variables, elevated procalcitonin and D‐dimer and low platelet count were more frequently observed in patients with severe disease. Elevated D‐dimer and low platelet count may be suggestive of systemic clotting activation with secondary fibrinolysis and platelet consumption. This finding confirms a pro‐thrombotic phenotype of SARS‐CoV‐2 infection, which is associated with the severity of the disease. 13 At this regard, a study including 449 patients with severe SARS‐CoV‐2 infection showed that patients with sepsis‐induced coagulopathy treated with low‐molecular weight heparin (LMWH) had a lower 28‐day mortality than non‐users (32.8% vs 52.4%, P = .017). 14 Based on this, the ISTH recommended that all SARS‐CoV‐2 infections should be treated with prophylactic doses of low‐molecular weight heparin to lower the thrombotic risk. 15 Further study is, however, necessary to establish whether this approach is effective and may be useful for all SARS‐CoV‐2 patients or only for those at risk of severe disease.
Finally, we found a significant association between the severity of COVID‐19 and serum levels of procalcitonin, a marker of systemic inflammation and sepsis severity, suggesting that systemic sepsis, probably related to bacterial sovra infection, may complicate the clinical course of SARS‐CoV‐2 infection. 16
The clinical relevance of our findings relies on the evidence that there is a latency between SARS‐CoV‐2 first clinical presentation and development of complications; thus, previous studies reported a median time from illness onset to hospitalization of 7‐11 days, 3 , 4 to ICU admission of 9 days 5 and to the development of ARDS of 8 days, 4 indicating that there may be a relatively long period between infection‐related symptoms onset and occurrence of severe complications. Interestingly, non‐survivor patients or those with ARDS had a higher latency period, suggesting that a delay in the care may lead to poorer outcomes. 4 , 5 Thus, early identification of variables associated with poor outcomes may be useful to plan more appropriate preventive therapy to reduce the risk of ARDS and ICU and eventually improve survival and also to optimize the allocation of healthcare resources, which may be very limited in some countries. Furthermore, outpatients presenting with one or more of these features may promptly be referred to hospital for management of the disease.
This analysis has limitations to acknowledge. As the majority of studies included Asian patients, these data need to be confirmed in other population living in Europe and United States, where the disease is rapidly spreading. Age, for example, is not associated with disease severity in China but may be a key issue for other country, such as Italy, where population is older and, apparently, at higher the risk of death. The same concept applies to the different clinical impact potentially elicited by comorbidities in young and old people. Data on the association between body weight or body mass index and the severity of infection are still lacking; thereby, no conclusion can be deduced on this aspect. Furthermore, in addition to the relative risk conferred by each single comorbidity, we cannot exclude that the concomitant presence of multiple conditions may further include the risk of severe disease.
In conclusion, data of this meta‐analysis show that comorbidities such as COPD, prior cardiovascular disease and diabetes are associated with SARS‐CoV‐2 severity. The association between these clinical characteristics and COVID‐19 severity may allow a better risk stratification in this patients’ population.
CONFLICT OF INTEREST
None related to this manuscript.
Supporting information
Figure S1
Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID‐19: A systematic review and meta‐analysis. Eur J Clin Invest. 2020;50:e13378. 10.1111/eci.13378
REFERENCES
- 1. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease. Circulation. 2020;141(20):1648‐1655. [DOI] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, J Am Med Assoc. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35‐53. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Wu S, Qin M, Jiang W, Liu X. The prevalence of cardiovascular comorbidities in COVID‐19, SARS and MERS: pooled analysis of published data. J Am Heart Assoc. 2020:e016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X, Pan X, Li Y, et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID‐19: a meta‐analysis and systematic review. Crit Care. 2020;24:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mauvais‐Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation and COVID‐19 outcomes. Endocrinology. 2020. 10.1210/endocr/bqaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng H, Cao JJ. ACE gene polymorphism and severe lung injury in patients with COVID‐19. Am J Pathol. 2020. 10.1016/j.ajpath.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penna C, Mercurio V, Tocchetti CG, Pagliaro P. Sex‐related differences in COVID‐19 lethality. Br J Pharmacol. 2020;1–11. 10.1111/bph.15207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120:949‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS‐CoV‐2 infection. Clin Chem Lab Med. 2020;58(7):1116‐1120. [DOI] [PubMed] [Google Scholar]
- 20. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Xin C, Xiong Z, et al. Clinical characteristics and outcomes of 421 patients with coronavirus disease 2019 treated in a mobile cabin hospital. Chest. 2020. 10.1016/j.chest.2020.05.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Y, Dong Y, Wang L, et al. Characteristics and prognostic factors of disease severity in patients with COVID‐19: The Beijing experience. J Autoimmun. 2020;112:102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Q, Xie L, Zhang W, et al. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020;45:609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Israelsen SB, Kristiansen KT, Hindsberger B, et al. Characteristics of patients with COVID‐19 pneumonia at Hvidovre Hospital, March‐April 2020. Dan Med J. 2020;67:A05200313. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


