Abstract
Objective
Individuals with pre-existing chronic illness have shown increased anxiety and depression due to COVID-19. Here, we examine the impact of the COVID-19 pandemic on emotional symptomatology and quality of life in individuals with Progressive Multiple Sclerosis (PMS).
Methods
Data were obtained during a randomized clinical trial on rehabilitation taking place at 11 centers in North America and Europe. Participants included 131 individuals with PMS. Study procedures were interrupted in accordance with governmental restrictions as COVID-19 spread. During study closure, a COVID Impact Survey was administered via telephone or email to all participants, along with measures of depressive symptoms, anxiety symptoms, quality of life, and MS symptomatology that were previously administered pre-pandemic.
Results
4% of respondents reported COVID-19 infection. No significant changes were noted in anxiety, quality of life, or the impact of MS symptomatology on daily life from baseline to lockdown. While total HADS-depression scores increased significantly at follow-up, this did not translate into more participants scoring above the HADS threshold for clinically significant depression. No significant relationships were noted between disease duration, processing speed ability or EDSS, and changes in symptoms of depression or anxiety. Most participants reported the impact of the virus on their psychological well-being, with a little impact on financial well-being. The perceived impact of the pandemic on physical and psychological well-being was correlated with the impact of MS symptomatology on daily life, as well as changes in depression.
Conclusions
Overall, little change was noted in symptoms of depression or anxiety or overall quality of life.
Keywords: COVID-19, Depression, Anxiety, Progressive multiple sclerosis, Quality of life
Introduction
Coronavirus disease 2019 (COVID-19) was declared a pandemic on March 11, 2020 by the World Health Organization [1]. Neurological involvement is common in COVID-19, with greater symptoms in more severe cases [2]. Individuals with underlying neurological impairment are vulnerable to infection, and those infected have worse outcomes [3].
Individuals with Multiple Sclerosis (MS) are typically on immunosuppressive/modulating medication placing them at-risk of infection from viruses [4] and are hypothetically at-risk for developing more severe forms of COVID-19 [5]. These individuals additionally have increased vulnerability to the neuropsychiatric concomitants of COVID-19, due to pre-existing neuropsychiatric symptomotology [6]. The COVID-19 pandemic has shown enormous psychological and social impact in the general population [7], not unlike other infectious diseases [8]. Mental health symptoms that can significantly impair functioning in otherwise healthy individuals [9], including stress, helplessness, and fear of becoming ill and dying, have been observed [10, 11]. The requirement to remain in quarantine has resulted in anger, confusion, anxiety, and stress [12]. A recent systematic review and meta-analysis reported a 32% prevalence of anxiety and 34% prevalence of depression in the general population [13] with higher rates in females [14–18] and individuals reporting symptoms consistent with COVID-19 and poor perceived health [18].
Pre-existing chronic illness is thus associated with increased psychiatric distress due to the spread of COVID-19 [18, 19], specifically increased stress, anxiety, and depression [7, 18, 20], placing individuals with MS in a uniquely vulnerable position to experience greater psychiatric symptomatology. We hypothesized that patients with Progressive Multiple Sclerosis (PMS) would demonstrate increased depression and anxiety and poorer QOL during the COVID-19 pandemic, as compared with prior to the pandemic.
Methods
Data for the current study were obtained during the course of a multi-arm, randomized, blinded, sham-controlled trial that includes a follow-up period. The parent study includes four arms with different combinations of Cognitive Rehabilitation (CR), Exercise (EX), Sham Cognitive Rehabilitation (CR-S), and sham exercise (EX-S). Participants are randomized to a study arm upon completion of baseline testing. Data are collected at 11 sites in 6 countries [Canada (1 site), US (2 sites), UK (2 sites), Denmark (1 site), Belgium (1 site), and Italy (4 sites)]. Outcome measures include neuropsychological assessment, Patient-Reported Outcomes (PROs), and neuroimaging. See Feinstein et al. [21] for the full study protocol.
Participants
Participants included 131 individuals with a clinically definite diagnosis of PMS (primary or secondary) of the 138 participants enrolled in the parent RCT. The mean age of the sample was 52 years (SD = 6.9), with a mean disease duration of 14.4 years (SD = 9.1). See Table 1 for demographic data. Given that these patients are generally the most impaired subtype of MS patients, they are thus the most likely to develop psychiatric symptomatology when facing a pandemic.
Table 1.
Sample demographics
| Demographic and clinical characteristics | (n = 131) |
|---|---|
| Age (in years), mean (SD) | 52.1 (6.9) |
| Education (in years), mean (SD) | 13.1 (3.1) |
| Female (%) | 63.4% |
| Country (%) | |
| Belgium | 6.9% |
| Canada | 12.2% |
| Denmark | 9.2% |
| United Kingdom | 20.6% |
| Italy | 44.2% |
| United States | 6.9% |
| Disease duration, mean (SD) | 14.4 (9.1) |
| Baseline SDMT score (z), mean (SD) | − 2.2 (0.79) |
| EDSS score, median (25th percentile, 75th percentile) | 6.0 (4, 6.5) |
Patients were recruited via specialized in and outpatient MS clinics, as well as via media advertising prior to the COVID-19 pandemic, and were at various points in study participation when study procedures were stopped at all sites due to the pandemic. Prior to initial study enrollment, all potential subjects completed a two-step screening procedure, including a pre-screening examination in person or via telephone to collect basic information and a detailed face-to-face screening for neurological, psychiatric, cognitive, and medical variables. Inclusion and exclusion criteria are summarized in Table 2 by the screening step.
Table 2.
Inclusion and exclusion criteria
| Criteria | Requirement | Screening |
|---|---|---|
| Inclusion criteria | ||
| Diagnosis | Clinically definite PMS | Telephone |
| Age | 25–65 years | Telephone |
| Ambulation | NOT wheelchair dependent (EDSS < 7) | Telephone |
| Processing speed impairment | SDMT Total Score ≥ 1.282 SD below published normative data (10th percentile) | In-person |
| Exclusion criteria | ||
| Substance abuse | Use of illicit drugs, PCP, LSD, Stimulants, Amphetamines, Barbiturates, etc. (Cannabis use was acceptable) | Telephone |
| Neurological history | A history of central nervous system disease other than PMS (e.g., stroke, Parkinson´s disease, traumatic brain injury) | Telephone |
| Severe mental illness | Psychotic symptoms, bipolar disorder, schizophrenia | Telephone |
| Medication use | Steroids use within the past 3 months | Telephone |
| Transport | Unable or unwilling to travel to the center for testing and training or requiring transportation by ambulance | Telephone |
| Medical contraindication | No medical clearance from family doctor | Telephone |
| Current exercise routine | Currently performing medium-to-high-intensity workouts according to the Exercise History Screening Questionnaire (GLTEQ score < 23) | Telephone |
| Visual acuity | Corrected near vision of at least 20/70 (to see the test materials). Severe nystagmus according to neurologist ratings | In-person |
| Depression | Beck Depression Inventory II Score ≥ 29 | In-person |
| Language comprehension | Token Test Score ≥ 29 | In-person |
| MRI compatibility (MRI sites only) | Failing the standard MRI screening form for MRI Compatibility | In-person |
Procedure
The parent RCT received ethics approval at all institutions and a modification was approved at all institutions for additional PROs, including a COVID Impact Survey, to be administered during lockdown.
Ongoing study procedures were interrupted at each individual data collection site in accordance with governmental restrictions as COVID-19 spread worldwide and all data collection sites were under lockdown orders. During the study closure, all sites contacted participants by telephone on a weekly basis to maintain contact with the participants and update them on any new information regarding the anticipated continuation of study procedures.
During this time, the study team developed a COVID Impact Survey, which was administered by a data collector via telephone or email to all enrolled participants between May 4, 2020 and July 5, 2020. All participants additionally completed selected Patient-Reported Outcomes (PROs) that were previously administered at study enrollment (baseline) to evaluate changes in depression, anxiety, quality of life (QOL), and MS symptomatology during the time period in which lockdown restrictions were in place. Survey administration occurred after lockdown orders and the resultant implications were evident across all data collection centers as lockdown was in place; this is an important methodological detail due to the fact that higher mean levels of psychiatric symptoms (stress, anxiety, and depression) have been observed after the sampled population began to experience the effects of stay at home orders [7]. The time between baseline PRO completion and lockdown survey completion varied (M = 9.5 months, SD = 4.1 months).
Assessments
Assessments in the current study included the COVID Impact Interview and several PROs administered at baseline and re-administered during lockdown.
The COVID Impact Interview was developed by the study team specifically for use in this study in an effort to evaluate the impact of the COVID-19 pandemic and lockdown orders on individuals with PMS across the participating 11 centers, representing 6 countries in North America and Europe. It consists of 22 questions related to self and family exposure to COVID-19, length of time under lockdown orders, activities during lockdown, disease symptomatology, and interactions with healthcare providers. A set of questions assessing the impact of the pandemic on psychological, financial, and physical well-being were included with responses recorded on an integer scale (0–10, with 0 being no impact and 10 being maximal impact). The survey was administered in the individual’s native language. Results were examined in response to each specific question.
The Hospital Anxiety Depression Scale (HADS) is widely used to assess psychological distress in non-psychiatric patients. It consists of two subscales, measured via 14 items, seven items for the anxiety subscale (HADS-Anxiety) and seven for the Depression (HADS-Depression) subscale [22]. Overall, it has demonstrated satisfactory psychometric properties in several different populations, including MS [23–26]. Each item is scored on a response scale with four alternatives ranging between 0 and 3 and a higher score indicates greater anxiety or depression. The HADS-depression cut-off for clinical depression was defined as scores ≥ 8.0 [27].
The Beck Depression Inventory -II (BDI-II) [28] is an easily administered, 21-item scale that assesses various aspects of depression, useful in determining the presence and severity of depressive symptoms. Each item is concerned with a specific aspect of depression (mood, motivation, and appetite) and contains four statements of graded severity expressing how a person might think or feel about that particular aspect of depression. The total score is the sum of all statements endorsed by the participant. A higher score indicates greater depression.
The Multiple Sclerosis Impact Scale (MSIS-29) is a disease-specific measure of the impact of MS. It consists of 29-items, 20 associated with a physical scale, and 9 associated with a psychological scale; the sum of each scale is transformed to a scale of 0–100 and higher scores indicating worse health [29]. Items ask about the impact of MS on day-to-day life in the past 2 weeks, rated on a five-point Likert scale. The MSIS-29 has strong reliability and validity in MS samples [29], with existing evidence supporting its responsiveness in rehabilitation trials [30].
The EuroQol (EQ5D) [31] is a widely used measure of QOL developed in Europe, often used in cost-effectiveness analyses. It evaluates QOL across five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
Analyses
Changes in responses from baseline to lockdown were evaluated using paired t tests and Wilcoxon signed-rank tests. Independent sample t tests were utilized to examine sex differences (male versus female) in response patterns. Pearson (or Spearman, when appropriate) correlation coefficients examined the relationships between the COVID-19 Impact Interview and changes in specific PROs as well the relationship between EDSS, MS-disease duration, baseline processing speed scores and changes in depression and anxiety.
Results
Longitudinal changes on PROs
Mean scores on the outcome measures across both time points are presented in Table 3. In regard to the impact of COVID-19 on MS symptomatology in daily life, no significant differences were noted on the MSIS-29 from baseline to lockdown. Two measures of depressive symptoms were administered. No significant differences were noted on the BDI-II from baseline to lockdown; however, a significant difference was noted on the HADS-Depression scale from baseline to lockdown (p = 0.033), with a small increase in depression symptoms noted at the lockdown follow-up (Table 3). Further analyses indicate that this difference was driven by a substantial increase in depressive symptoms in the sample from Belgium, while the remaining five countries show the similar levels of change (p < 0.001; Table 4). No significant difference was noted in regard to the number of patients meeting the HADS-depression cut-off for clinical depression, defined as scores ≥ 8.0. No significant difference was noted from baseline to lockdown on the HADS-Anxiety Scale or any of the EQ5D scales.
Table 3.
Mean responses on the BDI, HADS, and MSIS
| Variable | Baseline | Lockdown | P value |
|---|---|---|---|
| BDI total score | 11.3 (7.5) | 12.1 (9.2) | 0.329 |
| HADS-depression score | 5.8 (3.7) | 6.7 (4.6) | 0.033 |
| HADS anxiety score | 5.9 (4.3) | 6.0 (4.3) | 0.748 |
| MSIS-29 physical score | 45.3 (21.6) | 47.2 (22.4) | 0.595 |
| MSIS-29 mental score | 34.3 (22.7) | 35.1 (22.6) | 0.915 |
| EQ5D mobility | 0.707 | ||
| No problems | 16 (12.5) | 11 (8.8) | |
| Slight | 25 (19.5) | 27 (21.6) | |
| Moderate | 51 (39.8) | 59 (47.2) | |
| Severe | 36 (28.1) | 27 (21.6) | |
| Unable | 0 (0.0) | 1 (0.80) | |
| EQ5D self-care | 0.127 | ||
| No problems | 67 (52.3) | 57 (45.6) | |
| Slight | 35 (27.3) | 37 (29.6) | |
| Moderate | 21 (16.4) | 25 (20.0) | |
| Severe | 5 (3.9) | 6 (4.8) | |
| EQ5D usual activities | 0.709 | ||
| No problems | 22 (17.3) | 21 (16.8) | |
| Slight | 43 (33.9) | 31 (24.8) | |
| Moderate | 42 (33.1) | 60 (48.0) | |
| Severe | 20 (15.7) | 11 (8.8) | |
| Unable | 0 (0.0) | 2 (1.6) | |
| EQ5D pain | 0.082 | ||
| No problems | 35 (27.3) | 28 (22.4) | |
| Slight | 36 (28.1) | 36 (28.8) | |
| Moderate | 45 (35.2) | 42 (33.6) | |
| Severe | 9 (7.0) | 17 (13.6) | |
| Unable | 3 (2.3) | 2 (1.6) | |
| EQ5D anxiety/depression | 0.087 | ||
| No problems | 67 (52.3) | 60 (48.0) | |
| Slight | 38 (29.7) | 35 (28.0) | |
| Moderate | 20 (15.6) | 23 (18.4) | |
| Severe | 3 (2.3) | 5 (4.0) | |
| Unable | 0 (0.0) | 2 (1.6) | |
The average time between baseline PRO completion and lockdown survey completion was 9.5 months (SD = 4.1)
Table 4.
Difference from baseline to lockdown in PROs by country
| Total sample (n = 131) | Belgium (n = 9) | Canada (n = 16) | Denmark (n = 12) | England (n = 27) | Italy (n = 58) | US (n = 9) | P value | |
|---|---|---|---|---|---|---|---|---|
| BDI | − 0.72 (8.1) | 1.1 (6.2) | − 4.3 (10.8) | 1.6 (4.0) | − 1.7 (7.4) | − 0.14 (8.6) | 0.11 (4.9) | 0.40 |
| HADS-depression | − 0.79 (4.0) | − 6.7 (6.1) | − 0.13 (3.2) | 0.25 (2.8) | − 0.43 (2.6) | − 0.53 (4.1) | 0.25 (1.6) | < 0.001 |
| HADS-anxiety | − 0.16 (4.2) | − 1.4 (5.2) | − 0.93 (5.6) | 0.33 (3.4) | 0.08 (2.7) | 0.09 (4.6) | − 0.50 (2.8) | 0.88 |
| MSIS-physical | − 0.74 (16.5) | 0.56 (11.9) | − 10.1 (22.1) | 2.4 (12.7) | − 1.7 (11.1) | 0.55 (16.3) | 11.8 (20.6) | 0.05 |
| MSIS-Mental | 0.02 (19.7) | 0.61 (5.5) | − 7.2 (28.8) | 2.0 (16.4) | 2.8 (19.2) | 0.17 (20.1) | 1.6 (10.5) | 0.74 |
Sex differences
Independent sample t tests were utilized to examine sex differences (male versus female) in response patterns. No significant differences were noted between males and females in symptoms of depression and anxiety, or overall QOL.
COVID impact interview
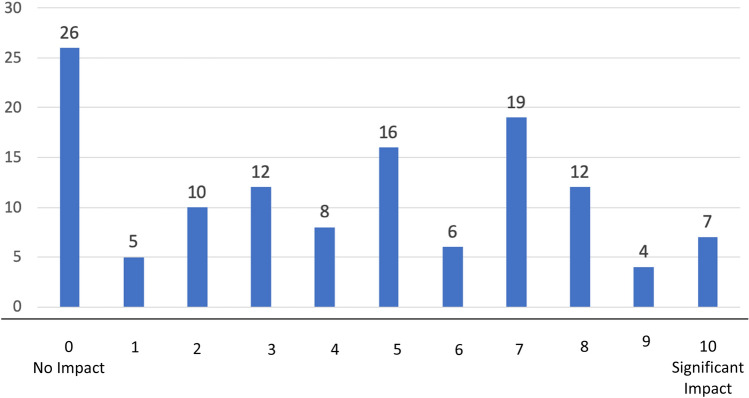
In regard to the impact of COVID-19 on the study population, only 5 of the 131 respondents reported that he/she had been infected with COVID-19, with 15 reporting infections in other family members. 31 individuals knew someone that died from the virus. The majority of participants reported some impact of the virus on their psychological well-being (Fig. 1), while little financial impact was reported.
Fig. 1.
Impact of COVID-19 on psychological well-being (frequency of responses)
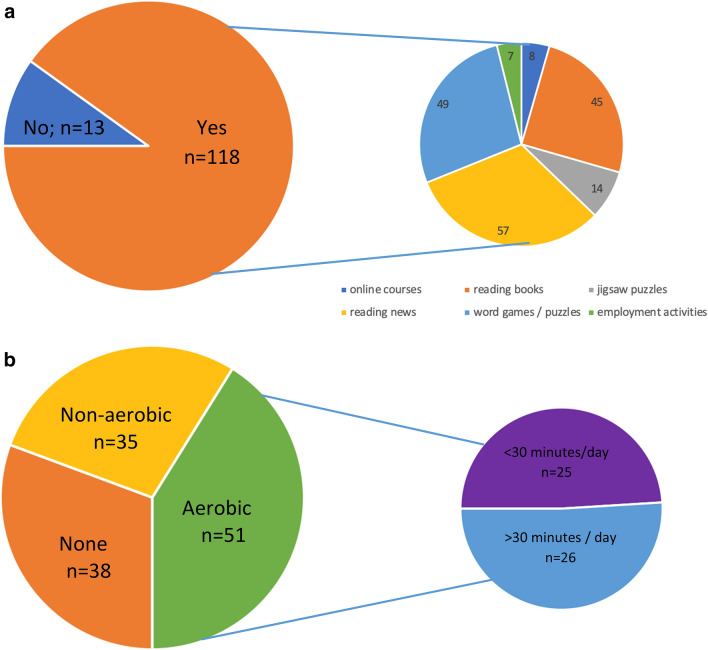
In regard to activities during lockdown, 90% of respondents reported undertaking some form of cognitive activity, while 71% reported participating in some form of physical activity (Fig. 2a, b). Overall, respondents reported a high level of social support (with 70% responding 8, 9, or 10 on a 10-point Likert scale). Only 57% of respondents reported any interaction with their medical team during lockdown orders, with a comparable proportion reporting MS symptom changes during the same time period (58%).
Fig. 2.
a Engagement in cognitive activities during lockdown. b. Engagement in physical activities during lockdown
With only 5 of the 131 respondents reporting COVID-19 infection, statistical significance between these respondents and the non-infected respondents could not reliably be determined. However, some identifiable differences in these five individuals are worth noting qualitatively. An increase from baseline to lockdown was noted in the MSIS mental score in those who were infected with COVID-19, with an increase of 15.1 (SD = 13.5) noted; this indicates a self-perceived worsening of challenges in daily life due to mental symptomatology. A similar decrement was noted in the MSIS-physical score, with an increase of 7.2 (SD = 20.07) noted. Depressive symptoms also appeared to be negatively impacted, with a 1-point increase on the BDI (SD = 7.6) and a 1.8-point (SD = 5.5) increase on the HADS depression.
Relationships between PROs and COVID responses
No significant relationships were noted between MS-disease duration, EDSS, or SDMT z-score (processing speed) and changes in depression and anxiety (range of r values: − 0.08 to 0.13).
Significant correlations were noted between differences in the MSIS-29 Mental Scale from baseline to lockdown and the degree to which the respondents felt the pandemic impacted their physical well-being (r = − 0.24, p = 0.009), psychological well-being (r = − 0.20, p < 0.03), and MS-disease course (r = − 0.21, p = 0.02). As the perceived impact of MS symptoms on mental functioning increased during lockdown, participants similarly reported a greater impact on physical and psychological well-being and MS-disease course. Significant correlations were also noted between differences in the HADS-depression scale and the degree to which the pandemic negatively influenced MS-disease course (r = − 0.19, p = 0.048) and the EQ5D Anxiety/Depression scale and the degree to which the respondent felt that the pandemic impacted his/her psychological well-being (r = − 0.20, p = 0.03).
Discussion
No statistically significant changes in perceived MS symptomatology were noted from baseline to the COVID follow-up conducted during lockdown in our sample of individuals with PMS. Despite the fact that the majority of participants reported some impact of the virus on their psychological well-being on the COVID Impact Interview, we saw little change in regard to symptoms of depression and anxiety and overall QOL on standardized PROs. The international composition of our sample indicates that these findings are largely consistent across widely dispersed geographical locations.
There are several potential explanations for this pattern of results. First, one must consider the impact of diligence in self-protection on psychological well-being. Others have hypothesized that individuals with a significant medical history may feel increased vulnerability to COVID-19 [34]. It is possible that individuals with PMS were diligent about protecting themselves from very early in the pandemic because of their increased risk of infection and subjective feelings of vulnerability. Their efforts for self-protection may have increased their level of comfort, because they were diligent in following safety precautions, thus mitigating their anxiety and depression. This may have resulted in less anxiety and depression symptoms than what might be expected under normal circumstances and seen in the general population.
Additionally, individuals with PMS already experience a substantial physical disability that often leads to some degree of isolation in daily life. Thus, the drastic societal changes in social interaction due to lockdown orders may have been less impactful for this population due to the fact that their activities have already been significantly restricted for quite some time. Social isolation has been shown to have a significant impact on mental health in numerous studies [32], with social isolation and loneliness being associated with depression in the general population [33]. It may be that our sample of individuals with PMS was already accustomed to some degree of social isolation, thus easing the transition to lockdown.
The impact of experience in living with medical uncertainly also cannot be overestimated. Studies conducted early in the COVID-19 outbreak in China concluded that fear of the unknown and uncertainty can lead to increased stress, anxiety, and depression [35]. Zandifar and colleagues similarly highlighted the role of unpredictability, uncertainty, and seriousness of the disease in such psychiatric symptomatology [36]. However, individuals with MS live with medical uncertainty from the time of diagnosis and thus have experience dealing with the associated discomfort. Individuals with PMS thus may not be experiencing the psychological discomfort that comes with such uncertainty in the face of COVID-19. The psychiatric symptomatology which they are experiencing is thus less than that which is seen in the general population.
Finally, the large majority of our sample additionally reported engagement in both cognitive and physical activities during lockdown. This is an encouraging finding and likely contributed to the little change observed in psychiatric symptomatology over the same time period. One of the aims of the parent RCT of the present study is to encourage a more active lifestyle and participants were all within some phase of the RCT when lockdown was initiated. Had the RCT run its full course prior to lockdown, engagement in cognitive and physical activities may have influenced changes in psychiatric symptomatology in a significantly positive way.
These same factors may be at play in the lack of significant differences seen in depression or anxiety between males and females in our PMS sample. This is contrary to that which is observed in the general population, in which females present with higher rates of anxiety and depression as compared with males [14–18]. Our sample is, indeed, 63% female, consistent with MS being more common in females. This larger proportion of females in which uncertainty may already be a normal component of life could potentially lead to less depression and anxiety in our female sample as compared to that which has been seen in the general population.
It is interesting to note that only 5 of the 131 respondents reported that he/she had been infected with COVID-19; this represents a 4% infection rate. This is, however, a higher infection rate than that which is seen in the general population within each country represented. The impact of the infection on MS symptoms was also quite evident, with those infected with COVID-19 showing worsening on both the MSIS-29 mental score (15-point increase) and the MSIS-29 physical score (7-point increase). This is compared to a change of less than 1 on each of these scores in the full sample, indicating that infection with COVID-19 had a tremendous impact on the MS-related symptomatology and daily limitations that individuals with PMS experience. The change in depression scores in this subgroup, however, was consistent with changes noted in the full sample.
No relationship was noted between baseline MS-disease-related variables (disease duration, processing speed ability, and EDSS) and changes in depression, anxiety, and QOL from baseline to lockdown. However, relationships were noted between changes in responses to the PROs and COVID Impact Interview. The perceived impact of the pandemic on physical and psychological well-being was correlated with the impact of MS symptomatology on daily life, as measured by the MSIS-29 mental scale, as well as changes in psychiatric symptomatology (HADS depression, EQ5D Anxiety/Depression). These relationships attest to the importance of one’s perception of the impact of the pandemic on standardized measures of disease symptomatology, emotional functioning, and QOL.
There are some limitations to the current study that deserve mention. Given that the full RCT through which these data were collected did not include a measure of stress, we did not measure changes in stress from baseline to lockdown. Given that elevated stress has been documented in the general population during the COVID-19 pandemic, these data would have been advantageous. Additionally, no questions were included regarding the severity of infection if an individual was indeed infected. We, therefore, could not examine the relationship between the severity of COVID-19 and changes in psychiatric symptomatology or the impact of MS on daily life. Another factor not examined in the current study was exposure to the news and potential misinformation. In the general population, depressive symptoms can be exacerbated by misinformation and fabricated reports about COVID-19 [15], and people who follow COVID-19 the most in the news experience more anxiety [37], but we were unable to examine this relationship in PMS. In addition, the lockdown follow-up was completed toward the end of the lockdown period across all sites. It is possible that the time in lockdown had afforded patients the time to adjust emotionally to the lockdown and thus exhibit less emotional symptomology. Sample bias could have also potentially impacted our pattern of results. The current sample engaged/or was engaging in a 3-month intensive training study; these individuals could potentially have higher levels of self-efficacy and/or resilience. The many strengths of the study, however, far outweigh these limitations. Specifically, the ongoing parent RCT allowed the comparison of pre-pandemic depression, anxiety, and QOL to the same ratings completed during lockdown in a fairly large sample of individuals with PMS in six different countries. These unique data thus provide comparative values that are rarely available.
Overall, findings indicate that individuals living with PMS through the COVID-19 pandemic are adapting well to date. That is, minimal change was noted from pre-COVID status to assessments conducted during COVID-19 lockdown on depression, anxiety, and QOL. Minimal changes were additionally noted in the impact of MS-related symptoms on daily life functioning on the limited measures utilized to assess this construct, with the exception of those infected with COVID-19. While the infection rate observed in our sample was higher than that which is seen in the general population, even those who contracted COVID-19 showed minimal change from pre-COVID depression, anxiety, and QOL to ratings of depression, anxiety, and QOL collected during lockdown.
Study funding
Supported by the MS Society of Canada (Grant # EGID3185).
Author contributions
NDC: design and conceptualized study; major role in the acquisition of data; interpreted the data; drafted the manuscript for intellectual content. MPA: major role in the acquisition of data; revised the manuscript for intellectual content. GB: major role in the acquisition of data; revised the manuscript for intellectual content. JC: major role in the acquisition of data; revised the manuscript for intellectual content. UD: major role in the acquisition of data; revised the manuscript for intellectual content. JD: design and conceptualization of study; Interpreted the data; revised the manuscript for intellectual content. CM: major role in the acquisition of data. NM: major role in the acquisition of data. PF: major role in the acquisition of data; revised the manuscript for intellectual content. MF: major role in the acquisition of data; revised the manuscript for intellectual content. JF: major role in the acquisition of data; revised the manuscript for intellectual content. MI: major role in the acquisition of data; revised the manuscript for intellectual content. RWM: major role in the acquisition of data; revised the manuscript for intellectual content. MAR: major role in the acquisition of data; revised the manuscript for intellectual content. BS: major role in the acquisition of data; revised the manuscript for intellectual content. AS: analyzed the data; assisted in interpretation of data; revised the manuscript for intellectual content. GC: revised the manuscript for intellectual content. AF: design and conceptualized study; Major role in the acquisition of data; revised the manuscript for intellectual content.
Compliance with ethical standards
Conflicts of interest
Nancy D. Chiaravalloti is on an Advisory Board for Akili Interactive and is a member of the Editorial Boards of Multiple Sclerosis Journal and Frontiers in NeuroTrauma. Maria Pia Amato received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Roche, Pharmaceutical Industries, and Fondazione Italiana Sclerosi Multiplav. Giampaolo Brichetto has no disclosures to report. Jeremy Chataway has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK MS Society, the US National MS Society, and the Rosetrees Trust. He is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK. He has been a local principal investigator for commercial trials funded by: Actelion, Biogen, Novartis, and Roche; has received an investigator grant from Novartis; and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, MedDay, Merck, and Roche. Ulrik Dalgas has received research support, travel grants, and/or teaching honorary from Biogen Idec, Merck Serono, Novartis, Bayer Schering, and Sanofi Aventis as well as honoraria from serving on scientific advisory boards of Biogen Idec and Genzyme. John DeLuca is an Associate Editor of the Archives of Physical Medicine and Rehabilitation, and Neuropsychology Review; received compensation for consulting services and/or speaking activities from Biogen Idec, Celgene, MedRhythms, and Novartis; and receives research support from Biogen Idec, National Multiple Sclerosis Society, Consortium of Multiple Sclerosis Centers, and National Institutes of Health. Cecilia Meza has no disclosures to report. Nancy B. Moore has no disclosures to report. Peter Feys is editorial board member of NNR and MSJ, provides consultancy to NeuroCompass and was board of advisory board meetings for BIOGEN. Massimo Filippi is the Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). Jennifer Freeman has been awarded research grants from the NIHR, UK. Matilde Inglese is Co-Editor for Controversies for Multiple Sclerosis Journal; received compensation for consulting services and/or speaking activities from Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme; and received research support from NIH, NMSS Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla. Robert W. Motl has no disclosures to report. Maria Assunta Rocca received speaker honoraria from Biogen Idec, Novartis, Teva Neurosciences, Merck Serono, Genzyme, Roche, Bayer, and Celgene, and receives research support from the Canadian MS Society and Fondazione Italiana Sclerosi Multipla. Brian Sandroff has no disclosures to report. Amber Salter is a statistical editor for Circulation: Cardiovascular Imaging. Gary Cutter is a member of Data and Safety Monitoring Boards for Astra-Zeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Horizon Pharmaceuticals, Hisun Pharmaceuticals, Mapi Pharmaceuticals Ltd, Merck, Merck/Pfizer, Opko Biologics, OncoImmune, Neurim, Novartis, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Teva pharmaceuticals, VielaBio Inc, Vivus, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee). He is on Consulting or Advisory Boards for Biodelivery Sciences International, Biogen, Click Therapeutics, Genzyme, Genentech, GW Pharmaceuticals, Klein-Buendel Incorporated, Medimmune, Medday, Neurogenesis Ltd, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Roche, and TG Therapeutics. Dr. Cutter is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham AL. Anthony Feinstein is on an Advisory Board for Akili Interactive and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press, Amadeus Press, and Glitterati Editions, and speaker’s honoraria from Novartis, Biogen, Roche, and Sanofi-Genzyme.
References
- 1.Centers for Disease Control. Coronavirus. https://www.cdc.gov/coronavirus/types.html. Accessed May 15, 2020.
- 2.Mao L, Wang M, Hu Y et al (2020) Neurologic manifestations of hospitalized patients with coronavirua disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed]
- 3.Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020 doi: 10.1212/WNL. [DOI] [PubMed] [Google Scholar]
- 4.Borrielloa G, Iannielloc A. COVID-19 occurring during Natalizumab treatment: a case report in a patient with extended interval dosing approach. Mult Scler Relat Disord. 2020 doi: 10.1016/j.msard.2020.102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzegar M, Mirmosayyeb O, Nehzat N, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhoundia FH, Sahraianb MA, Moghadasib AN. Neuropsychiatric and cognitive effects of the COVID-19 outbreak on T multiple sclerosis patients. Mult Scler Relat Disord. 2020;41:102–164. doi: 10.1016/j.msard.2020.102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozamiz-Etxebarria N, Dosil-Santamaria M, Picaza-Gorrochategui M, Idoiaga-Mondragon N. Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cad Saude Publica. 2020 doi: 10.1590/0102-311X00054020. [DOI] [PubMed] [Google Scholar]
- 8.Bao Y, Sun Y, Meng S, Shi J, Lu L. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020 doi: 10.1016/S0140-6736(20)30309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020 doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall RCW, Hall RCW, Chapman CMJ. The 1995 Kikwit Ebola outbreak: lessons hospitals and physicians can apply to future viral epidemics. Gen Hosp Psychiatry. 2008 doi: 10.1016/j.genhosppsych.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torales J, O’Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020 doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 12.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020 doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Heal. 2020;16(1):57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghanibashi-Mansourieh A. Assessing the anxiety level of Iranian general population during COVID-19 outbreak. Asian J Psychiatr. 2020 doi: 10.1016/j.ajp.2020.102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou SJ, Zhang LG, Wang LL, et al. Prevalence and socio-demographic correlates of psychological health problems in Chinese adolescents during the outbreak of COVID-19. Eur Child Adolesc Psychiatry. 2020 doi: 10.1007/s00787-020-01541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Ren Y, Yan F et al (2020) Psychological impact and predisposing factors of the coronavirus disease 2019 (COVID-19) pandemic on general public in China. SSRN Electron J. 10.2139/ssrn.3551415
- 17.Wang Y, Di Y, Ye J, Wei W. (2020) Study on the public psychological states and its related factors during the outbreak of coronavirus disease 2019 (COVID-19) in some regions of China. Psychol Heal Med. https://doi.org/10.1080/13548506.2020.1746817 [DOI] [PubMed]
- 18.Wang C, Pan R, Wan X et al (2020) Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed]
- 19.Holmes EA, O’Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Liang M, Li Y, et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feinstein A, Amato MP, Brichetto G, et al. Study protocol: Improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx) BMC Neurol. 2020 doi: 10.1186/s12883-020-01772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002 doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 24.Drageset J, Eide GE, Ranhoff AH. Anxiety and depression among nursing home residents without cognitive impairment. Scand J Caring Sci. 2013 doi: 10.1111/j.1471-6712.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 25.Annunziata MA, Muzzatti B, Altoé G. Defining hospital anxiety and depression scale (HADS) structure by confirmatory factor analysis: a contribution to validation for oncological settings. Ann Oncol. 2011 doi: 10.1093/annonc/mdq750. [DOI] [PubMed] [Google Scholar]
- 26.Iani L, Lauriola M, Costantini M. A confirmatory bifactor analysis of the hospital anxiety and depression scale in an Italian community sample. Health Qual Life Outcomes. 2014 doi: 10.1186/1477-7525-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honarmand K, Feinstein A. Validation of the hospital anxiety and depression scale for use with multiple sclerosis patients. Mult Scler. 2009 doi: 10.1177/1352458509347150. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. TX Psychol Corp: San Antonio; 1996. [Google Scholar]
- 29.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale (MSIS-29) a new patient-based outcome measure. Brain. 2001 doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 30.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry. 2005 doi: 10.1136/jnnp.2005.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balestroni G, Bertolotti G (2012) EuroQol-5D (EQ-5D): an instrument for measuring quality of life TT - L’EuroQol-5D (EQ-5D): uno strumento per la misura della qualità della vita. Monaldi Arch chest Dis. 10.4081/monaldi.2012.121 [DOI] [PubMed]
- 32.Taylor HO, Taylor RJ, Nguyen AW, Chatters L. Social isolation, depression, and psychological distress among older adults. J Aging Health. 2018 doi: 10.1177/0898264316673511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews T, Danese A, Wertz J, et al. Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Soc Psychiatry Psychiatr Epidemiol. 2016 doi: 10.1007/s00127-016-1178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018 doi: 10.1186/s13054-018-2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shigemura J, Ursano RJ, Morganstein JC, Kurosawa M, Benedek DM. Public responses to the novel coronavirus (2019-nCoV) in Japan: Mental health consequences and target populations. Psychiatry Clin Neurosci. 2019 doi: 10.1111/pcn.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zandifar A, Badrfam R. Iranian mental health during the COVID-19 epidemic. Asian J Psychiatr. 2020 doi: 10.1016/j.ajp.2020.101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. World Health O. Mental Health and Psychosocial Considerations during the COVID-19 Outbreak. Geneva