Abstract
The epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) contribute to an increased response rate, compared with chemotherapy, in patients with inhibitor-sensitive EGFR mutations. The present study evaluated the association between the maximum standardized uptake value (SUVmax) of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET/CT), as well as serum carcinoembryonic antigen (CEA) levels and EGFR mutations prior to treatment, in patients with non-small cell lung cancer (NSCLC). Patients with histologically confirmed NSCLC (n=167), who underwent an 18F-FDG PET/CT scan, EGFR mutation analysis and a serum CEA test participated in the present study. Multivariate logistic regression analysis was used to analyze predictors of EGFR mutations. Receiver-operating characteristic (ROC) curve analysis was performed to determine the efficient cut-off value. Survival rate analysis was evaluated according to SUVmax and EGFR mutation status. A decreased SUVmax and an increased CEA level was observed in patients with EGFR-mutations, compared with patients with wild-type primary lesions and metastatic lymph nodes. The exon 19 EGFR mutation was associated with increased SUVmax, compared with the exon 21 L858R mutation. The ROC analysis indicated that an 18F-FDG PET/CT uptake SUVmax >11.5 may be a predictor of the wild-type EGFR genotype and increased CEA levels (CEA >9.4 ng/ml) were associated with EGFR mutations. Furthermore, patients with no smoking history, low SUVmax of the primary tumor, metastatic lymph nodes and a high CEA level were significantly associated with EGFR mutation status. The results of the present study indicated that patients with advanced NSCLC, particularly Chinese patients, with decreased SUVmax and increased CEA levels are associated with EGFR mutations, which may serve as predictors for the EGFR-TKI therapeutic response.
Keywords: positron emission tomography, epidermal growth factor receptor, carcinoembryonic antigen, non-small cell lung cancer, exon mutation
Introduction
Lung cancer has been reported as the leading cause of cancer-associated mortality globally in the past 10 years (1,2). Non-small cell lung cancer (NSCLC) has been indicated to account for 80–85% of cancer cases, and the majority of patients have advanced stage or metastatic NSCLC at diagnosis (3,4). From the eastern cooperative oncology group 1594 trial, it was indicated that the overall survival (OS) rate of patients with NSCLC is 8–10 months if patients with advanced disease received chemotherapy alone (5). It has been reported that first-generation small molecule tyrosine kinase inhibitors (TKI) of the epidermal growth factor receptor (EGFR) are a notable factor in the treatment of advanced or metastatic NSCLC in patients with inhibitor-sensitive EGFR mutations (6,7). Numerous phase III studies demonstrated that compared with traditional chemotherapy, EGFR-TKIs, as first-line treatments, contributed to the protraction of progression-free survival (PFS) rate and to an increased response rate (RR) in patients with inhibitor-sensitive EGFR mutation (8–13). Furthermore, a previous study demonstrated that the median OS time was prolonged to 30 months, when patients with EGFR sensitive-mutations received chemotherapy and TKIs, compared with an OS time of 10 months in patients who were treated with chemotherapy alone (14). It has been reported that inhibitor-sensitive EGFR mutations are an important indicator of NSCLC response to TKI therapy (15). Therefore, the determination of EGFR mutation status is important for the optimization of NSCLC treatment. However, it has also been indicated that limited tissue size prevents determination of EGFR mutation status. It has been reported that, in 2013, only 32.8% of patients with NSCLC in China exhibited EGFR mutations (16). The population exhibiting optimal response to TKI treatment has been reported to be in non-smoking Asian female patients with adenocarcinoma (16). However, it has been indicated that 36% of patients exhibiting ≥3 of the aforementioned features did not develop an EGFR mutation (16). Therefore, it is imperative to identify novel prognostic indicators for the non-invasive detection of EGFR-mutation status.
Carcinoembryonic antigen (CEA) was first identified in 1965 in human colon cancer (17). It has been reported that 30–70% of patients with NSCLC, particularly those with advanced lung adenocarcinoma, exhibit elevated serum CEA levels (18–23). Previous studies reported that following gefitinib treatment, patients with NSCLC who exhibited increased CEA levels (>50 ng/ml) had an increased OS time (20). However, it has also been reported that an increased pre-treatment serum CEA level was associated with poor outcome in patients with NSCLC treated with erlotinib (18). Other studies indicated that CEA may be associated with EGFR mutation status (24–26). Thus far, researchers have not reached a consensus on the feasibility of serum CEA level as a predictor for the EGFR mutation status and the prognosis of NSCLC.
18F-fluorodeoxyglucose positron emission tomography (FDG PET) in addition to reduced dose computed tomography (CT) has been effectively employed for the staging of NSCLC (27). Furthermore, it has been reported that the primary maximum standardized uptake value (SUVmax) is associated with the status of EGFR mutation and that the tumor FDG uptake is a notable prognostic factor for NSCLC (28,29). Despite the SUVmax value being reported to be increased (≥6) in patients exhibiting wild-type EGFR, compared with patients exhibiting an EGFR mutation (26), no significant difference has been observed in 18F-FDG PET/CT uptake between patients exhibiting EGFR mutation and their wild-type counterparts (30).
The association of EGFR mutation status with FDG uptake and serum CEA level in NSCLC requires further investigation. The present study examined 18F-FDG PET/CT uptake and the CEA level in patients with NSCLC exhibiting different EGFR mutations, in order to predict the EGFR mutation status and optimize NSCLC treatment.
Materials and methods
Patients
A total of 454 patients with NSCLC were tested for CEA level and SUVmax in the Wuhan Cancer Center (Wuhan, China) and 167 were staged by using 18F-FDG PET/CT. Patient information (n=167) was collected by chart review, including age, sex, smoking status, pre-treatment serum CEA level (normal range, 0–5 ng/ml), histological type and clinical stage of the patient's tumors. The sample included 87 males (52.1%) and 80 females (47.9%), with their age ranging from 28–82 years (mean ± standard deviation 58.4±10.3 years). A total of 86 cases were <60 years of age and 81 cases were >60 years of age. The most common histological type was adenocarcinoma (97.0%), followed by squamous cell carcinoma (3.0%). The histopathological diagnoses were confirmed by means of CT-guided core-needle biopsy, ultrasound-guided percutaneous biopsy or bronchoscopic biopsy performed in the Wuhan Cancer Center. Tumor-Node-Metastasis (TNM) stages were recorded in all patients in accordance with the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual (31). Patients with stage I–IV NSCLC were examined for EGFR mutation status, serum CEA level and subjected to PET/CT for 18F-FDG uptake between January 2010 and October 2011 at the Cancer Center of the Union Hospital (Wuhan, China).
Patients with NSCLC were enrolled in the present study under the following inclusion criteria: i) Histological confirmation of NSCLC; ii) stage I–IV demonstrated by PET/CT and/or brain magnetic resonance imaging, and iii) underwent EGFR mutation detection and serum CEA level detection at diagnosis. Patients with active pneumonia or other types of infection and diabetes, which could have confounded the analysis, were not included in the present study. Patients were also categorized according to the exons of EGFR mutations. The EGFR mutations at exons 18–21 were detected using an EGFR 29 Mutations Detection kit (ADx-EG01; Amoy Diagnostics Co., Ltd.), according to the manufacturer's protocol. The present study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) and written informed consent was obtained from each participant prior to the initiation of any study-associated procedures.
18F-FDG PET/CT image acquisition and analysis
In accordance with the protocol of Union Hospital of Tongji Medical College, whole-body 18F-FDG PET/CT scans were conducted (32). All patients were asked to fast for ≥6 h prior to intravenous injection of 370 MBq of 18F-FDG and whole-body emission scans were obtained. The acquired PET data were reconstructed to volumetric images with a 2D-OSEM algorithm (2 iterations/16 subsets) in the Discovery LS PET/CT scanner (GE Healthcare) and (2 iterations/8 subsets) in the Biograph PET-CT scanner (Siemens Healthineers). Images were reconstructed with attenuation correction (CT-based).
All PET/CT scans were analyzed at the Union Hospital of Tongji Medical College by a radiologist and a nuclear physician with 8 and 5 years of PET experience, respectively. For each involved site, including the primary tumor, the metastatic lymph nodes and the distant metastases, a region of interest (ROI) was carefully drawn around the site of suspected lesions. The SUV was calculated using the standard formula normalized by body weight: SUV=cdc/(di/w), where cdc is the decay-corrected tracer tissue concentration (Bq/g), di is the injected dose (Bq), and w is the body weight of the patient (g). The physiological SUVs of lung tissue were 0.37–1.29, similar to those previously reported (33–35). The numerical value is associated with the differentiation of tumor cells, in addition to the activity and the degree of malignancy (36–38). In order to minimize variation and ensure reproducibility, sites where increased SUV value was considered as physiological uptake were excluded and the maximal pixel activity in the ROI was the SUVmax (34,35).
Metastatic lymph nodes were defined as lymph nodes with increased metabolic activity against the background of mediastinal structures, based on qualitative visual inspection. Only lesions with the longest axis ≥1.0 cm were included in the analysis to avoid partial volume effect. For patients with multiple metastatic lymph nodes, the mean SUVmax of all lymph nodes was used for subgroup analyses.
DNA extraction and quantitative PCR
The formalin-fixed and paraffin-embedded tumor tissues were collected from patients and DNA extraction performed with the QIAamp DNA Mini kits (Qiagen GmbH) according to the manufacturer's protocol. The tyrosine kinase domain of the EGFR coding sequence, i.e., exons 19 and exon 21, were amplified by independent rounds of PCR. The sequences of the primers used are presented in Table I. PCR was performed with an ADx-EG01 kit (Amoy Diagnostics Co., Ltd.) according to the manufacturer's protocol. A LightCycler® 480 real-time PCR machine (Roche Diagnostics) with the following thermocycling conditions: 95°C, 5 min; 95°C, 10 min; 15 cycles of 95°C, 25 sec; 64°C, 20 sec; and 72°C, 20 sec; followed by 31 cycles of 93°C, 25 sec 60°C, 35 sec; and 72°C, 20 sec. The relative expression levels were normalized to endogenous control and were expressed as 2−ΔΔct (39).
Table I.
Sequences of the primers used for PCR.
| Name | Sequences |
|---|---|
| Exon 19 | |
| Forward | 5′-GCAATATCAGCCTTAGGTGCGGCTC-3′ |
| Reverse | 5′-GCAATATCAGCCTTAGGTGCGGCTC-3′ |
| Exon 21 | |
| Forward | 5′-CTAACGTTCGCCAGCCATAAGTCC-3′ |
| Reverse | 5′-GCTGCGAGCTCACCCAGAATGTCTGG-3′ |
EGFR mutation analysis by immunohistochemistry
In the majority of cases, pathological tissue specimens for EGFR mutation analysis were obtained via surgical resection (n=8/167, 4.8%) and CT-guided core-needle biopsy (n=120/167, 71.9%). The remaining samples were harvested by ultrasound-guided percutaneous biopsy (n=18/167, 10.8%), and bronchoscopic biopsy (n=21/167, 12.6%). Immunohistochemical examination proceeded according to the standard avidin-biotin-peroxidase complex method using monoclonal rabbit antibodies against the exon 21 L858R EGFR mutation (cat. no. 3197) and the 15-bp E746-750 deletion in exon 19 (cat. no. 2085) (both from Cell Signaling Technology Inc.). Tissues were fixed in 4% formalin at room temperature for 8 h, and dehydrated by using increasing graded alcohol solutions (70, 90 and 100%) and xylene for 30 mins at room temperature before being embedded in paraffin. The paraffin-embedded tissue sections (5 mm thickness) were deparaffinized with xylene and rehydrated by using decreasing graded ethanol solutions (100, 95, 80 and 70%) for 30 min at room temperature. Antigens were retrieved by microwave for 15 min in EDTA buffer (pH 9.0). Sections were washed with TBS/Tween-20 (TBST) and then blocked with 5% bovine serum albumin at room temperature for 1 h. The rabbit monoclonal antibodies were applied as the primary antibody at a dilution of 1:100 at 4°C overnight. Slides were washed for 5 min in TBST and incubated at room temperature for 1 h with the respective horseradish peroxidase-conjugated anti-rabbit secondary antibody (cat. no. ab6721; Abcam) diluted with TBS in a ratio of 1:200. After washing, slides were incubated with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich; Merck KGaA) and immediately washed under running water after color development. Slides were counterstained with hematoxylin at room temperature for 3 min, mounted with dibutyl phthalate xylene and were observed under a light microscope at a ×100 magnification (Zeiss AG). Particular care was taken to ensure sufficient tumor tissues or cells, in terms of quality and quantity (>100 tumor cells), were available for later mutation detection.
A total of 29 mutations in 4 exons were observed, including 3 mutations in exon 18 (G719A, G719S and G719C), 19 deletions in exon 19, 2 mutations in exon 20 (S768I and T790M), 3 insertions in exon 20 and 2 mutations in exon 21 (L858R and L861Q). The EGFR mutation status of each patient was recorded as follows: Mutant (≥1 mutation) and wild-type (no mutation).
CEA level measurement
The serum CEA level was measured within 1 week prior to the initial diagnosis of NSCLC. Venous blood (5 ml) was drawn from all the patients with NSCLC early in the morning, and specimens were then promptly sent to the clinical laboratory of the Cancer Center of the Union Hospital, Tongji Medical College, within 30 min. Serum CEA level was quantitatively measured using the Roche Cobas E601 analyzer (Roche Diagnostics), by electro-chemiluminescence immunoassay following serum separation, according to the manufacturer's protocol. After serum separation, serum CEA level was quantitatively measured using electro-chemiluminescence immunoassay (ECLIA) kits, according to the manufacturer's instructions (Roche Diagnostics). The CEA level was categorized as normal when CEA <5 ng/ml and abnormal when CEA≥5 ng/l.
Statistical analyses
Statistical analyses were performed using SPSS 19.0 software (IBM Corp.). All data are expressed as the mean ± standard deviation. Age was a continuous variable and normally distributed, while SUVmax and CEA were abnormally distributed and expressed as the median and range. The smoking status, sex and AJCC stage were categorical variables. The Mann-Whitney U test was used to make comparisons between 2 groups and the χ2 test to compare the difference between patients with EGFR-mutant and EGFR wild-type. Receiver operating characteristic (ROC) curve analysis was conducted to obtain a cut-off value for SUV and CEA. On the basis of this value, SUV and CEA were categorized as low or high. To determine the prognostic markers, multivariate analyses were performed by using the logistic regression model on the basis of SUVmax and CEA. The odd ratios, at 95% confidence intervals (CI) were calculated and the P-values were derived from two-sided tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The demographic and clinical features of the 167 patients are indicated in Table II. According to the AJCC staging, 3 patients were classified as stage I, 5 as stage II, 16 as stage III and 143 as stage IV. Among the 167 subjects, 73 (43.7%) were positive for EGFR mutation and 94 (56.3%) for EGFR wild-type. The mutation subtypes included the L858R point mutation in exon 21 (n=40; 54.8%), followed by the exon 19 deletion (n=33; 45.2%). The medians for SUVmax were as follows: Primary lesion, 9.9 (7.3-16.1); and metastatic lymph nodes, 8.1 (5.7-11.3). The median CEA value was 7.3 (3.5-43.5). Of the 167 patients, 160 presented with lymph node metastases, of which 77 had an EGFR mutation and 83 did not. Additionally, among these 160 cases, 146 patients had mediastinal lymph node metastasis, 4 had cervical lymph node metastasis, 8 had supraclavicular lymph node metastasis and 2 had retroperitoneal lymph node metastasis.
Table II.
Clinicopathological characteristics of patients.
| Characteristics | Patients, n (%) (n=167) | Wild-type, n (%) (n=94) | Mutation, n (%) (n=73) | P-value |
|---|---|---|---|---|
| Mean age ± SD, years | 58.4±10.3 | 58.3±9.8 | 58.5±10.7 | 0.904 |
| Age, years | 0.756 | |||
| ≤60 | 86 (51.5) | 49 (52.1) | 36 (49.3) | |
| >60 | 81 (48.5) | 45 (47.9) | 37 (50.7) | |
| Sex | 0.876 | |||
| Male | 87 (52.1) | 48 (51.1) | 39 (53.4) | |
| Female | 80 (47.9) | 46 (48.9) | 34 (46.6) | |
| Smoking status | <0.001 | |||
| Never smoked | 102 (61.1) | 44 (46.8) | 58 (79.5) | |
| Regular smoker | 39 (23.3) | 33 (35.1) | 10 (13.7) | |
| Ex-smoker | 26 (15.6) | 17 (18.1) | 5 (6.8) | |
| AJCC stage | 0.202 | |||
| I | 3 (1.8) | 0 (0.0) | 3 (4.1) | |
| II | 5 (3.0) | 1 (1.1) | 4 (5.5) | |
| III | 16 (9.6) | 11 (11.7) | 5 (6.8) | |
| IV | 143 (85.6) | 82 (87.2) | 61 (83.6) | |
| Histology type | <0.001 | |||
| Squamous cell carcinoma | 5 (3.0) | 4 (4.3) | 1 (1.4) | |
| Adenocarcinoma | 162 (97.0) | 90 (95.7) | 72 (98.6) | |
| Median SUVmax, primary lesion | 9.9 | 15.3 | 8.1 | <0.001 |
| SUVmax range, primary lesion | 7.3-16.1 | 9.7-19.0 | 5.1-9.8 | <0.001 |
| SUVmax≤5 | 20 (12.0) | 6 (30.0) | 14 (70.0) | |
| 5<SUVmax≤10 | 53 (31.7) | 23 (43.3) | 30 (56.6) | |
| 10<SUVmax≤15 | 40 (24.0) | 14 (35.0) | 26 (65.0) | |
| SUVmax>15 | 54 (32.3) | 51 (94.4) | 3 (5.6) | |
| Median SUVmax, metastatic lymph nodes | 8.1 | 10.1 | 6.5 | <0.001 |
| SUVmax range, metastatic lymph nodes | 5.7-11.3 | 6.9-14.5 | 3.6-8.7 | <0.001 |
| SUVmax≤5 | 32 (19.2) | 8 (25.0) | 24 (75.0) | |
| 5<SUVmax≤10 | 76 (45.5) | 39 (51.3) | 37 (48.7) | |
| SUVmax>10 | 54 (32.3) | 47 (87.0) | 7 (13.0) | |
| Median CEA, ng/ml | 7.3 | 6.0 | 12.5 | 0.001 |
| CEA range, ng/ml | 3.5-43.5 | 3.4-29.5 | 4.3-76.0 | <0.001 |
| CEA≤5 | 59 (35.3) | 41 (69.5) | 18 (30.5) | |
| 5<CEA≤10 | 31 (18.6) | 24 (77.4) | 7 (22.6) | |
| 10<CEA≤15 | 10 (6.0) | 1 (10.0) | 9 (90.0) | |
| CEA>15 | 66 (39.5) | 28 (42.4) | 38 (57.6) |
SD, standard deviation; n, number; AJCC, American Joint Committee on Cancer; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen.
Association between clinical factors and EGFR mutation status
Among 73 patients, EGFR mutations were identified in 39 male patients (53.4%) and 34 female patients (46.6%). Among the 94 EGFR-wild-type patients (56.3%), 48 were male (51.1%) and 46 were female (48.9%) (Table II). A χ2 test showed there were no significant differences in EGFR mutation proportion between sex (P=0.876) and among different age groups (P=0.904), stages (P=0.202). Adenocarcinoma histology type tended to express EGFR mutations (P<0.001). In addition, EGFR mutation was associated with decreased SUVmax levels and increased CEA levels in non-smoking subjects, compared with EGFR-wild-type (P<0.001; Table II).
Multivariate analysis of predictive factors of EGFR mutation
Univariate analysis demonstrated that the histological type was associated with EGFR mutation, and patients with adenocarcinoma exhibited a significantly increased frequency of EGFR mutations (P<0.001; Table II). This increase may be due to the unbalanced patient number in the squamous cell carcinoma and adenocarcinoma groups. The multivariate logistic regression analysis revealed that smoking status, SUVmax in primary lesions, SUVmax in metastatic lymph nodes, and CEA classification were independent predictors of EGFR mutation. Additionally, non-smoking status and the high CEA value (10–15 ng/ml) were the most significant predictors of EGFR mutation (Table III). Patients with SUVmax >15 in primary lesions and SUVmax >10 in metastatic lymph nodes were less prone to mutation (P<0.001; Table III). 18F-FDG PET/CT images, histological and immunohistochemical results in a representative patient with EGFR status are indicated in Figs. 1–3.
Table III.
Multivariate analysis for predictive factors of epidermal growth factor receptor mutation.
| Factor | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Smoking status | |||
| Never-smokeda | 1.00 | – | – |
| Ex-smoker | 0.85 | 0.07-1.97 | 0.245 |
| Regular smoker | 0.71 | 0.11-1.70 | 0.224 |
| SUVmax, primary lesion | |||
| SUVmax≤5a | 1.00 | – | – |
| 5<SUVmax≤10 | 3.68 | 0.01-1.05 | 0.055 |
| 10<SUVmax≤15 | 6.33 | 0.01-0.52 | 0.012 |
| SUVmax>15 | 19.50 | 0.00-0.02 | <0.001 |
| SUVmax, metastatic lymph nodes | |||
| SUVmax≤5a | 1.00 | – | – |
| 5<SUVmax≤10 | 0.66 | 0.06-0.88 | 0.032 |
| SUVmax>10 | 0.85 | 0.02-0.64 | 0.013 |
| CEA, ng/ml | |||
| CEA≤5a | 1.00 | – | – |
| 5<CEA≤10 | 0.73 | 1.00-1.73 | 0.227 |
| 10<CEA≤15 | 1.16 | 0.61-56.94 | 0.127 |
| CEA>15 | 0.64 | 0.66-8.03 | 0.193 |
| Histology type | |||
| Squamous cell carcinomaa | 1.00 | – | – |
| Adenocarcinoma | 3.20 | 0.35-29.26 | 0.303 |
Hazard Ratio of the factor is set to 1. CI, confidence interval; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen.
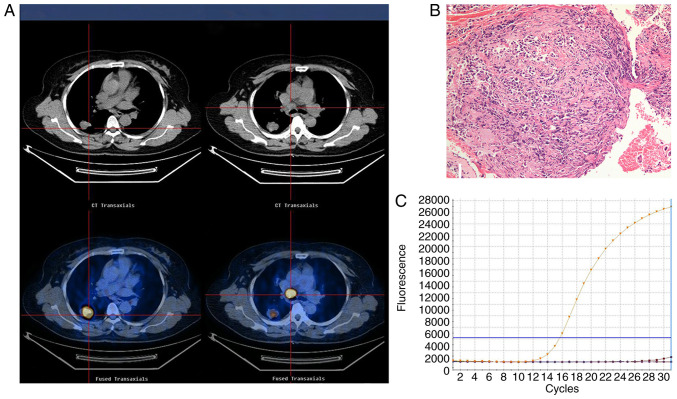
Figure 1.
Representative images of an adenocarcinoma with wild-type EGFR in a 55-year-old female who had never smoked with normal serum CEA levels (3.5 ng/ml). (A) 18F-FDG PET/CT in the axial plane and whole body maximum-intensity projection images, demonstrating abnormal FDG uptake in a left upper lobe tumor (SUVmax, 17.4) and the SUVmax of the mediastinal lymph node, 21.7. (B) Hematoxylin and eosin-stained tissue indicating adenocarcinoma features. Magnification, ×100. (C) Polymerase chain reaction confirmation of the EGFR wild-type. EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography.
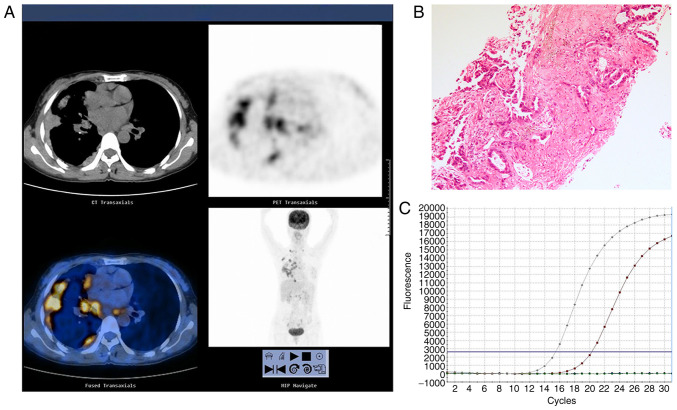
Figure 3.
Representative images of an adenocarcinoma with EGFR exon 21 mutation in a 48-year-old male who had never smoked with abnormal serum CEA levels (11.2 ng/ml). (A) 18F-FDG PET/CT in the axial plane and whole body maximum-intensity projection images, demonstrating abnormal FDG uptake in a right upper lobe tumor (SUVmax, 7.7) and the SUVmax of mediastinal lymph node was 7.9. (B) Hematoxylin and eosin-stained tissue indicating adenocarcinoma features. Magnification, ×100. (C) Polymerase chain reaction confirming the EGFR exon 21 mutation. EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography.
Association between EGFR status and serum CEA level
The median value of CEA of the EGFR wild-type group was significantly decreased compared with the EGFR mutation group (6.0 vs. 12.5; P=0.001). To evaluate whether the pre-treatment CEA level was associated with the EGFR status, patients were divided into four groups according to their pre-treatment CEA levels (CEA≤5, 5<CEA≤10, 10<CEA≤15 and CEA>15 ng/ml). A trend towards an increased incidence of EGFR mutation was observed in patients with increased CEA values (P<0.001; Table II).
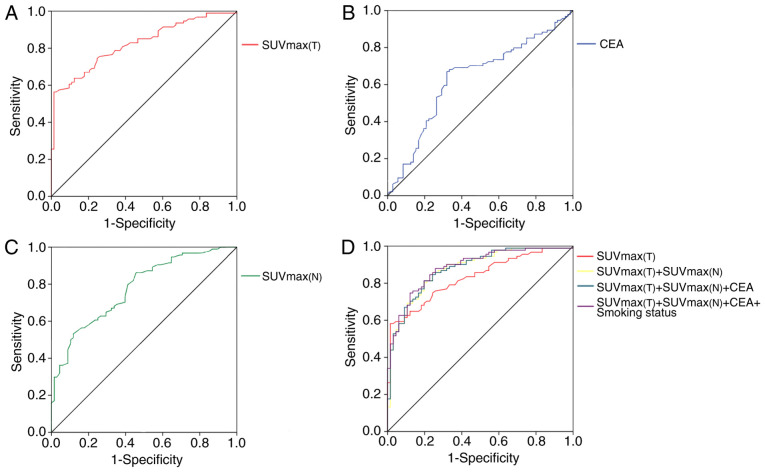
A ROC curve was analyzed to select a cut-off value for CEA level, which could be used to identify patients with an increased risk of EGFR mutations. A cut-off value of 9.6 was determined and ROC analysis of CEA levels indicated a sensitivity of 67.0%, a specificity of 68.1% and an area under the curve (AUC) of 0.632 (95% CI, 0.546–0.719) (Table IV). The frequency of EGFR mutations was increased in patients with CEA overexpression, compared with patients with decreased CEA level (40% vs. 11%; P=0.0010; Fig. 4B).
Table IV.
Comparative receiver operating characteristic analysis of predictive factors to discriminate epidermal growth factor receptor mutation.
| Predictive factors | AUC | 95% CI | Sensitivity, % | Specificity, % | P-value |
|---|---|---|---|---|---|
| SUVmax(T) | 0.830 | 0.768-0.892 | 87.7 | 63.8 | <0.001 |
| SUVmax(N) | 0.777 | 0.634-0.842 | 88.2 | 53.2 | <0.001 |
| CEA | 0.632 | 0.546-0.719 | 68.1 | 67.0 | <0.001 |
| SUVmax(T)+SUVmax(N) | 0.876 | 0.821-0.930 | 82.4 | 81.3 | <0.001 |
| SUVmax(T)+SUVmax(N)+CEA | 0.877 | 0.824-0.931 | 85.3 | 75.1 | <0.001 |
| SUVmax(T)+SUVmax(N)+CEA+smoking status | 0.886 | 0.835-0.937 | 82.1 | 80.3 | <0.001 |
SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; CI, confidence interval; AUC, area under the curve, SUVmax(T), SUVmax in primary lesions; SUVmax(N), SUVmax in metastatic lymph nodes.
Figure 4.
ROC curve analyses. (A) The sensitivity and specificity of primary lesions SUVmax for predicting the presence of EGFR mutations in patients with NSCLC. (B) Sensitivity and specificity of CEA value for predicting the presence of EGFR mutations in patients with NSCLC. (C) Sensitivity and specificity of metastatic lymph nodes SUVmax for predicting the presence of EGFR mutations in patients with NSCLC. (D) Comparative ROC curves of various factors for predicting EGFR mutation. EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; 18F-FDG PET/CT; SUVmax(T), SUVmax in primary lesions; SUVmax(N), SUVmax in metastatic lymph nodes; NSCLC, non-small cell lung cancer; ROC, receiver operating characteristic.
Association between EGFR mutation and the SUVmax in primary lesions
The median value of SUVmax in primary lesions [SUVmax(T)] was significantly increased in the EGFR wild-type group, compared with the EGFR mutant group (15.3 vs. 8.1; P<0.001). ROC curve analysis was performed to select a cut-off value for SUVmax(T), in order to identify patients with increased probability of EGFR mutations (Fig. 4A). ROC analysis indicated a cut-off value of 11.5 for SUVmax(T) with a specificity of 87.7%, a sensitivity of 63.8% and an AUC of 0.830 (95% CI, 0.768–0.892).
Association between EGFR status and metastatic lymph nodes
The median SUVmax values in metastatic lymph nodes of the EGFR wild-type group and mutation group were 10.1 and 6.5, respectively (P<0.001; Table II). The SUVmax of metastatic lymph nodes [SUVmax(N)] was a predictive value for EGFR gene mutation. ROC analysis was performed and a cut-off value of 9.8 for SUVmax(N) with specificity of 53.2%, a sensitivity of 88.2% and an AUC of 0.777 was determined (P<0.001; Table IV; Fig. 4C). When four factors which were SUVmax(T), SUVmax(N), CEA level and smoking status were all included, the AUC was increased to 0.886, compared with the AUC of primary tumor SUVmax, indicating that these factors can predict EGFR mutation status (Table IV; Fig. 4D).
The differences in CEA and SUVmax between EGFR gene mutations in exon 19 and 21
The association between each individual factor and the two types of EGFR mutation was analyzed. No significant difference was noted between the two mutation groups in terms of sex, age, smoking status, histological type or serum CEA levels. A significant difference in the SUVmax in primary lesions existed between the two groups (P=0.021; Table V). The median SUVmax was 10.6 in the EGFR exon 19 mutation group and 8.7 in the exon 21 mutation group in primary lesions.
Table V.
Association between clinical factors and epidermal growth factor receptor mutation status in exon 19 and 21.
| Characteristics | Exon 19 mutation, n (%) (n=33) | Exon 21 mutation, n (%) (n=40) | P-value |
|---|---|---|---|
| Age, years | |||
| ≤60 | 17 (51.5) | 19 (47.5) | 0.816 |
| >60 | 16 (48.5) | 21 (52.5) | |
| Sex | 0.876 | ||
| Male | 20 (60.6) | 19 (47.5) | |
| Female | 13 (39.4) | 21 (52.5) | |
| Smoking status | 0.805 | ||
| Never smoked | 26 (78.8) | 31 (77.5) | |
| Regular smoker | 7 (21.2) | 7 (17.5) | |
| Ex-smoker | 0 (0.0) | 2 (5.0) | |
| AJCC stage | 0.880 | ||
| I | 1 (3.0) | 2 (5.0) | |
| II | 2 (6.1) | 2 (5.0) | |
| III | 3 (9.1) | 2 (5.0) | |
| IV | 27 (81.8) | 34 (85.0) | |
| Histology type | 0.268 | ||
| Squamous cell carcinoma | 1 (3.0) | 0 (0.0) | |
| Adenocarcinoma | 32 (97.0) | 40 (100.0) | |
| Median SUVmax, primary lesion | 10.6 | 8.7 | 0.021 |
| SUVmax range, primary lesion | 7.2-12.7 | 5.0-10.2 | 0.057 |
| SUVmax≤5 | 4 (28.6) | 10 (71.4) | |
| 5<SUVmax≤10 | 12 (40.0) | 18 (60.0) | |
| 10<SUVmax≤15 | 14 (53.8) | 12 (46.2) | |
| SUVmax>15 | 3 (100.0) | 0 (0.0) | |
| Median SUVmax, metastatic lymph nodes | 6.7 (3.6-8.3) | 6.9 (4.0-9.5) | 0.960 |
| SUVmax range, metastatic lymph nodes | 0.920 | ||
| SUVmax≤5 | 11 (45.8) | 13 (54.2) | |
| 5<SUVmax≤10 | 15 (40.5) | 22 (59.5) | |
| SUVmax>10 | 3 (42.9) | 4 (57.1) | |
| Median CEA, ng/ml | 22.5 | 24.9 | 0.771 |
| CEA range, ng/ml | 5.6-53.0 | 4.5-91.2 | 0.780 |
| CEA≤5 | 8 (40.0) | 12 (60.0) | |
| 5<CEA≤10 | 6 (60.0) | 4 (40.0) | |
| 10<CEA≤15 | 5 (45.5) | 6 (54.5) | |
| CEA>15 | 14 (45.2) | 17 (54.8) |
SD, standard deviation; n, number; AJCC, American Joint Committee on Cancer; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen.
SUVmax and OS time
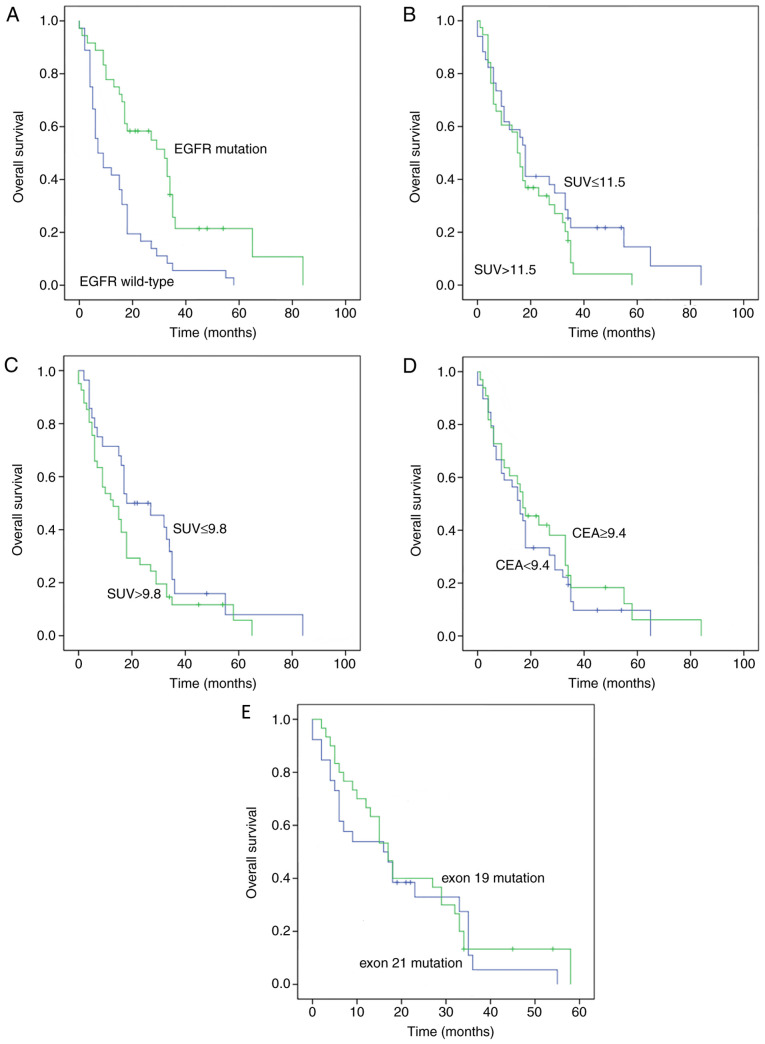
A total of 88 patients received EGFR-TKI treatment, and 73 patients developed EGFR mutations. The median OS time of all patients was 17.08 months. Patients with EGFR mutations had an increased OS time, compared with their EGFR wild-type counterparts (32.8 months vs. 7.8 months; P=0.001; Fig. 5). In terms of the SUVmax values in primary lesions (SUVmax ≤11.5 vs. SUVmax >11.5), the median OS time in the SUVmax ≤11.5 group was increased, compared with the SUVmax >11.5 group, but the difference was not statistically significant (18.6 months vs. 16.1 months; P=0.179). In terms of SUVmax values in metastatic lymph nodes (SUVmax ≤9.8 vs. SUVmax >9.8), the median OS time in the SUVmax ≤9.8 group was increased, compared with the SUVmax >9.8 group, but the difference was not significant (19.3 months vs. 13.1 months; P=0.079). The median OS time in the CEA ≤9.4 group was reduced, compared with the group with CEA level >9.4, but the difference was not statistically significant (16.4 months vs. 17.4 months; P=0.418). In terms of EGFR mutation type, the median OS time was increased in patients with the in-frame deletion in exon 19, compared with patients with exon 21 mutation (27.5 months vs. 24.3 months; P=0.532).
Figure 5.
Kaplan-Meier plot analyses. (A) OS time in terms of EGFR mutation. (B) OS time according to SUVmax in primary lesions. (C) OS time according to SUVmax in metastatic lymph nodes. (D) OS time according to CEA level. (E) OS time according to different EGFR exons. EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; OS, overall survival.
Discussion
In the present study, it was demonstrated that 18F-FDG PET/CT SUVmax and serum CEA levels prior to initial treatment were associated with EGFR mutations in patients with NSCLC. Patients with a reduced SUVmax in the primary lesions were more significantly associated with EGFR mutation, compared with the control group. The ROC analysis indicated that SUVmax serves as a predictor for EGFR mutation. Increased 18F-FDG PET/CT uptake (SUV≥11.5) may serve as a predictor of the wild-type EGFR genotype, whereas a reduced SUVmax (SUV<11.5) may be indicative of EGFR mutations. The present study demonstrated that metastatic lymph nodes in patients with EGFR mutations had significantly reduced SUVmax, compared with patients with EGFR wild-type. ROC analysis demonstrated that increased CEA levels (CEA≥9.4) were associated with EGFR gene mutation. Furthermore, multivariate analysis revealed that non-smoking status, low SUVmax of the primary lesions and high CEA levels were significantly associated with EGFR mutation status.
EGFR is a transmembrane receptor present on the cell surface (40). It has been reported that EGFR mutations occur in exon 19 and 21, and a number of studies have reported that EGFR mutation is associated with improved prognosis in TKI-treated patients (8,9,13). According to the Iressa Pan-Asian study, the objective RR was 71.2% when patients with EGFR-sensitive mutations received TKI treatment, while the RR was only 1.1% in patients with EGFR-wild-type receiving TKI treatment (8). A previous study indicated that the median OS time was prolonged to 30 months when patients with EGFR mutations received chemotherapy and TKIs, compared with 10 months in patients receiving chemotherapy alone (13). Therefore, the identification of the EGFR genotype is notable and may optimize treatment for patients with lung adenocarcinoma. However, it is sometimes difficult to obtain sufficient tumor tissues for genetic tests and, in some cases, invasive tests are not feasible. In these scenarios, non-invasive EGFR mutation detection is clinically desirable.
Previous studies reported different variations in the EGFR mutation rate according to region. Western countries have exhibited an EGFR mutation rate of 10%, while Asian countries have reported an EGFR mutation rate as high as 51.4% (41–43). Furthermore, an increased rate of EGFR mutation has been reported in non-smokers (60.7%) and females (61.1%). In the present study, it was indicated that among 167 patients with NSCLC, 73 (43.7%) exhibited EGFR mutations and 94 (56.3%) did not. The smoking status was demonstrated to be significantly associated with EGFR mutation frequency.
It was also indicated in the present study that the SUVmax of the primary lesion in 73 patients with EGFR mutation was significantly decreased (median SUVmax, 8.1), compared with the 94 patients with EGFR wild-type (median SUVmax, 15.3). ROC analysis revealed that high 18F-FDG PET/CT uptake (SUVmax ≥11.5) may serve as a predictor of the wild-type EGFR genotype. Nonetheless, the results of the present study were inconsistent with that of Putora et al (44), which reported that in 28 patients with lung adenocarcinoma, including 14 patients with EGFR mutation and 14 patients with wild-type EGFR, the mean SUVmax was 10.7 for EGFR-mutated adenocarcinoma cases and 9.9 for wild-type tumor cases. The study did not demonstrate any association between SUVmax values and EGFR mutation status. This could be due to the small size of the study. In the study by Ko et al (26), involving 132 patients with pulmonary adenocarcinoma, including 69 patients with EGFR mutation, it was reported that patients with SUVmax ≥6 had an increased probability of exhibiting EGFR mutations. In the study by Huang et al (45), which enrolled 77 patients with adenocarcinoma, including 49 patients with EGFR mutation and 28 patients with wild-type EGFR tumors, 18F-FDG PET/CT uptake was significantly increased in tumors with EGFR mutation (mean SUVmax, 10.5±4.7), compared with tumors with EGFR wild-type (mean SUVmax, 8.0±3.3). The ROC analysis of the aforementioned study indicated a cut-off value of SUVmax ≥9.5, which was predictive of EGFR mutation status. In contrast, the study by Mak et al (46) examined 100 patients with NSCLC, including 24 patients with EGFR mutations and patients with stage I–IV tumors (4 with stage IA, 2 with stage IB, 2 with stage IIIA, 5 with stage IIIB and 11 with stage IV), and demonstrated that patients with decreased SUVs had an increased probability of exhibiting EGFR mutations, compared with those with increased SUVs. Another study reported that increased SUVmax in the primary lesions was associated with EGFR wild-type, compared with their mutant counterparts (47). The multivariate analysis of the aforementioned study indicated that decreased SUVmax of the primary tumor was predictive of EGFR mutation (47). Furthermore, the ROC curve analysis of the study by Choi et al (47) identified a cut-off value of ≥5.0 to distinguish wild-type from mutant tumors. The present study demonstrated that low SUVmax (SUVmax ≤11.5) was associated with EGFR mutation and this result was in line with the data of two aforementioned studies (46,47). Additionally, the present study also demonstrated that the exon 19 mutation (median SUVmax, 10.6) was strongly associated with high SUVmax in comparison with the exon 21 mutation (median SUVmax, 8.7). However, this observation does not coincide with the results of Choi et al (47), which indicated that SUVmax is significantly decreased in the exon 19 mutation group, compared with the exon 21 mutation group.
One of the strengths of the present study was the inclusion of reliable clinical, tumor markers and imaging criteria for the prediction of EGFR mutation. In previous studies, the calculated AUC of SUVmax was 0.62–0.74 (48,49). Diagnostic efficiency of SUVmax alone has been reported to be insufficient, as Cho et al (49) indicated that the highest sensitivity of SUVmax alone was 79.3%. In the present study, ROC curve analyses were further applied to evaluate the diagnostic efficiency of SUVmax, CEA level and the combination of SUVmax, CEA level and smoking status, in order to differentiate between the EGFR mutation group and the wild-type group. In terms of EGFR mutation status prediction, the sensitivity and specificity of SUVmax, CEA level and smoking status alone did not exceed 80%. However, by combining clinical or serum factors with SUVmax to increase the AUC to 0.886, the sensitivity and specificity were >80%. It was also reported that patients with EGFR mutations had an increased OS time, compared with those with EGFR wild-type (32.8 months vs. 7.8 months; P=0.001). These observations were in accordance with those of previous studies (13,47,50–52).
The present study is different from previous studies in a number of aspects. Firstly, patients enrolled were primarily at stage III and IV of the disease, because EGFR-TKI treatment is used for late-stage tumors (12,53). Secondly, the data was analyzed in terms of different mutation types and were consistent with a previous study (54). The research of the present study demonstrated that following EGFR-TKI treatment, patients with advanced NSCLC with exon 19 deletion had an increased OS time, compared with those with L858R mutation of exon 21. Thirdly, the present data was collected from mainland China, in which EGFR mutation rate has been reported to be 43.7%, in contrast to previous studies conducted in the Taiwan region of China or Korea where a ~20% EGFR mutation rate in adenocarcinoma has been reported (26,45,46). The data of the present study were consistent with a previous study, which indicated that in 1,482 patients from Asian countries, the EGFR mutation rate was ~51.4% (41). Lastly, it was indicated that the SUVmax of primary pulmonary lesions and metastatic lymph nodes in mediastinal, supraclavicular regions and pelvic cavity was decreased in the EGFR mutation group, compared with the EGFR wild-type group. It has been reported that inter-tumor heterogeneity in EGFR mutations is a potential explanation for this phenomenon (55).
Serum CEA is frequently reported to be overexpressed in patients with NSCLC, particularly in adenocarcinoma cases. Additionally, patients with adenocarcinoma exhibit significantly increased mutation rates of EGFR, compared with their non-adenocarcinoma counterparts (56,57). The present study also revealed that patients with increased-serum CEA levels (≥12.5 ng/ml) at initial diagnosis were the ideal patient population for EGFR-TKI therapy, because this population was indicated to have an increased inhibitor-sensitive mutation rate (58). In a study involving 113 Chinese patients with adenocarcinoma, including 59 with EGFR mutations and 54 EGFR wild-type tumors, CEA level was significantly increased in tumors with EGFR mutations, compared with tumors with EGFR wild-type (55).
In the present study, the results were categorized by type of EGFR mutation and it was indicated that the mean SUVmax was significantly increased in the exon 19 group, compared with the exon 21 group. ROC analysis also demonstrated that increased CEA levels (CEA ≥9.4) were associated with EGFR gene mutation. A limitation of the present study was that it was of retrospective design, therefore selection bias was unavoidable and further investigation is required. Furthermore, indexes of SUVmax and CEA levels cannot replace conventional EGFR-mutation detection when adequate tumor tissue is available for DNA analysis. In conclusion, the present study indicated that in patients with advanced NSCLC, particularly Chinese patients, a decreased SUVmax and an increased CEA level are associated with EGFR mutation and may serve as predictors for responsiveness to EGFR-TKI therapy.
Figure 2.
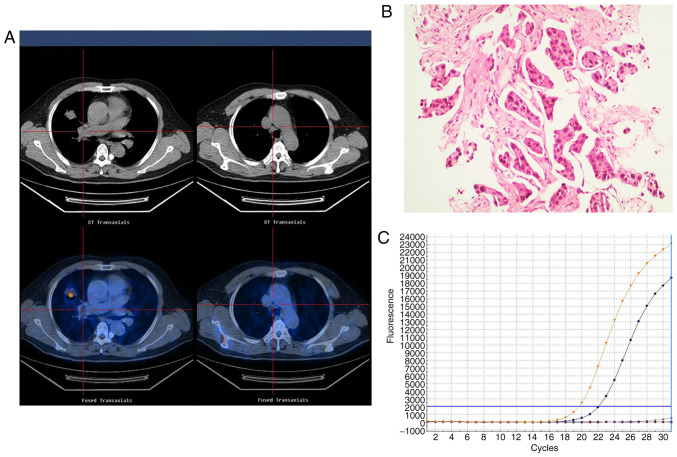
Representative images of an adenocarcinoma with EGFR exon 19 deletion in a 61-year-old male who had never smoked with abnormal serum CEA levels (9.8 ng/ml). (A) 18F-FDG PET/CT in the axial plane and whole body maximum-intensity projection images, demonstrating abnormal FDG uptake in a right upper lobe tumor (SUVmax, 10.6) and the SUVmax of mediastinal lymph node was 4.5. (B) Hematoxylin and eosin-stained tissue indicating adenocarcinoma features. Magnification, ×100. (C) Polymerase chain reaction confirming the EGFR exon 19 deletion. EGFR, epidermal growth factor receptor; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography-computed tomography.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature Science Foundation of China (grant nos. 30800283 and 81172595), the Postdoctoral foundation of China (grant no. 20100480905) and by the Postdoctoral special foundation of China (grant no. 201104440).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XG and CW analyzed the patient data and wrote the manuscript. RZ made substantial contributions to quality control of the study, and analyzed and described the figures. QC, YH and FT acquired the data and were involved in drafting the manuscript. JD and GW interpreted the data. XD conceived and designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, HUST (China). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S. Epidemiology of lung cancer in China. Thorac Cancer. 2015;6:209–215. doi: 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Akerley W, Bepler G, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Feigenberg SJ, Figlin RA, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2008;6:228–269. doi: 10.6004/jnccn.2008.0021. [DOI] [PubMed] [Google Scholar]
- 4.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Bria E, Milella M, Cuppone F, Novello S, Ceribelli A, Vaccaro V, Sperduti I, Gelibter A, Scagliotti GV, Cognetti F, Giannarelli D. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: A meta-analysis. Ann Oncol. 2011;22:2277–2285. doi: 10.1093/annonc/mdq742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loong HH, Kwan SS, Mok TS, Lau YM. Therapeutic strategies in EGFR mutant non-small cell lung cancer. Curr Treat Options Oncol. 2018;19:58. doi: 10.1007/s11864-018-0570-9. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al. First-SIGNAL: First-line single-agent iressa versus gemcitabine and cisplatin trial in non-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 10.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. The Lancet. Oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet. Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 14.Xue C, Hu Z, Jiang W, Zhao Y, Xu F, Huang Y, Zhao H, Wu J, Zhang Y, Zhao L, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77:371–375. doi: 10.1016/j.lungcan.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Jänne PA, Riely GJ, Ruiz MG, Giaccone G, Sequist LV, Johnson BE. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: Results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiala O, Pesek M, Finek J, Benesova L, Minarik M, Bortlicek Z, Topolcan O. Predictive role of CEA and CYFRA 21-1 in patients with advanced-stage NSCLC treated with erlotinib. Anticancer Res. 2014;34:3205–3210. [PubMed] [Google Scholar]
- 19.Yang ZM, Ding XP, Pen L, Mei L, Liu T. Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J Cancer Prev. 2014;15:3451–3455. doi: 10.7314/APJCP.2014.15.8.3451. [DOI] [PubMed] [Google Scholar]
- 20.Qin HF, Qu LL, Liu H, Wang SS, Gao HJ. Serum CEA level change and its significance before and after Gefitinib therapy on patients with advanced non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:4205–4208. doi: 10.7314/APJCP.2013.14.7.4205. [DOI] [PubMed] [Google Scholar]
- 21.Muley T, Dienemann H, Ebert W. CYFRA 21-1 and CEA are independent prognostic factors in 153 operated stage I NSCLC patients. Anticancer Res. 2004;24:1953–1956. [PubMed] [Google Scholar]
- 22.Barlesi F, Gimenez C, Torre JP, Doddoli C, Mancini J, Greillier L, Roux F, Kleisbauer JP. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med. 2004;98:357–362. doi: 10.1016/j.rmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Molina R, Filella X, Auge JM, Fuentes R, Bover I, Rifa J, Moreno V, Canals E, Viñolas N, Marquez A, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Bio. 2003;24:209–218. doi: 10.1159/000074432. [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Shimizu T, Ayabe T, Onitsuka T. Maximum SUV on positron emission tomography and serum CEA level as prognostic factors after curative resection for non-small cell lung cancer. Asia Pac J Clin Oncol. 2012;8:244–247. doi: 10.1111/j.1743-7563.2012.01549.x. [DOI] [PubMed] [Google Scholar]
- 25.Chiu CH, Shih YN, Tsai CM, Liou JL, Chen YM, Perng RP. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer. 2007;57:213–221. doi: 10.1016/j.lungcan.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Ko KH, Hsu HH, Huang TW, Gao HW, Shen DH, Chang WC, Hsu YC, Chang TH, Chu CM, Ho CL, Chang H. Value of 18F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging. 2014;41:1889–1897. doi: 10.1007/s00259-014-2802-y. [DOI] [PubMed] [Google Scholar]
- 27.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, von Schulthess GK, Steinert HC. Staging of nonsmall-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, Putnam JB, Herbst RS, Moran CA, Podoloff DA, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Onco. 2005;23:1136–1143. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 29.Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon JE, II, Patz EF., Jr Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 2008;26:1459–1464. doi: 10.1200/JCO.2007.14.3628. [DOI] [PubMed] [Google Scholar]
- 30.Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil Diaz C, Lopez-Picazo JM, Gurpide A, Zulueta JJ, Richter Echevarria JA, Perez Gracia JL. Role of [18F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:2058–2065. doi: 10.1007/s00259-014-2833-4. [DOI] [PubMed] [Google Scholar]
- 31.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 32.Lan XL, Zhang YX, Wu ZJ, Jia Q, Wei H, Gao ZR. The value of dual time point (18)F-FDG PET imaging for the differentiation between malignant and benign lesions. Clin Radiol. 2008;63:756–764. doi: 10.1016/j.crad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Chiu E, Rosenberg J, Gambhir SS. Standardized uptake value atlas: Characterization of physiological 2-deoxy-2-[18F]fluoro-D-glucose uptake in normal tissues. Mol Imaging Biol. 2007;9:83–90. doi: 10.1007/s11307-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 34.Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, Oguchi M, Shikata H, Taki S, Tonami H, Katsuda S, Yamamoto I. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: Correlation with histopathological features. Nucl Med Commun. 2000;21:707–714. doi: 10.1097/00006231-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, Wood DE. Lung cancer proliferation correlates with [F-18] fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–3844. [PubMed] [Google Scholar]
- 36.Song JY, Lee YN, Kim YS, Kim SG, Jin SJ, Park JM, Choi GS, Chung JC, Lee MH, Cho YH, et al. Predictability of preoperative 18F-FDG PET for histopathological differentiation and early recurrence of primary malignant intrahepatic tumors. Nucl Med Commun. 2015;36:319–327. doi: 10.1097/MNM.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 37.Ahn SJ, Park MS, Lee JD, Kang WJ. Correlation between 18F-fluorodeoxyglucose positron emission tomography and pathologic differentiation in pancreatic cancer. Ann Nucl Med. 2014;28:430–435. doi: 10.1007/s12149-014-0833-x. [DOI] [PubMed] [Google Scholar]
- 38.Purandare NC, Puranik A, Shah S, Agrawal A, Gupta T, Moiyadi A, Shetty P, Shridhar E, Jalali R, Rangarajan V. Common malignant brain tumors: Can 18F-FDG PET/CT aid in differentiation? Nucl Med Commun. 2017;38:1109–1116. doi: 10.1097/MNM.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 44.Putora PM, Fruh M, Muller J. FDG-PET SUV-max values do not correlate with epidermal growth factor receptor mutation status in lung adenocarcinoma. Respirology. 2013;18:734–735. doi: 10.1111/resp.12083. [DOI] [PubMed] [Google Scholar]
- 45.Huang CT, Yen RF, Cheng MF, Hsu YC, Wei PF, Tsai YJ, Tsai MF, Shih JY, Yang CH, Yang PC. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol. 2010;27:9–15. doi: 10.1007/s12032-008-9160-1. [DOI] [PubMed] [Google Scholar]
- 46.Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JA, Choi NC, Sequist LV. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist. 2011;16:319–326. doi: 10.1634/theoncologist.2010-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi YJ, Cho BC, Jeong YH, Seo HJ, Kim HJ, Cho A, Lee JH, Yun M, Jeon TJ, Lee JD, Kang WJ. Correlation between (18F)-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in advanced lung cancer. Nuclear Medicine Molecular Imaging. 2012;46:169–175. doi: 10.1007/s13139-012-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee EY, Khong PL, Lee VH, Qian W, Yu X, Wong MP. Metabolic phenotype of stage IV lung adenocarcinoma: Relationship with epidermal growth factor receptor mutation. Clin Nucl Med. 2015;40:e190–e195. doi: 10.1097/RLU.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 49.Cho A, Hur J, Moon YW, Hong SR, Suh YJ, Kim YJ, Im DJ, Hong YJ, Lee HJ, Kim YJ, et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer. 2016;16:224. doi: 10.1186/s12885-016-2251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang XT, Li LY, Mu XL, Cui QC, Chang XY, Song W, Wang SL, Wang MZ, Zhong W, Zhang L. The EGFR mutation and its correlation with response of gefitinib in previously treated Chinese patients with advanced non-small-cell lung cancer. Ann Oncol. 2005;16:1334–1342. doi: 10.1093/annonc/mdi340. [DOI] [PubMed] [Google Scholar]
- 51.Faehling M, Achenbach J, Staib P, Steffen U, Tessen HW, Gaillard VE, Brugger W. Erlotinib in routine clinical practice for first-line maintenance therapy in patients with advanced non-small cell lung cancer (NSCLC) J Cancer Res Clin Oncol. 2018;144:1375–1383. doi: 10.1007/s00432-018-2649-x. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi K, Hagiwara K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC) Target Oncol. 2013;8:27–33. doi: 10.1007/s11523-013-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Jänne PA, Joshi VA, McCollum D, Evans TL, Muzikansky A, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 54.Chen ZY, Zhong WZ, Zhang XC, Su J, Yang XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist. 2012;17:978–985. doi: 10.1634/theoncologist.2011-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang WT, Li Y, Ma J, Chen XB, Qin JJ. Serum carcinoembryonic antigen levels before initial treatment are associated with EGFR mutations and EML4- ALK fusion gene in lung adenocarcinoma patients. Asian Pac J Cancer Prev. 2014;15:3927–3932. doi: 10.7314/APJCP.2014.15.9.3927. [DOI] [PubMed] [Google Scholar]
- 56.Vincent RG, Chu TM, Fergen TB, Ostrander M. Carcinoembryonic antigen in 228 patients with carcinoma of the lung. Cancer. 1975;36:2069–2076. doi: 10.1002/cncr.2820360923. [DOI] [PubMed] [Google Scholar]
- 57.Vincent RG, Chu TM, Lane WW. The value of carcinoembryonic antigen in patients with carcinoma of the lung. Cancer. 1979;44:685–691. doi: 10.1002/1097-0142(197908)44:2<685::AID-CNCR2820440241>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Yang ZM, Ding XP, Pen L, Mei L, Liu T. Analysis of CEA expression and EGFR mutation status in non-small cell lung cancers. Asian Pac J Cancer Prev. 2014;15:3451–3455. doi: 10.7314/APJCP.2014.15.8.3451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.