Abstract
The current coronavirus disease 2019 (COVID‐19) pandemic is associated with a heavy burden on the mental and physical health of patients, regional healthcare resources, and global economic activity. Many patients with lung cancer are thought to be affected by this situation. Therefore, in this study, we aimed to evaluate the impact of COVID‐19 pandemic on lung cancer treatment scheduling. We retrospectively reviewed the medical records of lung cancer patients who were undergoing anticancer treatment at the National Hospital Organization Kyoto Medical Center (600 beds) in Kyoto, Japan, between 1 March 2020 and 31 May 2020. After the medical records were reviewed, the patients were assigned to one of two groups, depending on whether their lung cancer treatment schedule was delayed. We assessed the characteristics, types of histopathology and treatment, and the reason for the delay. A total 15 (9.1%) patients experienced a delay in lung cancer treatment during the COVID‐19 pandemic. Patients with a treatment delay received significantly more immune checkpoint inhibitor (ICI) monotherapy than patients without a treatment delay (P = 0.0057). On the contrary, no patients receiving molecular targeted agents experienced a treatment delay during the COVID‐19 pandemic period (P = 0.0027). The treatments of most of the patients were delayed at their request. We determined that 9.1% lung cancer patients suffered anxiety and requested a treatment delay during the COVID‐19 pandemic. Oncologists should bear in mind that patients with cancer have more anxiety than expected under unprecedented circumstances such as the COVID‐19 pandemic.
Keywords: COVID‐19, lung cancer, pandemic, SARS‐CoV‐2, treatment delay
We found that the lung cancer treatments of 9.1% of patients included in the study were delayed during the COVID‐19 pandemic. However, the treatments of most of these patients were delayed at their request. Oncologists should bear in mind that cancer patients tend to have more anxiety than expected under special circumstances such as the COVID‐19 pandemic.
Introduction
By the middle of July 2020, coronavirus disease 2019 (COVID‐19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was reported to have affected more than 15 000 000 people globally. 1 , 2 COVID‐19 is known to place a heavy burden on the mental and physical health of patients, regional healthcare resources, and global economic activity. In Japan, the government officially declared a state of emergency on 7 April 2020. 3 Daily coverage of the COVID‐19 pandemic has been negatively affecting the mental health of patients. Considering that patients with cancer may suffer worse respiratory outcomes than those without, 4 , 5 this pandemic is presumed to affect the daily routines of cancer patients and oncologists. Therefore, in this study, we aimed to evaluate the impact of the COVID‐19 pandemic on lung cancer treatment scheduling.
Methods
Patients
The period of the COVID‐19 pandemic has been defined as the time between 1 March 2020 and 31 May 2020. We retrospectively reviewed the medical records of lung cancer patients who were undergoing anticancer treatment at the National Hospital Organization Kyoto Medical Center (600 beds) in Kyoto, Japan, between 1 March 2020 and 31 May 2020. The review criteria were as follows: (i) pathologically confirmed lung cancer patients; (ii) patients who received treatment with cytotoxic chemotherapy ± immune checkpoint inhibitors (ICIs), ICI monotherapy, or molecular targeted agents; and (iii) patients who received treatment during the COVID‐19 pandemic period. After the medical records were reviewed, the patients were assigned to one of two groups, depending on whether their lung cancer treatment schedule was delayed. We assessed the characteristics, types of histopathology and treatment, and the reason for the delay.
The medical records of 165 patients who met the inclusion criteria were reviewed. The characteristics of the patients are summarized in Table 1. The patients were predominantly male (mean age: 70.2 ± 9.2 years). Regarding lung cancer type, the highest proportion of patients had adenocarcinoma, followed by squamous cell carcinoma, small‐cell lung cancer, and a type that was not otherwise specified. Of the 165 patients, 133 (80.6%) received their treatment at the outpatient clinic; 33 (20.0%), 76 (46.1%), and 56 (33.9%) patients had received cytotoxic chemotherapy ± ICIs, ICI monotherapy, and molecular targeted agents, respectively. This retrospective study was approved by the relevant institutional review board (approval number: 20‐022).
Table 1.
Characteristics of lung cancer patients undergoing treatment during the COVID‐19 pandemic period
| All patients | Patients with treatment delay | Patients without treatment delay | P‐value | |
|---|---|---|---|---|
| (n = 165) | (n = 15) | (n = 150) | ||
| Age | 70.2 ± 9.2 | 72.5 ± 9.2 | 70.0 ± 9.2 | 0.28 |
| Sex (female) | 62 (37.6) | 3 (20.0) | 59 (39.3) | 0.17 |
| Outpatient treatment | 136 (82.4) | 11 (73.3) | 125 (83.3) | 0.30 |
| Histopathology | ||||
| Adenocarcinoma | 99 (60.0) | 6 (40.0) | 93 (62.0) | 0.11 |
| Squamous cell carcinoma | 32 (19.4) | 5 (33.3) | 27 (18.0) | 0.17 |
| Small cell lung cancer | 18 (10.9) | 1 (6.7) | 17 (11.3) | >0.99 |
| Not otherwise specified | 15 (9.1) | 3 (20.0) | 12 (8.0) | 0.14 |
| Pleomorphic carcinoma | 1 (0.61) | 0 (0.0) | 1 (0.67) | >0.99 |
| Type of treatment | ||||
| Cytotoxic chemotherapy ± ICIs | 33 (20.0) | 3 (20.0) | 30 (20.0) | >0.99 |
| ICI monotherapy | 76 (46.1) | 12 (80.0) | 64 (42.7) | 0.0057 |
| Molecular targeted agents | 56 (33.9) | 0 (0.0) | 56 (37.3) | 0.0027 |
| Reason for treatment delay | ||||
| Doctor request | 2 (1.2) | 2 (13.3) | — | NE |
| Patient request | 12 (7.3) | 12 (80.0) | — | NE |
| Family request | 1 (0.61) | 1 (6.7) | — | NE |
Data are shown as number (%) or mean ± SD.
ICIs, immune checkpoint inhibitors; NE, not evaluated.
Statistical analysis
A chi‐square test or Fisher's exact test was applied to compare the categorical variables. In addition, the Mann‐Whitney U test was applied for the continuous variables. The condition for statistical significance was defined as a P‐value below 0.05. Statistical analyses were performed using SPSS v26.0 (IBM SPSS, Inc., Chicago, IL, USA).
Results
We reviewed the medical records of 165 patients who had undergone lung cancer treatment during the study period. The lung cancer treatments of a total of 15 patients (9.1%) were delayed during the COVID‐19 pandemic. Patients with delayed treatment received significantly more ICI monotherapy than patients without delayed treatment (P = 0.0057). By contrast, no patients who received molecular targeted agents experienced a treatment delay during the COVID‐19 pandemic period (P = 0.0027). Figure 1 shows the proportion of patients whose treatment was delayed according to the type of treatment. Of the 76 patients who received ICI monotherapy, 12 patients (15.8%) experienced a treatment delay. However, the treatments of most of the patients were delayed at their request. In addition, the doctors of two patients recommended delayed treatment, and one patient was advised by their family to delay treatment.
Figure 1.
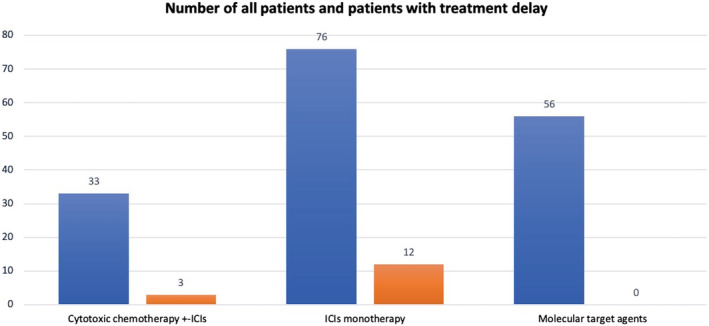
Proportion of patients who delayed treatment according to treatment type. The blue bar indicates the total number patients who received each type of treatment. The red bar indicates patients associated with delayed treatment.  All patients,
All patients,  Patients with treatment delay.
Patients with treatment delay.
Discussion
Here, we revealed that the lung cancer treatment schedules of 9.1% of lung cancer patients were altered during the COVID‐19 pandemic. In most cases, a treatment delay was requested by the patient, suggesting that lung cancer patients had more COVID‐19‐related anxiety than expected. Several studies have indicated that, compared to individuals with no history of cancer, cancer patients have a higher risk of death if they are infected with SARS‐CoV‐2 and develop COVID‐19. 4 , 5 In addition to host traits, hospital admission and recurrent hospital visits are also potential risk factors for SARS‐CoV‐2 infection. 6 Because the media has widely disseminated this information, many cancer patients are believed to experience anxiety regarding COVID‐19. Although several countries have advocated suggestions for cancer treatment during the COVID‐19 pandemic advocated from several countries, 7 , 8 a consensus has not been reached; thus, there is no generalized standard for cancer treatment during this pandemic.
In our study, among the patients receiving one of three types of treatment agents, a higher proportion of patients who received ICI monotherapy delayed their treatment.
Recently, an expert advocated weight‐based dosing of pembrolizumab. 9 According to their recommendation, patients should receive a 400 mg dose every six weeks instead of a 200 mg dose every three weeks. This can reduce the number hospital visits and lower the risk of infection. Various science‐based plans and ideas such as this will be required during a pandemic period. Furthermore, a recent study suggested that immunotherapy with ICIs might lead to exacerbation of COVID‐19 disease in patients with cancer. 10 Postponement of immunotherapy with ICIs is also another option in certain cancer patients.
This study had several limitations. First, this study was retrospectively conducted in a single center, which caused selection bias. Specifically, the proportion of delayed treatment cases is dependent on the severity of the epidemic in an area. Kyoto has been deemed to be a mild epidemic area. Thus, it is expected that areas where the severity of the COVID‐19 pandemic is higher will be associated with more patient requests for treatment delay. Second, because the COVID‐19 pandemic is unprecedented and ongoing, the study period may have been underestimated. Lastly, the effects of treatment delay on the prognosis of lung cancer are unknown. Nevertheless, our study provides insight into the COVID‐19 pandemic impact on cancer treatment.
In conclusion, our study revealed that 9.1% of lung cancer patients suffered anxiety and requested a treatment delay during the COVID‐19 pandemic. Therefore, oncologists should bear in mind that cancer patients tend to have more anxiety than expected under unprecedented circumstances such as the COVID‐19 pandemic.
Disclosure
All authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank Drs Yuki Yamamoto, Misato Okamura, and Akihiro Yasoda for their helpful feedback on this work.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19) situation reports. 2020. [Cited 23 Jul 2020.] Available from URL: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/.
- 2. Johns Hopkins University and Medicine . Coronavirus Resource Center. 2020. [Cited 23 Jul 2020.] Available from URL: https://coronavirus.jhu.edu/map.html. Accessed at July 23, 2020. [Google Scholar]
- 3. Ministry of Health, Labour and Welfare of Japan . Coronavirus Diseases (COVID‐19) situation. 2020. [Cited 23 Jul 2020.] Available from URL: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/newpage_00032.html.
- 4. Tian J, Yuan X, Xiao J et al Clinical characteristics and risk factors associated with COVID‐19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol 2020; 21: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang W, Guan W, Chen R e a. Cancer patients in SARS‐CoV‐2 infection: A nationwide analysis in China. Lancet Oncol 2020; 21: 335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu J, Ouyang W, Chua MLK, Xie C. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020; 6: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouyang W, Hu J, Zhang H, Xie C. The management of patients with lung cancer during the outbreak of coronavirus disease 2019. J Thorac Oncol 2020; 15: e106–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mauri D, Kamposioras K, Tolia M, Alongi F, Tzachanis D, International Oncology Panel and European Cancer Patient Coalition Collaborators . Summary of international recommendations in 23 languages for patients with cancer during the COVID‐19 pandemic. Lancet Oncol 2020; 21: 759–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein DA, Ratain MJ, Saltz LB. Weight‐based dosing of pembrolizumab every 6 weeks in the time of COVID‐19. JAMA Oncol 2020. May 27. 10.1001/jamaoncol.2020.2493 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Robilotti EV, Babady NE, Mead PA et al Determinants of COVID‐19 disease severity in patients with cancer. Nat Med 2020. Jun 24. 10.1038/s41591-020-0979-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


