Abstract
Since its eruption in China, novel coronavirus disease (COVID‐19) has been reported in most of the countries and territories (>200) of the world with ∼18 million confirmed cases (as of August 3, 2020). In most of the countries, COVID‐19 upsurge is uncontrolled with a significant mortality rate. Currently, no treatment effective for COVID‐19 is available in the form of vaccines or antiviral drugs and patients are currently treated symptomatically. Although the majority of the patients develop mild symptoms and recover without mechanical ventilation for respiratory management, severe respiratory illness develops in a significant portion of affected patients and may result in death. While the scientific community is working to develop vaccines and drugs against the COVID‐19 pandemic, novel alternative therapies may reduce the mortality rate. Recent use of stem cells for critically ill COVID‐19 patients in a small group of patients in China and subsequent Emergency Use Authorization of stem cells by Food and Drug Administration to Global Institute of Stem Cell Therapy and Research and Athersys has created excitement among the medical community. As a result, several clinical trials have been registered using stem cells for COVID‐19 treatment that aim to use different cell sources, dosage, and importantly diverse targeted patient groups. In this brief review, the possibilities of stem cell use in COVID‐19 patients and relevant challenges in their use have been discussed.
Keywords: acellular therapy, coronavirus, COVID‐19, mesenchymal stem cells, pandemic, stem cells
Abbreviations
- ARDS
severe acute respiratory distress syndrome
- AT‐MSCs
adipose tissue‐derived mesenchymal stem cells
- CB‐MSCs
cord blood‐derived mesenchymal stem cells
- COVID‐19
novel coronavirus disease
- CT‐MSCs
cord tissue‐derived mesenchymal stem cells
- DCs
dendritic cells
- EUA
Emergency Use Authorization
- GvHD
graft vs host disease
- IFN‐γ
interferon‐γ
- IL
interleukin
- MSCs
mesenchymal stem cells
- TGF‐β
transforming growth factor β
1. INTRODUCTION
Novel coronavirus disease (COVID‐19) is proliferating quickly worldwide and had been announced pandemic by the World Health Organization on March 11, 2020. Severe acute respiratory syndrome coronavirus (SARS‐CoV)‐19, the virus responsible for COVID‐19 is an enveloped, positive sense, single‐stranded RNA virus of the family Coronaviridae (Zhou, Yang, & Wang, 2020). It causes mild respiratory tract infection, fever, and cough in most of the patients. However, in a significant portion of the affected patients, these symptoms are accompanied by pneumonia and severe acute respiratory distress syndrome (ARDS). As a result, COVID‐19 is currently associated with a high mortality rate (∼697,700 deaths as of August 3, 2020). With over ∼6.06 million active cases of COVID‐19 (as of August 3, 2020; https://www.worldometers.info/coronavirus/), there is currently no vaccine or other antiviral treatment available. The affected patients are only left with symptomatic management of disease because the frantic search for an effective COVID‐19 treatment is not much successful. Drugs such as remdesivir, lopinavir/ritonavir, interferon β1 (IFN‐β1; H. Li, Wang, Xu, & Cao, 2020), convalescent plasma (Shen, Wang, & Zhao, 2020), and Food and Drug Administration (FDA) approved hydroxychloroquine (Cohen, 2020) are although under investigation but their safety and potential efficacy remain to be determined. Considering the extensive and continuous increase in patient numbers and resulting in substantial deaths, novel therapeutic strategies are required to reduce the mortality rate and to make the recovery better. Stem cell‐based regenerative medicine therapy may be an option for COVID‐19 patients (Leng, Zhu, & Hou, 2020; Liang et al., 2020). Stem cell‐based regenerative therapies were initiated recently in China (Leng et al., 2020; Liang et al., 2020) and approval of Emergency Use Authorization (EUA) of stem cell use by FDA for COVID‐19 patients has created an excitement among the medical community.
Mesenchymal stem cells (MSCs), a type of adult stem cells are found in various autologous and allogenic sources and have high proliferative potential and multilinage differentiation capacity. Previous studies have shown that the immunomodulatory properties of MSCs help in modulation of proliferation, activation, and function of various immune cells (Harrell, Sadikot, & Pascual, 2019) and thus are able to alter the innate and adoptive immune responses (S. Liu, Peng, & Qiu, 2020). MSCs were used previously for the treatment of graft vs host diseases (GvHDs) as well as for the treatment of other virus‐associated diseases such as immunologic abnormality in human immunodeficiency virus, chronic hepatitis in hepatitis B virus, and acute lung injury in influenza virus (Maytawan, Suradej, & Arunee, 2015). The immunomodulatory characteristics of MSCs indicate that MSCs can be used as a supportive treatment option for better recovery of critically ill COVID‐19 patients (Leng et al., 2020; Liang et al., 2020). The use of stem cells in COVID‐19 patients in China at the beginning of the pandemic suggests possible benefits for patients. Similarly, the FDA has recently granted EUA of stem cells for COVID‐19 patients. Considering initial promising results in a small group of critically ill COVID‐19 patients, a number of clinical trials have been registered using MSCs (Tables 1, 2, 3). These stem cell‐based trials for COVID‐19 will evaluate different sources, numbers, and patient groups for treatment. It is therefore imperative to understand the logic, associated mechanisms, and challenges for a successful stem cell therapy for COVID‐19 patients. As there is limited available data regarding stem cell use for COVID‐19 patients, the review has discussed a number of relevant implications imperative for understanding the logic, associated mechanisms, and relevant problems for a successful stem cell therapy for COVID‐19. The discussion of some relevant challenges and the stem cell‐based acellular therapies will be of particular interest for readers.
Table 1.
Current clinical trials “Recruiting” COVID‐19 patients for stem cells‐based therapies
| Trial number/identification | Condition or disease | Cell type used as an intervention | Phase |
|---|---|---|---|
| NCT04313322 | COVID‐19 | WJ‐MSCs | I |
| NCT04416139 | COVID‐19 | MSCs | II |
| NCT04336254 | COVID‐19 | Allogeneic human dental pulp stem cells | I |
| NCT04366323 | SARS‐CoV‐2 | Allogeneic and expanded adipose tissue‐derived MSCs | I/II |
| NCT04252118 | COVID‐19 | UC‐MSCs | I |
| NCT04366063 | COVID‐19 | MSCs | II, III |
| NCT04339660 | COVID‐19 | UC‐MSCs | I, II |
| NCT04392778 | COVID‐19, pneumonia, multiple organ failure | MSCs | I, II |
| NCT04355728 | COVID‐19, ARDS | UC‐MSCs | I, II |
| NCT04390139 | COVID‐19, ARDS | WJ‐MSCs | I, II |
| NCT04389450 | COVID‐19, ARDS | Allogeneic expanded placental mesenchymal‐like adherent stromal cells | II |
| NCT03042143 | ARDS | Human UC‐CD362 enriched MSCs | I/II |
| NCT04361942 | COVID‐19 pneumonia | MSCs | II |
| NCT04269525 | Viral pneumonia, ventilator‐associated | UC‐MSCs | II |
| NCT04333368 | SARS‐CoV‐2, ARDS | WJ‐MSCs | I, II |
| NCT04288102 | COVID‐19 | MSCs | II |
| ChiCTR2000030835 | Coronavirus disease 19 | WJ‐MSCs | I |
| ChiCTR2000030866 | Coronavirus disease 19 | UC‐MSCs | 0 |
| ChiCTR2000030300 | Coronavirus disease 19 infection, pulmonary fibrosis, viral pneumonia | UC‐MSCs | I |
| ChiCTR2000029990 | Coronavirus disease 19 infection, viral pneumonia | hMSCs | I/II |
| ChiCTR2000030020 | Coronavirus disease 19 infection, viral pneumonia | MSCs | II |
| ChiCTR2000029569, ChiCTR2000029572 | Coronavirus infection, pneumonia | UC‐MSCs | 0 |
| ChiCTR2000029580 | Coronavirus infection, pneumonia | MSCs | 0 |
| ChiCTR2000029606 | Coronavirus infection, pneumonia | Human menstrual blood‐derived stem cells | 0 |
| ChiCTR2000030835 | Coronavirus disease 19 infection, viral pneumonia | MSCs | NA |
| ChiCTR2000030866 | Coronavirus disease 19 infection, viral pneumonia | UC‐MSCs | 0 |
Abbreviations: ARDS, severe acute respiratory distress syndrome; COVID‐19, novel coronavirus disease; hMSC, human mesenchymal stem cell; MSC, mesenchymal stem cell; NA, not applicable; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; UC‐MSC, umbilical cord‐derived mesenchymal stem cell; WJ‐MSC, Wharton's jelly‐derived mesenchymal stem cell.
Table 2.
Current clinical trials “Enrolling” COVID‐19 patients for stem cells‐based therapies
| Trial number/identification | Condition or disease | Cell type used as an intervention | Phase |
|---|---|---|---|
| NCT04348435 | COVID‐19 | Allogeneic AdMSCs | II |
| NCT04382547 | COVID‐19, viral pneumonia | Allogenic‐pooled‐olfactory mucosa‐derived MSCs | I/II |
| NCT04349631 | COVID‐19 | AT‐MSCs (AdMSCs; autologous) | II |
Abbreviations: AdMSC, adipose‐derived mesenchymal stem cell; AT‐MSC, adipose tissue‐derived mesenchymal stem cell; COVID‐19, novel coronavirus disease; MSC, mesenchymal stem cell.
Table 3.
Current registered clinical trials “Not yet Recruiting” COVID‐19 patients for stem cells‐based therapies
| Trial number/identification | Condition or disease | Cell type used as intervention | Phase |
|---|---|---|---|
| NCT04428801 | COVID‐19 | Autologous adipose‐derived stem cells | II |
| NCT04302519 | COVID‐19 | Dental pulp‐MSCs | Early phase I |
| NCT04429763 | COVID‐19 | UC‐MSCs | II |
| NCT04315987 | COVID‐19 pneumonia | MSCs | II |
| NCT04349540 | COVID‐19 | Allogeneic hematopoietic stem cells | NA |
| NCT04390152 | Acute respiratory distress syndrome | WJ‐MSCs | I, II |
| NCT04348461 | COVID, respiratory distress syndrome | Allogeneic and expanded adipose tissue‐derived MSCs | II |
| NCT04371601 | COVID‐19 pneumonia | UC‐MSCs | I |
| NCT04362189 | COVID‐19 pneumonia | Allogeneic AT‐MSCs | II |
| NCT04393415 | COVID‐19 | Cord blood (CB) stem cells | NA |
| NCT04397796 | COVID | Allogenic BM‐MSCs | I |
| NCT04377334 | COVID, ARDS | Allogeneic BM‐MSCs | II |
| NCT04400032 | COVID‐19, ARDS | BM‐MSCs | I |
| NCT04398303 | COVID‐19 pneumonia | Allogenic human umbilical‐derived MSCs and allogenic human umbilical‐derived MSCs conditioned medium | I, II |
| NCT04345601 | COVID‐19, ARDS | BM‐MSCs | Early phase I |
| NCT04299152 | SARS pneumonia | CB stem cells | II |
| NCT04346368 | COVID‐19 | BM‐MSCs | I, II |
| NCT04273646 | COVID‐19, novel coronavirus pneumonia | UC‐MSCs | NA |
| ChiCTR2000030224 | Coronavirus disease 19 | MSC | II |
| ChiCTR2000030173 | Coronavirus disease 19 | UC‐MSCs | 0 |
| ChiCTR2000030261 | Coronavirus disease 19 infection, viral pneumonia | MSCs‐derived exosomes | 0 |
| ChiCTR2000030116 | Coronavirus disease 19 infection, respiratory distress syndrome, viral pneumonia | UC‐MSCs | II |
| ChiCTR2000030138 | Coronavirus disease 19 infection, viral pneumonia | UC‐MSCs | II |
| ChiCTR2000030088 | Coronavirus disease 19 infection, viral pneumonia | WJ‐MSCs | 0 |
| ChiCTR2000029816, ChiCTR2000029817 | Coronavirus disease 19 infection, pneumonia | CB‐NK cells + CB‐MSCs | 0 |
| NCT04276987 | Coronavirus | MSCs‐derived exosomes | I |
Abbreviations: ARDS, severe acute respiratory distress syndrome; AT‐MSC, adipose tissue‐derived mesenchymal stem cell; BM‐MSC, bone‐marrow‐derived mesenchymal stem cell; COVID‐19, novel coronavirus disease; MSC, mesenchymal stem cell; NK, natural killer; NA, not applicable; SARS, severe acute respiratory syndrome; UC‐MSC, umbilical cord‐derived mesenchymal stem cell; WJ‐MSC, Wharton's jelly‐derived mesenchymal stem cell.
2. POTENTIAL GROUPS OF COVID‐19 PATIENTS FOR STEM CELL THERAPY
Three groups of COVID‐19 patients, that is, (a) critically ill young patients, (b) critically ill older patients, and (c) those patients who are at high risk of infection due to other comorbidities, are potential candidates for stem cell‐based therapies. Current COVID‐19 data (https://www.worldometers.info/coronavirus/) indicates that a substantial portion (∼2%) of coronavirus‐affected people develops severe or critical symptoms. The inclusion criteria for critically ill COVID‐19 patients include respiratory rate ≥30 times/min, pulse oxygen saturation at rest ≤93%, the partial pressure of PaO2/FiO2 ≤ 300 mmHg, a requirement for mechanical ventilation and shock (S. Liu et al., 2020). Such critically ill COVID‐19 patients may require alternative therapeutic treatment options for better recovery. According to recent reports, stem cells administered in a small group of severely affected COVID‐19 patients showed beneficial effects (Leng et al., 2020; Liang et al., 2020). All patients in these studies showed improvement in lung function after stem cell administration (please see Section 5 for detail).
Older COVID‐19 patients with comorbidities such as diabetes, asthma, and heart diseases are also in danger as the highest morbidity was observed in this group. The immune system and regenerative potential of these patients are compromised with advancing age and disease, resulting in this segment of the population being badly hit by a coronavirus. Due to a compromised immune system in such patients, antibody production takes a longer time to fight the coronavirus and the patients often develop critical conditions of pneumonia and ARDS. Stem cell therapy may be particularly useful for such patients because the chances of recovery in such patients are significantly low. It is pertinent to mention that stem cell administration in this group of patients may also improve underlying morbidities.
3. POSSIBLE STEM CELL SOURCES AND TYPES FOR COVID‐19 PATIENTS
Stem cells are unspecialized cells in the body that have the potential to make more stem cells as well as differentiate into specialized cells of the body if appropriate signals are given in vitro or in vivo. Embryonic stem cells (ESCs) can be isolated from the inner cell mass of 5–8 days old embryos and possess high regenerative potential. However, the clinical use of ESCs is restricted due to a number of religious, ethical, and legal controversies. Adult stem cells can be isolated from neonatal sources (such as cord blood, cord tissue, placenta, and menstrual blood) as well as from adult tissues (such as bone marrow, adipose tissue, dental pulp, and peripheral blood) are used for these purposes. MSCs, a type of adult stem cells, are a special focus of stem cell‐related therapies currently due to their immunomodulatory and regenerative potential (Leng et al., 2020; Golchin, Seyedjafari, & Ardeshirylajimi, 2020). MSCs can be obtained in large numbers from autologous sources such as adipose tissue, bone marrow, and from allogenic sources such as cord blood and cord tissues. In addition, MSCs are multipotent, could be cryopreserved for multiple uses and thus are readily available at the time of care (Choudhery, Badowski, Muise, Pierce, & Harris, 2014).
Previously published clinical data of stem cell use against virus‐induced acute respiratory distress syndrome (ARDS) provides a hint for the success of stem cell‐based therapies for COVID‐19 infection. The selection of a suitable stem cell source and type is important for the treatment of COVID‐19 patients. The median time from first symptoms to death in COVID‐19 infection is ∼14 days (Lauer, Grantz, & Bi, 2020). This time period provides a narrow window of treatment opportunity and therefore starting therapy at an appropriate time is important especially for patients who are older and have other illnesses. However, no data is currently available regarding the time of the start of MSC therapy for maximum benefits; therefore, studies are required to evaluate the optimal period of the start of cell‐based therapies. Current registered clinical trials are primarily focused on the use of stem cells obtained from allogenic sources such as donated Wharton's jelly or umbilical cord tissues (Tables 1, 2, 3). Autologous stem cells are often preferred for cell‐based therapies. However, for active COVID‐19 patients, aspiration of adipose tissue or bone marrow and subsequent isolation of cells from these tissues does not seem to readily amenable to this purpose. A recent study by Rogers, Harman, and Bunnell (2020) provided some rationale for the clinical use of autologous adipose tissue‐derived MSCs (AT‐MSCs) for COVID‐19 patients. However, additional injury from aspiration of these tissues for AT‐MSC isolation makes this approach less desirable. Therefore, for active, severely ill patients, allogenic cell sources (being readily available) seems a better option for treatment. For COVID‐19 patients who are at high risk of developing a severe disease (older patients with comorbidities) due to COVID‐19 infection, autologous sources of stem cells such as adipose tissue and bone marrow could be used. For such patients, the time to initiate therapy is very important in order to boost the immune system. The biobanks, however, can play an important role in this regard by preserving the adipose tissue and bone marrow and making these tissues or cells available for patients at the time of care. It is pertinent to mention here that the use of stem cell therapy in critical patients in the intensive care unit (ICU) is not yet approved, therefore; precautions must be taken to ensure the safety of the patients.
A majority of registered stem cell clinical trials to treat COVID‐19 have proposed the use of MSCs as a treatment modality for such COVID‐19 patients. MSCs are a well‐characterized type of adult stem cells with ideal proliferative, differentiation, and immunomodulatory properties. MSCs are also without ethical issues and are available for allogenic or autologous use. MSCs regenerate and repair damaged tissues by transdifferentiation or secretion of various bioactive molecules to stimulate resident cells. MSCs are well known in the medical community for its promising anti‐inflammatory properties. Previously, few studies related to the use of stem cells to treat respiratory virus‐related lung injury have been conducted. Although reports are conflicting, the systemic administration of MSCs was protective for influenza respiratory infections (Khoury et al., 2020). Interestingly, studies found a greater protective effect of MSCs derived from bone marrow as compared to MSCs derived from umbilical cord tissues in influenza A infection (Loy, Kuok, & Hui, 2019).
4. WHY STEM CELLS COULD BE AN EFFECTIVE TREATMENT OPTION FOR COVID‐19 PATIENTS
Most COVID‐19 patients do not develop any major clinical symptoms during the early stages of infection. Common symptoms include mild or high temperature, cough, sore throat, muscle distress, and body pain. In a few patients, shortness of breath can lead to a sudden deterioration in the health of the patient during the later stages of the disease. In severe cases, immune system dysfunction is the major cause of death in patients as infection stimulates inflammatory cytokines that result in the respiratory system being overwhelmed by a storm of inflammatory cytokines such as interleukin 2 (IL‐2), IL‐6, granulocyte colony stimulating factor, IP10, MCP1, MIP1A, and tumor necrosis factor (TNF; Huang, Wang, & Li, 2020). In COVID‐19, the immune system seems unable to turn itself off and produces an excessive quantity of cytokines, thus producing an inimical environment for the infection (Figure 1). Such an unchecked inflammation caused by this cytokine storm compromises lung function and patients have difficulty in breathing and eventually die. The cytokine storm can lead to organ failure followed by edema, secondary infection, cardiac damage, and ARDS. MSCs are thought to balance the immune system and stop its overactivation. Such a balance of the immune system is very important, as complete shutting down of the immune system will affect the infection‐fighting ability of patients.
Figure 1.
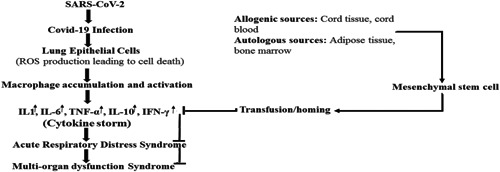
Cytokine storm modulation by mesenchymal stem cells. COVID‐19, novel coronavirus disease; IFN‐γ, interferon‐γ; IL, interleukin; ROS, reactive oxygen species; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TNF‐α, tumor necrosis factor α
Control of COVID‐19‐induced cytokine storm during infection can save patients, as shown by anecdotal reports of successful treatment of patients with anti‐IL‐6 receptor monoclonal antibodies. MSCs possess powerful anti‐inflammatory and immunomodulatory properties and therefore can also control cytokine storms by inhibiting overactivation of the immune system and by improving endogenous repair of injured tissues (Leng et al., 2020). MSCs possess special immunoregulatory properties that enable these cells to modulate the functions of various immune cells (Harrell et al., 2019).
MSCs are also able to alter the innate and adoptive immune responses (S. Liu et al., 2020). Previous studies indicated that MSCs can induce mature dendritic cells (DCs) into novel Jagged‐2 dependent regulatory DCs. These regulatory DCs not only play an important role in immune homeostasis but are immunosuppressive (Zhang et al., 2009; X. Liu et al., 2012). In one of the initial MSC‐based clinical studies for COVID‐19, it was found that the number of regulatory DC was increased significantly after MSC transplantation (Leng et al., 2020). Furthermore, MSC transplantation leads to decreased TNF‐α levels and increased IL‐10 levels in critically ill COVID‐19 patients in the MSC treatment group compared to the placebo control group.
The immunomodulatory properties of MSCs are due to low levels of MHC class 1 antigen concurrent with the release of IFN‐γ, indoleamine 2,3‐dioxygenase, transforming growth factor β, IL‐6, IL‐10, and prostaglandin E2 (Aggarwal & Pittenger, 2005; Jiang, Zhang, & Liu, 2005; Corcione, Benvenuto, & Ferretti, 2006; Spaggiari, Capobianco, Becchetti, Mingari, & Moretta, 2006; Rasmusson, Uhlin, Blanc, & Levitsky, 2007). With these properties MSCs can inhibit the differentiation of monocytes into DCs, can increase the ratio of regulatory cytokines to inflammatory cytokines, and can inhibit antibody production by B cells and proliferation of the natural killer cells.
The safety and effectiveness of MSCs in life‐threatening immune‐mediated inflammatory diseases such as GvHDs and systemic lupus erythematosus have previously been documented through various clinical trials. An overall increase in survival of GvHD patients was observed after MSC administration (Morata‐Tarifa, Macias‐Sanchez, Gutierrez‐Pizarraya, & Sanchez‐Pernaute, 2020). Furthermore, the efficacy of MSC has also been documented for the treatment of ARDS induced by H9N2 avian influenza viruses, and H5N1 infections in mice and humans (Chan et al., 2016; Y. Li et al., 2016).
If MSCs can reduce the incidence and severity of other virus‐related diseases (Maytawan et al, 2015), they might prevent overactivation of the immune system (Baron and Storb, 2012; Wei et al., 2013), lower levels of inflammatory substances and regenerate the damaged tissues in COVID‐19 patients. Systemic administration of MSCs results in homing to the pulmonary vascular bed where they release soluble factors such as anti‐inflammatory cytokines, antimicrobial peptides, angiogenic growth factors, and extracellular vesicles (Khoury et al., 2020) and thus could improve the pulmonary microenvironment, protect alveolar epithelial cells, prevent pulmonary fibrosis, and improve overall lung function (Leng et al., 2020). In addition, the repair of immune and respiratory epithelial cells can also occur by direct transfer of mitochondria from MSCs (Swati, Rituparna, Anurag, & Sujata, 2018). Previous studies have shown that mitochondrial transfer from MSCs can repair the tubular epithelial cells in diabetic nephropathy (Naoto, Kanna, Shin, & Mineko, 2019).
Angiotensin‐converting enzyme 2 (ACE2) is the main receptor for SARS‐COV‐2 (the virus that causes COVID‐19) expressed by many types of human cells such as alveolar type 2 cells and capillary epithelium cells. SARS‐COV‐2 infects these cells but not bone marrow, lymph nodes, thymus, spleen, and immune cells (such as T and B lymphocytes and macrophages) being negative for ACE2 (Hamming, Timens, & Bulthuis, 2004). This receptor plays an important role for the entry of SARS‐COV‐2 into these cells (Khoury et al., 2020). Recognition of the ACE2 receptor by viral spike protein is the first step in SARS‐COV‐2 pathogenesis. Another cellular serine protease TMPRSS2 is essential for the entry of the COVID‐19 virus into host cells (Hoffmann et al., 2020). Interestingly, a recent study indicated that MSCs are negative for ACE2 and TMRSS2 and thus could have a natural resistance to SARS‐COV‐2 (Leng et al., 2020). Previously published clinical data of MSC use for virus‐induced ARDS provides a hint for the success of stem cell‐based therapies for COVID‐19. Overall, the immunomodulatory and regenerative properties of MSCs can help in patient recovery by modulating the immune system and by repairing the damaged tissue in the lungs. Promising results of preclinical studies suggest MSCs are safe and effective therapeutic agents for a number of diseases and disorders. Based on recent promising results of MSC use in COVID‐19 patients in Chinese studies (Leng et al., 2020; Liang et al., 2020) and overall safety and effectiveness of its use in several American and European clinical trials, the FDA had granted permission of compassionate use of MSCs.
5. CURRENT STEM CELL‐BASED CLINICAL TRIALS FOR COVID‐19 PATIENTS
Although many countries including the USA, China, Italy, UK, France, Spain, Germany, Turkey, Iran, Brazil, and Jordon have proposed stem cell use for COVID‐19 patients, only a limited number of stem cell‐based studies are available currently to deduce solid limitations or potential of such therapy for COVID‐19. A study recently conducted in Beijing's YouAn Hospital, China, from January 23, 2020 to February 16, 2020 suggests a possible role of MSCs administration in COVID‐19 treatment (Leng et al., 2020). This single‐center open‐labeled study was performed on seven COVID‐19 patients of different degrees of severity (critically severe [n = 1], severe [n = 4], and common type [n = 2]). A single dose of MSCs (106 cells/kg body weight) was administered and patients were followed for 14 days. No adverse events in terms of allergic reactions or secondary infections were reported after MSC administration. Most importantly, pulmonary function and symptoms were significantly improved in all patients 2 days after MSC injection. In this study, treatment with MSCs increased the number of peripheral lymphocytes, decreased C‐reactive protein (CRP) levels, and numbers of overactivated cytokine secreting immune cells declined. Similarly, TNF‐α levels significantly decreased concurrently with an IL‐10 increase in patients treated with MSC as compared to the control group. All patients in the stem cell‐treated group survived but not in the control group. Overall, this study demonstrated the potential efficacy and safety of stem cell infusion in all seven patients.
In another case report, (Liang et al., 2020) human umbilical cord tissue‐derived MSCs (CT‐MSCs) were infused in a critically ill COVID‐19 patient. The 65 years old female patient had increased neutrophils, decreased numbers of lymphocytes, and was confirmed positive for SARS‐CoV‐2. Although the patient was treated with the antiviral drugs lopinavir/ritonavir, IFN‐α, and iv injection of moxifloxacin, methylprednisolone, and immunoglobulin, the patient developed a spastic cough, severe diarrhea, and electrolyte disturbance and was later diagnosed critically ill and was shifted to ICU and an invasive tracheal cannula was performed to decrease the respiratory distress. In addition, the noninvasive mechanical ventilator was also used to combat hypoxia and respiratory muscle fatigue of the patient. Stem cell therapy was proposed by the medical team and the patient was given three infusions of 5 × 107 CT‐MSCs 3 days apart. The patient responded to the MSC administration and within a week pneumonia was greatly relieved. The patient was able to be transferred from the ICU and further clinical investigation showed normal levels of laboratory indexes such as creatinine level, albumin, alanine aminotransferase, CRP, procalcitonin, d‐dimer. In addition, computed tomography images indicated relief in both the left and right lungs.
In another study, Sanchez‐Guijo et al. (2020) determined the safety and potential use of AT‐MSCs. In this study, 13 COVID‐19 adult patients under invasive mechanical ventilation were enrolled. All the patients previously received antiviral and/or anti‐inflammatory treatments including steroids, lopinavir/ritonavir, hydroxychloroquine and/or tocilizumab, and so forth. Ten out of thirteen patients received two doses (3 days after the first dose) of allogenic AT‐MSCs. The remaining two patients were given a single dose while the third patient received three doses of allogenic AT‐MSCs. Each dose contained 0.98 × 106 AT‐MSC/kg of the recipient's body weight. The study observed no cell therapy‐related adverse effects. While 70% of the patients exhibited clinical improvement and discharged from ICU, four patients remained intubated and two patients died. MSC therapy was related to a decrease in CRP, IL‐6, ferritin, lactate dehydrogenase, and d‐dimer and an increase in lymphocytes. The study indicates that the administration of AT‐MSCs is safe and can improve clinical outcomes in COVID‐19 patients.
Currently registered clinical trials (Tables 1, 2, 3) on the NIH US National Library of Medicine (Clinicaltrials.gov) and on the Chinese Clinical Trial Registry (http://www.chictr.org.cn/abouten.aspx) are using MSCs derived from various tissues such as CT‐MSCs, bone‐marrow‐derived MSCs, dental pulp‐derived MSCs, umbilical cord‐derived MSCs, AT‐MSCs, Wharton's jelly‐derived MSCs, and cord blood‐derived MSCs. Most of these trials have used MSCs from allogenic sources in order to provide the cells at the time of care. Some of these clinical trials aim to use MSC‐derived products such as exosomes (ChiCTR2000030261, NCT04276987). Overall, these registered trials aim to evaluate the safety and effectiveness of stem cell use for the treatment of COVID‐19.
6. MSC‐BASED ACELLULAR THERAPIES FOR COVID‐19
It has been shown that there are multiple mechanisms through which MSCs can exert their immunomodulatory effects. It was previously thought that MSCs promoted lung regeneration through engraftment and transdifferentiation. However, later studies supported the paracrine role of MSCs in lung regeneration. Although clinical findings suggest MSCs as promising candidates for the treatment of a number of diseases including ARDS, the safety, cell viability, and regulatory issues raises concerns about their use in patients.
Stem cell‐based acellular therapies are gaining significant attention by avoiding the controversies raised by stem cell‐based clinical use. It has been shown that MSCs exert their beneficial effects primarily by paracrine mechanisms in which they release extracellular vesicles such as microvesicles and exosomes. Exosomes contain a variety of chemokines, growth factors, messenger RNA, and microRNA. These products of extracellular vesicles have anti‐inflammatory and immunomodulatory properties and thus function as regulators of the immune system (Chen, Lai, & Lee, 2010; Lai, Tan, & The, 2012). In addition, exosomes also possess a tremendous regenerative potential for repair and regeneration of damaged organs and tissues. Interestingly, preclinical studies have shown encouraging effects of exosomes in animal models of ARDS and other respiratory and inflammatory diseases (Katsha et al., 2011; Lee, Park, & Lee, 2019; Tang et al., 2017; Zhu et al., 2014). Exosome injections in these studies showed reduced alveolar inflammation, enhanced edema clearance, and restoration of leaky epithelial membranes. These findings support a possible role for exosomes in managing the cytokine storm caused by inflammation.
In a recent nonrandomized, open‐label cohort study, 24 moderate to severe COVID‐19 patients were enrolled in a study that aimed to evaluate the safety and efficacy of allogenic bone marrow MSC‐derived exosomes (Sengupta et al., 2020). All of the enrolled patients were confirmed for SARS‐CoV‐2 infection by polymerase chain reaction testing. All patients received a single dose of exosomes (15 ml; please see Sengupta et al. [2020] for experimental details) and followed for 2 weeks to evaluate its safety and efficacy. No adverse effects related to exosome transplantation were observed in the study. In addition, in 83% of patients, the clinical status and oxygenation level was improved. Furthermore, absolute neutrophil counts and lymphocyte counts were significantly increased concurrently with a decrease in acute phase reactants such as CRP, ferritin, and d‐dimer reduction. Overall, the study suggested that allogenic bone marrow MSC‐derived exosomes are safe, can restore oxygenation, downregulate cytokine storms, and reconstitute immunity in severe COVID‐19 patients.
Currently, three studies using exosomes for COVID‐19 treatment have been registered on clinicaltrials.gov. One of these studies (NCT04276987) aims to utilize MSCs derived from allogenic adipose tissue and the other two studies, NCT04384445 and NCT04276987, have proposed the use of human amniotic fluid‐derived exosomes and T‐cell‐derived exosomes, respectively.
7. CHALLENGES
Despite hundreds of registered clinical trials and thousands of published papers, stem cell treatment remains controversial and currently is not FDA approved for most diseases and disorders. The FDA has only granted compassionate use for stem cell therapy and the ability to use an EUA outside of a clinical trial may be revoked at any time. The compassionate permission to use a drug refers to the use of an unproved new drug for severely ill patients in an emergency situation when no other effective drugs are available. According to the recent statement of the International Society for Stem Cell Research, “although stem cells are promising candidates for various diseases and disorders, currently there is no stem cell‐based approved approach for prevention or treatment of COVID‐19 infection” (ISSCR, 2020). Although the results of recent clinical studies for COVID‐19 are encouraging (Leng et al., 2020; Liang et al., 2020), but on a very small number of patients and without appropriate controls and therefore solid conclusions cannot be drawn. In addition, the treated groups were given stem cells in conjunction with conventional therapy and therefore it is questionable if the effect on patients is due to administered stem cells. Proper randomization, larger sample size, proper control groups with longer follow up in multicenter studies are therefore required to properly assess the effectiveness and safety of stem cell use for COVID‐19.
Due to the promising results of preclinical studies stem cell‐based therapeutics have a special interest for incurable diseases. However, these significant developments in the stem cell field also face the issues of immunogenicity and limited cell numbers. The clinical use of MSCs from autologous sources is the best approach in terms of safety and function, however, the production of clinically relevant number of stem cells requires a substantial amount of time which is not always feasible in an emergency situation like the current COVID‐19 emergency. As shown in Tables 1, 2, 3, most of the registered clinical trials for COVID‐19 have proposed the use of allogenic stem cell sources (e.g., placenta‐derived tissues). The use of allogenic stem cells in severe conditions (in the ICU) has not been proven through clinical studies. However, most of the COVID‐19 patients that the registered clinical trials aim to treat will be critically ill, often requiring mechanical ventilation for respiratory problems. Therefore, precautions must be taken while using the stem cells for such patients. Obtaining the required number of cells in a brief time span for treating the critically ill COVID‐19 patients is another challenge. In vitro expansion is time‐consuming and may reduce the functional potential of the expanded cells.
For different groups of patients, different sources of stem cells could be used. For critically ill older patients with comorbidities, readily available allogenic sources of cells seems the best option, however, for other patients at risk of developing COVID‐19 autologous sources such as adipose tissue could be used. For the isolation of clinical‐grade MSCs, harvested tissues must be processed in Good Manufacturing Practice (GMP) Compliant facilities. The availability of such GMP compliant cell processing facilities and the resultant supply of clinical‐grade MSCs is a major challenge for a number of countries, especially the developing and underdeveloped countries.
Rapid preparation of optimal numbers of clinical‐grade MSCs and provision at the time of care is another major challenge for stem cell‐based therapies. MSCs are scarce in primary tissues and therefore in vitro expansion of these cells is required to obtain the hundreds of millions of cells to be used as a therapeutic dose. Such cellular expansion in culture requires several weeks. Managing the time, the cost, GMP grade reagents, and proper quality testing in current lockdown conditions is another challenge. In addition, genomic stability and regenerative potential of expanded MSCs may be compromised and raises another concern about the safety of the expanded MSC used clinically.
Most registered clinical trials have proposed the use of MSCs due to their immunomodulatory activities. Use of MSCs in immunocompromised COVID‐19 patients, however, raises a concern regarding a greater risk of viral and other infections in such patients. Since the immunosuppressive effect of MSCs is nonspecific, both the alloantigen and viral antigen responses may be repressed leading to detrimental clinical outcomes. In addition, stem cell therapies are expensive and most people may not be able to afford it. Will it be possible for federal governments to offer such therapies for all patients if such therapies prove affective. Are institutes prepared to provide the required number of cells for such patients? It is only possible if stem cell banks start collecting the cells from allogenic tissues (neonatal/birth‐related tissues such as placenta, cord blood, cord tissue). However, the selection of donors during a pandemic is also challenging especially in countries where the COVID‐19 infection rate is high.
In the absence of proper control groups, the assessment of the safety of MSC treatment is difficult. The preparation of MSCs in laboratories compliant with FDA standards is of paramount importance. Along with the donors, the scientific staff should be strictly screened for the disease. The cells must be checked for any type of contamination and viability before use in patients. The issue of cell dose, cell number, route of administration, and cell passage number must be controlled for maximum efficacy and safety of cell use in patients.
8. LIMITATIONS
COVID‐19 is a new disease and there is limited information regarding its pathology and effective treatment. Due to the current prevalence of the disease, the relevant information regarding infection and mortality rate changes each day, and therefore the information regarding the total number of cases and the mortality rate described in this article may be changed in the near future. Currently, only a few studies have evaluated the use of MSCs for COVID‐19 patients and therefore we have discussed the results of limited studies in this review. Because of the absence of solid data from controlled, randomized, multicenter trials, confirmed conclusions about stem cell‐based therapies for COVID‐19 cannot be drawn. Although theoretically, MSCs could be used in COVID‐19 patients without the development of severe adverse effects, most of the information in this review is about critically ill COVID‐19 patients. Currently, MSCs‐based therapies are being used as an emergency protocol for the management of cytokine storm in critically ill COVID‐19 patients,. However, in the near future, such options may be expanded as a preventive remedy especially for elderly patients and patients who also have comorbidities. Due to limited available data, although the information regarding MSC‐based therapies is limited in this review article we have highlighted the arguments relevant to MSC use for COVID‐19.
9. CONCLUSIONS
Stem cells seem to have significant potential to treat COVID‐19. Such treatments may decrease the burden on the health system of countries by curing the patients quickly. Due to exponential increase in the number of deaths due to COVID‐19, scientists seem inclined to test any available interventions such as stem cell therapy. MSCs may possibly be a good option for COVID‐19 patients, as stem cells are readily available in large numbers from different tissues, MSC can be cryopreserved until needed, and MSC characteristics and potential have been studied widely in preclinical and clinical studies. However, there are certain apprehensions that must be addressed before starting such a therapy, and during and after the therapy. Furthermore, there is a need for discussion of results obtained from such trials in a rapid manner so that the FDA might fast‐track stem cell therapy as an approved emergency therapy for COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Choudhery MS, Harris DT. Stem cell therapy for COVID‐19: Possibilities and challenges. Cell Biol Int. 2020;44:2182–2191. 10.1002/cbin.11440
REFERENCES
- Aggarwal, S. , & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822. [DOI] [PubMed] [Google Scholar]
- Baron, F. , & Storb, R. (2012). Mesenchymal stromal cells: A new tool against graft‐versus‐host disease? Biology of Blood and Marrow Transplantation, 18(6), 822–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. C. , Kuok, D. I. , Leung, C. Y. , Hui, K. P. , Valkenburg, S. A. , Lau, E. H. , … Lee, J. W. (2016). Human mesenchymal stromal cells reduce influenza A H5N1‐associated acute lung injury in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 113, 3621–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. S. , Lai, R. C. , & Lee, M. M. (2010). Mesenchymal stem cell secretes microparticles enriched in pre‐microRNAs. Nucleic Acids Research, 38(1), 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery, M. S. , Badowski, M. , Muise, A. , Pierce, J. , & Harris, D. T. (2014). Donor age negatively impacts adipose tissue‐derived mesenchymal stem cell expansion and differentiation. Journal of Translational Medicine, 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. S. (2020). Hydroxychloroquine for the prevention of COVID‐19—Searching for evidence. New England Journal of Medicine, 383, 2020–586. 10.1056/NEJMe2020388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione, A. F. , Benvenuto, E. , & Ferretti. (2006). Human mesenchymal stem cells modulate B‐cell functions. Blood, 107(1), 367–372. [DOI] [PubMed] [Google Scholar]
- Golchin, A. , Seyedjafari, E. , & Ardeshirylajimi, A. (2020). Mesenchymal stem cell therapy for COVID‐19: Present or future. Stem Cell Reviews and Reports, 16(3), 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming, I. , Timens, W. , & Bulthuis, M. L. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology, 203(2), 631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, C. R. , Sadikot, R. , & Pascual, J. (2019). Mesenchymal stem cell‐based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells International, 2019, 4236973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , … Pöhlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , & Li, X. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSCR (2020). ISSCR statement regarding the marketing of unproven stem cell treatments for COVID‐19. Retrieved from https://www.isscr.org/news-publicationsss/isscr-news-articles/article-listing/2020/03/06/isscr-statement-regarding-the-marketing-of-unproven-stem-cell-treatments-for-covid-19
- Jiang, X. X. , Zhang, Y. , & Liu, B. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood, 105(10), 4120–4126. [DOI] [PubMed] [Google Scholar]
- Katsha, A. , Ohkouchi, S. , Xin, H. , Kanehira, M. , Sun, R. , Nukiwa, T. , & Saijo, Y. (2011). Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase‐induced emphysema model. Molecular Therapy, 19, 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury, M. , Cuenca, J. , Cruz, F. F. , Figueroa, F. E. , Rocco, P. R. M. , & Weiss, D. J. (2020). Current status of cell‐based therapies for respiratory virus infections. European Respiratory Journal, 55(6), 2000858. 10.1183/13993003.00858-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, R. C. , Tan, S. S. , & The, B. J. (2012). Proteolytic potential of the MSC exosome proteome: Implications for an exosome‐mediated delivery of therapeutic proteasome. International Journal of Proteomics, 2012, 971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer, S. A. , Grantz, K. H. , & Bi, Q. (2020). The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: Estimation and application. Annals of Internal Medicine, 172, 577–582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Park, J. , & Lee, J. W. (2019). Therapeutic use of mesenchymal stem cell‐derived extracellular vesicles in acute lung injury. Transfusion, 59(S1), 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng, Z. , Zhu, R. , & Hou, W. (2020). Transplantation of ACE2− mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging and Disease, 11(2), 216–228. 10.14336/AD.2020.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Wang, Y. M. , Xu, J. Y. , & Cao, B. (2020). Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jiehe He Huxi Zazhi, 43(3), 170–172. 10.3760/cma.j.issn.1001-0939.2020.03.004 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Xu, J. , Shi, W. , Chen, C. , Shao, Y. , Zhu, L. , … Han, X. (2016). Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus‐induced acute lung injury in mice. Stem Cell Research & Therapy, 7(159), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, B. , Chen, J. , Li, T. , Wu, H. , Yang, W. , Li, Y. , … Hu, M. (2020). Clinical remission of a critically ill COVID‐19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine, 99(31), e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Peng, D. , & Qiu, H. (2020). Mesenchymal stem cells as a potential therapy for COVID‐19. Stem Cell Research & Therapy, 11, 169. 10.1186/s13287-020-01678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Qu, X. , Chen, Y. , Liao, L. , Cheng, K. , Shao, C. , … Zhao, R. C. (2012). Mesenchymal stem/stromal cells induce the generation of novel IL‐10‐dependent regulatory dendritic cells by SOCS3 activation. Journal of Immunology, 189, 1182–1192. [DOI] [PubMed] [Google Scholar]
- Loy, H. , Kuok, D. I. T. , & Hui, K. P. Y. (2019). Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus‐associated acute lung injury. Journal of Infectious Diseases, 219, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytawan, T. , Suradej, H. , & Arunee, T. (2015). Mesenchymal stromal cells and viral infection. Stem Cells International, 2015, 8. 10.1155/2015/860950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata‐Tarifa, C. , Macias‐Sanchez, M. D. M. , Gutierrez‐Pizarraya, A. , & Sanchez‐Pernaute, R. (2020). Mesenchymal stromal cells for the prophylaxis and treatment of graft‐versus‐host disease‐a meta‐analysis. Stem Cell Research & Therapy, 11(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoto, K. , Kanna, N. , Shin, K. , & Mineko, F. (2019). Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Scientific Reports, 9(1), 5184. 10.1038/s41598-019-40163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson, I. M. , Uhlin, K. , Blanc, Le , & Levitsky, V. (2007). Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. Journal of Leukocyte Biology, 82(4), 887–893. [DOI] [PubMed] [Google Scholar]
- Rogers, C. J. , Harman, R. J. , & Bunnell, B. A. (2020). Rationale for the clinical use of adipose‐derived mesenchymal stem cells for COVID‐19 patients. Journal of Translational Medicine, 18, 203. 10.1186/s12967-020-02380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Guijo, S. , García‐Arranzb, M. , Lopez‐Parra, M. , Monederof, P. , Mata‐Martínezg, C. , Santosh, A. , … Del‐Pozo, J. L. (2020). Adipose‐derived mesenchymal stromal cells for the treatment of patients with severe SARS‐CoV‐2 pneumonia requiring mechanical ventilation. A proof of concept study. E ClinicalMedicine. Advance online publication. 10.1057/palgrave.kmrp.8500141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta, V. , Sengupta, S. , Lazo, A. , Woods, P. , Nolan, A. , & Bremer, N. (2020). Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells and Development, 29(12), 747–754. 10.1089/scd.2020.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C. , Wang, Z. , & Zhao, F. (2020). Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA, 323(16), 1582–1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari, G. M. , Capobianco, A. , Becchetti, S. , Mingari, M. C. , & Moretta, L. (2006). Mesenchymal stem cell‐natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL‐2‐induced NK‐cell proliferation. Blood, 107(4), 1484–1490. [DOI] [PubMed] [Google Scholar]
- Swati, P. , Rituparna, C. , Anurag, A. , & Sujata, M. (2018). Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. Journal of Biomedical Science, 25(1), 31. 10.1186/s12929-018-0429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. , Shi, L. , Monsel, A. , Li, X. , Zhu, H. , Zhu, Y. , & Qu, J. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang‐1 mRNA. Stem Cells, 35, 1849–1859. [DOI] [PubMed] [Google Scholar]
- Wei, X. , Yang, X. , Han, Z. P. , Qu, F. F. , Shao, L. , & Shi, Y. F. (2013). Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacologica Sinica, 34(6), 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Liu, R. , Shi, D. , Liu, X. , Chen, Y. , Dou, X. , … Liang, W. (2009). Mesenchymal stem cells induce mature dendritic cells into a novel Jagged‐2–dependent regulatory dendritic cell population. Blood, 113, 46–57. [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , & Wang, X. G. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. G. , Feng, X. M. , Abbott, J. , Fang, X. H. , Hao, Q. , Monsel, A. , … Lee, J. W. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells, 32, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]


