Dear Editor,
The recent publication by Choudhury and Mukherjee 1 in this journal depicted the interactions of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike glycoprotein with the cell surface Toll‐like receptors (TLRs) which is a relevant addition to the existing knowledge of coronavirus disease 2019 (COVID‐19) immunobiology.
In this fast‐spreading pandemic situation of COVID‐19, more than six lakhs peoples have died till date. The appropriate therapeutic treatment strategy, drug, or vaccine is still not available. Current clinical data manifests that the severity is due to the cytokine storm and overt immuno‐pathogenesis, causing inflammation in the lungs particularly at the alveolar tissue. 2 , 3 Activation of the human innate immune cells (macrophages, dendritic cells) through the binding of PAMP from SARS‐Cov‐2 to cell surface TLRs have been demonstrated to be a vital mediator of COVID‐19 immunopathogenesis. 4 Signaling pathways originated from TLR4 and TLR5 leading to the activation of mitogen‐activated protein kinases and/or nuclear factor‐κB induces the expression of proinflammatory cytokines. 5 Particularly in SARS‐CoV‐2 infection, major immunopathological consequences leading to death have resulted from the interaction of the SARS‐CoV‐2 antigens and human TLRs. 6 , 7 Precisely, viral spike protein binds with the extracellular domain of various TLRs including TLR1, TLR4, and TLR6, with the strongest binding with TLR4. 1 Considering the immense importance in the disease biology of COVID‐19, TLR‐SARS‐CoV‐2 interaction appears to be a suitable target for the conception of appropriate therapeutic strategy against the pandemic.
Being the crucial innate immune sensors and critical mediators of human immunity, manipulation of the activity of TLRs for restoring immune‐homeostasis in patients with COVID‐19 is currently under consideration for developing effective therapeutics. 6 Applying TLR‐agonists (eg, PUL‐042) for pre‐stimulation of immune repertoires are currently aimed to present as possible prophylaxis for the uninfected individuals while TLR‐antagonists (eg, M5049, hydroxychloroquine sulfate, imiquimod) are expected to cease the overt proinflammatory milieu (cytokine storm) in the infected symptomatic patients with COVID‐19 (as summarized in Figure 1). Most of the TLR antagonists can competitively inhibit the binding of spike protein/other viral PAMP to TLR and dampens the expression of the proinflammatory cytokines like interleukin‐1 (IL‐1), IL‐6, IL‐8, and tumor necrosis factor‐α. A number of TLR‐targeting immunotherapeutics are presently undergoing through different phases of the clinical trial are presented in Table 1. It is noteworthy to mention that in the recent past several TLR antagonists (eg, Eritoran, TAK‐242, NI‐0101, and SPA4) have already been explored successfully for reducing the inflammatory responses associated cancer, rheumatoid arthritis and other inflammatory diseases of human. 8 , 9 It is quite obvious that targeted manipulation of human TLRs also possesses the chance of unexpected outcomes. For example, inhibitors of TLRs could suppress interferon‐related responses which could increase the viral load. Therefore, suitable optimization of dose and duration of treatment are particularly important for the success of TLR‐targeted therapy. Most of the TLR‐antagonists currently undergoing through clinical trials are aiming to reduce the detrimental effects of the immune system by lowering the inflammatory mediators without causing a drastic change in their basal levels to maintain the immune homeostasis.
Figure 1.
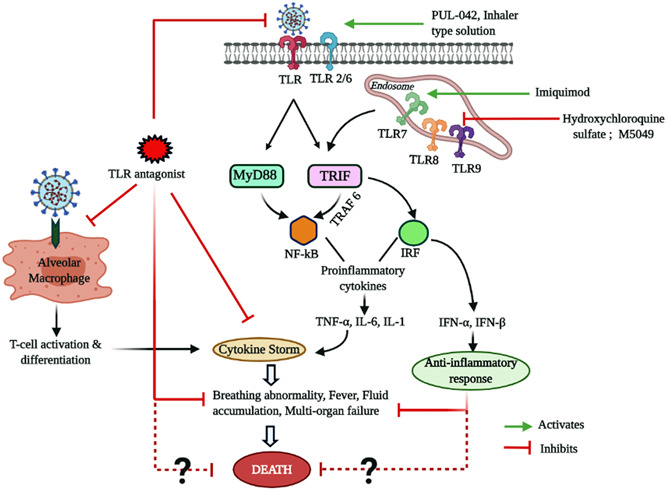
Therapeutic strategies for targeting Toll‐Like receptors (TLRs) against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). IFN, interferon; IL, interleukin; NF‐κB, nuclear factor κB
Table 1.
Therapeutic efficacy of TLR‐targeting agents against SARS‐CoV‐2
| Molecule/peptide/antibody | Mechanism of action | Clinical trial/developmental progress |
|---|---|---|
| PUL‐042, Inhaler type application | Acts as TLR 2/6/9 agonist. | Phase III trial |
| Polyinosinic:polycytidylic acid | Possesses agonist actions on TLR‐3. | Phase I trial |
| EC‐18, Enzychem Lifesciences, South Korea | Reduces the assembly of inflammatory cells due to fast removal of damage‐associated molecular pattern (DAMPs). | Undergoing thorough domestic trial |
| Hydroxychloroquine sulfate | Inhibits TLR7/9 signaling. | Phase III trial |
| M5049; small molecule developed by Merck, Germany | Blocks activation of TLR7 and 8. | Phase II trial |
| Imiquimod; a synthetic molecule | An immune‐stimulator that activates TLR7 and can be used to enhance the innate and adaptive immunity. | Preclinical and clinical trials are proposed |
| Chloroquine phosphate and chloroquine dihydrochloride | Blocks the activation of most of the intracellular TLRs. | Phase II trial |
Abbreviations: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TLR, Toll‐like receptor.
Intriguingly, a number of recent studies have also enumerated the design of multi‐epitope (B‐cell and T‐cell epitopes) peptide vaccines which can strongly bind to human TLR3 and/or TLR5 and can elicit an adequate level of immune response to circumvent the infection of SARS‐CoV‐2. 10 , 11 Interestingly, these vaccines are also expected to shape both B‐ and T‐cell mediated immune responses. All these studies do indicate selective targeting of human TLRs using immuno‐pharmacological agents and/or vaccines as promising options to stimulate the immune system of uninfected individuals or reducing the fatal “cytokine storm” in infected individuals, especially alleviating the inflammation of alveolar tissue, reducing fever and clinical refinement. However, validation of these new therapeutics is currently underway at various phases of clinical and we shall have to wait for a few more months to cheer the success of TLR‐targeted intervention approaches in curing the COVID‐19 pandemic.
REFERENCES
- 1. Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS‐CoV‐2 spike glycoprotein with ACE‐2 receptor homologs and human TLRs. J Med Virol. 2020:1‐9. 10.1002/jmv.25987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonam SR, Kaveri SV, Sakuntabhai A, Gilardin L, Bayry J. Adjunct immunotherapies for the management of severely ill COVID‐19 patients. Cell Reports Med. 2020;1:100016. 10.1016/j.xcrm.2020.100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lucchesi A, Silimbani P, Musuraca G, et al. Clinical and biological data on the use of hydroxychloroquine against SARS‐CoV‐2 could support the role of the NLRP3 inflammasome in the pathogenesis of respiratory disease. J Med Virol. 2020. 10.1002/jmv.26217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li H, Liu L, Zhang D, et al. Hypothesis SARS‐CoV‐2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517‐1520. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukherjee S, Huda S, Sinha Babu SP. TLR polymorphism in host immune response to infectious diseases: a review. Scand J Immunol. 2019;90:e12771. 10.1111/sji.12771 [DOI] [PubMed] [Google Scholar]
- 6. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355‐362. 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao W, Li T. COVID‐19: towards understanding of pathogenesis. Cell Res. 2020;30:367‐369. 10.1038/s41422-020-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Achek A, Yesudhas D, Choi S. Toll‐like receptors: promising therapeutic targets for inflammatory diseases. Arch Pharm Res. 2016;39:1032‐1049. 10.1007/s12272-016-0806-9 [DOI] [PubMed] [Google Scholar]
- 9. Mukherjee S, Karmakar S, Sinha Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20:193‐204. 10.1016/j.bjid.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide‐based subunit vaccine against novel coronavirus SARS‐CoV‐2. Microb Pathog. 2020;145:104236. 10.1016/j.micpath.2020.104236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhattacharya M, Sharma AR, Patra P, et al. Development of epitope‐based peptide vaccine against novel coronavirus 2019 (SARS‐COV‐2): immunoinformatics approach. J Med Virol. 2020;92:618‐631. 10.1002/jmv.25736 [DOI] [PMC free article] [PubMed] [Google Scholar]


