Abstract
Preclinical studies support the anticancer activity of statins; however, the existing clinical evidence is inconsistent and not definitive. Our study aimed at evaluating a postulated cancer chemo-sensitizing effect of statin (simvastatin) in a cohort of metastatic breast cancer (MBC) patients. We designed a prospective, single-centered, randomized, double blinded, placebo-controlled trial that encompassed MBC patients with an ECOG Performance Status Scale ≤2 and scheduled to be treated with a chemotherapy regimen consisting of carboplatin and vinorelbine every 3 weeks at Al-Baironi Hospital, Damascus, Syria. Patients were enrolled between August 2011 and July 2012 and randomly allocated to receive a 15-day course of either simvastatin (40 mg) or placebo seven days prior to the first day of each chemotherapy cycle and then continued for eight days in each individual cycle. Primary endpoints were objective response rate (ORR) and toxicity, and the secondary endpoint was overall survival (OS). Eighty-two patients met the inclusion criteria and consented. ORR (35% vs. 32.5%) and predominant toxicity and grade ≥3 neutropenia (occurred in 30% vs. 40% of the patients) were not significantly different between simvastatin and placebo groups, respectively. Over a median follow-up of 44 months (range, 10–60), median OS was 15 months in the simvastatin group and 17 the in placebo group (hazard ratio (HR) = 1.16, 95% CI (0.70–1.91), P=0.57). Elevated baseline values of high-sensitivity C-reactive protein (hsCRP >10 mg/l), lactate dehydrogenase (LDH >480 U/L), and chemotherapy being ≥2nd line were significantly associated with shorter OS for the total cohort in both Univariate and multivariate analyses. Our data prove a safe profile of simvastatin at 40 mg per day combined with carboplatin and vinorelbine in MBC patients but without any beneficial increase of tumor sensitivity to chemotherapy. Moreover, we demonstrated a strong clinical advantage of baseline values of hsCRP and LDH as useful prognostic tools in MBC patients. This trial is registered with ISRCTN12964275.
1. Introduction
Breast cancer is a major public health problem for women worldwide [1]. Early diagnosis and advances in treatments (i.e., chemotherapy, hormone therapy, and targeted therapies) have led to remarkable increases in survival rates. Nevertheless, a substantial number of patients will still develop metastases during the course of their disease [2]. Based on the currently available therapeutic options, cure of metastatic breast cancer (MBC) is a rather elusive goal, and palliative care can only help maintain quality of life while possibly prolonging survival [3]. Developing new therapeutic approaches for MBC is a time, patience, and diligence demanding research area [4]. Identifying new uses for established multimodes of action drugs (i.e., statins, metformin, and aspirin), also known as “drug repositioning”, represents a less costly and time sparing evolving approach [5].
Statins, or 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors, are well-established cholesterol-lowering agents commonly used in the primary and secondary prevention of cardiovascular disease [5]. Moreover, statins also inhibit the synthesis of essential isoprenoid intermediates required for activation of various intracellular signaling proteins known to play indispensable role(s) in multiple cellular processes, suggesting a pleiotropic nature of statins' effects. Beyond their lipid lowering properties, statins possess anti-inflammatory, antioxidant, and antiproliferative effects, which fed the growing interest in the therapeutic potential of statins in multiple treatment areas including oncology [6]. “Statin repositioning” was supported by in vitro and in vivo studies that have shown a wide range of anticancer activities, including induction of apoptosis, inhibition of tumor cell proliferation, and reduction of invasiveness and metastasis [6–8]. In addition, observational studies have provided substantial evidence that statin use is associated with a reduction in cancer incidence and mortality in several cancer types including breast cancer [9–11]. A few clinical studies have shown a positive role of lipophilic statins both as neoadjuvant therapy (i.e., before surgery) [12, 13] and in secondary prevention in breast cancer survivors [14]. Nevertheless, despite the growing evidence of synergistic effects of statins with chemotherapeutic drugs in other cancer types [15], no clinical studies investigated combination(s) of statins with standard treatment protocols in breast cancer.
Therefore, we designed this study to investigate the chemosensitizing effects of short-term treatment of simvastatin, at clinically relevant doses (40 mg), in MBC patients receiving a combination of carboplatin and vinorelbine. Although not universally accepted as the standard treatment for MBC [16], vinorelbine in combination with carboplatin is adapted in the clinical practice for MBC patients in “Al-Baironi” the major oncology Hospital in Syria. Since inflammation is a critical component of tumor progression and due to the well-established anti-inflammatory properties of statins [17], we sought to investigate the impact of statins on some inflammatory markers (high-sensitivity C-reactive protein (hsCRP) and lactate dehydrogenase (LDH)) and their prognostic potential in predicting therapeutic outcomes in MBC.
2. Materials and Methods
2.1. Trial Design and Eligibility
This was a prospective, single-centered, randomized, double blinded, placebo-controlled study. The study protocol was approved by the scientific research ethics committee at the Faculty of Pharmacy, Damascus University. The eligibility criteria were as follows: female patients attending the breast cancer unit at Al-Baironi Hospital, with confirmed diagnosis of metastases (stage IV) prior to commencing chemotherapy course consisting of carboplatin and vinorelbine; age between 20 and 75 years; adequate function of major organs (including cardiac, hepatic and renal functions); and an ECOG Performance Status score ≤2. Pregnant patients and those with previous treatment with statins or carboplatin and vinorelbine within 30 days of the study entry were excluded. All patients provided written informed consent and enrolled between August 2011 and July 2012. All treatments were double blinded to assure that neither oncologists were involved the study nor do participants know which type of preparation is administered. The follow-up lasted until death or the cutoff date of July 2017. Primary endpoints were objective response rate (ORR) and toxicity, and the secondary endpoint was overall survival (OS) over the follow-up period.
2.2. Treatment Protocol
Statin or placebo was provided in outpatient setting. Patients were assigned (1 : 1 or 2 : 2 ratio) to each treatment group ((carboplatin and vinorelbine) plus simvastatin or placebo) using randomization with metastasis sites as stratification factors. Simvastatin (40 mg) and placebo (provided in containers of identical shape and sequentially numbered) were generous gifts from ALFARES Pharmaceuticals Co. (Damascus, Syria). Chemotherapy regimen was conducted every 3 weeks according to the hospital protocol as follows: carboplatin (carboplatin “Ebewe”), area under the curve (AUC) 4, intravenously on day 1, and vinorelbine (Navelbine®) intravenously (25 mg/m2) or orally (60 mg/m2) on days 1 and 8 of each cycle. Simvastatin (40 mg) or placebo was administered orally once daily for 15 days, starting seven days prior to the first day of each chemotherapy cycle and continued to the eighth day to ensure that both chemotherapy and simvastatin have eight days of overlapping (i.e., as a combination therapy) and then followed by one-week rest.
Additionally, patients with bone metastases were treated with zoledronic acid via intravenous infusion (4 mg every 4 weeks), and palliative radiotherapy was allowed for brain metastases if needed. Study treatment was supposed to be continued in the absence of progression or until another termination criterion was met, including unacceptable toxicity, consent withdrawal, loss to follow-up, or death.
2.3. Response and Toxicity Assessment
Each patient underwent an initial evaluation within one week prior to commencing treatment. Evaluation encompassed full medical history, physical examination, chest X-ray, computed tomography (CT) scan, bone scan, magnetic resonance image (MRI) scan, and laboratory analyses, including complete blood counts (CBCs), creatine kinase (CK), creatinine, alanine aminotransferase (ALT), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), hsCRP, LDH and tumor markers; carcinoembryonic antigen (CEA), and cancer antigen 15-3 (CA15-3). With the exception of tumor markers, laboratory tests were performed at each cycle before chemotherapy administration on day 1, whereas TC, LDL-C, and HDL-C were repeated on day 8. To assess tumor progression, physical examination, tumor markers, and radiological studies were conducted at baseline and every three cycles, and bone scan was repeated by the end of the sixth cycle. Patients' response was classified according to the response evaluation criteria in solid tumor (RECIST) (version 1.1) as follows: complete response (CR), complete disappearance of clinical evidence of disease for a minimum of 8 weeks; partial response (PR), decrease in tumor burden ≥30%; stable disease (SD), decreased by <30% or increased by <20%; progressive disease (PD), increase in tumor burden by ≥20%; and nonevaluable response, due to specific reasons (e.g., early death or toxicity). ORR was calculated based on both CR + PR. Treatment related-toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4. In case of chemotherapy-related toxicity, including grade 2 neutropenia before the start of each cycle, treatment was delayed for 1 week. For grade 3/4 neutropenia, granulocyte colony-stimulating factor (G-CSF) was administered subcutaneously for 3 days at a dose of 5 μg/kg. For simvastatin toxicity, treatment was planned to be discontinued if the serum transaminase was of more than 3 times the upper limit of the reference range or if the CK concentration was more than 5 times the upper limit of the reference range. OS was defined as time from study entry to death from any cause.
2.4. Statistical Analysis
We calculated the sample size of our study based on Simon's randomized Phase 2 design. According to Simon's method for calculating “Sample Size per Treatment for Binary Outcomes and 0.90 Correct Selection Probability,” the objective response rate for the chemotherapy line used in our study was previously reported by Iaffaioli (1995) to be approximately 41% (18); therefore, an increase of greater than 41 % would provide evidence of simvastatin effect. The intent was to enroll at least 74 patients in the two arms of the study in order to detect a 15 % absolute increase which would certainly indicate a superior response rate with 90% power and a two-sided type I error rate of 5%.
Statistical analysis was performed using Graphpad Prism® (version 5) except for Cox proportional hazard regression models that were performed using SPSS® (version 22) to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for both univariate and multivariate analyses. Between-group comparisons were performed using the chi-squared test for categorical variables, unpaired T test for normally distributed data, and Mann–Whitney test for data that were not normally distributed (hsCRP and LDH). Within each group, comparisons for lipid values (pre-vs. postchemotherapy) were performed using paired T test. Median overall survival was estimated using Kaplan–Meier analysis. Statistical significance was tested using the log-rank test, and two-tailed P value <0.05 was considered significant. The median follow-up was estimated using reverse censoring for overall survival.
3. Results
3.1. Patient Characteristics
The eighty-two MBC patients who enrolled were randomly assigned to treatment groups: 41 patients to the carboplatin and vinorelbine plus simvastatin and 41 patients to the carboplatin and vinorelbine plus placebo. The two treatment groups were well balanced in terms of their baseline characteristics as shown in Table 1.
Table 1.
Baseline patients' characteristics.
| Characteristics | Chemotherapy + simvastatin (n = 41) | Chemotherapy + placebo (n = 41) | P value | Total (n = 82) | |
|---|---|---|---|---|---|
| N (%) | n (%) | ||||
| Age (years) | |||||
| Median | 47 | 49 | 0.73 | 47.5 | |
| Range | 28–74 | 24–71 | 24–74 | ||
|
| |||||
| BMI (kg/m2) | |||||
| <18.5 | 1 (2.44) | 0 (0) | 0.46 | 1 (1.21) | |
| 18.5–24.9 | 9 (21.95) | 11 (26.83) | 20 (24.39) | ||
| 25–29.9 | 15 (36.59) | 19 (46.34) | 34 (41.46) | ||
| ≥30 | 16 (39.02) | 11 (26.83) | 27 (32.92) | ||
|
| |||||
| No. of metastatic sites | |||||
| 1 | 23 (56.10) | 17 (41.46) | 0.23 | 40 (48.78) | |
| 2 | 17 (41.46) | 20 (48.78) | 37 (45.12) | ||
| 3 | 1 (2.44) | 4 (9.75) | 5 (6.09) | ||
|
| |||||
| Site of metastases | |||||
| Bone | 5 (12.20) | 6 (14.63) | 0.54 | 11 (13.41) | |
| Liver | 8 (19.51) | 3 (7.32) | 11 (13.41) | ||
| Lung | 4 (9.76) | 3 (7.32) | 7 (8.54) | ||
| Brain | 0 (0) | 1 (2.44) | 1 (1.22) | ||
| Skin/chest wall | 2 (4.88) | 2 (4.88) | 4 (4.88) | ||
| Lymph node | 4 (9.76) | 2 (4.88) | 6 (7.32) | ||
| Multiple sites | 18 (43.9) | 24 (58.54) | 42 (51.22) | ||
|
| |||||
| ECOG-PS | |||||
| 0 | 4 (9.75) | 4 (9.75) | 0.84 | 8 (9.75) | |
| 1 | 29 (70.73) | 31 (75.61) | 60 (73.17) | ||
| 2 | 8 (19.50) | 6 (14.63) | 14 (17.07) | ||
|
| |||||
| Hormone receptor | |||||
| ER+ PR+ | 14 (34.15) | 13 (31.71) | 0.46 | 27 (32.92) | |
| ER− PR− | 18 (43.90) | 21 (51.22) | 39 (47.56) | ||
| ER+ PR− | 2 (4.88) | 4 (9.76) | 6 (7.32) | ||
| ER− PR+ | 5 (12.20) | 1 (2.44) | 6 (7.32) | ||
| Unknown | 2 (4.88) | 2 (4.88) | 4 (4.88) | ||
|
| |||||
| HER 2 | |||||
| HER+ | 30 (73.17) | 26 (63.41) | 0.6 | 56 (68.29) | |
| HER− | 9 (21.95) | 13 (31.71) | 22 (26.82) | ||
| Unknown | 2 (4.88) | 2 (4.88) | 4 (4.88) | ||
|
| |||||
| Chemotherapy line | |||||
| 1st line | 18 (43.90) | 19 (46.34) | 0.37 | 37 (45.12) | |
| 2nd line | 17 (41.46) | 19 (46.34) | 36 (43.90) | ||
| ≥3rd line | 6 (14.63) | 3 (7.32) | 9 (10.97) | ||
The median age of all patients was 47.5 years (range 24–74 years). The ECOG-performance status was 1 in the majority of patients (73.17%). Fifty-six patients (68.29%) were positive for HER2 (human epidermal growth factor receptor-2), and 39 patients (47.56%) were ER/PR negative (estrogen receptor/progesterone receptor). Forty-two patients (51.22%) had two or more sites of metastases. The chemotherapy regimen was the first line in 37 patients (45.12%) and the second in 36 (43.9%).
3.2. Treatment Outcomes
Of the 82 patients enrolled in our study, 80 patients (97.6%) received at least 1 cycle of chemotherapy (median 4.5 cycles; range, 1–12 cycles). The remaining 2 patients (2.4%) withdrew consent. Only 77 patients were assessable for response by the end of the chemotherapy course, as 3 patients were classified not evaluable for response due to early death (n = 2) and chemotherapy toxicity (n = 1), as illustrated in Figure 1.
Figure 1.
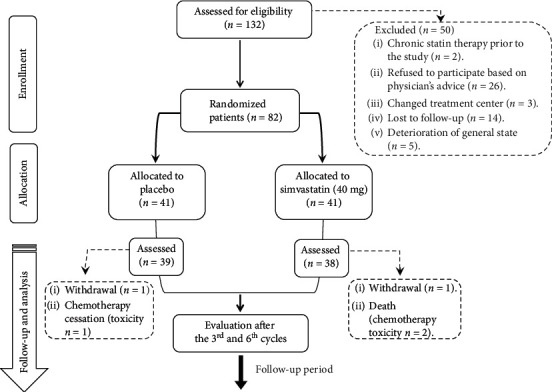
CONSORT flow chart.
In the simvastatin group, one patient had a complete response, 13 patients had a partial response, and the ORR was 35%. Similarly, four patients had a complete response, nine patients had a partial response, and the ORR was 32.5% in the placebo group. No significant differences were found between the two treatment groups with regard to the response assessment results (P=0.57) (Table 2).
Table 2.
Efficacy outcomes.
| Best response | Chemotherapy + simvastatin (n = 40) | Chemotherapy + placebo (n = 40) | P value |
|---|---|---|---|
| n (%) | |||
| Complete response | 1 (2.5) | 4 (10) | 0.57 |
| Partial response | 13 (32.5) | 9 (22.5) | |
| Stable disease | 10 (25) | 11 (27.5) | |
| Progressive disease | 14 (35) | 15 (37.5) | |
| Not evaluable | 2 (5) | 1 (2.5) | |
The assessment did not include the withdrawn consent patients (n = 2)
All patients who received at least one dose of therapy were assessable for toxicity. Most common grade 3 or higher adverse events were neutropenia (30% and 40% in the simvastatin and placebo group, respectively) and anemia (20% in both groups). The addition of simvastatin did not result in clinically significant increase in chemotherapy-related toxicities (no cases of CK elevated ≥ five times the upper limit of the normal range or ALT ≥ three times the upper limit of the normal range), as shown in Table 3.
Table 3.
Most common grades 1 to 4 adverse events of chemotherapy and adverse events of special interest to simvastatin.
| Adverse events n (%) | Chemotherapy + simvastatin (n = 40) | Chemotherapy + placebo (n = 40) | ||
|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | |
| Anemia | 34 (85) | 8 (20) | 32 (80) | 8 (20) |
| Thrombopenia | 5 (12.5) | 1 (2.5) | 4 (10) | 2 (5) |
| Neutropenia | 22 (55) | 12 (30) | 18 (45) | 16 (40) |
| Clinical hemorrhage | — | — | 1 (2.5) | — |
| Injection site reaction | 7 (17.5) | — | 5 (12.5) | — |
| Rash | — | — | 1 (2.5) | — |
| Left ventricle function | 1 (2.5) | — | — | 1 (2.5) |
| Stomatitis | — | — | 1 (2.5) | — |
| Creatinine elevation | 6 (15) | — | 5 (12.5) | — |
| Simvastatin special adverse events | — | |||
| ALT elevation∗ | 12 (30) | — | 13 (32.5) | — |
| CK elevation# | 1 (2.5) | — | 4 (10) | — |
∗None of the elevated levels of ALT exceed more than 3 times the upper limit of the reference range. #None of the elevated levels of CK exceed more than 5 times the upper limit of the reference ranges P value >>0.05 for all comparisons
By the end of a median follow-up period of 44 months (range, 10–60), an overall 65 fatalities were recorded. Median OS was not significantly different between the simvastatin group (15 months) and the placebo group (17 months) (HR = 1.16, 95% CI (0.70–1.91), P=0.57), as depicted in Figure 2.
Figure 2.
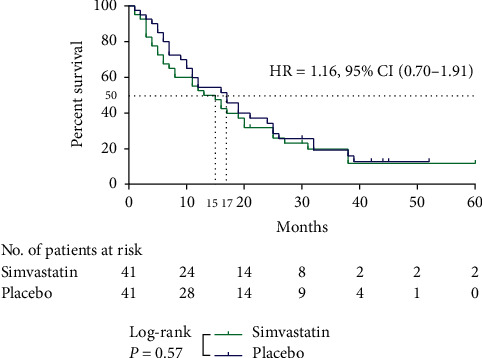
Survival for 60 months. The survival curve with reference to treatment groups (simvastatin vs. placebo) for MBC patients who were treated with palliative chemotherapy (carboplatin and vinorelbine). Median survival was estimated during a 60-month follow-up period using the Kaplan–Meier method. Statistical significance was assessed using the log-rank test, and two-tailed P value of <0.05 was considered significant.
We analyzed the difference(s) of overall survival outcomes between subgroups classified according to baseline characteristics including age, ECOG-PS, hormone receptors status, HER2 status, number of metastatic sites, chemotherapy line's grade, and baseline levels of CEA, CA15-3, hsCRP, and LDH. Univariate Cox models of survival revealed significantly shorter survival of MBC patients when they were <50 years old (P=0.026), having two metastatic sites or more (P=0.02), elevated baseline levels of hsCRP (P=0.002), LDH (P=0.002),CEA (P=0.016), or chemotherapy being ≥2nd line (P=0.04). CA15-3 levels, ECOG-PS, HER2, and hormone receptors status did not significantly the impact survival (P > 0.05). After adjusting for other factors, multivariate Cox models proved that only elevated baseline levels of hsCRP (HR = 2.168, 95% CI (1.299–3.616), P=0.003) or LDH (HR = 2.213, 95% CI (1.273–3.845), P=0.005) and chemotherapy being ≥2nd line (HR = 1.766, 95% CI (1.067–2.923), P=0.027) were still significantly predictive for shorter survival. Table 4 demonstrates detailed results for univariate and multivariate analyses.
Table 4.
Factors associated with overall survival for total cohort.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| hsCRP (mg/l) | ||||
| >10 vs. ≤ 10 | 2.210 (1.339–3.647) | 0.002 | 2.168 (1.299–3.616) | 0.003 |
|
| ||||
| LDH (U/l) | ||||
| >480 vs. ≤ 480 | 2.335 (1.351–4.036) | 0.002 | 2.213 (1.273–3.845) | 0.005 |
|
| ||||
| CEA (ng/ml) | ||||
| >5 vs. ≤ 5 | 1.842 (1.119–3.033) | 0.016 | ||
|
| ||||
| CA15-3 (U/ml) | ||||
| >30 vs. ≤ 30 | 1.289 (0.673–2.469) | 0.444 | ||
|
| ||||
| Age (years) | ||||
| ≥50 vs. <50 | 0.555 (0.330–0.932) | 0.026 | ||
|
| ||||
| ECOG-PS | ||||
| 1/2 vs. 0 | 2.352 (0.939–5.893) | 0.068 | ||
|
| ||||
| HER2 | ||||
| (−) vs. (+) | 1.009 (0.583–1.747) | 0.973 | ||
|
| ||||
| Hormone receptors (HRs) | ||||
| (−) vs. (+) | 0.681 (0.412–1.124) | 0.133 | ||
|
| ||||
| No. of metastatic sites | ||||
| ≥2 sites vs. 1 site | 1.802 (1.095–2.965) | 0.020 | ||
|
| ||||
| Chemotherapy line | ||||
| ≥2nd line vs. 1st line | 1.692 (1.025–2.795) | 0.040 | 1.766 (1.067–2.923) | 0.027 |
3.3. Impact of Simvastatin on Lipids and Inflammatory Markers
Neither TC nor LDL-C levels were significantly different at baseline between the two groups. However, simvastatin clearly induced a substantial drop in TC and LDL-C levels throughout the chemotherapy cycles in comparison with placebo (P ≪ 0.05) (see Table 1 in the Supplementary Material). Thorough follow-up of serum TC, LDL-C and HDL-C levels and pre- and postchemotherapy administration at each cycle revealed a significant decrease in TC and LDL-C levels at day 8 of each chemotherapy cycle, except for the sixth, in both simvastatin and placebo groups (summarized in Table 5). Noteworthy, following the one-week drug break, all patients recovered TC and LDL-C levels to within at least 77% of their baseline by the start of the next cycle, and this pattern persisted over all treatment cycles. Serum HDL-C levels did not differ significantly between the two groups at any cycle after simvastatin exposure.
Table 5.
Differences in TC, LDL-C, and HDL-C levels during each chemotherapy cycle.
| Variable | Simvastatin | Placebo | ||
|---|---|---|---|---|
| Differences between 1st and 8th day of each chemotherapy cycle | ||||
| Mean (%) | P value | Mean (%) | P value | |
| TC (mg/dl) | ||||
| Cycle 1 | −15.67 (−9.54%) | 0.007 | −18.13 (−8.57%) | 0.003 |
| Cycle 2 | −15.91 (−9.07%) | 0.013 | −20.47 (−9.43%) | 0.0002 |
| Cycle 3 | −13.58 (−7.81%) | 0.018 | −13.52 (−6.6%) | 0.017 |
| Cycle 4 | −16.05 (−9.01%) | 0.013 | −19.45 (−8.71%) | 0.002 |
| Cycle 5 | −14.29 (−8.43%) | 0.016 | −20.13 (−9.16%) | 0.001 |
| Cycle 6 | −8.17 (−4.85%) | 0.3 | −7 (−3.26%) | 0.3 |
|
| ||||
| LDL-C (mg/dl) | ||||
| Cycle 1 | −10.42 (−11.75%) | 0.006 | −16 (−12.73%) | 0.001 |
| Cycle 2 | −16.85 (−16.77%) | 0.0006 | −8.71 (−6.7%) | 0.014 |
| Cycle 3 | −10.02 (−9.84%) | 0.018 | −9.84 (−7.85%) | 0.006 |
| Cycle 4 | −11.41 (−10.94%) | 0.008 | −15.59 (−11.4%) | 0.0002 |
| Cycle 5 | −6.29 (−6.44%) | 0.2 | −7.31 (−5.53%) | 0.056 |
| Cycle 6 | −7.17 (−7.31%) | 0.29 | −3.85 (−2.82%) | 0.6 |
|
| ||||
| HDL-C (mg/dl) | ||||
| Cycle 1 | 0.52 (1.06%) | 0.5 | −3.47 (−6.77%) | 0.1 |
| Cycle 2 | 0.06 (0.12%) | 0.9 | −4.65 (−8.58%) | 0.06 |
| Cycle 3 | 0.03 (0.07%) | 0.9 | −0.55 (−1.12%) | 0.8 |
| Cycle 4 | 0.22 (0.46%) | 0.9 | 0.23 (0.45%) | 0.9 |
| Cycle 5 | −0.21 (−0.45%) | 0.9 | −5.31 (−9.38%) | 0.03 |
| Cycle 6 | 0.23 (0.48%) | 0.9 | −0.46 (−0.84%) | 0.8 |
No significant changes were observed in the levels of inflammatory markers (hsCRP and LDH) during the study in any of the treatment groups (see Table 2 in the Supplementary Material).
4. Discussion
Recently, the cholesterol lowering-independent or “pleiotropic” effects of statins have gained greater recognition, particularly in the area of cancer therapeutics [7]. Cumulative in vivo evidence and observational clinical studies suggest a therapeutic potential of statins in different cancer models [17]. Nevertheless, translating these findings into clinical studies faces multidimensional challenges comprising which statin to use, the timing (when or at what stage of cancer progression), the temporal frame (short-term versus long-term), and what cytotoxic agents/chemotherapy line, hormonal, or radiotherapy with which should a particular type of statin is coadministered. To our knowledge, this study represents the first clinical trial to utilize short-term simvastatin at therapeutically relevant dose (40 mg) in combination with carboplatin and vinorelbine in metastatic breast cancer patients.
The combination of carboplatin and vinorelbine is a one of the palliative treatments for MBC in Al-Baironi Hospital. Our study confirms the effectiveness of carboplatin plus vinorelbine with an ORR of 33.75%, a median OS of 16 months, and 35% grade ≥3 neutropenia for all patients. Iaffaioli et al. reported their experience with a same therapy regimen, the ORR was 41%, median OS was 16 months, and the principal toxicity was myelotoxicity and grade 3/4 leukopenia in 46% of advanced breast cancer patients [18].
Expectedly, the lipid lowering effect of simvastatin was evident by a significant decrease in total cholesterol and LDL-C levels compared with that of placebo. These findings suggest a good compliance with the study intervention and confirm the effectiveness of simvastatin in lowering lipids within a short-term (15 days) exposure frame.
Surprisingly, a significant drop in total cholesterol (range −3.26% to −9.43%, P ≪ 0.05) and LDL-C (range −2.82% to −12.73%, P ≪ 0.05) levels was observed between the first and eighth days of each of the treatment cycles in patients who received chemotherapy plus placebo. These observed simvastatin-independent changes in cholesterol levels are consistent with in vitro experiments, where that in acute myeloid leukemia (AML) samples exhibited abnormally increased demands for cholesterol following the exposure to cytotoxic agents (daunorubicin or cytarabine), a phenomenon described as “defensive adaptation” of cancerous cells to increase chemoresistance [19].
Within the 15-day simvastatin exposure in each cycle, no changes in hsCRP level was observed, suggesting that a dose of 40 mg of simvastatin for this short-term duration may not have been sufficient to exhibit anti-inflammatory benefit in MBC patients. These findings contradict others in coronary artery disease (CAD) patients, where short-term exposure of simvastatin lowered hsCRP [20]. We should note here that the hsCRP baseline levels are rather different between the two patient populations, (median [lower-upper quartile]; 8.18 [3.93–22.11] in MBC vs. 2.8 [1.3–4.8] mg/l or 1.1 [0.8–2.5] in female and male CAD patients, respectively). A significant reduction in serum CRP in breast cancer patients may demand a higher dose and/or longer duration of statin therapy (e.g. at least 3 months) to demonstrate a similar effect to that observed in CAD patients [21].
Despite the promising in vitro and in vivo evidence that provided support to antitumor effect(s) of statins in a variety of human malignancies [15], our findings prove that clinically relevant dose of simvastatin, added to carboplatin and vinorelbine course, has no clinical benefit in terms of outcome (i.e., ORR and median OS) in MBC patients. However, treatment with simvastatin in MBC proved to be very well tolerated, as no significant chemotherapy toxicity or simvastatin adverse effects were recorded. These findings are in agreement with a study by kim and colleagues that investigated a combination of statins with capecitabine and cisplatin in advanced gastric cancer, as no increase in progression free survival was reported [22]. Similarly, gemcitabine-simvastatin at 40 mg daily vs. gemcitabine-placebo resulted in no significant difference in time to progression in advanced pancreatic cancer patients [23]. The overall null results in our study and others may stem from statins' conflicting properties; on the one hand, they have antiproliferative effects, but on the other hand, they exhibit immune tolerance-promoting properties during tumor development. Therefore, statins might be concurrently inhibiting and promoting tumor growth [24]. Another explanation may arise from the pulsatile administration of statin (15 days every cycle), which may not be enough in terms of cholesterol deprivation of cancerous cells.
We decided the dose-level of simvastatin (40 mg) in combination with the carboplatin and vinorelbine course based on an observation that low concentrations of statins were capable of inducing apoptosis of microvascular endothelial cells and lowering VEGF serum levels, implicating a possible antiangiogenic role for statin in cancer treatment [22, 25]. Of interest, statins were proved to induce apoptosis through activation of the JNK-signaling pathway, and since inhibition of JNK activation is a major mechanism beyond tumor resistance to platinums and Vinca alkaloid, the addition of statins to either of these drugs is speculated to help overcome chemoresistance [26, 27].
There have been only a few reports on prognostic factors in patients with metastatic disease [28]. Notably, not all studies agreed on the same set of risk factors explaining variation in prognosis following breast cancer metastasis [29]. Some studies have shown that age, number of metastatic sites, ER/PR and HER2 status, ECOG-PS, and baseline values of CEA and CA15-3 are valuable prognostic factors [28–31]. Nevertheless, our study did not provide support for a significant influence of any of these factors on survival in multivariate analysis. Variations in prognostic factors in terms of the patients' selection, presence of clinical covariates, rate of patients' lost to follow-up, lines of chemotherapy, and statistical method for analysis [29, 32] may explain the differences between our results and those from previous ones.
Nevertheless, our findings prove that increased baseline serum concentrations of hsCRP may serve as a predictor for poor prognosis among MBC patients. This result is consistent with that of Albuquerque et al.'s study [33], Murri et al.'s study [34], and Petekkaya et al.'s study [35].
On the other hand, Swenerton et al. were the first to report the clinical importance of serum lactate dehydrogenase in predicting survival of MBC patients [32]. Our results reinforced that elevated serum LDH levels significantly correlate with poorer survival among MBC patients [35,36].
No global consensus exists regarding the ideal treatment strategy for MBC. Thus, once the first line failed, second and later lines are adapted, reflecting the clinically challenging picture of progressive disease [2]. Our data showed that the grade of chemotherapy line had significant impact on survival of MBC patients.
5. Conclusions
The present study is the first, to the best of our knowledge, to investigate the proposed chemosensitizing effect of simvastatin added on to carboplatin and vinorelbine in metastatic breast cancer. Adding simvastatin to this combination did not seem to provide any additional clinical benefit and also did not result in any significant increase in toxicity. We were able to show that inflammatory markers, such as CRP and LDH, can be used to predict prognosis in patients with metastatic breast cancer. We recognize our study's limitations concerning the generalization of the conclusions based on data originated from a small-sized sample of MBC patients receiving one line of chemotherapy. Larger population-based studies on different chemotherapy agents are needed to confirm or refute any prognostic significance in MBC patients.
Acknowledgments
We acknowledge ALFARES Pharmaceuticals Co. for their generous donation of simvastatin and placebo. Our gratitude goes to the late Professor Mohammad Mahgoub Gairoudi, who passed away in April of 2014, for his invaluable help and facilitation of periodic lab work. We thank the medical staff, doctors, Mohammad Kadri, Moosheer Alammar, Mhd Adel Haykal, and Alhadi Alseoudi, and the nursing staff, particularly Ms. Gufran hussien alfayoumi, at the Breast Cancer Unit, Al-Baironi Hospital. Finally, this work could not have been realized without the cooperation of the patients and their families to whom we dedicate this work. The study was funded by the Damascus University.
Abbreviations
- ALT:
Alanine aminotransferase
- AML:
Acute myeloid leukemia
- AUC:
Area under the curve
- CA15-3:
Cancer antigen 15–3
- CAD:
Coronary artery disease
- CBC:
Complete blood counts
- CEA:
Carcinoembryonic antigen
- CIs:
Confidence intervals
- CK:
Creatine kinase
- CR:
Complete response
- CT:
Computed tomography
- ER/PR:
Estrogen receptor/progesterone receptor
- G-CSF:
Granulocyte colony-stimulating factor
- HDL-C:
High-density lipoprotein cholesterol
- HER2:
Human epidermal growth factor receptor-2
- HMG-CoA:
3-Hydroxy-3-methylglutaryl-Coenzyme A
- HRs:
Hazard ratios
- hsCRP:
High-sensitivity C-reactive protein
- LDH:
Lactate dehydrogenase
- LDL-C:
Low-density lipoprotein cholesterol
- MBC:
Metastatic breast cancer
- MRI:
Magnetic resonance image
- ORR:
Objective response rate
- OS:
Overall survival
- PD:
Progressive disease
- PR:
Partial response
- RECIST:
Response evaluation criteria in solid tumor;
- SD:
Stable disease
- TC:
Total cholesterol.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study protocol was approved by the Scientific Research Ethics Committee at the Faculty of Pharmacy, Damascus University (Damascus, Syria; Number: 10, Date: November 26, 2013), and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Our study adhered to CONSORT guidelines. All eligible patients gave signed informed consent.
Disclosure
The funder had no role in the data acquisition, analysis or interpretation; or in the preparation of this manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
LAY was involved in the concept and design, data interpretation, and co-writing and revisions of the manuscript. HA was responsible for the eligibility assessment, data acquisition and interpretation, follow-up of enrolled patients, and co-writing of the manuscript. MS was involved in the eligibility assessment, treatment supervision, and follow-up of enrolled patients. All authors read the final manuscript and gave their approval. LAY contributed equally to this work. Hiba Alarfi and Lama A. Youssef have contributed equally to this work, and this entitles Dr. Youssef a co-first authorship.
Supplementary Materials
Table 1: serum levels of TC, LDL TC, LDL-C, and HDL-C were measured on day 1 and day 8 of each cycle, and comparisons were made between simvastatin and placebo groups (DOCX 15 kb). Table 2: serum levels of hsCRP and LDH throughout and LDH were measured in each cycle, and comparisons were made between simvastatin and placebo groups (DOCX 13 kb).
References
- 1.Ferlay J., Colombet M., Soerjomataram I., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Roché H., Vahdat L. T. Treatment of metastatic breast cancer: second line and beyond. Annals of Oncology. 2011;22(5):1000–1010. doi: 10.1093/annonc/mdq429. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F., Harbeck N., Fallowfield L., Kyriakides S., Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2012;23(7):vii11–vii19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F. Metastatic breast cancer patients: the forgotten heroes! The Breast. 2009;18(5):271–272. doi: 10.1016/j.breast.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Gronich N., Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nature Reviews Clinical Oncology. 2013;10(11):625–642. doi: 10.1038/nrclinonc.2013.169. [DOI] [PubMed] [Google Scholar]
- 6.Jiang P., Mukthavaram R., Chao Y., et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. British Journal of Cancer. 2014;111(8):1562–1571. doi: 10.1038/bjc.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klawitter J., Shokati T., Moll V., Christians U., Klawitter J. Effects of lovastatin on breast cancer cells: a proteo-metabonomic study. Breast Cancer Res. 2010;12(2) doi: 10.1186/bcr2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso D. F., Farina H. G., Skilton G., Gabri M. R., De Lorenzo M. S., Gomez D. E. Reduction of mouse mammary tumor formation and metastasis by lovastatin, an inhibitor of the mevalonate pathway of cholesterol synthesis. Breast Cancer Research and Treatment. 1998;50(1):83–93. doi: 10.1023/a:1006058409974. [DOI] [PubMed] [Google Scholar]
- 9.Graaf M. R., Beiderbeck A. B., Egberts A. C. G., Richel D. J., Guchelaar H.-J. The risk of cancer in users of statins. Journal of Clinical Oncology. 2004;22(12):2388–2394. doi: 10.1200/jco.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen S. F., Nordestgaard B. G., Bojesen S. E. Statin use and reduced cancer-related mortality. New England Journal of Medicine. 2012;367(19):1792–1802. doi: 10.1056/nejmoa1201735. [DOI] [PubMed] [Google Scholar]
- 11.Cardwell C. R., Hicks B. M., Hughes C., Murray L. J. Statin use after diagnosis of breast cancer and survival. Epidemiology. 2015;26(1):68–78. doi: 10.1097/ede.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 12.Garwood E. R., Kumar A. S., Baehner F. L., et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Research and Treatment. 2010;119(1):137–144. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjarnadottir O., Romero Q., Bendahl P.-O., et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Research and Treatment. 2013;138(2):499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- 14.Higgins M. J., Prowell T. M., Blackford A. L., et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast Cancer Research and Treatment. 2012;131(3):915–924. doi: 10.1007/s10549-011-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae Y. K., Yousaf M., Malecek M. K. Statins as anti-cancer therapy; Can we translate preclinical and epidemiologic data into clinical benefit? Discovery Medicine. 2015;20(112):413–427. [PubMed] [Google Scholar]
- 16.Gradishar W. J., Anderson B. O., Balassanian R., et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2018;16(3):310–320. doi: 10.6004/jnccn.2018.0012. [DOI] [PubMed] [Google Scholar]
- 17.Van Wyhe R. D., Rahal O. M., Woodward W. A. Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer. 2017;9:559–565. doi: 10.2147/BCTT.S148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iaffaioli R., Tortoriello A., Facchini G., et al. A phase II study of carboplatin and vinorelbine as second-line treatment for advanced breast cancer. British Journal of Cancer. 1995;72(5):1256–1258. doi: 10.1038/bjc.1995.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banker D. E., Mayer S. J., Li H. Y., Willman C. L., Appelbaum F. R., Zager R. A. Cholesterol synthesis and import contribute to protective cholesterol increments in acute myeloid leukemia cells. Blood. 2004;104(6):1816–1824. doi: 10.1182/blood-2004-01-0395. [DOI] [PubMed] [Google Scholar]
- 20.Plenge J. K., Hernandez T. L., Weil K. M., et al. Simvastatin lowers C-reactive protein within 14 days. Circulation. 2002;106(12):1447–1452. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 21.Arun B. K., Gong Y., Liu D., et al. Phase I biomarker modulation study of atorvastatin in women at increased risk for breast cancer. Breast Cancer Research and Treatment. 2016;158(1):67–77. doi: 10.1007/s10549-016-3849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S. T., Kang J. H., Lee J., et al. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. European Journal of Cancer. 2014;50(16):2822–2830. doi: 10.1016/j.ejca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Hong J. Y., Nam E. M., Lee J., et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemotherapy and Pharmacology. 2014;73(1):125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 24.Lee K. J., Moon J. Y., Choi H. K., et al. Immune regulatory effects of Simvastatin on regulatory T cell-mediated tumour immune tolerance. Clinical and Experimental Immunology. 2010;161(2):298–305. doi: 10.1111/j.1365-2249.2010.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alber H. F., Dulak J., Frick M., et al. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. Journal of the American College of Cardiology. 2002;39(12):1951–1955. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- 26.Brantley-Finley C., Lyle C. S., Du L., et al. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochemical Pharmacology. 2003;66(3):459–469. doi: 10.1016/s0006-2952(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Lan T., Hou J., et al. Atorvastatin sensitizes human non-small cell lung carcinomas to carboplatin via suppression of AKT activation and upregulation of TIMP-1. The International Journal of Biochemistry & Cell Biology. 2012;44(5):759–769. doi: 10.1016/j.biocel.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Chang J., Clark G. M., Allred D. C., Mohsin S., Chamness G., Elledge R. M. Survival of patients with metastatic breast carcinoma. Cancer. 2003;97(3):545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]
- 29.Jung S. Y., Rosenzweig M., Sereika S. M., Linkov F., Brufsky A., Weissfeld J. L. Factors associated with mortality after breast cancer metastasis. Cancer Causes & Control. 2012;23(1):103–112. doi: 10.1007/s10552-011-9859-8. [DOI] [PubMed] [Google Scholar]
- 30.Khanfir A., Lahiani F., Bouzguenda R., Ayedi I., Daoud J., Frikha M. Prognostic factors and survival in metastatic breast cancer: a single institution experience. Reports of Practical Oncology & Radiotherapy. 2013;18(3):127–132. doi: 10.1016/j.rpor.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Trufero J., de Lobera A. R., Lao J., et al. Serum markers and prognosis in locally advanced breast cancer. Tumori Journal. 2005;91(6):522–530. doi: 10.1177/030089160509100613. [DOI] [PubMed] [Google Scholar]
- 32.Swenerton K. D., Legha S. S., Smith T., et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Research. 1979;39(5):1552–1562. [PubMed] [Google Scholar]
- 33.Albuquerque K. V., Price M. R., Badley R. A., et al. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. European Journal of Surgical Oncology (EJSO) 1995;21(5):504–509. doi: 10.1016/s0748-7983(95)96935-7. [DOI] [PubMed] [Google Scholar]
- 34.Murri A. M. A., Bartlett J. M. S., Canney P. A., Doughty J. C., Wilson C., McMillan D. C. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. British Journal of Cancer. 2006;94(2):227–230. doi: 10.1038/sj.bjc.6602922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petekkaya I., Unlu O., Roach E. C., et al. Prognostic role of inflammatory biomarkers in metastatic breast cancer. Journal of the Balkan Union of Oncology. 2017;22(3):614–622. [PubMed] [Google Scholar]
- 36.Brown J. E., Cook R. J., Lipton A., Coleman R. E. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clinical Cancer Research. 2012;18(22):6348–6355. doi: 10.1158/1078-0432.ccr-12-1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: serum levels of TC, LDL TC, LDL-C, and HDL-C were measured on day 1 and day 8 of each cycle, and comparisons were made between simvastatin and placebo groups (DOCX 15 kb). Table 2: serum levels of hsCRP and LDH throughout and LDH were measured in each cycle, and comparisons were made between simvastatin and placebo groups (DOCX 13 kb).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


