Abstract
Aim
As the COVID‐19 pandemic evolves, human milk banks world‐wide continue to provide donor human milk to vulnerable infants who lack access to mother's own milk. Under these circumstances, ensuring the safety of donor human milk is paramount, as the risk of vertical transmission of SARS‐CoV‐2 is not fully understood. Here, we investigate the inactivation of SARS‐CoV‐2 in human milk by pasteurisation and the stability of SARS‐CoV‐2 in human milk under cold storage.
Methods
SARS‐CoV‐2 was experimentally inoculated into human milk samples from healthy donors or into a control medium. Triplicates of each sample were layered onto uninfected cells after Holder pasteurisation (63°C for 30 min), heating to 56°C for 30 min, or after 48 h of storage at 4°C or −30°C. Infectious titres of virus were determined at 72 h post‐infection by endpoint titration.
Results
Following heating to 63°C or 56°C for 30 min, replication competent (i.e. live) SARS‐CoV‐2 was undetected in both human milk and the control medium. Cold storage of SARS‐CoV‐2 in human milk (either at 4°C or −30°C) did not significantly impact infectious viral load over a 48 h period.
Conclusion
SARS‐CoV‐2 is effectively inactivated by Holder pasteurisation, suggesting that existing milk bank processes will effectively mitigate the risk of transmission of SARS‐COV‐2 to vulnerable infants through pasteurised donor human milk. The demonstrated stability of SARS‐CoV‐2 in refrigerated or frozen human milk may assist in the development of guidelines around safe expressing and storing of milk from COVID‐19 infected mothers.
Keywords: COVID‐19, Holder pasteurisation, pasteurised donor human milk, SARS‐CoV‐2, viral inactivation
What is already known on this topic
SARS‐CoV‐2 has not been shown to be transmitted via human milk.
Holder pasteurisation, a common process in milk banks worldwide, inactivates many viruses in human milk.
Demonstrating the safety of pasteurised donor human milk ensures vulnerable infants will continue to have access to donor milk during the SARS‐CoV‐2 pandemic.
What this paper adds
Holder pasteurisation (the usual process used) inactivates SARS‐CoV‐2 virus that has been experimentally inoculated into human milk.
Holder pasteurisation effectively mitigates theoretical risks of SARS‐CoV‐2 transmission to vulnerable infants through donor milk.
Freezing human milk experimentally inoculated with SARS‐CoV‐2 does not prevent infectivity of SARS CoV2, although some reduction in virus titre (infectious amount) occurs.
As the COVID‐19 pandemic evolves, human milk banks world‐wide continue to provide donor human milk to vulnerable infants who lack access to mother's own milk. 1 Under these circumstances, ensuring the safety of donor human milk is paramount, as the risk of vertical transmission of SARS‐CoV‐2 is not fully understood. 2 Although case series have reported that SARS‐CoV‐2 was not detected in breast milk, 3 there have been recent case reports 4 , 5 of SARS‐CoV‐2 detected in breast milk from women with symptomatic COVID‐19 infection. Expressed milk may theoretically become contaminated from maternal respiratory secretions or via skin, although there is no evidence to suggest that breast milk is a means of transmission of SARS‐CoV‐2 to infants. Respiratory transmission from mother to infant remains the more likely transmission route. Consistent with available data, international guidelines recommend mothers with COVID‐19 continue to provide breast milk for their babies as the benefits of breast milk outweigh risks of virus transmission. 2 Although low, the potential risk of SARS‐CoV‐2 transmission through human milk is concerning for milk banks globally. 1
Here, we investigate the inactivation by pasteurisation of SARS‐CoV‐2 experimentally inoculated in to human milk and the stability of SARS‐CoV‐2 in human milk under cold storage (freezing or refrigeration).
Methods
Frozen (14 weeks) and freshly expressed (<12 h) human milk was obtained from healthy donors to Australian Red Cross Lifeblood Milk.
For the assessment of viral inactivation by pasteurisation, SARS‐CoV‐2 10 5 50% tissue culture infectious dose (TCID50)/mL was diluted 1:10 in previously frozen human milk or non‐supplemented minimum essential medium (MEM, Gibco, Life Technologies, CA, USA) (control). The TCID50 is a standard measure of infectious virus titre. Triplicates of each sample were layered onto uninfected cells after being subjected to Holder pasteurisation (63°C for 30 min), under‐pasteurisation (56°C for 30 min) or were unpasteurised. Endpoint titration of samples was performed on Vero cells in sextuplicate, and theTCID50 determined at 72 h post‐infection using standard methods. 6
Freshly expressed milk from two donors and MEM (control) was similarly inoculated with SARS‐CoV‐2 to assess virus stability under cold storage conditions. Infectious titres of samples were measured at the time of inoculation, and after 48 h of storage at 4°C or −30°C.
This project was approved by the Australian Red Cross Lifeblood Milk Human Research Ethics Committee (Clifford 27042020).
Results
Following heating to 63°C or 56°C for 30 min, SARS‐CoV‐2 replication competent (i.e. live) virus was undetected in both milk and MEM (Fig. 1a) representing, at minimum, a 3.5‐log reduction. After 48 h there was no reduction in SARS‐CoV‐2 infectious titre in milk samples kept at 4°C, and a 0.41 log‐unit reduction in samples frozen at −30°C (Fig. 1b). In the control media (MEM), there was a 0.02 and 0.25 log‐unit reduction in infectious titres of samples stored at 4°C and −30°C, respectively. Whilst freezing of milk and MEM samples resulted in a slight reduction in infectious titre, viable virus was still recovered after 48 h of storage.
Fig 1.
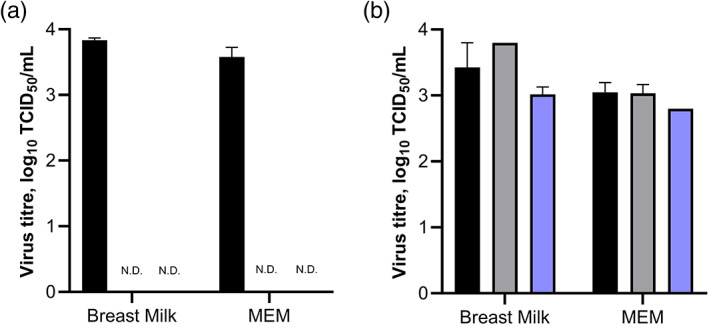
Viability of SARS‐CoV‐2 in breast milk. (a) Inactivation by pasteurisation. Previously frozen human breast milk or MEM was inoculated with SARS‐CoV‐2. Infectious titres were determined in triplicates of each sample that were subjected to Holder (63°C) pasteurisation (ND), under‐pasteurisation (56°C) (ND) or were unpasteurised (black). (b) Stability under cold storage conditions. Freshly expressed breast milk (n = 2) or MEM (n = 3) was inoculated with SARS‐CoV‐2. Infectious titres of samples were determined at 0 h post‐inoculation (black), and after 48 h of storage at either +4°C (grey) or −30°C (blue). Infectious titres for all experiments were determined using endpoint titration on Vero cells in sextuplicate. Error bars indicate the standard error of the mean. MEM, minimum essential medium; ND, not detected; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TCID50, 50% tissue culture infectious dose.
Discussion
To date, there is no evidence that SARS‐CoV‐2 is transmitted vertically via breast milk. The World Health Organization recommends that, based on current evidence, women with COVID‐19 should continue to breastfeed. 7 Only viral RNA (not infectious virus) has been detected in human milk, 4 , 5 and there have been no reports to suggest that viral RNA detected by molecular methods in breast milk is capable of causing COVID‐19 in the breastfeeding infant. The risk that SARS‐CoV‐2 may be transmitted to infants through donor milk is therefore largely theoretical.
In a pandemic, there is nevertheless a significant risk that pasteurised donor human milk (PDHM) supply may be interrupted due to concerns around milk safety. This has occurred where previous viral epidemics have resulted in donor milk use being interrupted for vulnerable infants. 1 It is therefore imperative to conclusively demonstrate that PDHM remains safe for this patient population in the SARS‐CoV‐2 pandemic.
Milk banks already employ many risk mitigation strategies to ensure the safety of donor milk for recipient infants, with donor selection criteria, serological and/or viral nucleic acid screening, validated frozen transport methods, microbial testing and pasteurisation. 8 For SARS‐CoV‐2, this includes specific questions to screen for symptoms of COVID‐19, deferral of donors with COVID‐19 or close contact with COVID‐19, and pasteurisation of donor milk. Holder pasteurisation (62.5°C for 30 min) is the most common pasteurisation method among milk banks world‐wide, and has been shown to inactivate most enveloped viruses, including MERS‐CoV 9 and SARS‐CoV‐1. 10
Our findings confirm that SARS‐CoV‐2 in human milk can be effectively inactivated by Holder pasteurisation, and that existing milk bank processes will effectively mitigate the risk of transmission of SARS‐CoV‐2 to vulnerable infants through PDHM. These findings are consistent with a recent study that reported SARS‐CoV‐2 is inactivated by heat treatment, 10 in different matrices. It should be noted that our study involved experimental inoculation of SARS‐CoV‐2 in to human milk, and thus may not be fully representative of the behaviour of the virus if present in the breast milk of a mother with COVID‐19. However, the high infectious titre of virus used for inoculation of samples in the current study is representative of a worst case scenario, if virus was found to be present in human milk.
Additionally, this is the first study to assess the stability of experimentally inoculated SARS‐CoV‐2 in human milk under common storage conditions. We show that cold storage of SARS‐CoV‐2 (either by refrigeration or freezing) does not significantly impact infectious viral load over a 48 h period. Results seen in the refrigerated samples are consistent with a recent study (not involving human milk) that found SARS‐CoV‐2 to be highly stable at 4°C in the environment. 11 As storage does not significantly impact on infectious viral load in human milk, it is particularly important for mothers with COVID‐19 to ensure that their expressed breast milk does not become contaminated (either from skin or respiratory secretions) with SARS‐CoV‐2. These findings may therefore assist in the development of guidelines around safe expressing and storing milk from COVID‐19 infected mothers.
Conclusion
In conclusion, the findings of this study provide evidence to confirm that common risk mitigation strategies (primarily Holder pasteurisation) employed by milk banks world‐wide are sufficient to ensure the safety of PDHM, and thus supports the continued provision of PDHM to vulnerable infants.
Acknowledgements
The authors thank Christine Sulfaro (Australian Red Cross Lifeblood), Nicole Stevens (Australian Red Cross Lifeblood), Lifeblood Milk donors, and members of the Serology and Virology Division (SAViD) Area Diagnostic Virology Laboratory and Kirby Institute UNSW for their assistance with this study.
Laura D Klein and William Rawlinson are joint senior authors.
Conflict of interest: None declared.
References
- 1. Shenker N, Aprigio J, Arslanoglu S et al. Maintaining safety and service provision in human milk banking: A call to action in response to the COVID‐19 pandemic. Lancet Child Adolesc. Health 2020; 4: 484–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Pregnancy & Breastfeeding: Information about Coronavirus Disease. Atlanta, Georgia, USA: CDC; 2019. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prepare/pregnancy-breastfeeding.html [accessed 9 June 2020].
- 3. Lackey KA, Pace RM, Williams JE et al. SARS‐CoV‐2 and human milk: What is the evidence? Matern. Child Nutr. 2020: e13032. 10.1111/mcn.13032 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groß R, Conzelmann C, Müller JA et al. Detection of SARS‐CoV‐2 in human breastmilk. Lancet 2020; 395: 1757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tam PCK, Ly KM, Kernich ML et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID‐19). Clin. Infect. Dis. 2020; ciaa673. 10.1093/cid/ciaa673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938; 27: 493–7. [Google Scholar]
- 7. World Health Organization . Breastfeeding and COVID‐19. Geneva: WHO; 2020. Available from: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 [accessed 24 June 2020].
- 8. Clifford V, Sulfaro C, Lee J, Pink J, Hoad V. Development and evaluation of formal guidelines for donor selection for human milk banks. J. Paediatr. Child Health 2020. 10.1111/jpc.14909. [DOI] [PubMed] [Google Scholar]
- 9. van Doremalen N, Bushmaker T, Karesh WB, Munster VJ. Stability of Middle East respiratory syndrome coronavirus in milk. Emerg. Infect. Dis. 2014; 20: 1263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005; 194: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chin AWH, Chu JTS, Perera MRA et al. Stability of SARS‐CoV‐2 in different environmental conditions. Lancet Microbe 2020; 1: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]


