Dear Editor,
Coronaviruses (CoVs) are enveloped positive‐sense RNA viruses that can infect a wide variety of species, including human beings and companion animals. The superior genetic plasticity of this virus family is responsible for an extensive host range and results from the accumulation of point mutations and recombination events (Buonavoglia et al. 2006, Brownlie & Sibley 2020). During the last two decades, three zoonotic CoVs have emerged that cross species barriers: severe acute respiratory syndrome coronavirus (SARS‐CoV), Middle East respiratory syndrome coronavirus (MERS‐CoV) and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Decaro et al. 2020, Malik et al. 2020, Tiwari et al. 2020). All of these CoVs originated at the human‐animal interface created by progressive deforestation, forest encroachment, anthropization of natural environments and consumption of endangered wildlife (Decaro et al. 2020). SARS‐CoV‐2 (initially named 2019‐nCoV) emerged from the Huanan seafood market in Wuhan, China. Preliminary genetic analysis suggested bats as the probable reservoir of SARS‐CoV‐2 (Malik et al. 2020). Even though SARS‐CoV‐2 like CoVs has been isolated from Malayan Pangolins, the likelihood of pangolins acting as the intermediate host is low (Tiwari et al. 2020).
CoVs are classified into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus (Fig. 1). Canine and feline CoVs (CCoV and FCoV) are mostly alphacoronaviruses, while zoonotic CoVs, such as SARS‐CoV, MERS‐CoV and SARS‐CoV‐2 that infect human beings, are betacoronaviruses (Brownlie & Sibley 2020). CCoV is an enterotropic virus found in dogs that causes mild to severe diarrhoea in pups. It has two different genotypes, designated type I (CCoV‐I) and type II (CCoV‐II) (Decaro & Buonavoglia 2011). However, a highly virulent and pathogenic variant of CCoV (pantropic CCoV) identified later, caused fatal systemic disease in pups (Buonavoglia et al. 2006, Zicola et al. 2012). The pups infected with pathogenic variant of CCoV exhibited clinical signs such as fever, inappetence, lethargy, haemorrhagic diarrhoea, vomiting and neurologic signs (ataxia, seizures) (Buonavoglia et al. 2006). Apart from the enterotropic CCoVs (Alphacoronavirus), dogs reportedly harbour the canine respiratory coronavirus (CRCoV), a virus genetically and antigenically unrelated to other CoVs. CRCoV is a unique species and belongs to the Betacoronavirus genus (Erles et al. 2003, Decaro & Buonavoglia 2011). CRCoV is now considered as a common and widespread cause of canine infectious respiratory disease complex (CIRDC). It is associated with mild respiratory disease characterised by clinical signs such as nasal discharge and persistent cough (Priestnall 2020).
FIG 1.
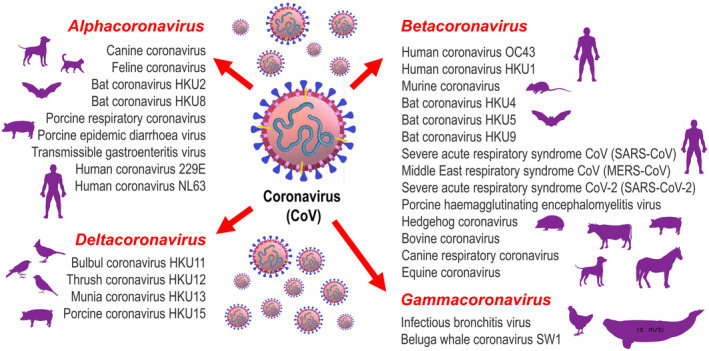
Classification of CoV genera – Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus. Some examples from each genus are shown
FCoV belongs to the genus Alphacoronavirus, and avirulent enteric FCoV strains can cause mild diarrhoea in cats. Kittens infected with FCoV have also reported to occasionally exhibit upper respiratory tract signs (Addie & Jarrett 1992). In a small percentage of cats, however, a mutant variant of FCoV causes feline infectious peritonitis, a fatal immune‐mediated disease (Barr 1998, Hartmann 2005). FCoVs comprise the avirulent and hyper‐virulent biotypes, referred as feline enteric coronavirus and feline infectious peritonitis virus, respectively (Addie et al. 2020). The clinical signs of feline infectious peritonitis vary depending upon the involvement of organs like liver, pancreas, kidneys and eyes. CNS involvement has also been reported in some cases (Hartmann 2005).
SARS‐CoV‐2, the aetiological agent responsible for coronavirus disease 2019 (COVID‐19) belongs to the genus Betacoronavirus. This novel virus is related to the highly pathogenic CoVs, SARS‐CoV and MERS‐CoV (Chen et al. 2020). Betacoronaviruses that infect canines, felines and other animals have a limited correlation with SARS‐CoV‐2; however, several companion and wild animals have tested positive for SARS‐CoV‐2 infection in the past several weeks, indicating the possibility of human‐to‐animal transmission. To date, pet dogs, pet cats, tigers, lions and minks tested positive for SARS‐CoV‐2 (American Veterinary Medical Association 2020, Gollakner & Capua 2020, Halfmann et al. 2020, Wageningen Bioveterinary Research 2020). SARS‐CoV‐2 RNA and antibodies were detected in 2 of 15 tested asymptomatic dogs (Pomeranian and German shepherd) who lived with COVID‐19‐positive owners in Hong Kong SAR. According to genetic analysis, the virus retrieved from these dogs resembled that of human SARS‐CoV‐2, evidencing human‐to‐animal transmission of SARS‐CoV‐2; however, animal‐to‐human transmission remains uncertain (Almendros & Gascoigne 2020, Sit et al. 2020, Mallapaty 2020b). The low susceptibility of dogs to SARS‐CoV‐2 infection was confirmed by x‐ray structures of the human host receptor angiotensin‐converting enzyme 2 (ACE2) bound to the receptor‐binding domain of the SARS‐CoV‐2 spike protein; dogs displayed relatively low levels of ACE2 in the respiratory tract (Zhai et al. 2020).
Further, we analysed the complete genome sequences of SARS‐CoV and SARS‐CoV‐2 of human and animal origin belonging to the genus Betacoronavirus and other CoVs of animal origin (canine and feline) within the genus Alphacoronavirus (Fig. 2). Upon sequence alignment using ClustalW in MEGA software and phylogenetic analysis using Splits Tree software, we found that CoVs of animal origin dispersed in separate clades. CoV isolates belonging to a particular subgenus of the Betacoronavirus genus clustered in their respective clades. Similarly, canine and feline CoVs belonging to the genus Alphacoronavirus formed a clade distant from those of Betacoronavirus isolates. Canine and feline CoVs had a common ancestral origin and were considerably different from SARS‐CoVs as they shared only 44.0–44.5% similarity with SARS‐CoV and SARS‐CoV‐2 at the nucleotide level. The canine betacoronavirus CRCoV clustered in the Embecovirus subgenus, whereas the SARS‐CoV and SARS‐CoV‐2 isolates clustered in the Sarbecovirus subgenus clade. CRCoV shared 45.8‐46.2% similarity with SARS‐CoV‐2. On the other hand, SARS‐CoV‐2 isolated from tiger (MT065033) and mink (MT396266) were highly similar to human SARS‐CoV‐2 isolates, sharing 99.6‐99.9% similarity. Similarity indices between SARS‐CoV‐2 and other CoV species of animal origin within the Sarbecovirus subgenus, such as pangolin CoVs (MP789; MT081071), SARS‐like bat CoVs (CoVZXC21; MG772934) and bat CoVs (RaTG13; MN996532), varied between 86.6 and 96.3%. Moreover, SARS‐CoVs and MERS‐CoV showed 80.6‐81.1% and 51% similarity, respectively, with SARS‐CoV‐2 at the nucleotide level (Fig. 2). Our analysis demonstrated a high divergence between canine/feline CoVs and SARS‐CoV‐2.
FIG 2.
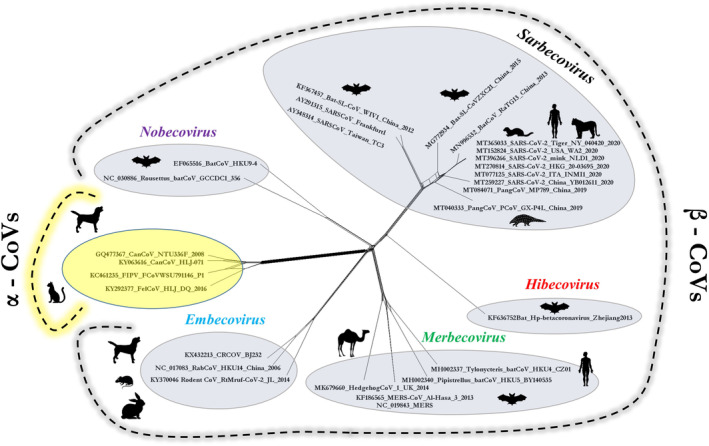
Splits Tree Analysis of complete genomes of CoVs. Complete genome‐based phylogenetic analysis (Splits Tree 4.0) of SARS‐CoV‐2 and SARS‐CoVs of human and animal origin (Betacronavirus) and other CoVs of canine‐feline origin (Alphacoronavirus). The analysis included all five defined subgenera of Betacoronavirus: Sarbecovirus, Embecovirus, Merbecovirus, Nobecovirus and Hibecovirus. Isolates from Alphacoronavirus and Betacoronavirus genera are highlighted in yellow and grey, respectively
Wageningen Bioveterinary Research (WBVR) has recently confirmed SARS‐CoV‐2 infection in minks at two mink farms in the Netherlands. The affected minks showed signs of respiratory illness, and it is suspected that COVID‐19‐positive employees have infected them (Wageningen Bioveterinary Research 2020). An experimental study assessed the susceptibility of certain animal species to SARS‐CoV‐2, revealing that ferrets and cats were highly susceptible to SARS‐CoV‐2 (Shi et al. 2020). In contrast, dogs exhibited low susceptibility, whereas ducks, chickens and pigs were not infected at all. The same study also reported the transmission of SARS‐CoV‐2 from infected to naïve cats via respiratory droplets, indicating an active airborne infection (Shi et al. 2020). These results, however, cannot be extrapolated to natural conditions since a very high viral load was used to induce infection in the animals. As the infected cat did not show any clinical symptoms, the potential for animal‐to‐human transmission was considered negligible (Rodriguez‐Morales et al. 2020, Mallapaty 2020a). However, pet owners, especially cat owners, have to take sufficient precautionary measures to keep their pets safe from SARS‐CoV‐2 infection. Most cases of SARS‐CoV‐2 infection in companion animals, such as dogs and cats, are linked to COVID‐19‐positive owners. During the SARS outbreak in 2003, some cats were found to be positive for SARS‐CoV; however, evidence was lacking to substantiate the transmission of SARS‐CoV from domestic cats to humans (Rodriguez‐Morales et al. 2020; Mallapaty 2020a).
At the time of submission of this manuscript, several domestic cats have been tested positive for SARS‐CoV‐2 worldwide; namely, two in the USA and one each in Hong Kong, Belgium and France (American Veterinary Medical Association 2020, OIE 2020). The two cats that tested positive for SARS‐CoV‐2 in USA were symptomatic and had respiratory illnesses that lasted 8 and 10 days, thus becoming the first case of symptomatic SARS‐CoV‐2 infection in companion animals (Newman et al. 2020). The infected cats developed respiratory disease characterised by lethargy, loss of appetite, sneezing, coughing, watery nasal discharge and clear ocular discharge (Newman et al. 2020). Similarly, the cat tested positive for SARS‐CoV‐2 in France exhibited mild respiratory and digestive signs (anorexia and vomiting) (Sailleau et al. 2020). Hence, we can conclude that SARS‐CoV‐2 infection in cats is predominantly associated with respiratory illness similar to that seen in human beings.
A serological survey in the cat population in Wuhan, China, identified virus‐neutralising antibodies in cats (11 out of 102 tested) (Zhang et al. 2020). However, in another serological study that evaluated 9 cats and 12 dogs living in close contact with COVID‐19‐positive owners, all animals tested negative for SARS‐CoV‐2 antibodies (Temmam et al. 2020). Surveillance systems relying on a single serological test for CoVs can result in antigenic cross‐reactivity and diagnostic errors; therefore, two tests are required for disease confirmation. Previous studies have demonstrated antigenic cross‐reactivity between S1 proteins of FCoV type 1 and porcine epidemic diarrhoea virus (Zhao et al. 2019).
Following sporadic reports of SARS‐CoV‐2 infection in dogs, Goumenou et al. (2020) emphasised the possibility of dogs acting as intermediate hosts in transmitting the disease to humans. The authors further linked the exponential increase in COVID‐19‐positive cases in Northern Italy to the increased contact between dogs and Italian families (Goumenou et al. 2020). However, such claims require the support of valid evidence. As of now, only two dogs have been tested positive for SARS‐CoV‐2 infection (American Veterinary Medical Association 2020). Deng et al. (2020) performed a large‐scale serological survey to detect SARS‐CoV‐2‐specific antibodies in 35 animal species, including dogs and cats; however, all 1914 serum samples were tested negative for SARS‐CoV‐2 antibodies (Deng et al. 2020). Similarly, IDEXX, a leading veterinary diagnostic company, has tested thousands of samples collected from dogs and cats for SARS‐CoV‐2 antibodies but also without success (IDEXX 2020).
The high genetic similarity of CoVs isolated from horseshoe bat (Rhinolophus affinis) and pangolin (Manis javanica) to SARS‐CoV‐2 supports the suggestion that bats and pangolins serve as viral reservoirs for SARS‐CoV‐2. The increasing fear of pets transmitting SARS‐CoV‐2 to humans has already resulted in large‐scale pet abandonment in some places. These inhumane activities can significantly affect animal well‐being and welfare. As the number of COVID‐19 cases is increasing day by day, there might be an unprecedented increase in SARS‐CoV‐2 infections in several animal species due to human‐to‐animal transmission. Even though disease surveillance systems are monitoring the progression of COVID‐19 in humans, minimal data are available on the prevalence of SARS‐CoV‐2 infection in animals. Considering the possible impact of animal reservoirs on the transmission of SARS‐CoV‐2, there is an urgent need for establishing One Health surveillance systems that bring animals under a common surveillance umbrella. Understanding the mutation of feline enteric coronavirus into feline infectious peritonitis virus may provide an insight into the pathogenicity of SARS‐CoV‐2 (Olsen et al. 2020). Attaining knowledge of animal CoVs by studying their transmission, pathogenesis, ecology and evolution is of paramount significance in our journey to understand how zoonotic CoVs cross species barriers and adapt to new host species.
Conflict of interest
No conflicts of interest to declare.
References
- Addie, D. D. & Jarrett, O. (1992) A study of naturally occurring feline coronavirus infections in kittens. Veterinary Record 130, 133‐137. 10.1136/vr.130.7.133 [DOI] [PubMed] [Google Scholar]
- Addie, D. , Houer, L. , Maitland, K. , et al (2020) Effect of cat litters on feline coronavirus infection of cell culture and cats. Journal of Feline Medicine and Surgery 22, 350‐357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros, A. & Gascoigne, E. (2020) Can companion animals become infected with Covid‐19? Veterinary Record 186, 419‐420 [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association . (2020) https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets. Accessed May 11, 2020.
- Barr, F. (1998) Feline infectious peritonitis. Journal of Small Animal Practice 39, 501‐504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie, J. & Sibley, D. (2020) What can animal coronaviruses tell us about emerging human coronaviruses? Veterinary Record 186, 446‐448 [DOI] [PubMed] [Google Scholar]
- Buonavoglia, C. , Decaro, N. , Martella, V. , et al (2006) Canine coronavirus highly pathogenic for dogs. Emerging Infectious Diseases 12, 492‐294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Li, X. , Li, S. , et al (2020) How related is SARS‐CoV‐2 to other coronaviruses? Veterinary Record 186, 496 [DOI] [PubMed] [Google Scholar]
- Decaro, N. & Buonavoglia, C. (2011) Canine coronavirus: not only an enteric pathogen. Veterinary Clinics of North America: Small Animal Practice 41, 121‐132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Martella, V. , Saif, L. J. , et al (2020) COVID‐19 from veterinary medicine and one health perspectives: what animal coronaviruses have taught us. Research in Veterinary Science 131, 21‐23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, J. , Jin, Y. , Liu, Y. , et al (2020) Serological survey of SARS‐CoV‐2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transboundary and Emerging Diseases 67, 1745‐1749. 10.1111/tbed.13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles, K. , Toomey, C. , Brooks, H. W. , et al (2003) Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310, 216‐223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollakner, R. & Capua, I. (2020) Is COVID‐19 the first pandemic that evolves into a panzootic? Veterinaria Italiana 56, 7‐8 [DOI] [PubMed] [Google Scholar]
- Goumenou, M. , Spandidos, D. A. & Tsatsakis, A. (2020) Possibility of transmission through dogs being a contributing factor to the extreme Covid‐19 outbreak in North Italy. Molecular Medicine Reports 21, 2293‐2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , et al (2020) Transmission of SARS‐CoV‐2 in domestic cats. The New England Journal of Medicine. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, K. (2005) Feline infectious peritonitis. Veterinary Clinics of North America: Small Animal Practice 35, 39‐79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDEXX . (2020) www.idexx.com/en/about-idexx/news/no-covid-19-cases-pets. Accessed May 11, 2020.
- Malik, Y. S. , Sircar, S. , Bhat, S. , et al (2020) Emerging novel coronavirus (2019‐nCoV)‐current scenario, evolutionary perspective based on genome analysis and recent developments. The Veterinary Quarterly 40, 68‐76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty, S. (2020a) Coronavirus can infect cats – dogs, not so much. Nature. 10.1038/d41586-020-00984-8 [DOI] [PubMed] [Google Scholar]
- Mallapaty, S. (2020b) Dogs caught coronavirus from their owners, genetic analysis suggests. Nature. 10.1038/d41586-020-01430-5 [DOI] [PubMed] [Google Scholar]
- Newman, A. , Smith, D. , Ghai, R. R. , et al (2020) First reported cases of SARS‐CoV‐2 infection in companion animals – New York, March‐April 2020. MMWR. Morbidity and Mortality Weekly Report 69, 710‐713. 10.15585/mmwr.mm6923e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2020) https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/. Accessed May 11, 2020.
- Olsen, M. , Cook, S. E. , Huang, V. , et al (2020) Perspectives: potential therapeutic options for SARS‐CoV‐2 patients based on feline infectious peritonitis strategies: central nervous system invasion and drug coverage. International Journal of Antimicrobial Agents 55, 105964 10.1016/j.ijantimicag.2020.105964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall, S. L. (2020) Canine respiratory coronavirus: a naturally occurring model of COVID‐19? Veterinary Pathology, 57(4), 467‐471. 10.1177/0300985820926485 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Dhama, K. , Sharun, K. , et al (2020) Susceptibility of felids to coronaviruses. Veterinary Record 186, e21 10.1136/vr.m1671 [DOI] [PubMed] [Google Scholar]
- Sailleau, C. , Dumarest, M. , Vanhomwegen, J. , et al (2020) First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transboundary and Emerging Diseases. 10.1111/tbed.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , et al (2020) Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus. Science 368, 1016‐1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. C. , Brackman, C. J. , Ip, S. M. , et al (2020) Infection of dogs with SARS‐CoV‐2. Nature. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmam, S. , Barbarino, A. , Maso, D. , et al (2020) Absence of SARS‐CoV‐2 infection in cats and dogs in close contact with a cluster of COVID‐19 patients in a veterinary campus. bioRxiv. 10.1101/2020.04.07.029090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, R. , Dhama, K. , Sharun, et al (2020). COVID‐19: animals, veterinary and zoonotic links. Veterinary Quarterly, 40(1), 169–182. 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wageningen Bioveterinary Research . (2020) https://www.wur.nl/en/Research-Results/Research-Institutes/Bioveterinary-Research/show-bvr/COVID-19-detected-on-two-mink-farms.htm. Accessed May 11, 2020.
- Zhai, X. , Sun, J. , Yan, Z. , et al (2020) Comparison of SARS‐CoV‐2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. Journal of Virology, 94(15), e00831–20. 10.1128/JVI.00831-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Huang, K. , et al (2020) SARS‐CoV‐2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 10.1101/2020.04.01.021196 [DOI] [Google Scholar]
- Zhao, S. , Li, W. , Schuurman, N. , et al (2019) Serological screening for coronavirus infections in cats. Viruses 11, 743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicola, A. , Jolly, S. , Mathijs, E. , et al (2012) Fatal outbreaks in dogs associated with pantropic canine coronavirus in France and Belgium. Journal of Small Animal Practice 53, 297‐300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgements
All authors acknowledge their respective Institutes and Universities. This compilation is a review article written, analysed and designed by its authors and required no substantial funding to be stated. All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest. KS and KD involved in the conceptualization, design, interpretation of data and manuscript drafting. SS and YSM involved in the genetic analysis, manuscript drafting and reviewing. RKS involved in checking and approving the final version of the manuscript. All authors agree to be accountable for its contents.
Contributor Information
Y. S. Malik, Email: malikyps@gmail.com.
K. Dhama, Email: kdhama@rediffmail.com.


