Abstract
Low levels of serum albumin may increase the risk of infections and mortality in critically ill patients. We tested the hypothesis that admission hypoalbuminemia predicted infectious complications and poor outcome in subjects with acute intracerebral hemorrhage (ICH).
We analyzed a single center cohort of ICH patients collected between 1994 and 2015. Pneumonia, urinary tract infection and sepsis were retrospectively identified, according to validated criteria. Serum albumin was measured on admission and hypoalbuminemia was defined as total albumin ≤ 3.5 g/dL. The association between albumin levels, infections and mortality at 90 days was tested with multivariable logistic regression analyses.
A total of 2010 patients were included (median age 74 years, 54.5% males) of whom 444 (22.1%) had hypoalbuminemia on admission and 763 (38%) died within 90 days. The frequency of pneumonia, urinary tract infection and sepsis was 19.9%, 15.1% and 2.7% respectively. Hypoalbuminemic patients had lower admission Glasgow coma scale, higher frequency of intraventricular hemorrhage and were more likely to have a history of chronic kidney or liver disease. After adjustment for potential confounders, hypoalbuminemia was an independent predictor of pneumonia (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.34-2.33, p <0.001) and sepsis (OR 2.29, 95% CI 1.22-4.30, p=0.010). Low levels of albumin were also independently associated with higher mortality at 90 days (OR 1.78, 95% CI 1.30-2.44, p<0.001).
In conclusion, early hypoalbuminemia is common and predicts poor outcome in ICH patients. Increased susceptibility to pneumonia and sepsis may be the pathophysiological mechanism underlying this association.
Keywords: Stroke, Intracerebral Hemorrhage, Albumin, Pneumonia, Sepsis, Outcome
INTRODUCTION
The presence of low levels of serum albumin on admission is a strong validated predictor of poor outcome in critically ill patients [1, 2]. Early hypoalbuminemia is also associated with poor outcome in ischemic stroke and subarachnoid hemorrhage [3–5] whereas the frequency and clinical relevance of serum albumin levels in patients with acute intracerebral hemorrhage (ICH) remains unknown. Furthermore, the biological mechanisms underlying the association between circulating albumin and unfavorable outcome in stroke patients are poorly characterized. Previous reports suggested an inverse relationship between serum albumin and the risk of infections in hospitalized patients with ischemic stroke or other non-neurological critical illnesses [6–9]. This may be because besides the well known functions in fluid-electrolyte albumin may also play an immunomodulatory role [10, 11]. Circulating albumin interacts with multiple inflammatory mediators, promotes neutrophils degranulation and enhances phagocytosis [12, 13]. Thus, a suboptimal level of serum albumin could reduce the efficiency of the immune system leading to higher propensity to develop infectious complications. Infections are a frequent complication and a major cause of morbidity and mortality in stroke patients and may therefore represent the pathophysiological mechanism underlying the association between low serum albumin and poor outcome. However, there is great heterogeneity in how clinicians diagnose infectious complications [14] and most of the previously published studies on this topic are limited by the lack of standardized criteria for their assessment.
In this study we aimed at investigating the frequency and clinical relevance of admission hypoalbuminemia in patients admitted for ICH. In particular, we tested the hypothesis that the presence of early hypoalbuminemia is independently associated with increased risk of infectious complications and unfavorable outcome.
METHODS
Study Design and Patient Selection
The Institutional Review Board (IRB) approved all the aspects of the study. We obtained informed written or verbal consent from patients or family members or the acquisition of consent was waived by the IRB.
Patients with spontaneous ICH admitted at Massachusetts General Hospital from 1994 to 2015 were retrospectively analyzed [15]. For the present analysis we selected patients with: 1) diagnosis of acute non traumatic ICH on non-contrast CT scan 2) serum albumin measurement obtained on admission. We excluded subjects with 1) traumatic intracranial hemorrhage 2) brain lesions like intracranial tumor, aneurysm or other vascular malformation presumed to be the cause of the bleeding 3) hemorrhagic transformation of acute ischemic stroke 4) missing data on infectious complications during hospitalization or missing data on long-term outcome.
Image acquisition and analysis
The diagnosis of acute ICH was based on axial non-contrast CT images acquired with the following protocol: 5 mm thickness slices, 120-140 kVp, 10-500 mA. Trained readers reviewed all the scans for determination of ICH location (Lobar, deep and cerebellar) and presence of intraventricular hemorrhage (IVH). Admission ICH volume was determined with semi-automated computer assisted technique (Analyze Direct 11.0 software). Intracerebral hemorrhage expansion was defined as ICH growth greater than 30% or 6 mL from baseline ICH volume [15].
Clinical Variables
Demographic and clinical variables and information on past medical history were collected through retrospective review of hospital charts. We assessed the presence of the following conditions: history of hypertension, diabetes mellitus, hypercholesterolemia, chronic liver disease, chronic kidney disease (defined as estimated glomerular filtration rate < 60 mL/min per 1.73 m2) [16], antiplatelet therapy and oral anticoagulation as previously reported [15].We also collected data on pre-stroke functional status and functional dependence was defined as requiring assistance in at least one instrumental activity of daily living before the index ICH [17]. Serum albumin was measured within 48 h from stroke onset and hypoalbuminemia was defined as admission serum albumin ≤ 3.5 g/dL [18, 19].
Given the great heterogeneity in the definition of low serum albumin [1, 2, 9], we repeated all the analyses using two different cutoffs for the definition of hypoalbuminemia (3.4 g/dL and 4.0 g/dL).
Infections and Mortality
The presence of infectious complications during the hospital stay was determined according to validated criteria with retrospective review of hospital charts, laboratory and radiological tests performed by two investigators (AM, SM). Both the investigators were blinded to serum albumin levels and outcome. Pneumonia was diagnosed in case of typical symptoms of lower respiratory tract infection with confirmatory chest x-ray changes [20]. The diagnosis of urinary tract infection was based on the presence of positive urine culture without evidence of contaminating bacteria [21]. The following criteria for sepsis were adopted: presence of a documented source of infection associated with evidence of acute organ dysfunction [22].
The case-fatality rate at 90 days was assessed through follow-up telephone calls and querying of the Social Security Death Index (SSDI) national database as previously described [23].
Statistical Analysis
Categorical variables were expressed as count (%) and continuous variables as median (interquartile range, IQR) and compared using the χ2 test or Mann–Whitney U test respectively.
We used a logistic regression analysis to investigate the association between serum albumin and infectious complications, adjusting the model for known predictors of infections in stroke patients [24, 25]. The influence of hypoalbuminemia on mortality was studied with multivariable logistic regression as well, adjusting for previously reported independent predictors of mortality in ICH [26]. All the multivariable regression models were repeated including all the variables with p value < 0.1 in univariate analysis. The predicted probability of developing pneumonia or sepsis was obtained combining individual level data with estimates from the multivariable logistic regression model and analyzed as a continuous variable ranging from 0 to 1.
The threshold for statistical significance was set at p values < 0.05 and the analyses were performed using the statistical software SPSS v. 21, 2012 (www.spss.com).
RESULTS
A total of 2010 subjects met the inclusion criteria for the present analysis (median age 74 years, 54.5% males). Missing albumin led to the exclusion of 340 patients whereas 53 patients were excluded because of missing clinical or outcome data. Compared with patients included in the analysis, excluded subjects were older, had higher admission GCS and were less likely to have IVH, liver disease and medical history of diabetes mellitus. All the remaining variables were similar between the two groups (all p >0.10).
Hypoalbuminemia was present in 444 (22.1%) subjects and a total of 400 (19.9%) and 54 (2.7%) patients developed pneumonia and sepsis respectively. The case fatality rate at 90 days was 38%.
Hypoalbuminemic patients had a lower GCS at presentation and a higher frequency of IVH. In addition, they had a higher prevalence of chronic liver and renal disease and were more frequently on antiplatelet treatment. The comparison between patients with and without hypoalbuminemia is shown in table 1.
Table 1.
Comparison between patients with and without admission hypoalbuminemia (n = 2010 )
| Hypoalbuminemia | |||
|---|---|---|---|
| NO (n = 1566 ) | YES (n = 444 ) | p | |
| Age, median (IQR), y | 74 (64 - 82) | 75 (66 -82) | 0.175 |
| Sex, male, n (%) | 852 (54.4) | 243 (54.7) | 0.904 |
| History of hypertension, n (%) | 1230 (78.5) | 350 (78.8) | 0.897 |
| History of diabetes, n (%) | 332 (21.2) | 113 (25.5) | 0.057 |
| History of hypercholesterolemia, n (%) | 593 (37.9) | 115 (25.9) | <0.001 |
| History of chronic liver disease, n (%) | 35/1340 (2.6) | 27/299 (9.0) | <0.001 |
| History of chronic kidney disease, n (%) | 493 (31.5) | 183 (41.2) | <0.001 |
| Prior dependence, n (%) * | 192/1211 (15.9) | 55/313 (17.6) | 0.462 |
| Antiplatelet treatment, n (%) | 767 (49.0) | 245 (55.2) | 0.021 |
| Warfarin treatment, n (%) | 326 (20.8) | 107 (24.1) | 0.138 |
| Baseline ICH volume, median (IQR), mL | 18 (6 - 47) | 21 (6 - 53) | 0.174 |
| ICH expansion, n (%) ** | 175/1027 (17.0) | 36/210 (17.1) | 0.971 |
| Admission GCS, median (IQR) | 14 (7 - 15) | 11 (6 - 15) | <0.001 |
| ICH location | 0.334 | ||
| Lobar, n (%) | 656 (41.9) | 173 (39.0) | |
| Deep, n (%) | 728 (46.5) | 224 (50.5) | |
| Infratentorial, n (%) | 182 (11.6) | 47 (10.6) | |
| IVH presence, n (%) | 771 (49.2) | 244 (55.0) | <0.001 |
| Intubation, n (%) | 525/1547 (33.9) | 137/432 (31.7) | 0.386 |
| Surgery, n (%) | 94/1560 (6.0) | 34 (7.7) | 0.194 |
| Pneumonia, n (%) | 282 (18.0) | 118 (26.6) | <0.001 |
| Urinary tract infection, n (%) | 238 (15.2) | 66 (14.9) | 0.863 |
| Sepsis, n (%) | 36 (2.3) | 18 (4.1) | 0.043 |
| 90-day mortality, n (%) | 554 (35.4) | 209 (47.1) | <0.001 |
IQR indicates inter-quartile range; ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; IVH, intraventricular hemorrhage.
Pre-stroke dependence was defined as requiring assistance in at least one instrumental activity of daily living.
ICH expansion was defined as growth >30% or > 6 mL from baseline volume.
Infectious complications and 90-day mortality
Hypoalbuminemia was associated with higher prevalence of pneumonia and sepsis in univariate analysis (table 1). After adjustment for multiple potential confounders, the presence of hypoalbuminemia remained an independent predictor of pneumonia (odds ratio, [OR] 1.76, p <0.001) and sepsis (OR 2.29, p = 0.010) as summarized in table 2. This association remained significant when serum albumin was analyzed as a continuous variable (table 2). Each 1 g/dL increase in the concentration of serum albumin was indeed associated with a 43% and 53% reduction in the risk of pneumonia and sepsis respectively.
Table 2.
Multivariable analysis of infectious complications
| MODEL 1 | ||||||
|---|---|---|---|---|---|---|
| Pneumonia | UTI | Sepsis | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Hypoalbuminemia | 1.76 (1.34-2.33) | <0.001 | 1.05 (0.75-1.47) | 0.772 | 2.29 (1.22-4.30) | 0.010 |
| Total albumin | 0.67 (0.53-0.83) | <0.001 | 0.84 (0.64-1.10) | 0.213 | 0.57 (0.35-0.95) | 0.032 |
| MODEL 2 | ||||||
| Pneumonia | UTI | Sepsis | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Hypoalbuminemia | 1.67 (1.21-2.30) | 0.002 | 1.06 (0.72-1.56) | 0.786 | 3.63 (1.76-7.48) | <0.001 |
| Total albumin | 0.73 (0.57-0.95) | 0.018 | 0.82 (0.61-1.11) | 0.200 | 0.39 (0.22-0.67) | 0.001 |
Model 1 was adjusted for age, admission GCS, baseline ICH volume, IVH, ICH location, intubation and surgery.
Model 2 was adjusted for age, admission GCS, baseline ICH volume, IVH, ICH location, intubation, surgery, history of hypercholesterolemia, history of diabetes mellitus, antiplatelet treatment, chronic liver disease and chronic kidney disease.
GCS indicates Glasgow Coma Scale; ICH, intraverebral hemorrhage; IVH intraventricular hemorrhage; OR, odds ratio; CI, confidence interval.
The unadjusted case-fatality rate at 90 days was significantly higher in patients with low albumin levels (47.1% vs 35.4%, p <0.001). Early hypoalbuminemia remained associated with increased risk of 90-day mortality (OR 1.95, p < 0.001) after accounting for known predictors of outcome in ICH and other confounders (table 3). The strength of the relationship between hypoalbuminemia and mortality was reduced by the inclusion of sepsis and pneumonia in the multivariable regression model (OR 1.43, p = 0.023).
Table 3.
Multivariable analysis of 90-day mortality
| MODEL 1 | MODEL 2 | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Hypoalbuminemia | 1.78 (1.30-2.44) | <0.001 | 1.95 (1.35-2.82) | <0.001 |
| Total albumin | 0.67 (0.52-0.87) | 0.002 | 0.69 (0.52-0.92) | 0.012 |
Model 1 was adjusted for age, admission GCS, baseline ICH volume, IVH and ICH location.
Model 2 was adjusted for age, admission GCS, baseline ICH volume, IVH, ICH location, history of hypercholesterolemia, history of diabetes mellitus, antiplatelet treatment, chronic liver disease and chronic kidney disease.
GCS indicates Glasgow Coma Scale; ICH, intraverebral hemorrhage; IVH intraventricular hemorrhage; OR, odds ratio; CI, confidence interval.
We repeated all the analyses after exclusion of patients with a medical history of chronic liver or renal disease. In this population, hypoalbuminemia remained associated with increased susceptibility to pneumonia (OR 1.72, p = 0.005) and sepsis (OR 2.67, p = 0.031) and predicted unfavorable outcome as well (OR for 90-day mortality 1.99, p = 0.012). All these associations remained significant also when liver disease was analyzed as a categorical variable with the additional unavailable category.
Hypoalbuminemia was an independent predictor of pneumonia (OR 1.74, p = 0.001), sepsis (OR 2.57, p = 0.011) and three-months mortality (OR 1.79, p = 0.002) also after exclusion of patients on anticoagulant treatment.
We confirmed all the results using two different definitions of hypoalbuminemia as well (serum albumin ≤ 3.4 g/dL and serum albumin ≤ 4.0 g/dL).
Finally, increasing serum albumin was associated with lower risk of pneumonia and sepsis in a linear, dose-response relationship (p for trend < 0.001) as represented in figure 1.
Figure 1.
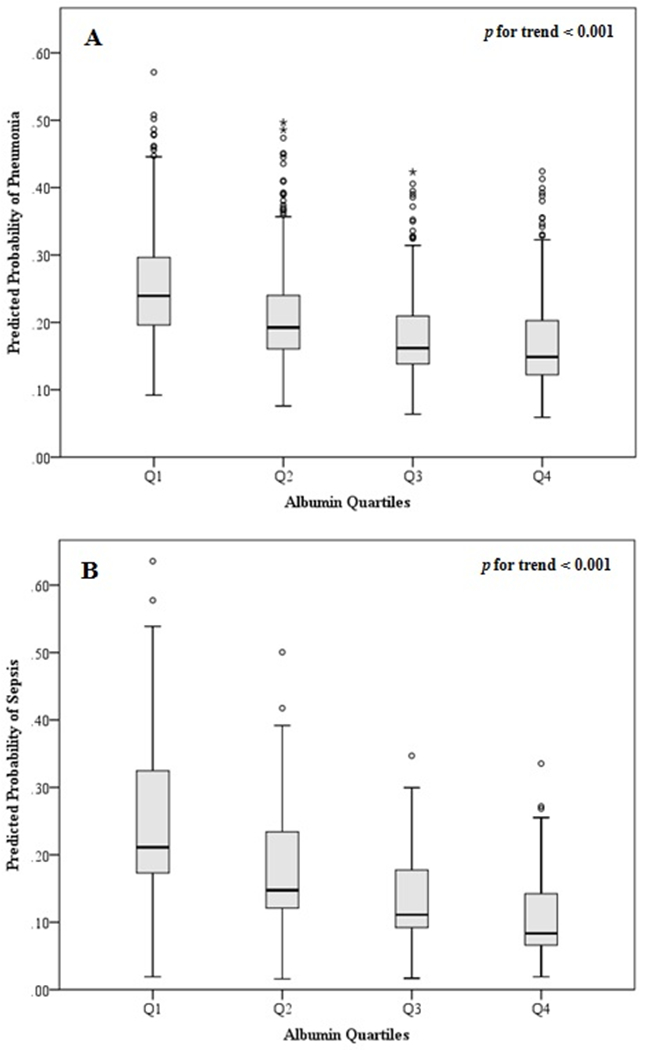
Association between admission albumin level and risk of pneumonia and sepsis.
Predicted probability of pneumonia (A) and sepsis (B) stratified by albumin quartiles.
P value for trend was calculated with the Jonckheere-Terpstra test.
DISCUSSION
In this single-center cohort of ICH patients we found that low levels of serum albumin on admission independently predict unfavorable outcome. We also provide novel data regarding the pathophysiological mechanism underlying this relationship. Early hypoalbuminemia was an independent predictor of pneumonia and sepsis and infectious complications may therefore be the biological mechanism by which hypoalbuminemia is associated with increased mortality.
In our cohort, hypoalbuminemic patients were more likely to have intraventricular extension of the bleeding and lower admission GCS. Circulating albumin is a negative acute phase reactant [5, 18] and hypoalbuminemia could therefore be a surrogate marker of ICH severity. However the association between albumin and 90-day mortality remained significant after adjustment for different measures of ICH severity and known predictors of outcome [26]. Hypoalbuminemia may also arise from metabolic stress response and poor nutritional support [27, 28]. Circulating albumin has a half life ranging from 18 to 20 days and serum albumin was measured in the hyperacute phase in our study [18]. Thus it appears unlikely that hypoalbuminemia simply reflects an acute drop in serum albumin that is proportional to ICH severity. Chronic liver disease or kidney disease may also be relevant confounders [29, 30]. Again, however, our findings remained significant after accounting for these comorbidities in multivariable analysis. Finally, hypoalbuminemia remained an independent predictor of infections and mortality in a sensitivity analysis excluding patients with hepatic or renal dysfunction.
Another possibility is that hypoalbuminemic patients are more sensitive to vitamin k antagonists because of reduced protein binding or impaired synthesis of coagulation factors secondary to chronic liver disease [31, 32]. However early hypoalbuminemia was not associated with larger ICH volume or higher frequency of ICH expansion, suggesting that the relationship between low albumin and unfavorable outcome is not explained by increased extent of bleeding. Furthermore, low albumin on admission predicted pneumonia, sepsis and 90-day mortality also after exclusion of subjects on anticoagulant therapy.
Our findings corroborate a previously reported association between hypoalbuminemia and increased mortality in a small cohort of ICH patients [33]. We also report novel data showing an association between low serum albumin and increased risk of pulmonary infections and sepsis whereas there was no association with urinary tract infections. The pathophysiology of inflammation and immunodepression after stroke is complex [34] and the exact mechanism of any association between albumin and infections is not yet clear.
Accumulating evidence suggested an immunomodulatory role of circulating albumin [10, 35, 36]. Albumin is indeed the main transporter of several inflammatory mediators, binds bacterial lipopolysaccharide and promotes phagocytosis [12, 13, 37] . Low levels of serum albumin may therefore increase the susceptibility to infectious complications. Albumin could also be a marker of poor nutritional status and hypoalbuminemia might be a simple marker of malnutrition which in turn is a major cause of impaired immune response and a strong predictor of nosocomial infections [38, 39].
In line with this, admission hypoalbuminemia was linked to the risk of stroke-associated pneumonia in a large cohort of ischemic stroke patients [7]. Infections are common in ICH patients and independently associated with poor outcome [24]. Thus, an increased propensity to develop pneumonia and sepsis may explain the association between hypoalbuminemia and poor outcome in ICH patients.
From a clinical and therapeutic point of view, there is no current evidence to support albumin administration in patients with cerebrovascular diseases. Despite the association between hypoalbuminemia and poor outcome, a recently completed randomized trial showed no benefit of albumin infusion in patients with acute ischemic stroke [40]. However, our findings might still have relevant therapeutic implications. Unlike ischemic stroke, ICH still lacks an acute phase treatment proven to provide benefit [41]. Infections are a common complication of the subacute phase of the disease and represent an appealing therapeutic target [42, 43]. Albumin is a commonly available biomarker that may improve the prediction of infectious complications, leading to preventive strategies in ICH patients. Finally, accurate prediction of prognosis is a key aspect of ICH care [44] and the presence of admission hypoalbuminemia may help clinicians in identifying those patients at high risk of unfavorable outcome.
Some limitations should be acknowledged when interpreting our study. First, our results derive from a single-center retrospective analysis, with ICH patients collected over a long time course, during which multiple changes in ICH management occurred. Second, given the hospital based setting of our analysis pre-stroke albumin levels were not available. Therefore we were not able to investigate the possibility of an acute drop in serum albumin concentration as a consequence of acute intracranial bleeding. Third, serum albumin may also impact ICH outcome through other biological mechanisms like increased perihematomal edema, heart failure or other cardio-pulmonary and renal complications [45, 46] .
CONCLUSION
Admission hypoalbuminemia is common in ICH and identifies patients at high risk of infectious complications and poor outcome. Prospective studies are needed in order to confirm our findings and provide further insights into the biological mechanisms underlying these associations.
Acknowledgments
Sources of funding
This study was supported by the following awards from the NINDS: 5R01NS073344, K23AG02872605, K23 NS086873, R01NS059727.
None of the funding entities had any involvement in study design; data collection, analysis, and interpretation; writing of the manuscript; or decision to submit the study for publication.
Disclosures and Conflicts of Interest
Joshua N. Goldstein reports research funding from Boehringer Ingelheim, Pfizer, and Portola; Advisory Board for Prolong Pharmaceuticals.
Footnotes
Ethical standards
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
REFERENCES
- 1.Caironi P, Gattinoni L (2009) The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus 7:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson JP, Wolmarans MR, Park GR (2000) The role of albumin in critical illness. Br J Anaesth 85:599–610. [DOI] [PubMed] [Google Scholar]
- 3.Idicula TT, Waje-Andreassen U, Brogger J, et al. (2009) Serum albumin in ischemic stroke patients: the higher the better. The Bergen Stroke Study. Cerebrovasc Dis 28:13–7. [DOI] [PubMed] [Google Scholar]
- 4.Dziedzic T, Slowik A, Szczudlik A (2004) Serum albumin level as a predictor of ischemic stroke outcome. Stroke 35:e156–8. [DOI] [PubMed] [Google Scholar]
- 5.Behrouz R, Godoy DA, Topel CH,et al. (2016) Early Hypoalbuminemia is an Independent Predictor of Mortality in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 25:230–6. [DOI] [PubMed] [Google Scholar]
- 6.Cho Y-M, Choi I-S, Bian R-X, et al. (2008) Serum albumin at admission for prediction of functional outcome in ischaemic stroke patients. Neurol Sci 29:445–449. [DOI] [PubMed] [Google Scholar]
- 7.Dziedzic T, Pera J, Klimkowicz A, et al. (2006) Serum albumin level and nosocomial pneumonia in stroke patients. Eur J Neurol 13:299–301. [DOI] [PubMed] [Google Scholar]
- 8.Berger B, Gumbinger C, Steiner T, et al. (2014) Epidemiologic features, risk factors, and outcome of sepsis in stroke patients treated on a neurologic intensive care unit. J Crit Care 29:241–248. [DOI] [PubMed] [Google Scholar]
- 9.Vincent J-L, Dubois M-J, Navickis RJ, Wilkes MM (2003) Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg 237:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler DS, Giuliano JS, Lahni PM, et al. (2011) The immunomodulatory effects of albumin in vitro and in vivo. Adv Pharmacol Sci 2011:691928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantin AM, Paquette B, Richter M, Larivee P (2000) Albumin-mediated Regulation of Cellular Glutathione and Nuclear Factor Kappa B Activation. Am J Respir Crit Care Med 162:1539–1546. [DOI] [PubMed] [Google Scholar]
- 12.Gorudko IV, Grigorieva DV, Shamova EV, et al. (2014) Hypohalous acid-modified human serum albumin induces neutrophil NADPH oxidase activation, degranulation, and shape change. Free Radic Biol Med 68:326–334. [DOI] [PubMed] [Google Scholar]
- 13.Rabaglia JL, Gonzalez R, Moore EE, Harken AH (2002) Pooled human albumin primes neutrophils. J Card Surg 17:209–13. [DOI] [PubMed] [Google Scholar]
- 14.Kishore AK, Vail A, Chamorro A, et al. (2015) How Is Pneumonia Diagnosed in Clinical Stroke Research?: A Systematic Review and Meta-Analysis. Stroke 46:1202–1209. [DOI] [PubMed] [Google Scholar]
- 15.Morotti A, Phuah C-L, Anderson CD, et al. (2016) Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke 47:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster AC, Nagler EV, Morton RL, Masson P (2016) Chronic Kidney Disease. Lancet. [DOI] [PubMed] [Google Scholar]
- 17.Katz S (1983) Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31:721–7. [DOI] [PubMed] [Google Scholar]
- 18.Powner DJ (2011) In my opinion: Serum albumin should be maintained during neurocritical care. Neurocrit Care 14:482–488. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph JL, Jones RN, Levkoff SE, et al. (2009) Derivation and Validation of a Preoperative Prediction Rule for Delirium After Cardiac Surgery. Circulation 119:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CJ, Kishore AK, Vail A, et al. (2015) Diagnosis of Stroke-Associated Pneumonia: Recommendations From the Pneumonia in Stroke Consensus Group. Stroke 46:2335–40. [DOI] [PubMed] [Google Scholar]
- 21.Wilson ML, Gaido L (2004) Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 38:1150–8. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Deutschman CS, Seymour CW, et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–10. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biffi A, Halpin A, Towfighi A, et al. (2010) Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 75:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord AS, Langefeld CD, Sekar P, et al. (2014) Infection after intracerebral hemorrhage: Risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke 45:3535–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy SB, Moradiya Y, Shah J, et al. (2016) Nosocomial Infections and Outcomes after Intracerebral Hemorrhage: A Population-Based Study. Neurocrit Care 1–7. [DOI] [PubMed] [Google Scholar]
- 26.Hemphill JC, Bonovich DC, Besmertis L, et al. (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32:891–7. [DOI] [PubMed] [Google Scholar]
- 27.Hübner M, Mantziari S, Demartines N, et al. (2016) Postoperative Albumin Drop Is a Marker for Surgical Stress and a Predictor for Clinical Outcome: A Pilot Study. Gastroenterol Res Pract 2016:8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preiser J-C, van Zanten ARH, Berger MM, et al. (2015) Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care 19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh NS, Merkler AE, Schneider Y, et al. (2016) Discharge Disposition After Stroke in Patients With Liver Disease. Stroke 48:476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahalom G, Schwartz R, Schwammenthal Y, et al. (2009) Chronic Kidney Disease and Clinical Outcome in Patients With Acute Stroke. Stroke 40:1296–303. [DOI] [PubMed] [Google Scholar]
- 31.Tripodi A, Mannucci PM (2011) The coagulopathy of chronic liver disease. N Engl J Med 365:147–156. [DOI] [PubMed] [Google Scholar]
- 32.Rolan PE (1994) Plasma protein binding displacement interactions--why are they still regarded as clinically important? Br J Clin Pharmacol 37:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limaye K, Yang JD, Hinduja A (2016) Role of admission serum albumin levels in patients with intracerebral hemorrhage. Acta Neurol Belg 116:27–30. [DOI] [PubMed] [Google Scholar]
- 34.Meisel C, Schwab JM, Prass K, et al. (2005) Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 6:775–786. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Martinez R, Andreola F, Mehta G, et al. (2015) Immunomodulatory and antioxidant function of albumin stabilises the endothelium and improves survival in a rodent model of chronic liver failure. J Hepatol 62:799–806. [DOI] [PubMed] [Google Scholar]
- 36.Tang S, Leung JCK, Abe K, et al. (2003) Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 111:515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo V, García-Martinez R, Salvatella X (2014) Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol 61:396–407. [DOI] [PubMed] [Google Scholar]
- 38.Schneider SM, Veyres P, Pivot X, et al. (2004) Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr 92:105–11. [DOI] [PubMed] [Google Scholar]
- 39.Schaible UE, Kaufmann SHE (2007) Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med 4:0806–0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin RH, Yeatts SD, Hill MD, et al. (2016) ALIAS (Albumin in Acute Ischemic Stroke) Trials. Stroke 47:2355–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morotti A, Goldstein JN (2016) Diagnosis and Management of Acute Intracerebral Hemorrhage. Emerg Med Clin North Am 34:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klehmet J, Harms H, Richter M, et al. (2009) Stroke-induced immunodepression and post-stroke infections: Lessons from the preventive antibacterial therapy in stroke trial. Neuroscience 158:1184–1193. [DOI] [PubMed] [Google Scholar]
- 43.Chamorro Á, Urra X, Planas AM (2007) Infection after acute ischemic stroke: A manifestation of brain-induced immunodepression. Stroke 38:1097–1103. [DOI] [PubMed] [Google Scholar]
- 44.Hwang DY, Dell CA, Sparks MJ, et al. (2016) Clinician judgment vs formal scales for predicting intracerebral hemorrhage outcomes. Neurology 86:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belayev L, Saul I, Busto R, et al. (2005) Albumin Treatment Reduces Neurological Deficit and Protects Blood–Brain Barrier Integrity After Acute Intracortical Hematoma in the Rat. Stroke 36:326–31. [DOI] [PubMed] [Google Scholar]
- 46.Balami JS, Buchan AM (2012) Complications of intracerebral haemorrhage. Lancet Neurol 11:101–18. [DOI] [PubMed] [Google Scholar]


