To the Editor,
In general, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) replication in the host reaches its peak in the first week of infection, decreasing rapidly afterward, while some level of immunity is build up. Yet, the infection seems to follow a distinctive course in some individuals, reactivating after the apparent resolution of symptoms. 1 , 2 , 3 We report here the first case to be disclosed of a more vigorous coronavirus disease 2019 (COVID‐19) recurrence with SARS‐CoV‐2 RNA redetection and late antibody response, and also the first to address COVID‐19 recurrence in Brazil.
On 21 April a 26‐year‐old man residing in the metropolitan area of Rio de Janeiro, Brazil, without risk factors for COVID‐19 reported headache and prostration without respiratory symptoms. Two days later, he had respiratory material collected (oropharynx and nasopharynx swabs) for the SARS‐CoV‐2 investigation. Samples were extracted by Quick‐DNA/RNA Viral MagBead Automated Kit (Zymo Research, CA) and tested for SARS‐CoV‐2 by quantitative reverse transcription‐polymerase chain reaction (RT‐qPCR) using Allplex 2019‐nCoV Assay (Seegene Inc, Seoul, Korea). The cycle threshold (C t) values from the qRT‐PCR were measured and the sample tested positive for SARS‐CoV‐2 RNA (E C t: 31.5; RdRp C t: 34.1; N C t: 31.5). Being a mild infection, the patient remained in isolation for 14 days at home, recovering after 3 days of symptoms onset. On 5 May, after a new respiratory swab collection, the viral RNA was no longer detected. Immunoglobulin M (IgM)/IgA and IgG antibodies were negative by COVID‐19 enzyme‐linked immunosorbent assay (ELISA) test (Vircell, Spain). However, 1 month later, on 6 June, the symptoms returned more acutely and included fever (101.3 F), cough, headache, myalgia, arthralgia, anosmia, and fatigue, and lasted for 2 weeks. SARS‐CoV‐2 RNA detection reversed to positive (E C t: 19.9; RdRp C t: 20.8; N C t: 22.7) on 8 June and remained positive in another testing on 16 June (E C t: 32.8; RdRp C t: 33.4; N C t: 34.5), although SARS‐CoV‐2 antibodies remained negative. A chest computed tomography scan performed on 18 June showed typical findings of multiple patchy ground‐glass opacities in the lungs (Figure 1).
Figure 1.
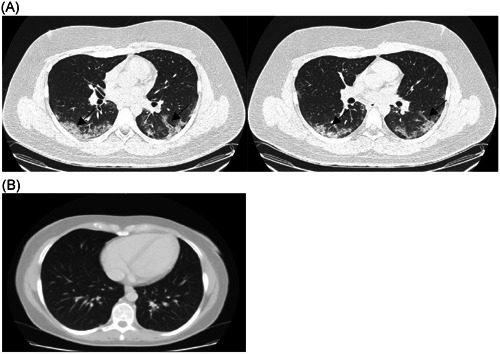
A, Chest computed tomography (CT) images of the patient showing multiple patchy ground‐glass opacities (arrows). B, Normal chest CT image (available at: https://doi.org/10.6001/actamedica.v23i1.3270)
During this second episode of COVID‐19, the patient was treated on an outpatient basis with azithromycin plus analgesics and antipyretics for 6 days when symptoms subsided. On 22 June, IgA/IgM and IgG antibodies were detected in serum and only N gene was detected by RT‐qPCR (C t: 36.6) and finally, on 29 June, no viral genes were further detected. SARS‐CoV‐2 viremia was also investigated after recurrence on 8 and 16 June, showing negative results. The RT‐qPCR and ELISA methodologies used were maintained throughout this investigation.
Different reports indicate that reactivation or reinfection by SARS‐CoV‐2 is possible, although the event appears to be unusual. 4 Although cases vary in terms of serological data, the timing of reactivation and clinics, patients who retested positive to SARS‐CoV‐2 generally have a mild or asymptomatic course, 5 , 6 which is perhaps the result of some level of immunity, while symptomatic reactivation is rare but may happen. 7 Our patient, on the other hand, presented a more potent form of COVID‐19 after more than 40 days from the first mild infection, and with a detectable antibody response only after the second infectious episode. Our hypothesis is that the first mild infection was not sufficient to build up a detectable humoral response, 8 which occurred only after 14 days of a second more severe episode. In addition, the absence of detectable antibodies in the first episode may have allowed for a new infection, rather than a recurrence. However, as we did not investigate viral genetics at different times, such a statement is hypothetical.
A limitation of this study is the absence of cell culture assays, which could indicate the presence of infectious particles. Also, a false‐positive result in the first RT‐qPCR test cannot be excluded, so that the patient only became infected with SARS‐CoV‐2 afterward. 4 However, given the (a) high specificity of RT‐qPCR test; (b) presentation of symptoms coinciding with the positive RT‐qPCR; and (c) viral detection in close family members living in the same residence during COVID‐19 symptoms (data not shown); such false result is unlikely. Regarding the ELISA tests used (S: 100%, E: 92.5%), 9 although its accuracy is compatible do LFA tests, the latter presents more inconsistencies, being useful as a screening tool in the absence of ELISA and RT‐PCR. 10
In this paper, we describe a COVID‐19 recurrence from a mild to a moderate form after convalescence, with RT‐qPCR turning positive and antibody detection after more severe symptoms. These findings, although summarized in a case report, raise questions about the influence of the severity of the infection on the immune response and the host's susceptibility, which can have important epidemiological consequences, and should be better understood.
FUNDING INFORMATION
Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: PQ2; Laboratório Contraprova Análises, Ensino e Pesquisas LTDA.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests
ETHICS STATEMENT
The study was approved by the University Hospital Ethical Committee of the Fluminense Federal University (register 30926020.2.0000.5243).
ACKNOWLEDGMENTS
This paper was supported by Laboratório Contraprova Análises, Ensino e Pesquisas LTDA. Varella RB was partially supported by National Council for Scientific and Technological Development—CNPq.
REFERENCES
- 1. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS‐CoV‐2 in patients recovered from COVID‐19. J Med Virol. 2020. 10.1002/jmv.26056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen D, Xu W, Lei Z, et al. Recurrence of positive SARS‐CoV‐2 RNA in COVID‐19: A case report. Int J Infect Dis. 2020;93(Apr01):297–299. 10.1016/j.ijid.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yee G, Pan Z, Pan Y, et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80(5):e14–e17. 10.1016/j.jinf.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang H, Wang Y, Tong Z, Liu X. Retest positive for SARS‐CoV‐2 RNA of “recovered” patients with COVID‐19: persistence, sampling issues, or re‐infection? J Med Virol. 2020. 10.1002/jmv.26114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanan L, Xu D, Ye G, et al. Positive RT‐PCR test results in patients recovered from COVID‐19. JAMA. 2020;323(15):1502–1503. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao H, Ruan L, Liu J, Liao W. The clinical characteristic of eight patients of COVID‐19 with positive RT‐PCR test after discharge. J Med Virol. 2020. 10.1002/jmv.26017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loconsoleoconsole D, Passerini F, Palmieri VO, et al. Recurrence of COVID‐19 after recovery: a case report from Italy. Infection. 2020;May(16):1–3. 10.1007/s15010-020-01444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin YC, Cheng CY, Chen CP, Cheng SH, Chang SY, Hsueh PR. A case of transient existence of SARS‐CoV‐2 RNA in the respiratory tract with the absence of anti‐SARS‐CoV‐2 antibody response. Int J Infect Dis. 2020;96:464–466. 10.1016/j.ijid.2020.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Clinical performance of different SARS‐CoV‐2 IgG antibody tests. J Med Virol. 2020. 10.1002/jmv.26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ong DSY, de Man SJ, Lindeboom FA, Koeleman JGM. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin Microbiol Infect. 2020;26(8):1094.e7–1094.e10. 10.1016/j.cmi.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]


