Abstract
The pandemic of the new coronavirus disease‐2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), initially described in China, is challenging the health care systems of all countries. Every emerging disease raises many questions with a scarcity of answers since all its characteristics are still being discovered. In the case of SARS‐CoV‐2, most of the literature comes from adult patients. Children seem to be less affected. Pediatric patients diagnosed with COVID‐19 disease usually suffer a mild illness, with a low risk of complications, or mortality. Defining the role of children in the transmission of SARS‐CoV‐2 is critical as some national infection control decisions involving children, such as school closures or social distancing, will probably impact the dynamics of the virus. To aid in the knowledge of COVID‐19 in children, this study presents an expert review of the literature published from 1 January to 28 May 2020, including peer‐reviewed and preprint nonpeer‐reviewed studies, along with some relevant articles afterward, summarizing ten key points that characterize the disease in children.
Keywords: children, COVID‐19, epidemiology, novel coronavirus, SARS‐CoV‐2
1. BACKGROUND
An outbreak of a new coronavirus disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was described in Wuhan (China) in December 2019. The first case in a child was reported on 20 January 2020 in Shenzhen (China). 1 On 11 March 2020, the WHO declared the new coronavirus disease 2019 (COVID‐19) a pandemic. As of 28 May 2020, more than 350 000 patients have died worldwide.
Most literature has focused on adult disease, but this information might not be transferable to children. 2 , 3 Diagnostic limitations during the COVID‐19 pandemic and data loss due to low‐quality retrospective studies have probably led to clinicians attending children to use unreliable information. In addition, the recent use of social media permits the sharing of data but might carry a risk of misinformation, disseminating unproven practices. This review is an effort by authors to collect and briefly present published literature in pediatrics to shed light on the topic.
2. METHODS
This expert review was performed by searching published articles through PubMed using the following search terms: COVID‐19, 2019‐nCoV, SARS‐CoV‐2, or novel coronavirus, along with the term child or children. We searched for articles from 1 January to 20 April, 2020 (just 3 months after the first reported case in pediatrics). A total of 243 studies were collected. Two different authors separately (LEG and DAA) screened titles and abstracts for potential eligibility. These eligible articles included patients less than 18 years old, and for those containing descriptions of symptoms, laboratory signs, and radiology features. Only case series with a number of more than 5 were included. Finally, 62 studies were included. Also, a manual search of some references in the selected articles was completed. Due to constant updating, some non‐peer‐reviewed preprint manuscripts were also reviewed. Furthermore, while this review was being written, relevant pediatric papers have been published, which were considered important to be included, increasing the number of included studies to 92. Confidence intervals (CIs) of proportions were calculated using the Wald test included in the Stata version 15, College Station, TX.
3. EPIDEMIOLOGY
The average age reported of COVID‐19 confirmed cases in most countries is around 50 years old, with small differences depending on the demographic characteristics of each country (Figure 1). Most of the data from several countries place the prevalence of confirmed cases in children around 1% to 2% of all diagnosed cases, 4 , 5 which is strikingly low compared to infections caused by other respiratory viruses. The most recent official report on the epidemiology of COVID‐19 in Spain (18 May 2020), describes among the total notified cases only 0.3% cases in the groups under 10 years old and 0.3% cases in the group 10 to 19 years old. 6 Subsequent studies in the late stage of the epidemic in China have suggested a similar transmission in children. 7 , 8 The data from Iceland offer an accurate view of the age distribution, because the screening included the asymptomatic population. 9 This study describes a lower prevalence in population screening in children under 10 years of age compared with adolescents and adults (0/848 [0%] vs 100/12 232 [0.8%], respectively). Targeted diagnosis also showed a parallel trend: 6.7% vs 13.7% confirmed cases among children less than 10 years old and those 10 years of age or older, respectively. This has been similarly reported in a screening study carried out in Vo, a small population next to Padua, Italy. 10
Figure 1.
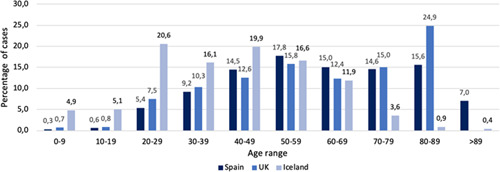
Age distribution of SARS‐CoV‐2 confirmed cases in different countries. Percentages are calculated from the total of confirmed cases in each country. In the case of the UK, the group aged 80 to 89 includes more than 89 years old. In the case of Iceland, the 0 to 9 group includes 0 to 12 years old; the 10 to 19 group includes 13 to 17 years old; the 20 to 29 group includes 18 to 29 years old. Source: Spain (Spanish Ministry of Health; 26/4/2020), UK (Public Health England; 23/4/2020), Iceland (The Directorate of Health and The Department of Civil Protection and Emergency Management, Iceland; 26/4/2020). SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
Seroprevalence studies are being performed in different countries and settings. A preprint study conducted in Geneva (Switzerland) showed an increasing seroprevalence throughout April, from 6.1% to 9.7%. 11 The seroprevalence in the 5 to 19‐year‐old group (6.1%) did not differ (P = .12) from that in the 20 to 49‐year‐old group (8.4%). However the first group did not include children under 5 years old, and it included a broad range of ages. A similar study conducted in Spain (ENE‐COVID19 study) between 27 April to 10 May, coordinated by the Spanish Ministry of Health, has shown a global seroprevalence of 5%. 12 One of the most interesting aspects of this study is the low seroprevalence among children: the younger the age group, the lower the percentage of seroprevalence (1.1% in <1 year old; 2.2% in 1‐4 years old; 3% in 5‐9 years old; 3.9% in 10‐14 years old, and 3.8% in 15‐19 years old).
In several contact‐tracing studies, children do not seem to be the usual source of infection in most cases. 13 , 14 Based on the data currently published, it seems that children have not been a major vector for transmission in the current pandemic, but further information is needed to draw clear conclusions. Whether this lower propensity to acquire and transmit the infection is due to biological resistance or due to less exposure is still a question to be answered.
To evaluate the real impact of COVID‐19 among children, screening strategies, including serological studies, are essential, since children usually have nonsevere symptoms or are even asymptomatic, which implies that they are underdiagnosed in studies following targeted diagnosis strategies. These data will be key to determining the role of children in the transmission of SARS‐CoV‐2 and, as a consequence, adopting decisions regarding nonpharmacologic preventive approaches.
To summarize, the prevalence of COVID‐19 disease in children is lower than in adults. Although initially children were supposed to have a relevant role in the transmission of the infection, several studies suggest that they do not have such an important position.
4. SYMPTOMS
Clinical features seem to be mild in comparison with adults. 15 At the time of writing, at least 3473 COVID‐19 cases had been reported in children (Table 1), but detailed data remain scarce. The best‐established features are the presence or absence of fever and cough, but these have only been recorded in 31% of children in case reports. Fever is the most frequent symptom (58.3%), followed by cough (47.3%) and sore throat (18.3%). Rhinorrhea (15.9%) and gastrointestinal symptoms (12.7%) are also frequent. Some data in adults have established fever (71%‐83%) and cough (65%‐80%) as the most commonly reported symptoms. 15 , 16 Other symptoms in adults, such as headaches or myalgia, are not usually reported in children. Non‐mild disease (defined as pneumonia or need for hospitalization) or a more severe illness accounted for 33.3% and 9.1% of all the cases reported, respectively, in this review.
Table 1.
Case series of COVID‐19 in pediatric patients. Symptoms, laboratory and radiology features. Update: May 24, 2020
| Authors | Wei 16 | Feng 17 | Chen 18 | Cai 19 | Zhou 20 | Wang 21 | Xia 22 | Tang 23 | Liu 24 | Xu 25 | Zhang 26 | Lu 27 | Sun 28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 9 | 15 | 31 | 10 | 9 | 31 | 20 | 26 | 6 | 10 | 34 | 171 | 8 |
| Symptoms | |||||||||||||
| Fever | 4/7b | 5 | 14 | 7 | 4 | 20 | 12 | 11 | 6 | 6 | 26 | 71 | 6 |
| Cough | 2/7 | 1 | 13 | 6 | 2 | 14 | 13 | 12 | 6 | 5 | 20 | 83 | 6 |
| Sore throat | ND | 0 | 2 | 4 | 0 | 2 | 1 | ND | ND | 4 | ND | 79 | ND |
| Rhinorrhea | 2/7 | 1 | 22 | 2 | 1 | 2 | 3 | 2 | ND | 2 | ND | 13 | ND |
| Gastrointestinal | ND | 0 | 2 | ND | 0 | 3 | 5 | 2 | 4 | 2 | 4 | 15 | 5 |
| Non‐mild disease | 0/7 | 12 | 12 | 4 | 4 | 14 | 20 | 18 | 5 | 0 | ND | 111 | 8 |
| Severe or critical | 0/7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | ND | 4 | 8 |
| Chronic disease | ND | ND | ND | ND | ND | ND | 7 | 0 | 0 | ND | 4 | ND | 1 |
| Laboratory | |||||||||||||
| Lymphocytosis | ND | ND | 17 | 1 | 6 | ND | 3 | 25 | 0 | 0 | 17 | ND | 2 |
| Lymphocytopenia | ND | ND | 0 | 0 | 0 | 2 | 7 | 1 | 4 | 0 | 0 | 6 | 1 |
| Normal CRP | ND | ND | 27 | 7 | ND | 27/30 | 11 | 21 | ND | 10 | 14 | 138 | 3 |
| High PCT | ND | ND | ND | 0 | ND | 1/28 | 16 | 0 | ND | 5 | 0 | 105 | 5 |
| Radiology | |||||||||||||
| Chest X‐ray | |||||||||||||
| Normal | ND | ND | ND | 6 | ND | ND | ND | 8 | ND | 10 | ND | ND | ND |
| Unilateral opacities | ND | ND | ND | 4 | ND | ND | ND | 11 | ND | 0 | ND | ND | ND |
| Bilateral opacities | ND | ND | ND | 0 | ND | ND | ND | 7 | ND | 0 | ND | ND | ND |
| Chest CT | |||||||||||||
| Normal | ND | 6 | 20 | ND | 1 | 17 | 4 | 8 | 1/5 | 10 | 6 | 60/169 | 0 |
| Unilateral opacities | ND | ND | 8 | ND | ND | ND | 6 | 11 | 0 | 0 | 14 | 32/169 | 2 |
| Bilateral opacities | ND | ND | 3 | ND | ND | ND | 10 | 7 | 4/5 | 0 | 14 | 77/169 | 6 |
| Authors | Shen 29 | Zhu 30 | Zheng 31 | Qiu 32 | Su 33 | USA CDC 15 | Tagarro 5 | UK RCPCH 34 | Parri 35 | Garazzino 36 | Shekerdemian 37 | TOTAL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 9 | 10 | 25 | 36 | 9 | 2572 | 41 | 83 | 100 | 168 | 48 | 3473 | ||
| Symptoms | PROPORTION (%) | CI, 95% | ||||||||||||
| Fever | 3 | 4 | 13 | 13 | 2 | 163/291 | ND | 61/76 | 54 | 138 | ND | 643/1102 (58.3) | 55.4 − 61.2 | |
| Cough | 1 | 3 | 11 | 7 | 1 | 158/291 | ND | 31/76 | 44 | 82 | ND | 521/1102 (47.3) | 44.3 − 50.2 | |
| Sore throat | 1 | 0 | ND | 2 | 0 | 71/291 | ND | 3/76 | 4 | 9 | ND | 182/996 (18.3) | 16.0 − 20.7 | |
| Rhinorrhea | 0 | ND | 2 | ND | 0 | 21/291 | ND | 20/76 | 22 | 45 | ND | 160/1008 (15.9) | 13.7 − 18.2 | |
| Gastrointestinal | 2 | 0 | 3 | 2 | 0 | 37/291 | ND | 11/76 | 10 | 31 | ND | 138/1086 (12.7) | 10.8 − 14.8 | |
| Non‐mild disease | 2 | 5 | 17 | 19 | 0 | 147/745 | 25 | ND | 21 | 33 | 34 | 511/1535 (33.3) | 30.9 − 35.6 | |
| Severe or critical | 0 | 1 | 2 | 0 | 0 | ND | ND | ND | 2 | 16 | 33 | 68/749 (9.1) | 7.2 − 11.3 | |
| Chronic disease | ND | ND | 2 | ND | ND | 80/345 | 11 | 32/76 | 27 | 33 | 40 | 237/897 (26.4) | 23.6 − 29.4 | |
| Laboratory | ||||||||||||||
| Lymphocytosis | 2 | 2 | 15 | ND | 1 | ND | ND | ND | ND | ND | ND | 91/207 (44.0) | 37.3 − 50.7 | |
| Lymphocytopenia | 0 | 0 | 10 | 11 | 2 | ND | ND | ND | 14/57 | ND | ND | 58/331 (17.5) | 13.8 − 21.9 | |
| Normal CRP | 7 | 10 | ND | 35 | 9 | ND | ND | ND | ND | 74/121 | ND | 393/525 (74.8) | 70.9 − 78.3 | |
| High PCT | ND | 0/8 | ND | 6 | 0 | ND | ND | ND | 4/23 | ND | ND | 142/383 (37.1) | 32.3 − 42.0 | |
| Radiology | ||||||||||||||
| Chest X‐ray | ||||||||||||||
| Normal | ND | ND | ND | ND | ND | ND | ND | ND | 15/35 | ND | ND | 39/81 (48.1) | 37.6 − 58.8 | |
| Unilateral opacities | ND | ND | ND | ND | ND | ND | ND | ND | 6/35 | ND | ND | 21/42 (50.0)d | 35.5 − 64.4 | |
| Bilateral opacities | ND | ND | ND | ND | ND | ND | ND | ND | 14/35 | ND | ND | 21/42 (50.0)d | 35.5 − 64.4 | |
| Chest CT | ||||||||||||||
| Normal | 7 | 5 | 8 | 17 | 5 | ND | ND | ND | ND | ND | ND | 175/447 (39.1) | 34.7 − 43.7 | |
| Unilateral opacities | 2 | 3 | 5 | ND | 1 | ND | ND | ND | ND | ND | ND | 84/222 (37.8)d | 31.7 − 44.3 | |
| Bilateral opacities | 0 | 2 | 12 | ND | 3 | ND | ND | ND | ND | ND | ND | 138/222 (62.2)d | 55.6 − 68.2 | |
Note: Symptoms, laboratory, and radiology features. Update: 24 May 2020.
Abbreviations: CDC, Centers for Disease Control and Prevention (US); CI, confidence interval; CRP, C‐reactive protein; CT, computed tomography; GI, gastrointestinal; RCPCH, Royal College of Pediatrics and Child Health (UK); ND, no data; PCT, procalcitonin.
Only case series with N > 5 are included in this table.
A fraction means that there is no available data for the total N in the case series.
Non‐mild disease includes a presentation as pneumonia in case reports from China and Italy (Parri and Garazzino) and the need for hospitalization in CDC and Tagarro reports. Severe or critical cases are also included in those characterized as a non‐mild disease.
Percentages are calculated from the total cases with abnormal chest X‐ray or abnormal computed chest tomography, respectively.
Notably, some singular presentations that could be associated with SARS‐CoV‐2 infection have been described in children, such as infection‐induced chilblains 39 in adolescents and young adults. By mid‐April, an increase in episodes similar to Kawasaki disease and/or toxic shock syndrome was reported in several countries (eg, UK, US, France, Italy, or Spain). It was initially named Pediatric multisystem inflammatory syndrome (PIMS) temporally associated with COVID‐19 by the Royal College of Paediatrics and Child Health (RCPCH). 40 Due to the temporal coincidence with the SARS‐CoV‐2 pandemic an association with COVID‐19 has been proposed. The Centers for Disease Control and Prevention (CDC), the European Centre for Disease Prevention and Control (ECDC), WHO, and RCPCH have published their own case definitions. 40 , 43 The main characteristics of the case series published to date are shown in Table 2. 44 , 48
Table 2.
Case series (n > 5) describing pediatric inflammatory multisystem syndrome temporally associated with COVID‐19
| Verdoni et al 44 | Belhadjer et al 45 | Riphagen et al 46 | Toubiana et al 47 | Chiotos et al 48 | |
|---|---|---|---|---|---|
| n = 10 | n = 35 | n = 8 | n = 17 | n = 6 | |
| Country | Italy | France and Switzerland | UK | France | US |
| Date of diagnosis | 18/2‐20/04/2020 | 22/03‐30/4/2020 | 10 d in mid‐April, 2020 | 27/4‐7/5/2020 | ND |
| Age, median (range) | 7.3 (2‐16) | 10 (2‐16) | 8 (4‐14) | 7.5 (3‐16) | 7.5 (5‐14) |
| Sex (male) | 7 (70%) | 18 (51%) | 5 (63%) | 7 (42%) | 1 (17%) |
| Symptoms | |||||
| Fever | 10 (100%) | 35/35 (100%) | 8 (100%) | 17 (100%) | 7 (100%) |
| Rash | 7 (70%) | 20 (57%) | 4 (50%) | 13 (76%) | 2 (33%) |
| Abdominal pain | ND | ND | 6 (75%) | 17 (100%) | 3 (50%) |
| Vomiting/diarrhea | 6 (60%) | 29 (83%) | 7 (88%) | 16 (94%) | 5 (83%) |
| Intensive care | |||||
| Shock | 5 (50%) | 28 (80%) | 8 (100%) | 11 (65%) | 6(100%) |
| Cardiac involvement | 6 (60%) | 35 (100%) | 7 (88%) | 12 (71%)a | 5 (83%) |
| Inotrope support | 2 (20%) | 28 (80%) | 8 (100%) | 10 (59%) | 5 (83%) |
| Mechanical ventilation | ND | 22 (62%) | ND | 10 (59%) | 3 (50%) |
| Mortality | 0 (0%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) |
| SARS‐CoV‐2 | |||||
| Positive PCRb | 2 (20%) | 12 (34%) | 2 (25%) | 7 (41%) | 3 (50%) |
| Positive serology | 8 (80%) | 30 (86%) | 8 (100%) | 14 (88%) | 5/5 (100%) |
Note: Cardiac involvement: coronary aneurism, ejection fraction decreased, mitral valve regurgitation, or pericardial effusion.
Abbreviations: COVID‐19, coronavirus disease‐2019; ND, not described; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Considered as myocarditis.
SARS‐CoV‐2 PCR from nasopharyngeal/oropharyngeal swab.
To date, some cases of neonatal SARS‐CoV‐2 infection have been reported. 49 , 50 Most were asymptomatic or had mild symptoms, but some cases progressed to a severe infection. 51 , 52 The earliest diagnosed patient using molecular diagnosis from a nasopharyngeal specimen was a 36‐hour‐old newborn. 53 Breastfeeding has not been discouraged by most scientific societies (eg, WHO, UNICEF, Spanish Society of Neonatology, or Academy of Breastfeeding Medicine). To note, recently the detection of SARS‐CoV‐2 in human breast milk by RT‐PCR has been published, which deserves further studies. 54
Some questions about vertical transmission have been raised after the publication of three newborns born to mothers with SARS‐CoV‐2 infection who presented positive IgM against SARS‐CoV‐2 at birth, but a negative SARS‐CoV‐2 PCR, 55 , 56 and the detection of SARS‐CoV‐2 RNA in the placenta of pregnancies with COVID‐19. 57 However, these data should be interpreted with caution.
In summary, pediatric patients with a SARS‐CoV‐2 infection usually develop mild disease. However, the increasing number of patients with PIMS, who usually have a severe presentation, deserves a detailed analysis to establish the best definition and treatment. Regarding vertical transmission, currently, there is not enough information and further studies are needed.
5. LABORATORY
Typical COVID‐19 laboratory markers in adults are not prevalent in children, but the vast majority (Table 1) do not document information about laboratory tests, so inaccurate extrapolation from adult literature is frequent. Leukocyte counts are often normal, but lymphocytosis is frequent (44%) in children with COVID‐19. Lymphocytopenia has been reported as the most common sign in blood count for adults, 58 , 59 but it is only present in 17.5% of children. It has been related to poorer prognosis in adults, so perhaps the low prevalence in our review might be explained by a high frequency of mild cases (66.7% in this review). Procalcitonin levels seem to be greater (37.1%) than adults but reference ranges were not clearly defined. C‐reactive protein is within the normal range in about 74.8% of children, but in contrast, it remained normal in only 37.5% (3/8 cases) of children requiring intensive care. 29 Liver enzymes are frequently normal in pediatric patients, 20 , 23 , 26 in contrast to adults. Remarkably, 50% of children in a case series of severe and critically ill patients presented abnormal liver function. 29 Other typical markers in adults such as high lactate dehydrogenase, ferritin, D‐dimer, or interleukin‐6 are not evident in children but may be altered in severe and critical patients. In our experience, from 43 confirmed pediatric cases who underwent a blood test, 30% presented lymphocytes below 1200/mm3 and 70% a D‐dimer above 700 mg/dL. 60
So, some analytical markers to take into account when evaluating a child with confirmed or suspected COVID‐19, are lymphocyte count, D‐dimer, C‐reactive protein, procalcitonin, and liver enzymes. However, their cutoff points in COVID‐19 and the association with severe disease is not as well defined as in adults.
6. RADIOLOGY
Most data in children are provided by computed chest tomography (CCT) studies from China (Table 1). From our perspective, it is surprising that CCT has become the COVID‐19 gold standard in radiology diagnosis in children in some regions as radiation concerns might exist with doubtful medical benefit. In Spain, CCT is not recommended by pediatric guidelines in mild and moderate COVID‐19. 61 Few data are available from chest radiographs in children with COVID‐19. No abnormalities in radiographs are shown in 48.1% of cases. Unilateral or bilateral infiltrates in CCT are found in 60.9% of children. Bilateral ground‐glass opacities are the most prevalent findings. In addition, patchy shadows and consolidations are frequent.
7. MICROBIOLOGICAL DIAGNOSIS
Accurate and reliable diagnosis of SARS‐CoV‐2 infections remains the cornerstone of the public health strategy for disease containment. The virus nucleic acid real time‐polymerase chain reaction (RT‐PCR) test has become the current standard diagnostic method, using specimens collected via nasopharyngeal swab. 53 Most patients have high viral loads in upper respiratory specimens soon after symptom onset which peak in the first few days before declining. 54 A study including 57 children showed that symptomatic infants had higher nasopharyngeal SARS‐CoV‐2 viral loads (measured as cycle threshold) than older children. 55 Recent data from a German study indicate that viral loads in the very young (age group 0‐6 years) do not significantly differ from those of adults. 62 Whether these results are associated with different time points of testing during the infection or with host biology peculiarities deserves further analysis.
Lower respiratory tract secretions, such as bronchoalveolar lavage fluid or bronchial aspirate, are more sensitive for diagnosis, 48 and should be tested in undiagnosed critically ill children with pneumonia. In newborns born to mothers with SARS‐CoV‐2 infection a combined throat/nasopharynx, PCR should be done first at 24 hours of age and again at 48 hours of age. Some infants have had a negative test at 24 hours only to have a positive test at a later time. 63
However, there are some limitations regarding nucleic acid tests, including low throughput and high rates of false‐negative results. These may be caused by a very early or late collection of the sample, inadequate or insufficient viral material in the specimen, laboratory error during sampling, or restrictions on sample transportation. 57 So, if a negative result is obtained from a child with a high index of suspicion for COVID‐19 virus infection the patient should be retested.
Detection of specific serum antibodies for SARS‐CoV‐2 by automated chemiluminescence immunoassays or enzyme‐linked immunosorbent assays, could provide an alternative solution and compensate for the limitations of the RT‐PCR, especially in the late stages of the disease. The majority of children with the multisystem inflammatory syndrome have serologic evidence of infection, but approximately a third or fewer tested positive for SARS‐CoV‐2 by PCR. 45
To put it briefly, SARS‐CoV‐2 PCR of the nasopharyngeal swab is considered the gold standard diagnostic test for acute COVID‐19 disease. However, due to its suboptimal sensitivity, a retest of the same specimen or even invasive specimens may be considered for nonconfirmed cases. On the other hand, SARS‐CoV‐2 serology has a relevant diagnostic role in the late stages of the disease, including PIMS, or for seroprevalence studies.
8. COINFECTIONS
Children with COVID‐19 might be coinfected with other respiratory viruses and bacteria. Few case series in children have reported relevant data on this topic. Remarkably, two different studies found that 40% to 50% of pediatric patients were coinfected. Influenza virus and Mycoplasma pneumoniae were the most frequently documented pathogens. In addition, respiratory syncytial virus (RSV), parainfluenza, adenovirus, Epstein‐Barr virus, or cytomegalovirus have been described. 23 , 27 , 64 Also, Jiang et al 65 documented two children with SARS‐CoV‐2 confection with human metapneumovirus (2/2), RSV (1/2), and Mycoplasma (1/2). In contrast, other case series did not document coinfections with respiratory viruses such as influenza, parainfluenza or RSV. 20 , 26 The clinical relevance of coinfections is an issue that may have important implications.
9. COMORBIDITIES
At the time of writing this article, 12 studies have recorded information on underlying conditions, which is less than 26% of the total patients in this review. Among patients with reliable information, 26.4% had at least one comorbidity. The CDC in 6 April 2020 reports established that chronic lung disease (including asthma) is the most prevalent preexisting condition (50%), followed by cardiovascular disease (31%) and immunosuppression (12.5%). In addition, a study highlighted that 40/48 (83%) patients less than 21 years old admitted to a pediatric intensive care unit (PICU) had significant preexisting comorbidities. 38
Of note, immunosuppressive therapy has not been linked to poorer prognosis in small pediatric case series. D'Antiga 66 described three children in a postliver transplant period positive for SARS‐CoV‐2 with mild disease and absence of lung affection in Lombardia (Italy). Turner et al 67 focused on children affected by inflammatory bowel disease on immunosuppression and described eight cases of COVID‐19 in these children who only experienced mild symptoms. Melgosa et al 68 described 16 children with chronic renal pathologies diagnosed with COVID‐19 in Spain. Of these, six had end‐stage kidney disease (three transplant recipients and three on chronic hemodialysis), and the severity was mild in all the patients.
Balduzzi et al 69 have described an Italian cohort of 5 children with malignancy positive for SARS‐CoV‐2. All patients recovered from a mild course. 69 However, there are insufficient and controversial data in children with hematology‐oncology malignant diseases. Sun et al 29 describe the clinical features of severe pediatric patients requiring intensive care. One out of three critical children in this study was suffering acute lymphocytic leukemia when infected with COVID‐19. A flash survey circulated on 16 March 2020 involving reports of children on anticancer treatment from 25 countries collected only nine cases of COVID‐19, including eight children with asymptomatic or mild disease and one who had just been diagnosed. 70 A multicenter study involving all pediatric oncology units in Madrid (Spain) calculated a COVID‐19 prevalence of 1.3% (15 confirmed cases) among children with cancer. 71 The clinical characteristics were milder than those described in adult patients with cancer. On the other hand, as of 16 April 2020, there were 33 confirmed COVID‐19 cases from French pediatric oncology centers, of which five (15%) were admitted to PICU. 72 There were no fatal cases at the time of publication.
In summary, comorbidities in children do not appear to be a relevant risk factor among children with COVID‐19 in studies focused on cohorts of children with specific chronic conditions. However, as is shown below, a relevant percentage of children with COVID‐19 admitted to PICU have some comorbidity. Anyway, due to the vulnerability of patients with chronic conditions, a special caution seems to be recommended when attending these children during SARS‐CoV‐2 epidemics.
10. SEVERE AND CRITICAL CASES
The vast majority of symptomatic children recover from COVID‐19 within 1 to 2 weeks. In contrast to adults, severe COVID‐19 infection in children is not frequent. Some of the proposed hypotheses as to why there is different severity among children compared with adults are 73 , 74 : (a) different angiotensin‐converting enzyme 2 expressions in cell membranes 75 ; (b) better control of viral replication through innate immunity; (c) different inflammatory signaling pathways; (d) preexisting immunity to common coronaviruses 76 ; (e) differences in clotting function; and (f) lower comorbidities. However, none of these hypotheses has been validated.
A study from China included 2143 children with microbiology‐confirmed (34%) or clinically suspected COVID‐19 (66%). Severe (defined as hypoxic) or critical cases were documented in 5.8% and 2.8% of the total SARS‐CoV‐2 confirmed patients, respectively. Children aged less than 1 year had the highest prevalence of severe and critical disease (10.6%), and 53% of children in PICUs were infants. However, as a limitation, this group had the highest proportion of clinically suspected disease, so other viruses may have led to severe disease. Of note, children aged 1 to 5 years might have a poorer prognosis (7.3% had the severe and critical disease) compared with children more than 5 years, and adolescents (3%‐4.2%).
The CDC report included prognostic information, but hospitalization status was declared in only 29% of children. 15 Hospitalization was more frequent among children aged less than 1 year and 5.2% of infants required intensive care admission. In addition, 33% of children in intensive care units were aged less than 1 year. Patients with underlying conditions also required more frequent hospitalization than healthy children. Out of hospitalized patients, 77% were children with chronic conditions which stand in contrast to 12% of non‐hospitalized COVID‐19 infected children.
Information on children with COVID‐19 requiring intensive care is scarce and incomplete. Deterioration starts typically after 7 to 10 days of clinical course. Some previously described clinical case series in this review documented children needing admission to PICU 5 , 25 , 28 , 29 , 32 but most series did not. Liu et al 25 reported 6 children and 1 patient 3 years of age that required intensive care. Lu et al 28 included 171 children with confirmed SARS‐CoV‐2 infection, and three required intensive care. All three had comorbidities. A 14‐year‐old adolescent with intussusception died of multiple organ failure. Zheng et al 32 described 25 children with COVID‐19, and two of them required PICU admission and invasive mechanical ventilation (IMV). Remarkably, both patients were infants and had congenital heart diseases. At the time of publishing that paper no deaths were reported. Also, a Spanish report described 41 children affected by COVID‐19 disease, of whom four were transferred to PICU. 5 Only one patient required IMV and no deaths were reported.
Sun et al 29 described a case series of eight children with COVID‐19 focused on those requiring intensive care. All patients were admitted with tachypnea, but only 6/8 had fever and cough. Of note, only one patient had preexisting conditions. Two patients underwent IMV. A cytokine storm was common in these patients and especially in those critically ill. A multicenter study involving 46 PICU located in the US and Canada included 48 confirmed COVID‐19 infections between 14 March and 3 April 2020. 38 Respiratory symptoms were the most common presentation (73% of all cases), with 18 (38%) cases requiring IMV. At the time of publishing that study, two patients (4%) had died and 15 (31%) were still hospitalized.
So, although severe disease is not common in children with COVID‐19, some patients develop a clinical deterioration that requires intensive care. A retrospective view of the pandemic in children shows two different periods in the manifestations of COVID‐19 severe disease in children: a first period during the first weeks of the pandemic, with respiratory failure as the main manifestation, and a second period, 2 to 4 weeks after the peak of incidence, with patients developing PIMS with hemodynamic dysfunction.
11. MORTALITY
Many asymptomatic or mildly symptomatic patients are not microbiologically confirmed nor reported, so caution is required when analyzing prognostic data in children.
The global case fatality rate in adults has been established at 4%, but many geographical variations have been established. As far as we know, by 27 April, at least 22 children and adolescents (<18 years old) had died from COVID‐19 (2 in China, 1 in Italy, 6 in Spain, 3 in the US, 8 in the UK, and 2 in Colombia), but an official global data source focusing on pediatric cases at the time of writing is not available. Regarding PIMS, as of 11 May, five fatality cases have been reported (1 in France, 1 in the UK, and 3 in the US). 42
12. TREATMENT
Currently, the main treatment for COVID‐19 disease is supportive care, ensuring adequate oxygenation and nutritional support for the patient. The specific treatment has focused on two different strategies 77 : (a) antiviral treatment aimed at controlling viral replication, which could be useful mainly in early stages of the infection and (b) immunomodulatory treatment to control the deleterious effects of an excessive inflammatory response, which occurs in the intermediate and late stage of the disease. To note, experience and knowledge of pharmacokinetics in some of these drugs are scarce in children. For this reason, it is convenient to make rational use of the drugs under study, assessing the risk‐benefit individually. Some national pediatric societies have published clinical guidelines on the treatment of COVID‐19 in children. 61 , 78 The Spanish Society of Pediatrics has proposed specific treatments according to the severity and risk factors of the patient, highlighting a cautious approach (Table 3). 61
Table 3.
Proposed management of COVID‐19 in children
| Severity | Chest X‐ray | Treatment | Management |
|---|---|---|---|
| Mild: No hypoxemia, no distress, or mild distress | Not indicated except for risk groups | Supportive care (1) (2) | Discharge at home except for risk groups (individualize) |
| Moderate: Hypoxemia or moderate distress | No signs of lung infection | Supportive care (1) | Hospital admission without antiviral treatment (2) |
| Signs of lung infection | Supportive care (1) | Hospital admission | |
| Consider systemic steroids if hypoxemia | |||
| Consider the compassionate use of remdesivir (2) | |||
| Severe (ICU): Severe hypoxemia, severe distress, or hemodynamic dysfunction | Signs of lung infection | Supportive care (1) | Hospital admission |
| Consider the compassionate use of remdesivir (2) | |||
| Consider systemic steroids and/or tocilizumab |
Note: Adapted from the Guidance of The Clinical Management of Pediatric Patients with SARS‐CoV‐2 Infection from the Spanish Society of Pediatrics. 61 (1) Consider empirical antibiotic therapy if bacterial coinfection is suspected. (2) Consider the use of antimicrobials against SARS‐CoV‐2 other than remdesivir only in the context of clinical trials.
Abbreviations: COVID‐19, coronavirus disease‐2019; ICU, intensive care unit; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Regarding the antiviral treatments proposed, they target different stages of the viral replication cycle. 79 Among antimicrobials 77 (eg, lopinavir/ritonavir, chloroquine/hydroxychloroquine, or ivermectin), only remdesivir has proved to be of clinical benefit in a clinical trial including patients more than or equal to 18 years old. 80 In terms of anti‐inflammatory drugs, some guidelines have included systemic steroids to be considered for COVID‐19 disease. 81 Tocilizumab, an anti‐IL‐6 antibody, is being evaluated in several studies. 82 Currently, some clinical trials are evaluating different drugs in children: hydroxychloroquine in PanCOVID19 trial (EudraCT2020‐001156‐18) or remdesivir in children more than or equal to 12 years old (NCT04292730).
Consequently, until specific evidence on the best pharmacological approach for COVID‐19 in children is developed, supportive care continues to be the backbone of the management. Depending on the stage of the disease, antiviral treatment, or immunomodulatory drugs may be considered, always balancing the individual's risk to benefit ratio.
CONFLICTS OF INTEREST
The authors declare that there are no conflict of interests to disclose.
ACKNOWLEDGMENTS
We thank Megan Yoder for kindly reviewing the manuscript, César García‐Vera for guiding authors on statistical work, and Alasdair Munro and Don't Forget The Bubbles team for inspiring this review. This study did not receive any funding. DAA is funded by the Spanish Ministry of Health – Instituto de Salud Carlos III (ISCIII) and cofunded by the European Union (FEDER) (Contrato Río Hortega CM18/00100).
Escosa‐García L, Aguilera‐Alonso D, Calvo C, Mellado MJ, Baquero‐Artigao F. Ten key points about COVID‐19 in children: The shadows on the wall. Pediatric Pulmonology. 2020;55:2576–2586. 10.1002/ppul.25025
REFERENCES
- 1. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review [published online ahead of print April 22, 2020]. JAMA Pediatr. 2020. [DOI] [PubMed] [Google Scholar]
- 3. Yagnik PJ, Umscheid J, Khan AW, Ali M, Bhatt P, Desai PH. Pediatric characteristics of 2019 novel coronavirus: review of available published literature. Clin Pediatr. 2020;59:849‐852. [DOI] [PubMed] [Google Scholar]
- 4. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain [published online ahead of print April 08, 2020]. JAMA Pediatr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spanish Ministry of Health . Actualización no 109. Enfermedad por el coronavirus (COVID‐19). Situación en España. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_109_COVID-19.pdf. Accessed May 18, 2020.
- 7. Liu J, Liao X, Qian S, et al. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26(6):1320‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID‐19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;0(0):911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med. 2020;382:2302‐2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of COVID‐19 outbreak in the municipality of Vo, Italy [published online ahead of print April 18, 2020]. medRxiv. 2020.
- 11. Stringhini S, Wisniak A, Piumatti G, et al. Repeated seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in a population‐based sample from Geneva, Switzerland [published online ahead of print May 06, 2020]. medRxiv. 2020.
- 12. Instituto de Salud Carlos III M de SM de C e I . Estudio Ene‐COVID19: Primera Ronda Estudio Nacional De Sero‐epidemiología De La Infección Por SARS‐COV‐2 en España. 2020. https://www.ciencia.gob.es/stfls/MICINN/Ministerio/FICHEROS/ENECOVID_Informe_preliminar_cierre_primera_ronda_13Mayo2020.pdf
- 13. Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (Covid‐19) in the French Alps, 2020. Clin Infect Dis. 2020;71:825‐832. 10.1093/cid/ciaa424; https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa424/5819060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu Y, Bloxham CJ, Hulme KD, et al. Children are unlikely to have been the primary source of household SARS‐CoV‐2 infections [published online ahead of print March 30, 2020]. medRxiv. 2020.
- 15. Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tahvildari A, Arbabi M, Farsi Y, et al. Clinical features, diagnosis, and treatment of COVID‐19: a systematic review of case reports and case series [published online ahead of print April 03, 2020]. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 17. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. J Am Med Assoc. 2020;323(13):1313‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 Children with 2019 novel coronavirus infection. Zhonghua er ke za Zhi = Chinese J Pediatr. 2020;58:E007. [DOI] [PubMed] [Google Scholar]
- 19. Chen C, Cao M, Peng L, et al. Coronavirus disease‐19 among children outside Wuhan, China [published online ahead of print March 24, 2020]. SSRN Electron J. 2020. [Google Scholar]
- 20. Cai Jiehao, Xu Jing, Lin Daojiong, et al. Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features [published online ahead of print February 28, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yun Zhou. Clinical Features and Chest CT Findings of Coronavirus Disease 2019 in Infants and Young Children ‐ PubMed. Zhongguo Dang Dai Er Ke Za Zhi. 2020;143:684‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi = Chinese J Pediatr. 2020;58(4):E011. [DOI] [PubMed] [Google Scholar]
- 23. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang A, Xu W, shen min, et al. A retrospective study of the clinical characteristics of COVID‐19 infection in 26 children [published online ahead of print March 10, 2020]. medRxiv. 2020.
- 25. Liu W, Zhang Q, Chen J, et al. Detection of Covid‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang C, Gu J, Chen Q, et al. Clinical characteristics of 34 children with coronavirus disease‐2019 in the West of China: a multiple‐center case series [published online ahead of print March 16, 2020]. medRxiv. 2020.
- 28. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55(6):1430‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;0(0):689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China – the character of children with COVID‐19. Emerg Microbes Infect. 2020;9(1):707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Royal College of Paediatrics and Child Health . COVID‐19 ‐ service evaluation and audit on the care needs of children admitted to hospital (England)‐RCPCH. 2020 https://www.rcpch.ac.uk/key-topics/covid-19. Accessed April 29, 2020.
- 36. Parri N, Lenge M, Buonsenso D Children with Covid‐19 in pediatric emergency departments in Italy [published online ahead of print May 01, 2020]. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 37. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25(18):2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units [published online ahead of print May 11, 2020]. JAMA Pediatr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;0(0):489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Royal College of Paediatrics and Child Health . Guidance ‐ Paediatric multisystem inflammatory syndrome temporally associated with COVID‐19: RCPCH. 2020. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19
- 41. World Health Organization . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID‐19. 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 42. European Centre for Disease Prevention and Control (ECDC) . Rapid risk assessment: Paediatric inflammatory multisystem syndrome and SARS ‐CoV‐2 infection in children. 2020. https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment
- 43. Centers for Disease Control and Prevention . Multisystem Inflammatory Syndrome in Children (MIS‐C) Associated with Coronavirus Disease 2019 (COVID‐19). https://emergency.cdc.gov/han/2020/han00432.asp. Accessed May 14, 2020.
- 44. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;0(0):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic [published online ahead of print May 17, 2020]. Circulation. 2020;120:048360. [DOI] [PubMed] [Google Scholar]
- 46. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toubiana J, Poirault C, Corsia A, et al. Outbreak of Kawasaki disease in children during COVID‐19 pandemic: a prospective observational study in Paris, France [published online ahead of print May 14, 2020]. medRxiv. 2020.
- 48. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the COVID‐19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9(3):393‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;174:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: a retrospective, single‐centre, descriptive study. Lancet Infect Dis. 2020;559‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coronado Munoz A, Nawaratne U, McMann D, Ellsworth M, Meliones J, Boukas K. Late‐onset neonatal sepsis in a patient with Covid‐19. N Engl J Med. 2020;382:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis. 2020;52:427‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang LF J. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020;71:853‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Groß R, Conzelmann C, Müller JA, et al. Detection of SARS‐CoV‐2 in human breastmilk [published online ahead of print May 21, 2020]. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA ‐ J Am Med Assoc. 2020:E1‐E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. J Am Med Assoc. 2020:E1‐E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of COVID‐19: SARS‐CoV‐2 RNA on the fetal side of the placenta in pregnancies with COVID‐19 positive mothers and neonates at birth [published online ahead of print May 18, 2020]. Am J Obstet Gynecol MFM. 2020:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Qian JY, Wang B, Liu BC. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. de Ceano‐Vivas M, Martín‐Espín I, Del Rosal T, et al. SARS‐CoV‐2 infection in ambulatory and hospitalised Spanish children. Arch Dis Child. 2020;105:808‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spanish Ministry of Health . Manejo clínico del COVID‐19: atención hospitalaria. 2020.
- 62. Jones TC, Mühlemann B, Veith T, et al. An analysis of SARS‐CoV‐2 viral load by patient age [published online ahead of print June 9, 2020]. medRxiv. 2020.
- 63. Puopolo KM, Hudak ML, Kimberlin DW, Cummings J Initial guidance: management of infants born to mothers with COVID‐19 ‐ American Academy of Pediatrics Commmittee on Fetus and Newborn [published online ahead of print April 2, 2020]. 2020.
- 64. Wu Q, Xing Y, Shi L, et al. Co‐infection and other clinical characteristics of COVID‐19 in children. Pediatrics. 2020;146:e20200961. [DOI] [PubMed] [Google Scholar]
- 65. Jiang S, Liu P, Xiong G, et al. Coinfection of SARS‐CoV‐2 and multiple respiratory pathogens in children. Clin Chem Lab Med. 2020;1160‐1161. [DOI] [PubMed] [Google Scholar]
- 66. D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26:832‐834. [DOI] [PubMed] [Google Scholar]
- 67. Turner D, Huang Y, Martín‐de‐Carpi J, et al. COVID‐19 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the paediatric IBD Porto group of ESPGHAN [published online ahead of print March 31, 2020]. J Pediatr Gastroenterol Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Melgosa M, Madrid A, Alvárez O, et al. SARS‐CoV‐2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020;20:1521‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID‐19 outbreak in a pediatric transplant and hemato‐oncology center embedded within a COVID‐19 dedicated hospital in Lombardia, Italy. Estote parati [published online ahead of print April 20, 2020]. Bone Marrow Transpl. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hrusak O, Kalina T, Wolf J, et al. Flash survey on severe acute respiratory syndrome coronavirus‐2 infections in paediatric patients on anticancer treatment. Eur J Cancer. 2020;132:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Rojas T, Pérez‐Martínez A, Cela E, et al. COVID‐19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer. 2020;67:e28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. André N, Rouger‐Gaudichon J, Brethon B, et al. COVID‐19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67:e28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cristiani L, Mancino E, Matera L, et al. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55:2000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. García‐Salido A. Three hypotheses about children COVID19 [published online ahead of print April 01, 2020]. Pediatr Infect Dis J. 2020. [DOI] [PubMed] [Google Scholar]
- 75. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. J Am Med Assoc. 2020;323:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020;181(7):1489‐1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical‐therapeutic staging proposal. J Hear Lung Transpl. 2020;39:405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with COVID‐19/SARS‐CoV‐2 [published online ahead of print April 22, 2020]. J Pediatric Infect Dis Soc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review [published online ahead of print April 13, 2020]. J Am Med Assoc. 2020. [DOI] [PubMed] [Google Scholar]
- 80. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — preliminary report [published online ahead of print May 22, 2020]. N Engl J Med. 2020. [DOI] [PubMed]
- 81. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Intensive Care Med. 2020;28:1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roumier M, Paule R, Groh M, Vallee A, Ackermann F Interleukin‐6 blockade for severe COVID‐19 [published online ahead of print April 22, 2020]. medRxiv. 2020.


