Abstract
To provide a comprehensive and systematic analysis of demographic characteristics, clinical symptoms, laboratory findings, and imaging features of coronavirus disease 2019 (COVID‐19) in pediatric patients. A meta‐analysis was carried out to identify studies on COVID‐19 from 25 December 2019 to 30 April 2020. A total of 48 studies with 5829 pediatric patients were included. Children of all ages were at risk for COVID‐19. The main illness classification ranged as: 20% (95% confidence interval [CI]: 14%‐26%; I 2 = 91.4%) asymptomatic, 33% (95% CI: 23%‐43%; I 2 = 95.6%) mild and 51% (95% CI: 42%‐61%; I 2 = 93.4%) moderate. The typical clinical manifestations were fever 51% (95% CI: 45%‐57%; I 2 = 78.9%) and cough 41% (95% CI: 35%‐47%, I 2 = 81.0%). The common laboratory findings were normal white blood cell 69% (95% CI: 64%‐75%; I 2 = 58.5%), lymphopenia 16% (95% CI: 11%‐21%; I 2 = 76.9%) and elevated creatine‐kinase MB 37% (95% CI: 25%‐48%; I 2 = 59.0%). The frequent imaging features were normal images 41% (95% CI: 30%‐52%; I 2 = 93.4%) and ground‐glass opacity 36% (95% CI: 25%‐47%; I 2 = 92.9%). Among children under 1 year old, critical cases account for 14% (95% CI: 13%‐34%; I 2 = 37.3%) that should be of concern. In addition, vomiting occurred in 33% (95% CI: 18%‐67%; I 2 = 0.0%) cases that may also need attention. Pediatric patients with COVID‐19 may experience milder illness with atypical clinical manifestations and rare lymphopenia. High incidence of critical illness and vomiting symptoms reward attention in children under 1 year old.
Keywords: 2019‐nCoV, children, coronavirus, COVID‐19, meta‐analysis, SARS‐CoV‐2
Highlights
To provide a comprehensive characterization of coronavirus disease 2019 (COVID‐19) in pediatric patients.
The majority of pediatric cases with COVID‐19 have milder illness with atypical clinical manifestations and rare lymphopenia.
It is worth noting that the high incidence of critical illness and vomiting in children under 1 year old.
Our evidence‐based data will help formulate strategies for early clinical identification and epidemic control of COVID‐19 in children.
1. INTRODUCTION
An outbreak of the coronavirus disease 2019 (COVID‐19) is spreading rapidly around the world, which is caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection. As of 26 July 2020, the COVID‐19 pandemic has resulted in approximately 15 785 641 confirmed cases, including 640 016 deaths worldwide. 1 In the early stage, the majority of cases were concentrated in middle‐aged and old people. Under the ongoing pandemic situation, children cases are showing an increasing trend in many countries of the world: In China, a large cohort study on the epidemiological characteristics of children with COVID‐19 included 2135 cases 2 ; In the United States, it was reported that 74 children were admitted to pediatric intensive care units in 19 states; On a global scale, it was estimated 176 190 children infected with SARS‐CoV‐2 by 6 April 2020. 3 One meta‐analysis found that fever and cough occurred in adults up to 92.8% and 63.4%, respectively. 4 Another study showed lymphopenia occurred in 57.4% of an adult patient with COVID‐19. 5 Although some previous studies have demonstrated that SARS‐CoV‐2 infection affects adults and children differently, 6 , 7 , 8 the data of a systematic meta‐analysis on characteristics of children with COVID‐19 are still lacking. Thus, in our research, we reviewed and analyzed the studies and reported cases from 25 December 2019 to 30 April 2020 to provide evidence‐based data involving clinical manifestations of COVID‐19 in pediatric patients. It will help to formulate policies on controlling SARS‐CoV‐2 transmission among children for pediatricians and public health specialists.
2. METHODS
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement and was registered in PROSPERO as CRD42020191099.
2.1. Search strategies
To identify studies pertaining clinical, laboratory, and imaging features in children with COVID‐19, we systematically searched from PubMed, Web of Science, Chinese Wanfang and China National Knowledge Infrastructure databases for relevant articles published through 30 April 2020. Moreover, additional records identified from World Health Organization (WHO)/Center for Disease Control reports, medRxiv, bioRxiv, SSRN, and Google Scholar. We also reviewed the references of all identified articles to identify additional studies. Search terms were as follows: novel coronavirus, nCoV, SARS2 vaccine, Wuhan coronavirus vaccine, 2019 novel coronavirus, novel coronavirus 2019, 2019‐nCoV, COVID‐19, Wuhan coronavirus, Wuhan pneumonia and SARS‐CoV‐2. These terms were used in combination with “AND” or “OR.” The literature review was performed independently by two investigators (XJC and TQZ), with a third reviewer (YMS) resolving any disputes as needed.
2.2. Inclusion criteria
We included published papers (cohort studies, case series, and case reports) that given available data about the demographic information, clinical, laboratory, and image features in children with COVID‐19. Pediatric population was defined as under 18 years old. SARS‐CoV‐2 nucleic acid was teased by RT‐PCR in accordance with the WHO guideline. 9 The level of laboratory test items was determined according to the following standards: normal white blood cell: 5.5 to 12.0 × 109/L, leukocytosis: more than 12.0 × 109/L, leukopenia: less than 5.5 × 109/L, lymphopenia: less than 1.2 × 109/L, high PCT: more than 0.046 ng/mL, high CRP: more than 10 mg/L, high LDH: more than 300 U/L, high ALT: more than 45 U/L, high AST: more than 50 U/L, high Creatinine: more than 62 μmol/L, high blood urea nitrogen: more than 7.1 mmol/L, high CK: more than 170 U/L, high CK‐MB: more than 25 U/L, high D‐dimer: more than 0.55 mg/L. We excluded studies that did not report original data or clear diagnostic criteria, and no relevant outcome.
2.3. Data collection
Two authors (XJC and TQZ) independently extracted relevant information, including first author, publication year, sex (male, %), country, number of COVID‐19 patients, age distribution (ratio of <1, 1‐5, 6‐10, 11‐15, and >15 years) and contact history. The severity of COVID‐19 was defined according to the clinical characteristics, laboratory testing, and chest imaging, including asymptomatic infection, mild, moderate, severe and critical cases. 10 The diagnostic criteria were as follows: Asymptomatic infection: The child had no clinical symptoms or signs, chest imaging was normal, and only 2019‐nCoV nucleic acid test was positive; mild: the acute upper respiratory tract infection was the main manifestations and some children may only have digestive symptoms. Some children may have no fever. Physical examination shows no auscultatory abnormalities; Moderate: with pneumonia, most of children have fever and cough, which are dry cough at first and then phlegm cough. Some of them may have wheezing, but without obvious hypoxia such as shortness of breath, sputum or dry snoring and/or wet snoring can be heard in the lungs. Some children only found lung lesions on chest computed tomography (CT) without any clinical symptoms and signs, which is called subclinical type; Severe: Early onset of respiratory symptoms such as fever and cough, some can be accompanied by gastrointestinal symptoms, disease progression often occurs in about 1 week, with hypoxia performance, and dyspnea occurs, oxygen saturation is less than 0.92; critical: children may rapidly progress to acute respiratory distress syndrome or respiratory failure. They may also develop shock, coagulation dysfunction or other multiple organ dysfunction, which may endanger their lives. Moreover, we extracted clinical performance data including fever, cough, sore throat, tachycardia, rhinorrhea, nasal congestion, tachypnea, diarrhea, vomiting, myalgia or fatigue, hypoxemia, and chest pain. Clinical laboratory results were extracted based on blood routine tests, liver function tests, renal function measurement, cardiac function, inflammatory factors and D‐dimer. Distributions for all imaging: normal, ground‐glass opacity (GGO), local patchy shadowing, bilateral patchy shadowing, white lung change and pleural effusion.
2.4. Quality assessment
We used the quality assessment tool for case series studies published by the National Institutes of Health to assess the methodological quality of included studies. 11 We scored 0 or 1 point for each item according to the criteria and added scores for all items to generate an overall quality score that ranged from 0 to 8. Based on the overall score, we classified studies as low (≥7), moderate (5‐6), or high risk of bias (≤4). Any disagreement was resolved through discussion by all investigators.
2.5. Statistical analysis
We performed data analysis using metan packages in STATA (version 12.0; Stata Corp, College Station, TX). Statistical heterogeneity between studies was evaluated with Cochran's Q test and the I 2 statistic. The I 2‐value of less than 50% was equivalent to no heterogeneity, whereas values greater than 50% was equivalent to large heterogeneity among studies. Freeman‐Tukey double arcsine transformation was used to stabilize the variance of specific prevalence rates between the included studies. 12 To account for the potential heterogeneity of studied population in each study reflected by different geographic locations, ethnicity, age group, and so forth, a random‐effect model using the DerSimonian and Laird method was used to aggregate effect sizes to estimate the overall pooled prevalence and corresponding 95% confidence intervals (CIs). To explore the reasons for heterogeneity, subgroup analyses were applied based on the study type (noncase reports vs case reports). The Begg's funnel plot was performed to evaluate the publication bias. P value of less than .05 was identified as statistically significant.
3. RESULTS
3.1. Study selection, general characteristics, and quality assessment
A total of 2048 relevant studies were collected from databases and other sources based on the search strategies. We used Endnote Software (Version X7; Thompson Reuters, CA) to remove 992 duplicate studies. According to the title and abstract, 1003 relevant studies were excluded, and then, five studies were removed by reading the full text. Finally, a total of 48 studies 2 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 were included in this meta‐analysis in according with the inclusion criteria. The PRISMA flow diagram of the included studies is listed in Figure 1. The general characteristics of the included studies are shown in Table 1. One study was from Singapore, one from Korea, one from Spain, one from America, and one from Iranian. The rest of the studies were all from China. The number of patients ranged from 1 to 2490, and male patients ranged from 0 to 1408. Twenty‐nine studies were case reports, sixteen studies were case series, and the rest three studies were cohort studies. Total scores of the included studies ranged from 3 to 8 and mean scores were 5.69. The percentage scores were used for ordinal categorization of the studies as low quality (≤4, 25%), medium quality (5‐6, 43.8%), and high quality (≥7, 31.2%). The detailed results on quality assessment are listed in the Supporting Information material 1 (Table S1).
Figure 1.
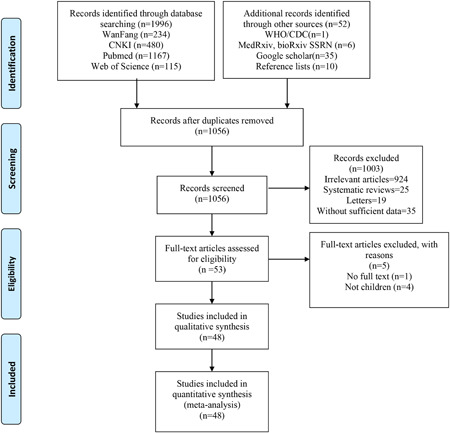
Flow diagram for the included studies
Table 1.
Characteristics of included studies
| Author | Journal | Case number (N) | Male (n) | Female (n) | Study location | Study design | Total score |
|---|---|---|---|---|---|---|---|
| Bialek et al 59 | MMWR Morb Mortal Wkly Rep | 2490 | 1408 | 1082 | America | Case series | 6 |
| Dong et al 2 | Pediatrics | 2143 | 1213 | 930 | China | Case series | 7 |
| Ma et al 13 | Chin J Contemp Pediatr | 115 | 73 | 42 | Wuhan | Case series | 6 |
| Wang et al 14 | Chin J Pediatr | 34 | 14 | 20 | Shenzhen | Case series | 5 |
| Wang et al 15 | Chin J Pediatr | 31 | NA | NA | Six provinces in north China | Case series | 7 |
| Cai et al 16 | Clin Infect Dis | 10 | 4 | 6 | Shanghai | Case report | 4 |
| Feng et al 17 | Chin J Pediatr | 15 | 5 | 10 | Shenzhen | Case report | 7 |
| Su et al 18 | Emerg Microbes Infect | 9 | 3 | 6 | Jinan | Case report | 7 |
| Zhou et al 19 | Chin J Contemp Pediatr | 9 | 4 | 5 | Shenzhen | Case report | 6 |
| Wei et al 20 | JAMA | 9 | 2 | 7 | Wuhan | Case report | 5 |
| Liu et al 21 | NEJM | 6 | 2 | 4 | Wuhan | Case report | 7 |
| Cai et al 22 | Chin J Pediatr | 1 | 1 | 0 | Shanghai | Case report | 3 |
| Zhang et al 23 | Chin J Pediatr | 1 | 0 | 1 | Hubei | Case report | 3 |
| Ji et al 24 | World J Pediatr | 2 | 2 | 0 | Zhejiang | Case report | 4 |
| Zhang et al 25 | Chin J Contemp Pediatr | 2 | 0 | 2 | Hunan | Case report | 3 |
| Wang et al 26 | Chin J Contemp Pediatr | 1 | 1 | 0 | Wuhan | Case report | 5 |
| Zhao et al 27 | Zhejiang Medicine | 1 | 1 | 0 | Zhejiang | Case report | 4 |
| Zeng et al 28 | Chin J Pediatr | 1 | 1 | 0 | Wuhan | Case report | 4 |
| Zhang et al 29 | J Med Virol | 3 | 3 | 0 | Tianjin | Case report | 4 |
| Zeng et al 30 | JAMA Pediatrics | 3 | 3 | 0 | Wuhan | Case report | 7 |
| Kam et al 31 | Clin Infect Dis | 1 | 1 | 0 | Singapore | Case report | 4 |
| Wu et al 32 | Pediatrics | 74 | 44 | 30 | Shandong | Case series | 6 |
| Xing et al 33 | J Mircobiol Immunol Infect | 3 | 2 | 1 | Shandong | Case report | 6 |
| Park et al 34 | J Korean Med Sci | 1 | 0 | 1 | Korea | Case report | 3 |
| Xia et al 35 | Pediatr Pulmonol | 20 | 13 | 7 | Wuhan | Case series | 5 |
| Tagarro et al 36 | JAMA Pediatr | 41 | 18 | 23 | Spain | Case series | 6 |
| Zhu et al 37 | Pediatr Pulmonol | 10 | 5 | 5 | Suzhou | Case report | 6 |
| Qiu et al 38 | Lancet Infect Dis | 36 | 23 | 13 | Zhejiang | Cohort study | 7 |
| Yu et al 39 | Pre‐print | 82 | 51 | 31 | Wuhan | Case series | 6 |
| liu et al 40 | Chin J Nosocomiol | 91 | 56 | 35 | Wuhan | Case series | 7 |
| Ma et al 41 | BMC Med | 50 | 28 | 22 | Wuhan | Cohort study | 7 |
| Wang et al 42 | Pre‐print | 74 | 38 | 36 | Wuhan | Case series | 7 |
| Li et al 43 | Radiol Practice | 30 | 18 | 12 | Wuhan | Case series | 6 |
| Lu et al 44 | Pediatr Infect Dis J | 110 | 59 | 51 | Wuhan | Cohort study | 8 |
| Tang et al 45 | Pre‐print | 26 | 17 | 9 | Shenzhen | Case series | 7 |
| Zheng et al 46 | Curr Med Sci | 25 | 14 | 11 | Wuhan | Case series | 8 |
| Shen et al 47 | Pediatr Pulmonol | 9 | 3 | 6 | Huna | Case report | 6 |
| Sun et al 48 | World J Pediatr | 8 | 6 | 2 | Wuhan | Case report | 5 |
| Golnar et al 49 | J Pediatr Rev | 9 | 6 | 3 | Iranian | Case report | 4 |
| Liu et al 50 | J Infect | 4 | 2 | 2 | Wuhan | Case report | 7 |
| Wu et al 51 | Chin J Contemp Pediatr | 23 | 9 | 14 | Jiangxi | Case series | 8 |
| Tan et al 52 | Chin J Contemp Pediatr | 13 | 4 | 9 | Changsha | Case report | 6 |
| Yang et al 53 | Journal of Shandong University (Health Sciences) | 10 | 3 | 7 | Shandong | Case report | 6 |
| Zhang et al 54 | Journal of Shandong University (Health Sciences) | 10 | 3 | 7 | Shandong | Case report | 6 |
| Xu et al 55 | Nat Med | 10 | 6 | 4 | Guangzhou | Case report | 6 |
| Zhang et al 56 | World Latest Medicine Information | 1 | 0 | 1 | Yunnan | Case report | 6 |
| Chen et al 57 | Chin J Pediatr | 1 | 1 | 0 | Wuhan | Case report | 4 |
| Lu et al 58 | NEJM | 171 | 104 | 67 | Wuhan | Case series | 6 |
3.2. Characteristics of COVID‐19 in children
3.2.1. Demographical characteristics
Figure 2 presents a summary of the age distribution of all participants. Among all the children cases, 17% (95% CI: 15%‐18%; I 2 = 48.6%) was under 1 year old at diagnosis, 24% (95% CI: 19%‐29%; I 2 = 80.6%) 1 to 5 years old, 25% (95% CI: 19%‐31%; I 2 = 88.7%) 6 to 10 years old, 20% (95% CI: 16%‐24%; I 2 = 82.7%) 11 to 15 years old and 18% (95% CI: 8%‐28%; I 2 = 96.7%) were 15 years old or more.
Figure 2.
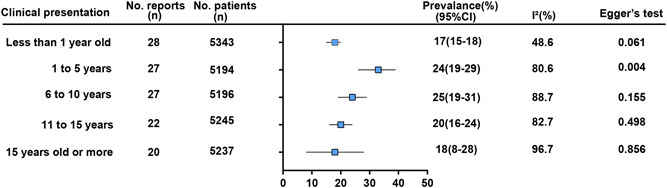
Summary results of the age distribution of all participants
Gender was shown as the proportion of males (M). Male gender of the children with COVID‐19 was 55% (95%CI: 53%‐58%; I 2 = 33.4%; P = .030). The proportion of cases with known contact history was estimated at 72% (95% CI: 64%‐80%; I 2 = 90.1%; P = .000).
3.2.2. Illness severity
Figure 3 presents a summary of the illness severity of all patients. Patients were divided into six groups as indicated based on disease severity from light to severe, mainly including: asymptomatic, mild, moderate, severe, critical, and death. Included patients were classified by their clinicians as asymptomatic 20% (95% CI: 14%‐26%; I 2 = 91.4%), mildly 33% (95% CI: 23%‐43%; I 2 = 95.6%), moderate 51% (95% CI: 42%‐61%; I 2 = 93.4%), severely 7% (95% CI: 4%‐11%; I 2 = 90.2%), critically 5% (95% CI: 2%‐9%; I 2 = 84.5%), and death 0% (95% CI: 0%‐0%; I 2 = 94.9%).
Figure 3.
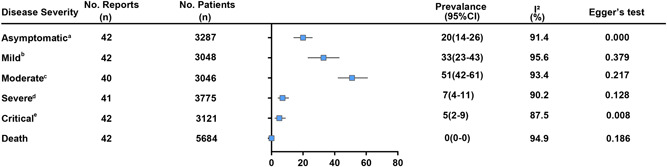
Summary results of illness severity in children with COVID‐19. The definition of illness severity was mentioned as follows: (A) without any clinical symptoms and signs. Chest imaging examination was normal, while the 2019‐nCoV nucleic acid test is positive. B, The main manifestations were acute upper respiratory tract infection and some children may have only digestive symptoms. Physical examination shows no auscultatory abnormalities. C, With pneumonia, some cases may have no clinical symptoms and signs, but chest CT shows lung lesions, which are subclinical. D, The disease usually progresses in about 1 week, and dyspnea occurs, oxygen saturation is less than 92%. E, Children can quickly progress to acute respiratory distress syndrome (ARDS) or respiratory failure, multiple organ dysfunction can be life‐threatening. COVID‐19, coronavirus disease 2019; CT, computed tomography
Among children under 1‐year old cases, asymptomatic 6% (95% CI: 5%‐13%; I 2 = 24.3%), mildly 54% (95% CI: 49%‐59%; I 2 = 0.0%), moderate 36% (95% CI: 27%‐45%; I 2 = 4.0%), severely 7% (95% CI: 4%‐11%; I 2 = 34.3%), and critically 14% (95% CI: 13%‐34%; I 2 = 37.3%) (Table 2).
Table 2.
Comparison of meta‐analysis results among different ages patients with COVID‐19
| Study | Present study | Present study | Rodriguez‐Morales AJ 4 | Michael C Grant 60 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 y | 0‐18 y | >18 y | >16 y | |||||||||||||
| No. reports | No. patients | Prevalence (%) | I 2 (%) | No. reports | No. patients | Prevalence (%) | I 2 (%) | No. reports | No. patients | Prevalence (%) | I 2 (%) | No. reports | No. patients | Prevalence (%) | I 2 (%) | |
| Male | 11 | 27 | 46 (22‐66) | 33.7 | 36 | 5838 | 55 (53‐58) | 33.4 | 22 | 2874 | 55.9 (51.6‐60.1) | 66.1 | NA | NA | NA | NA |
| Asymptomatic | 11 | 433 | 6 (5‐13) | 24.3 | 42 | 3287 | 20 (14‐26) | 91.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Mild | 10 | 395 | 54 (49‐59) | 0 | 42 | 3048 | 33 (23‐43) | 95.6 | NA | NA | NA | NA | NA | NA | NA | NA |
| Moderate | 10 | 395 | 36 (27‐45) | 4 | 40 | 3046 | 51 (42‐61) | 93.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Severe | 12 | 499 | 7 (4‐11) | 34.3 | 41 | 3775 | 7 (4‐11) | 90.2 | NA | NA | NA | NA | NA | NA | NA | NA |
| Critical | 11 | 404 | 14 (13‐34) | 37.3 | 42 | 3121 | 5 (2‐9) | 87.5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Death | 0 | 0 | NA | NA | 42 | 5684 | 0 (0‐0) | 94.9 | 7 | 632 | 13.9 (6.2‐21.5) | 91.4 | NA | NA | NA | NA |
| Fever | 11 | 24 | 53 (30‐76) | 0 | 48 | 1494 | 51 (45‐57) | 78.9 | 13 | 735 | 92.8 (89.4‐96.2) | 82.4 | 138 | 21701 | 78 (75‐81) | 94 |
| Cough | 9 | 19 | 30 (2‐58) | 0 | 45 | 1435 | 41 (35‐47) | 81 | 13 | 735 | 63.4 (48.0‐78.8) | 97.1 | 138 | 21682 | 57 (54‐60) | 94 |
| Sore throat | 7 | 10 | NA | NA | 38 | 1040 | 16 (7‐25) | 91.6 | 5 | 308 | 11 (2.8‐19.2) | 85.4 | 78 | 11721 | 12 (10‐14) | 88 |
| Tachycardia | 5 | 7 | NA | NA | 35 | 950 | 12 (3‐21) | 93.9 | NA | NA | NA | NA | NA | NA | NA | NA |
| Rhinorrhea | 8 | 17 | 21 (5‐43) | 0 | 36 | 990 | 14 (8‐19) | 75.4 | NA | NA | NA | NA | 36 | 10656 | 8 (5‐12) | 97 |
| Nasal congestion | 6 | 9 | 50 (20‐99) | 0 | 33 | 623 | 17 (6‐27) | 87.2 | NA | NA | NA | NA | 10 | 2584 | 5 (3‐7) | 78 |
| Tachypnea | 6 | 10 | 33 (20‐57) | 0 | 29 | 1034 | 9 (4‐14) | 87.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Diarrhea | 7 | 10 | NA | NA | 42 | 1250 | 8 (6‐11) | 47 | 6 | 457 | 6.1 (2.4‐9.7) | 62.1 | 93 | 11707 | 10 (8‐12) | 93 |
| Vomiting | 7 | 12 | 33 (18‐67) | 0 | 42 | 1238 | 7 (5‐10) | 50.4 | NA | NA | NA | NA | 26 | 4959 | 4 (2‐8) | 94 |
| Myalgia or Fatigue | 6 | 9 | NA | NA | 42 | 1253 | 12 (7‐17) | 77.7 | 11 | 446 | 29.4 (19.8‐39.0) | 80.7 | 78 | 13385 | 31 (27‐35) | 95 |
| Hypoxemia | 5 | 7 | NA | NA | 33 | 623 | 3 (1‐4) | 0 | 8 | 656 | 45.6 (10.9‐80.4) | 99.5 | NA | NA | NA | NA |
| Chest pain | 5 | 7 | NA | NA | 34 | 673 | 3 (0‐5) | 0 | NA | NA | NA | NA | 30 | 3510 | 7 (4‐10) | 92 |
| Leukocytosis | NA | NA | NA | NA | 38 | 907 | 10 (7‐14) | 63.1 | 7 | 487 | 16.8 (5.5‐28.0) | 93.1 | NA | NA | NA | NA |
| Leukopenia | NA | NA | NA | NA | 42 | 978 | 19 (14‐25) | 80.9 | 8 | 517 | 18.7 (8.5‐28.8) | 94.5 | NA | NA | NA | NA |
| Lymphopenia | 5 | 9 | 33 (24‐47) | 0 | 39 | 795 | 16 (11‐21) | 76.9 | 8 | 511 | 43.1 (18.9‐67.3) | 98 | NA | NA | NA | NA |
| High PCT | 7 | 10 | NA | NA | 29 | 709 | 36 (21‐51) | 97.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| High CRP | 9 | 15 | 42 (6‐78) | 0 | 32 | 651 | 19 (13‐26) | 79.3 | 6 | 332 | 58.3 (21.8‐94.7) | 98.9 | NA | NA | NA | NA |
| High LDH | 4 | 7 | 50 (15‐69) | 0 | 24 | 301 | 29 (20‐39) | 69.8 | 5 | 341 | 57.0 (38.0‐76.0) | 92.6 | NA | NA | NA | NA |
| High ALT | 7 | 25 | 47 (25‐69) | 0 | 32 | 686 | 11 (7‐15) | 38.5 | 2 | 128 | 24.1 (13.5‐34.6) | 42.8 | NA | NA | NA | NA |
| High AST | 7 | 14 | 33 (20‐67) | 0 | 28 | 529 | 18 (13‐23) | 48.6 | 3 | 169 | 33.3 (26.3‐40.4) | 0 | NA | NA | NA | NA |
| High Creatinine | 5 | 20 | NA | NA | NA | NA | NA | NA | 3 | 169 | 4.5 (1.0‐8.0) | 10.2 | NA | NA | NA | NA |
| High Blood urea nitrogen | 4 | 18 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| High CK | 2 | 3 | NA | NA | 17 | 109 | 9 (1‐17) | 33.2 | 2 | 140 | 21.3 (3.2‐39.4) | 81.4 | NA | NA | NA | NA |
| High CK‐MB | 4 | 21 | 88 (71‐94) | 8.5 | 23 | 228 | 37 (25‐48) | 59.0 | NA | NA | NA | NA | NA | NA | NA | NA |
| High D‐dimer | 4 | 5 | NA | NA | 24 | 194 | 11 (8‐14) | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Normal Imaging | 8 | 13 | 42 (6‐78) | 0 | 38 | 902 | 41 (30‐52) | 93.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Ground‐glass opacity | 8 | 14 | 50 (20‐80) | 0 | 39 | 898 | 36 (25‐47) | 92.9 | 10 | 584 | 68.5 (51.8‐85.2) | 99.1 | NA | NA | NA | NA |
| Local patchy shadow | 7 | 11 | 42 (6‐78) | 0 | 35 | 928 | 26 (21‐32) | 58.2 | 7 | 316 | 25 (5.2‐44.8) | 96.4 | NA | NA | NA | NA |
| Bilateral patchy shadow | 7 | 11 | 40 (13‐55) | 0 | 34 | 814 | 28 (21‐35) | 73.8 | 7 | 508 | 70.7 (50.4‐91.0) | 98.7 | NA | NA | NA | NA |
| White lung change | NA | NA | NA | NA | 32 | 653 | 2 (0‐4) | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
| Pleural effusion | NA | NA | NA | NA | 35 | 769 | 2 (0‐3) | 0 | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviation: COVID‐19, coronavirus disease 2019.
3.2.3. Clinical presentation
Figure 4 presents a summary of the clinical presentation of all children. The most common clinical manifestations were fever 51% (95% CI: 45%‐57%; I 2 = 78.9%), cough 41% (95% CI: 35%‐47%; I 2 = 81.0%), sore throat 16% (95% CI: 7%‐25%; I 2 = 91.6%), tachycardia 12% (95% CI: 3%‐21%; I 2 = 93.9%), rhinorrhea 14% (95% CI: 8%‐19%; I 2 = 75.4%), nasal congestion 17% (95% CI: 6%‐27%; I 2 = 87.2%), tachypnea 9% (95% CI: 4%‐14%; I 2 = 87.4%), diarrhea 8% (95% CI: 6%‐11%; I 2 = 47.0%), vomiting 7% (95% CI: 5%‐10%; I 2 = 50.4%), myalgia or fatigue 12% (95% CI: 7%‐17%; I 2 = 77.7%), hypoxemia 3% (95% CI: 1%‐4%; I 2 = 0.0%) and chest pain 3% (95% CI: 0%‐5%; I 2 = 0.0%).
Figure 4.
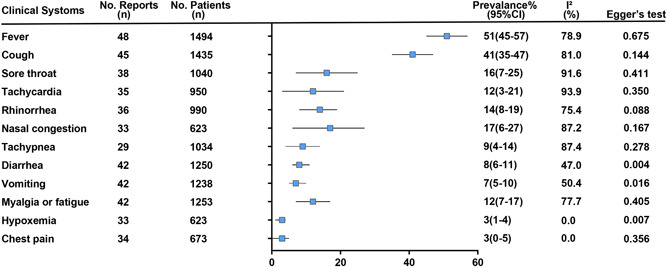
Aggregated results of clinical presentation in children with COVID‐19. COVID‐19, coronavirus disease 2019
Among children under 1‐year old cases, fever 53% (95% CI: 30%‐76%; I 2 = 0.0%), cough 30% (95% CI: 2%‐58%; I 2 = 0.0%), rhinorrhea 21% (95% CI: 5%‐43%; I 2 = 0.0%), nasal congestion 50% (95% CI: 20%‐99%; I 2 = 0.0%), tachypnea 33% (95% CI: 20%‐57%; I 2 = 0.0%) and vomiting 33% (95% CI: 18%‐67%, I 2 = 0.0%) (Table 2).
3.2.4. Laboratory examination
Laboratory examination results, which including blood routine test, liver function tests, renal function measurement, cardiac function, inflammatory factors, and D‐dimer, are shown in Figure 5. With respect to laboratory findings, the proportion of normal white blood cells in COVID‐19 patients in children was 69% (95% CI: 64%‐75%; I 2 = 58.5%). Leukocytosis (>12 × 109/L) was observed in 10% (95% CI: 7%‐14%; I 2 = 63.1%) of patients, and 19% (95% CI: 14%‐25%; I 2 = 80.9%) patient had leukopenia (<5.5 × 109/L). The proportion of patients with lymphopenia was 16% (95% CI: 11%‐21%; I 2 = 76.9%). The proportion of patients with high PCT 36% (95% CI: 21%‐ 51%; I 2 = 97.0%), CRP 19% (95% CI: 13%‐26%; I 2 = 79.3%) and LDH 29% (95% CI: 20%‐39%; I 2 = 69.8%), respectively. ALT and AST are indicative parameters for liver function, the high ALT and AST patients was 11% (95% CI: 7%‐15%; I 2 = 38.5%) and 18% (95% CI: 13%‐23%; I 2 = 48.6%), respectively. Creatine‐kinase (CK) and CK‐MB are indicative parameters for cardiac function, the high CK and CK‐MB was 9% (95% CI: 1%‐17%; I 2 = 33.2%) and 37% (95% CI: 25%‐48%; I 2 = 59.0%), respectively. We also evaluated the patients had elevated D‐dimer, result revealed that nearly 11% (95% CI: 8%‐14%; I 2 = 0.0%).
Figure 5.
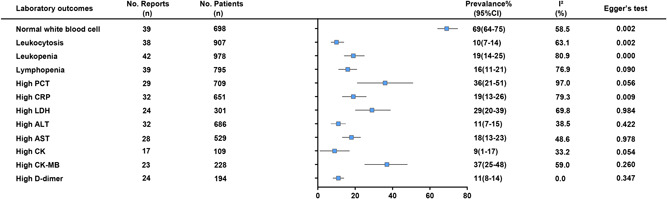
Summary results of laboratory examination in children with COVID‐19. COVID‐19, coronavirus disease 2019
Among children under 1‐year old cases, the proportion of lymphopenia was 33% (95% CI: 24%‐47%; I 2 = 0.0%). The ratio of elevated CRP and LDH were 42% (95% CI: 6%‐78%; I 2 = 0.0%) and 50% (95% CI: 15%‐69%; I 2 = 0.0%), respectively. The high ALT and AST patients were 47% (95% CI: 25%‐69%; I 2 = 0.0%) and 33% (95% CI: 20%‐67%; I 2 = 0.0%), respectively. The proportion of elevated CK‐MB was 88% (95% CI: 71%‐94%; I 2 = 8.5%) (Table 2).
3.2.5. Imaging features
Imaging features for patients were summarized in Figure 6. All pediatric patients with normal imaging was 41% (95% CI: 30%‐52%; I 2 = 93.4%), GGO 36% (95% CI: 25%‐47%; I 2 = 92.9%), local patchy shadowing 26% (95% CI: 21%‐32%; I 2 = 58.2%), bilateral patchy shadowing 28% (95% CI: 21%‐35%; I 2 = 73.8%), white lung change was 2% (95% CI: 0%‐4%; I 2 = 0.0%) and pleural effusion 2% (95% CI: 0%‐3%; I 2 = 0.0%).
Figure 6.
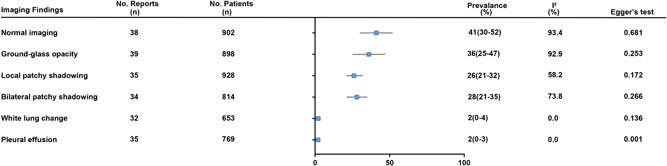
Pooled results of imaging features in children with COVID‐19. COVID‐19, coronavirus disease 2019
Among children under 1‐year old cases, the proportion of normal imaging was 42% (95% CI: 6%‐78%, I 2 = 0.0%). The GGO accounted for 50% (95% CI: 20%‐80%; I 2 = 0.0%) of lung abnormality. Local and bilateral patchy shadowing were 42% (95% CI: 6%‐78%; I 2 = 0.0%) and 40% (95% CI: 13%‐55%; I 2 = 0.0%), respectively (Table 2).
3.2.6. Publication bias
Publication bias was evaluated by Begg's test, which suggested that there was no notable evidence of publication bias except for 1 to 5 year (P = .004); asymptomatic (P = .000); critical (P = .008); diarrhea (P = .004); vomiting (P = .016); hypoxemia (P = .007); normal white blood cell (P = .002); leukocytosis (P = .002); leukopenia (P = .000); high CRP (P = .009) and pleural effusion (P = .001).
3.2.7. Subgroup analysis
Tables S2 to S6 present the results of subgroup analyses. Noncase reports included case series and cohort studies, case reports only referred to the study design of the case report. The findings were consistent in all subgroup analyses except for the 6 to 10 years, critical and tachycardia subgroups.
4. DISCUSSION
We comprehensively examined the demographic, clinical characteristics, laboratory, and imaging features among pediatric patients with COVID‐19 and further made a meta‐analysis with literature studies. We also compared the characteristics of SARS‐CoV‐2 infection in children under 1 year old, children between 0 and 18 years old, as well as in adults (Table 2). The main findings are as follows: first, SARS‐CoV‐2 was susceptible to all age groups of children, the most common clinical manifestations were fever and cough and the majority of them had experienced asymptomatic, mild and moderate illness; second, children were more likely to have normal leukocyte counts, whereas lymphocytosis occurred infrequently; Third, the incidence of critical illness and vomiting symptoms was high in children under 1 year old.
The pandemic of COVID‐19 affected all age groups in children based on the current studies. 3 , 6 , 61 Our result was consistent with those previous studies that there was no significant difference in age distribution of SARS‐CoV‐2 infection among children. The most common clinical manifestations of COVID‐19 pediatric patients were fever (51%) and cough (41%) from our meta‐analysis, which were lower than the fever (78.0%‐92.8%) and cough (57.0%‐63.4%) in adults. 4 , 5 , 60 These findings further suggested that children's clinical symptoms were not typical when compared with adults. The frequency of severe illness was 7% in children with COVID‐19, which was lower than that in adults (25.6%). 5 One possible reason might be that children are less likely to have underlying diseases such as diabetes, hypertension, or cardiovascular disease. In addition to the above reason, the fact that innate immune response declines with age could also be important for the difference. 62 The percentage of asymptomatic in children with COVID‐19 was 20% and deserves full attention to control the ongoing pandemic.
For the laboratory examination, normal leukocyte counts were up to 70% in children, which was as similar as 69.7% in adults. 63 The reduction of lymphocytes in children was only 16%, but in adults, it went up to 43.1%, 4 57.4%, 5 and 56%, 63 respectively, reported by previous meta‐analysis. The reason for these differences may be related to the immune response of different organisms to novel coronavirus. The level of CK‐MB was raised in 37% of all children, which is one of the classical biomarkers of cardiotoxicity. Subgroup analysis further revealed CK‐MB was elevated in nearly 88% in children under 1 year old. These results suggested that we should pay special attention to the myocardial damage in children, especially for those under 1year old. It should be noted that the activity of CK‐MB was detected by immunosuppressive method, which is easy to be affected by the peripheral blood creatine‐kinase BB (CK‐BB). In addition, the blood‐brain barrier in children, especially in infants, is not fully developed, and more CK‐BB will appear in the peripheral blood, resulting in the high level of CK‐MB activity. 64 , 65 , 66 In the future, specific studies are still needed to explain the causes and effects of CK‐MB elevation in children.
The CT manifestations of COVID‐19 in pediatric patients are diverse and lack specificity depending on the severity of disease or clinical classification. Normal imaging occurred in 41% pediatric patients in our meta‐analysis, which are similar to the other two reports with the largest number of pediatric cases so far, the proportion of normal lung CT was 35% (60/171) and 57% (66/115), respectively, 13 , 58 but, it was infrequent in adults, the rate of normal imaging was only 10%. 67 GGO was the most common performance in children presenting lung abnormality, which most located in the lower lung, outer band, near the pleura, and the scope was small compared with adults. 13 , 15 , 17 , 29 , 35 , 68 , 69 Pediatric patients with GGO was 36%, significantly lower than adults with 80% to 83%. 5 , 67 This may be related to the fact that COVID‐19 in children shows milder than adults.
The rate of critical illness in children under 1 year old was 14%, higher than 5% in all children. These results suggested that clinicians should pay more attention to changes in disease activity in children under 1 year old. A total of 33% and 7% suffer from vomiting in children under 1 year old and all children, respectively. Based on all the above facts, it was worth noting that the proportion of infants developing critical cases is relatively high, and the initial symptom often include vomiting. However, due to the limited sample size, large, well‐designed studies are still needed to confirm these findings.
5. LIMITATION
This meta‐analysis has several limitations that should be addressed. First, few cohort studies available for inclusion, and most of them come from China. Second, it's hard to standardize the results of laboratory testing and radiographic imaging from different data sources. Third, more detailed patient information in children under 1 year old is not available in large sample studies at the time of analysis, the results need to be further updated.
6. CONCLUSION
The COVID‐19 pandemic affects all age groups in children and appears to be a mild illness. The common presenting complaints with COVID‐19 are nonspecific symptoms, such as fever and cough. Normal leukocyte counts and infrequent of lymphopenia tend to be the laboratory characteristics. High incidence of critical illness and vomiting symptoms were as the main features in children under 1 year old. Characteristics of COVID‐19 in children and adults are different and thus special criteria is still needed for more studies to identify.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed to the intellectual content of this manuscript and approved the final manuscript as submitted. (a) Conception and design: Chunquan Cai, Yongming Shen; (b) collection, assembly of data: Xiaojian Cui, Tongqiang Zhang; (c) administrative support: Ping Si, Yongsheng Xu; (d) data analysis and interpretation: Zhihu Zhao, Wei Guo, Wenwei Guo, Jiayi Zhang; (e) search literatures: Cuicui Dong, Jiafeng Zheng, Ren Na, Lisheng Zheng, Wenliang Li, Zihui Liu, Jinhu Wang, Jia Ma; (f) manuscript writing: all authors; (g) final approval of manuscript: all authors.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant number: 81771589), the Key Project of Tianjin Health Care Professionals (Grant number: 16KG166), and the Program of Tianjin Science and Technology Plan (Grant number 18ZXDBSY00170).
Cui X, Zhao Z, Zhang T, et al. A systematic review and meta‐analysis of children with coronavirus disease 2019 (COVID‐19). J Med Virol. 2021;93:1057–1069. 10.1002/jmv.26398
Xiaojian Cui, Zhihu Zhao, Tongqiang Zhang, and Wei Guo contributed equally to this work.
Contributor Information
Yongsheng Xu, Email: xxyyss@126.com.
Ping Si, Email: kangsiping@live.cn.
Yongming Shen, Email: shenymtj@sina.com.
Chunquan Cai, Email: 15122656313@126.com.
DATA AVAILABILITY STATEMENT
The original data can be requested reasonably.
REFERENCES
- 1. WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.who.int/
- 2. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 Among Children in China. Pediatrics. 2020;145(6):e202007 [DOI] [PubMed] [Google Scholar]
- 3. Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID‐19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Public Health Manage Pract: JPHMP. 2020;26(4):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80(6):656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui X, Zhang T, Zheng J, et al. Children with coronavirus disease 2019: a review of demographic, clinical, laboratory, and imaging features in pediatric patients. J Med Virol. 2020. 10.1002/jmv.26023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandao MB. Clinical manifestations of children with COVID‐19: a systematic review. Pediatr Pulmonol. 2020;55:1892‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Technical‐guidance for 2019‐nCoV RT‐PCR. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technicalguidance/laboratory-guidance
- 10. Society of Pediatrics CMA, Editorial Board CJoP . [Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children (first interim edition)]. Zhonghua Er Ke Za Zhi. 2020;58(3):169‐174. [DOI] [PubMed] [Google Scholar]
- 11. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974‐978. [DOI] [PubMed] [Google Scholar]
- 13. Ma YL, Xia SY, Wang M, Zhang SM, Du WH, Chen Q. [Clinical features of children with SARS‐CoV‐2 infection: an analysis of 115 cases]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(4):290‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang XF, Yuan J, Zheng YJ, et al. [Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen]. Zhonghua Er Ke Za Zhi. 2020;58:E008. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Ju XL, Xie F, et al. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China]. Zhonghua Er Ke Za Zhi. 2020;58(4):269‐274. [DOI] [PubMed] [Google Scholar]
- 16. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020:ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feng K, Yun YX, Wang XF, et al. [Analysis of CT features of 15 children with 2019 novel coronavirus infection]. Zhonghua Er Ke Za Zhi. 2020;58(4):275‐278. [DOI] [PubMed] [Google Scholar]
- 18. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID‐19. Emerg Microbes Infect. 2020;9(1):707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y, Yang GD, Feng K, et al. [Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323(13):1313‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu W, Zhang Q, Chen J, et al. Detection of Covid‐19 in children in early january 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai JH, Wang XS, Ge YL, et al. [First case of 2019 novel coronavirus infection in children in Shanghai]. Zhonghua Er Ke Za Zhi. 2020;58(2):86‐87. [DOI] [PubMed] [Google Scholar]
- 23. Zhang YH, Lin DJ, Xiao MF, et al. [2019 novel coronavirus infection in a three‐month‐old baby]. Zhonghua Er Ke Za Zhi. 2020;58(3):182‐184. [DOI] [PubMed] [Google Scholar]
- 24. Ji LN, Chao S, Wang YJ, et al. Clinical features of pediatric patients with COVID‐19: a report of two family cluster cases. World J Pediatr. 2020;16:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang GX, Zhang AM, Huang L, et al. [Twin girls infected with SARS‐CoV‐2]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):221‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Wang D, Chen GC, Tao XW, Zeng LK. [SARS‐CoV‐2 infection with gastrointestinal symptoms as the first manifestation in a neonate]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruihong Z, Xiaomin S, Kaijin X, Jifang S. 1 case of novel coronavirus pneumonia in children. Zhejiang Med J. 2020;42(4):305‐306. 2020. [Google Scholar]
- 28. Zeng LK, Tao XW, Yuan WH, Wang J, Liu X, Liu ZS. [First case of neonate with COVID‐19 in China]. Zhonghua Er Ke Za Zhi. 2020;58(4):279‐280. [DOI] [PubMed] [Google Scholar]
- 29. Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020;92:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020.174(7):722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID‐19) with high viral load. Clin Infect Dis. 2020;71:847‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Q, Xing Y, Shi L, et al. Co‐infection and other clinical characteristics of COVID‐19 in children. Pediatrics. 2020;146:e20200961. [DOI] [PubMed] [Google Scholar]
- 33. Xing YH, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol, Immunol Infection. 2020;53:473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35(11):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2020:e201346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55(6):1430‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu H, Cai Q, Dai X, Liu X, Sun HJM The clinical and epidemiological features and hints of 82 confirmed COVID‐19 pediatric cases aged 0‐16 in Wuhan, China. 2020.
- 40. Jie L, Wan‐jun L, Zhi‐hong D, et al. Clinical and epidemiological characteristics of 91 children conformed with COVID‐19. Chin J Nosocomiol. 2020;30(11):1‐5. [Google Scholar]
- 41. Ma H, Hu J, Tian J, et al. A single‐center, retrospective study of COVID‐19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Zhu F, Wu J, et al. Epidemiological and Clinical Characteristics of 74 Children Infected with SARS‐CoV‐2 in Family Clusters in Wuhan, China. 2020.
- 43. Qian L, Xue‐hua P, Zi‐yan S, Jian‐bo S. Clinical and imaging characteristics of children with corona virus disease 2019 (COVID‐19). Radiol Practic. 2020;35(3):277‐280. [Google Scholar]
- 44. Lu Y, Li Y, Deng W, et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Pediatr Infect Dis J. 2020;39:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang A, Xu W, Chen P, Li G, Liu Y, Liu LJM A retrospective study of the clinical characteristics of COVID‐19 infection in 26 children. 2020.
- 46. Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, et al. COVID‐19 infection in Iranian children: a case series of 9 patients. 2020;8(2):139‐144.
- 50. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu HP, Li BF, Chen X, et al. [Clinical features of coronavirus disease 2019 in children aged <18 years in Jiangxi, China: an analysis of 23 cases]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(5):419‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan X, Huang J, Zhao F, Zhou Y, Li JQ, Wang XY. [Clinical features of children with SARS‐CoV‐2 infection: an analysis of 13 cases from Changsha, China]. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(4):294‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y, Zhan L, Huaru X, et al. Epidemiological and clinical characteristics of 10 children with coronavirus disease (COVID‐19) in Jinan City. J Shandong University (Health Sciences). 2020;58(4):36‐39. [Google Scholar]
- 54. Xiaoguo Z, Yan M, Jiaan X, Zhongfa Z. Clinical characteristics of novel coronavirus pneumonia in children in Jinan. J Shandong University (Health Sciences). 2020;58(3):62‐64. [Google Scholar]
- 55. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xia Z, Li L, Xiaomei L. Clinical analysis of 1 novel coronavirus infecting pneumonia cases in Yunnan. World Latest Medicine Informatio [J]. 2020;20(32). [Google Scholar]
- 57. Chen F, Liu ZS, Zhang FR, et al. Frist case of severe childhood novel coronavirus pneumonia in China. Zhonghua er ke za zhi = Chinese J Pediatr. 2020;58:E005. [DOI] [PubMed] [Google Scholar]
- 58. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Team CC‐R. Coronavirus disease 2019 in children—United States, February 12‐April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grant MC, Geoghegan L, Arbyn M, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS‐CoV‐2; COVID‐19): a systematic review and meta‐analysis of 148 studies from 9 countries. PLoS One. 2020;15(6):e0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Molloy EJ, Bearer CF. COVID‐19 in children and altered inflammatory responses. Pediatr Res. 2020. 10.1038/s41390-020-0881-y [DOI] [PubMed] [Google Scholar]
- 62. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3,062 COVID‐19 patients: a meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan H, Nan‐nan X. Diagnostic value of myocardial troponin I, CK‐MB, and C reactive protein in children with viral myocarditis. Chinese J Lab Diagn. 2014;18(04):611‐612. [Google Scholar]
- 65. Jiaqi Y, Yanping f, Aisheng L, Yongge L, Xiaoge L. The difference between the normal value of serum creatine kinase MB in children and that in adults. Lab Med Clinic. 2018;2(02):230‐231. [Google Scholar]
- 66. Rong Z, Zhen‐nan D, Jin D, Guang‐hong G, Xin‐yu W. Clinical evaluation of serum creatine phosphokinase isoenzyme in healthy children at different ages. J Chinese Pla Postgraduate Med Sch. 2010;31(4):337‐338. [Google Scholar]
- 67. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID‐19) CT findings: a systematic review and meta‐analysis. J Am Coll Radiol. 2020;17:701‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Duan YN, Zhu YQ, Tang LL, Qin JCT. features of novel coronavirus pneumonia (COVID‐19) in children. Eur Radiol. 2020;30:4427‐4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma H, Shao J, Wang Y, Zhai A, Zheng N, Li QJCJR. High resolution CT features of novel coronavirus pneu monia in children [J]. Environ Sci Technol. 2020;54:1005‐1201.31904951 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The original data can be requested reasonably.


