Abstract
Objective
There is minimal evidence describing outcomes for emergency department (ED) patients with suspected coronavirus disease 2019 (COVID‐19) infection who are not hospitalized. The study objective was to assess 30‐day outcomes (ED revisit, admission, ICU admission, and death) for low‐risk patients discharged after ED evaluation for COVID‐19.
Methods
This was a retrospective cohort study of patients triaged to a COVID‐19 surge area within an urban ED and discharged between March 12 and April 6. Physicians were encouraged to discharge patients if they were well‐appearing with few comorbidities. Data were collected from review of medical records and phone follow‐up, and the analysis was descriptive.
Results
Of 452 patients, the median age was 38, and 61.7% had no comorbidities. Chest radiographs were performed for 50.4% of patients and showed infiltrates in 14% of those tested. Polymerase chain reaction testing was performed for 28.3% of patients during the index ED visit and was positive in 35.9% of those tested. Follow‐up was achieved for 75.4% of patients. ED revisits occurred for 13.7% of patients. The inpatient admission rate at 30 days was 4.6%, with 0.7% requiring intensive care. Median number of days between index ED evaluation and return for admission was 5 (interquartile range 3–7, range 1–17). There were no known deaths.
Conclusions
A minority of low‐risk patients with suspected COVID‐19 will require hospitalization after being discharged home from the ED. Outpatient management is likely safe for well‐appearing patients with normal vital signs, but patients should be instructed to return for worsening symptoms including labored breathing. Future work is warranted to develop and validate ED disposition guidelines.
Keywords: COVID, COVID‐19, discharge, disposition, emergency medicine, emergency medicine practice, guidelines, pandemic, safety
1. INTRODUCTION
1.1. Background
The World Health Organization has called the current severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic the “defining health crisis of our time.” 1 After its discovery in December 2019, the virus spread to metropolitan areas across the globe, leading to early and sustained community spread in certain areas including metropolitan Los Angeles. Despite large numbers of people being infected, little work has been done to characterize outcomes among relatively low‐risk patients with suspected or confirmed illness who present to the emergency department or outpatient settings.
1.2. Importance
Published guidance suggests that disposition home is appropriate for patients with mild symptoms and few comborbidities. 2 , 3 However, there is minimal evidence describing post‐discharge outcomes for patients who are well appearing at the time of ED evaluation. Prognostic evidence from China suggests that 80% of patients have mild illness, 15% require oxygen, and 5% require ICU monitoring. 4 Currently in the United States, however, it is unknown how often patients evaluated in the ED seek subsequent care for worsening symptoms after their initial evaluation and how often they undergo monitoring in the inpatient and ICU settings.
1.3. Goals of this investigation
The primary goal of this investigation was to describe outcomes for low‐risk patients discharged home from the ED, including rates of ED revisit, inpatient admission, and ICU admission within 30 days.
2. METHODS
2.1. Study design and setting
This study is reported in accordance with the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) statement. 5 This was a retrospective cohort study of consecutive patients with suspected coronavirus disease 2019 (COVID‐19) in whom clinical and phone follow‐up data had been compiled as a quality improvement initiative during the early COVID‐19 pandemic. The study was conducted at an urban ED at an academic medical center in the Los Angeles metropolitan area with ≈90,000 visits per year.
2.2. Selection of participants
Patients were selected for inclusion in the study if they were evaluated for influenza‐like illness suggestive of COVID‐19 in a temporary surge tent between March 12, 2020 and April 6, 2020 and discharged home. Patients were excluded from the study if they had been seen in our ED within the prior 7 days, if they did not complete ED evaluation (ie, left without being seen, left against medical advice, or eloped), or if they were asymptomatic according to clinician documentation.
The Bottom Line
The majority of patients diagnosed with coronavirus disease 2019 from US emergency departments are discharged and subsequent ED revisit, admission, ICU admission, and death rates are unknown. This retrospective study of 30‐day follow up in 452 ED patients reported that a minority of low‐risk patients require subsequent hospital admission.
2.3. Treatment and disposition pathway
Patients were assessed by emergency physicians or physician assistants who were given guidelines to (1) minimize testing (ie, avoid unnecessary blood tests and advanced imaging) and (2) optimize time spent in educating patients about COVID‐19, its typical progression, isolation instructions, and return precautions. Testing in the tent itself was limited to SARS‐CoV‐2 polymerase chain reaction (PCR) nasopharyngeal swabs, chest radiographs, and electrocardiograms; however, some patients initially triaged to the tent were ultimately sent to the main ED for a more comprehensive workup including blood testing at the discretion of the clinician. COVID‐19 PCR tests were performed initially through the Los Angeles County Department of Public Health and Quest Diagnostics. Later during the study period and after approval by the Federal Drug Administration, Cedars‐Sinai Medical Center's laboratory performed COVID‐19 PCR tests on site rather than sending them to the County or Quest. The clinician's decision to test for COVID‐19 was informed by evolving guidelines from the Centers for Disease Control and Prevention and the Los Angeles County Department of Public Health. Results of COVID‐19 testing performed in the ED were not available at the time of the ED visit. Outpatient management of suspected COVID‐19 cases was encouraged if patients met criteria listed in Box 1, regardless of whether COVID‐19 testing was performed. These criteria were developed by our department leadership after review of available guidance in March 2020, including 1 relevant peer‐reviewed publication and a report from the World Health Organization. 6 , 7 Suggested discharge criteria were communicated to ED clinicians during a 10‐minute presentation during departmental grand rounds and also through distribution by email. In the same presentation, hospitalization was discouraged given the absence of proven prophylactic or therapeutic treatment options and to preserve inpatient resources for an anticipated wave of higher acuity patients.
1. Box 1: Discharge criteria for patients with suspected COVID‐19 infection
Resting pulse oximeter ≥ 92%
No respiratory distress or tachypnea (respiratory rate ≤ 20) at rest
≤ 60 years of age
Ambulatory
No significant comorbidities
No more than mild disease on chest radiograph (if obtained)
Ability to return within 24 hours if worse
Patient preference for outpatient management
2.4. Data collection
ED administrative staff obtained data used in this study during a quality improvement initiative designed to ascertain outcomes of patients cared for in the ED's surge tent during the early COVID‐19 pandemic. Data collection involved review of the electronic health record (EHR) as well as phone follow‐up to patients to characterize follow‐up care received and the total duration of symptoms. Data collection was performed via a combination of automatic data exportation through Epic reports (most structured data elements) and human entry into a spreadsheet (vital signs, number of days of symptoms prior to ED visit) by 2 operations team members with experience in healthcare delivery (AJH: emergency medical technician; NCG, physician assistant). These case reviews were performed as independent, unblinded reviews. One physician member of the team (CTB) supervised data collection. Two physicians (CTB and SST) reviewed cases that included outcomes of interest to ensure accurate reporting.
Data elements collected from the EHR include patient characteristics (age, sex, race, ethnicity, comorbidities); visit characteristics (day of week, arrival time, length of stay, emergency severity index triage score); clinical characteristics (chief complaint, vital signs, duration of symptoms prior to ED visit), and testing performed in the ED (chest radiography, electrocardiogram, influenza test, COVID‐19 PCR test, and any blood work). The number of comorbidities was generated by counting certain conditions (hypertension, cardiovascular disease, lung disease, chronic kidney disease, obesity, and immunosuppression) documented in the patient's past medical history. The chief complaint was extracted as documented by the triage nurse. Because it was common for triage chief complaints to include free‐text or multiple complaints, 1 physician member of the research team (CTB) consolidated the chief complaints using the following stepwise process: (1) counted first‐listed chief complaints and listed them in order of most frequent to least frequent; (2) consolidated similar complaints (eg, combined “fever” with “chills”; combined “influenza‐like illness” with “flu”); and (3) combined remaining chief complaints occurring <8 times into clinically relevant categories (eg, combined rhinorrhea and ear pain).
Through phone follow‐up at 30 days after the visit, patients (or caregivers) were asked to provide information about any care received after the ED visit, including dates of subsequent ED visits, inpatient admissions, ICU admissions, and/or deaths. The patient was also asked to recall the total duration of their symptoms and/or the date that symptoms resolved. If symptoms were reported as still ongoing, symptom duration was capped at >30 days. If the patient or caregiver was not reachable after 2 attempts by phone, the team sought information about vital status and timing of any subsequent care through our medical center's EHR and its CareEverywhere feature. Any patient care activity >30 days after the index visit was considered to be confirmation the patient survived the 30‐day follow‐up period. County coroner data were queried using an online tool. Complete death certificate data were not available at the time of publication because of proximity of the visit date and preparation of the manuscript.
All data were stored in a password‐protected database that was maintained on an encrypted server.
2.5. Outcomes
The primary outcomes of interest were ED revisits, inpatient admissions, ICU admissions, and deaths within 30 days of the index visit. Secondary outcomes were measured through patients’ self‐reported duration of symptoms including symptom onset and symptom resolution.
2.6. Analysis
We performed a descriptive analysis of patient and visit characteristics using STATA version 13.1 (StataCorp, College Station, TX). Additionally, 2 research team members (CTB and AJH) reviewed cases that resulted in ED revisits to determine reasons that patients cited for returning to the ED and described their findings in the supplementary appendix. The institutional review board approved this study as non‐human subjects research because we utilized an already‐existing ED operations database.
3. RESULTS
Of 504 patients screened, 452 patients met inclusion criteria (Figure 1). The median age was 38 (interquartile range [IQR] 30–51.5); 98.7% of patients were adults, 50.9% were women, and 61.7% had no significant comorbidities. The median duration of symptoms at the time of index ED evaluation was 4 days (IQR 2–7, range 0–60). The most commonly abnormal vital sign was the heart rate >100 in 103/452 (22.8%) patients, followed by respiratory rate >20 in 36/452 (8.0%) patients. Temperature of >100.4°F was present in the ED in 19/452 (4.2%), and all patients had oxygen saturation of 92% or higher. Chest radiographs were the most commonly ordered test, performed in 228/452 (50.4%) patients with infiltrates present on 32/452 (7.1%) of patients, which was 32/228 (14.0%) of those imaged. Nasopharyngeal PCR testing for SARS‐CoV‐2 was performed in 128/452 (28.3%) of patients the during the ED visit and returned positive results in 46/452 (10.2%), which was 46/128 (35.9%) of those tested. A total of 62/452 (13.7%) patients in our sample had positive COVID‐19 testing during the 30 days following the index visit. See Table 1 for additional patient characteristics and clinical outcomes for the study population.
FIGURE 1.
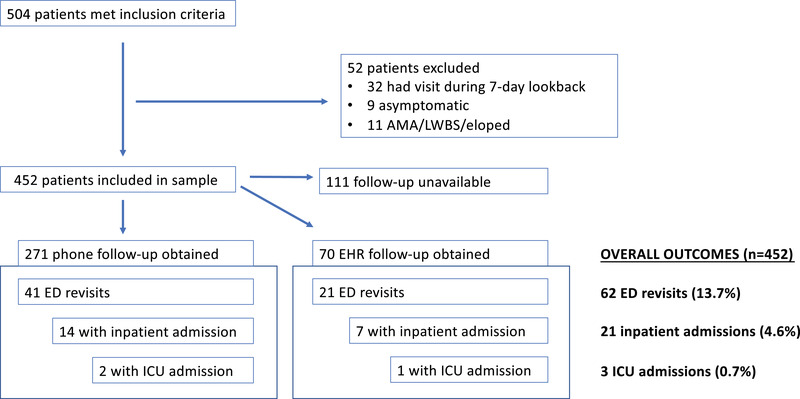
Flowsheet demonstrating numbers of patients excluded, follow‐up success, and outcomes for patients in the study cohort. AMA, against medical advice; LWBS, left without being seen.
TABLE 1.
Patient characteristics and clinical outcomes
| Characteristics | N = 452 (%) | |
|---|---|---|
| Age | 0–17 | 6 (1.3) |
| 18–34 | 180 (39.8) | |
| 35–49 | 136 (30.1) | |
| 50–64 | 89 (19.7) | |
| 65–79 | 41 (9.1) | |
| Sex | Female | 230 (50.9) |
| Male | 222 (48.1) | |
| Race | White | 313 (69.2) |
| Black | 67 (14.8) | |
| Asian | 26 (5.8) | |
| Missing | 46 (10.2) | |
| Ethnicity | Non‐Hispanic | 360 (79.6) |
| Hispanic | 86 (19.0) | |
| Missing | 6 (0.3) | |
| Number of comorbidities | 0 | 279 (61.7) |
| 1 | 111 (24.6) | |
| 2 | 34 (7.5) | |
| 3 or more | 28 (6.2) | |
| Time of arrival | 7 am‐3 pm | 232 (51.3) |
| 3 pm‐11 pm | 220 (48.7) | |
| Day of week | Weekday | 362 (80.1) |
| Weekend | 90 (19.9) | |
| Length of stay (minutes) | <90 | 179 (39.6) |
| 90–179 | 61 (13.5) | |
| 180–269 | 184 (40.7) | |
| 270+ | 28 (6.2) | |
| Emergency severity index triage score | 2 | 2 (0.4) |
| 3 | 119 (26.3) | |
| 4 | 329 (72.8) | |
| 5 | 2 (0.4) | |
| Chief complaint | Influenza‐like illness | 182 (40.3) |
| Cough | 121 (26.7) | |
| Fever or chills | 58 (12.8) | |
| COVID‐19 related | 30 (6.6) | |
| Shortness of breath or wheezing | 26 (5.8) | |
| Sore throat | 9 (1.9) | |
| Other upper respiratory symptoms (rhinorrhea, ear pain) | 8 (1.8) | |
| Chest pain | 8 (1.8) | |
| Other (diarrhea, body aches, malaise, headache) | 10 (2.2) | |
| Abnormal triage vital signs | Systolic blood pressure < 90 | 0 (0) |
| Heart rate > 100 | 103 (22.8) | |
| Respiratory rate > 20 | 36 (8.0) | |
| Temperature > 100.4°F | 19 (4.2) | |
| Oxygen saturation < = 91% on room air | 0 (0) | |
| Days of symptoms prior to index ED visit | <5 | 238 (52.7) |
| 5 to 10 | 143 (31.6) | |
| >10 | 56 (12.4) | |
| Missing | 15 (3.3) | |
| Testing done during index ED visit | Chest X‐ray | 228 (50.4) |
| EKG | 51 (11.3) | |
| Influenza test | 28 (6.2) | |
| COVID‐19 test in ED | 128 (28.3) | |
| Blood work | 57 (12.6) | |
| Results | Infiltrate on chest X‐ray | 32 (7.1) |
| Influenza test positive | 0 (0) | |
| ED COVID test positive | 46 (10.1) | |
| Any COVID test positive during 30‐day follow‐up period | 62 (13.7) | |
| Total duration of symptoms | <10 days | 67 (14.8) |
| 10–30 days | 1422 (31.4) | |
| >30 days | 443 (9.75) | |
| Missing | 199 (44.0) | |
| Outcomes | ED re‐visit within 30 days | 62 (13.7) |
| Hospital admission within 30 days | 21 (4.6) | |
| ICU admission within 30 days | 3 (0.7) | |
| Median days before discharge and return for admission | 5 (interquartile range 3–7) | |
| Missing | 111 (24.6) |
Thirty‐day follow up was available for 341/452 (75.4%) patients: 271/452 (60.0%) through phone follow‐up and an additional 70/452 (15.4%) through documentation in the EHR. Patient‐reported total duration of symptoms was a median of 15 days (IQR 9–17). Revisits to the ED within 30 days occurred for 62/452 (13.7%) of patients. Returns resulting in hospitalization within 30 days occurred in 21/452 (4.6%) of patients, with 3/452 (0.7%) admitted to the ICU. For patients who returned and were hospitalized within 30 days, the median number of days from the index visit to return for admission was 5 (IQR 3–7, range 1–17) and median total duration of symptoms prior to the admission was 10 (IQR 9–12, range 3–17). A majority (16/21 = 76.2%) of the patients who returned and required hospitalization were diagnosed with COVID‐19 by PCR testing within 30 days of the index ED visit. To our knowledge, the mortality rate was zero.
4. LIMITATIONS
The study is limited, first, by its retrospective nature and potential for biases introduced by incomplete clinician documentation. Second, follow‐up information (by either phone or subsequent clinical documentation) was available for only 75.4% of patients in the sample, which may have led to underestimated rates of hospitalization or mortality. Third, death certificate data were not available because of the proximity of clinical care and preparation of this manual, and mortality may have thus been underestimated. Fourth, unlike other reports focusing on patients with PCR‐proven COVID‐19, the patients in this study comprised a more heterogenous population with PCR testing performed in 45.3% with only 30.2% of those tested being positive for SARS‐CoV‐2. Fifth, members of the team reviewing ED revisits were not blinded to the study's intent, which may have introduced bias into assessment of those visits. Lastly, the study's generalizability to other settings is limited by the data coming from a single site. Because of site‐specific characteristics, it is possible that other ED sites with different availability of access to transportation and/or those who lack medical complexity may have different rates of outcomes than our own.
5. DISCUSSION
In this retrospective cohort study of 452 ambulatory patients with symptoms consistent with COVID‐19, we provide preliminary evidence that relatively young patients with reassuring pulse oximetry, respiratory rate, general appearance, and lack of significant comorbidities can self‐manage their conditions outside of the hospital. Our sample had a lower rate of ED revisits compared to the 20% rate that has been published in peer‐reviewed studies of all‐comers to the ED. 8 Only a small proportion of our patients were hospitalized within 30 days (4.6%) and few required intensive care (0.7%). These outcomes provide preliminary evidence that relatively healthy patients tend to fare well after ED evaluation for COVID‐19.
The clinical progression of disease in our cohort was similar to earlier publications describing maximal symptom severity at 8–10 days after symptom onset. 9 The median interval from ED discharge to ED return of 5 days is also reassuring and argues against COVID‐19 as a rapidly progressive illness or one associated with sudden onset of respiratory decompensation in younger and healthy populations. We also describe the median total duration of illness in our population to be 15 days, similar to those with “mild disease” in China. 9
Before this study, only 2 decision support tools were available to help clinicians assess the prognosis of patients with suspected viral pneumonia infection; however, neither tool is directly applicable to the US ED population during the COVID‐19 pandemic. The first is the MuLBSTA score, which requires the patient to have had chest radiography (to characterize presence or absence of infiltrate) and blood work (to determine the absolute lymphocyte count) and was developed prior to the spread of COVID‐19. 10 The second is the Brescia‐COVID Respiratory Severity Scale, which requires serial chest radiography evaluation in patients with illness of at least 7 days and has not yet been validated outside of Italy. 11 To our knowledge, our study is the first to evaluate outcomes among ED patients with suspected COVID‐19 infection in an environment in which physicians were given disposition guidelines. Of note, adherence to guidelines was not enforced nor audited. However, our clinical experience suggests that they have good face validity and could be a good candidate for formal testing in a future prospective trial.
In summary, low‐risk patients with suspected COVID‐19 who present to the ED can be safely discharged home. Some patients will experience progression of symptoms in the ensuing week that require hospitalization for supportive therapy, typically at 10 days of symptomatic illness. ED clinicians should explain to patients that they may experience worsening symptoms after the ED visit, such as labored breathing, which should prompt them to seek follow‐up assessment by a medical professional.
AUTHOR CONTRIBUTIONS
Sam S. Torbati and Carl T. Berdahl conceived the study. Sam S. Torbati and Carl T. Berdahl supervised data collection. NCG and AJR undertook data collection and management. Carl T. Berdahl managed quality control. Carl T. Berdahl and Sam S. Torbati drafted the manuscript, and all authors contributed substantially to its revision. Carl T. Berdahl and Sam S. Torbati take responsibility for the paper as a whole.
Supporting information
Supplement Table A1. List of outcomes of interest at 30 days (ED revisit; inpatient hospital admission, and/or ICU admission) with accompanying descriptions of cases, including trends in oxygen saturation and patient rationale for returning to the emergency department
Biography
Carl T. Berdahl, MD, is Assistant Professor of Medicine and Emergency Medicine at Cedars Sinai Medical Center, Los Angeles, CA.

Berdahl CT, Glennon NC, Henreid AJ, Torbati SS. The safety of home discharge for low‐risk emergency department patients presenting with coronavirus‐like symptoms during the COVID‐19 pandemic: A retrospective cohort study. JACEP Open. 2020;1:1380–1385. 10.1002/emp2.12230
Funding and support: This study was internally funded by Cedars‐Sinai Medical Center.
Supervising Editor: Angela Lumba‐Brown, MD.
REFERENCES
- 1.World Health Organization. WHO's Director General Calls on G20 to Fight, Unite, and Ignite Against COVID‐19. World Health Organization. https://www.who.int/news-room/detail/26-03-2020-who-s-director-general-calls-on-g20-to-fight-unite-and-ignite-against-covid-19. Published 2020. Accessed 5 May 2020.
- 2. Yee J, Unger L, Zadravecz F, et al. Novel coronavirus 2019 (COVID‐19): Emergence and implications for emergency care. J Am Coll Emerg Physicians Open. 2020;1(2):63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chavez S, Long B, Koyfman A, Liang SY. Coronavirus Disease (COVID‐19): a primer for emergency physicians. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID‐19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. Feb 24. 10.1001/jama.2020.2648. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 5. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019‐Ncov) Infection is Suspected: Interim Guidance. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 8. Duseja R, Bardach NS, Lin GA, et al. Revisit rates and associated costs after an emergency department encounter: a multistate analysis. Ann Intern Med. 2015;162(11):750‐756. [DOI] [PubMed] [Google Scholar]
- 9. Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 10. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duca A, Piva S, Foca E, Latronico N, Rizzi M. Calculated decisions: Brescia‐COVID Respiratory Severity Scale (BCRSS)/Algorithm. Emerg Med Pract. 2020;22(5 Suppl):CD1‐CD2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table A1. List of outcomes of interest at 30 days (ED revisit; inpatient hospital admission, and/or ICU admission) with accompanying descriptions of cases, including trends in oxygen saturation and patient rationale for returning to the emergency department


