The coronavirus disease 2019 (COVID‐19) outbreak has led to a pandemic with an overwhelming impact on daily living and the health care system. Major life‐threatening complications may occur. 1 Although most patients suffer from respiratory symptoms, different neurological symptoms have been described either directly or indirectly caused by COVID‐19. 2 Within the movement disorder spectrum, myoclonus has been observed as a COVID‐19‐related feature in several patients. 3 , 4 , 5
Case Report
We report a patient with severe myoclonic jerks, cerebellar ataxia, and mild neurocognitive symptoms following severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. This 44‐year‐old male without relevant medical history or medication use presented with a new‐onset tremulous body sensation, slurred speech, and unsteady gait. Two weeks prior he developed fever, mild respiratory complaints, and anosmia with a positive SARS‐CoV‐2 reverse‐transcription polymerase chain reaction on nasopharyngeal swab. The fever and respiratory complaints resolved (with azithromycin monotherapy) 1 week prior to presentation. Clinical neurological examination showed action‐induced myoclonic jerks in the face, arms, and trunk (Video S1). The myoclonic jerks were sensitive for tactile and auditory stimuli, without enhanced startle response or hyperekplexia. Examination of eye movements revealed horizontal saccadic intrusions and a transient ocular flutter (lasting < 24 hours), but no clear opsoclonus. The speech was stuttering, suggestive of a speech‐activated myoclonus. Finger‐to‐nose and heel‐to‐shin tests showed evidence of ataxia. Prominent gait ataxia and titubation were noted. Tendon reflexes were symmetrical and brisk, with normal plantar responses and no frontal release signs. Sensory abnormalities were absent. On the behavioral level, we noted attention and memory deficits (with amnesia for several conversations during hospitalization), perseveration, impulsivity, some anxiety, hypervigilance, and insomnia without disorientation or apraxia. Detailed cognitive testing was not possible because of COVID‐19 isolation measures.
Extensive diagnostic testing was performed with chest computed tomography showing bilateral multifocal infectious abnormalities with a ground‐glass pattern strongly suggestive of COVID‐19 pneumonia (Fig. 1). Gadolinium‐enhanced magnetic resonance imaging of the brain and spinal cord were unremarkable. Cerebrospinal fluid (CSF) analysis showed a normal cell count with normal total protein and glucose levels. CSF oligoclonal bands were absent and IgG index was not elevated. Polymerase chain reaction for herpes simplex virus, varicella zoster virus, and SARS‐CoV‐2 in CSF were negative. Testing for autoimmune and paraneoplastic antineuronal antibodies (including anti‐Ri, type 2 antineuronal nuclear antibody (ANNA‐2); anti‐Hu, type 1 antineuronal nuclear antibody (ANNA‐1); anti‐NMDA‐R, N‐methyl‐D‐aspartate receptor; anti‐GABA‐B‐R, gamma‐aminobutyric acid type B receptor; anti‐AMPAR, alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor; anti‐CASPR2, contactin‐associated protein‐like 2; anti‐LGI1, leucine‐rich glioma‐inactivated 1; anti‐GAD65 antibodies, glutamic acid decarboxylase 65) in both serum and CSF were negative. Additional blood tests excluded other infectious or autoimmune diseases. Screening for an occult neoplasm using whole‐body 18F‐fluorodeoxyglucose positron emission tomography combined with a low‐dose computed tomography scan, dermatological screening, and testicular echography was negative.
FIG 1.
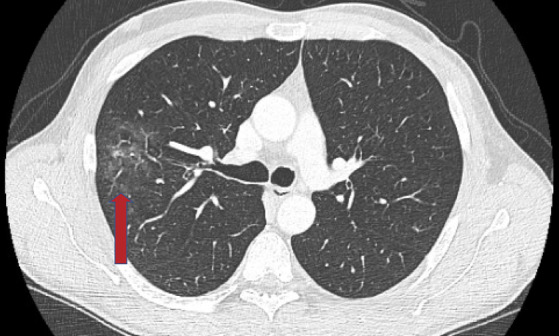
This chest computed tomography scan shows infectious abnormalities with a ground glass pattern (arrow) strongly suggestive of coronavirus disease 2019.
The clinical nadir of the neurological syndrome was reached 3 days after onset, followed by a plateau phase lasting 4 days. At day 7, corticosteroid therapy (methylprednisolone 1 g intravenously daily for 5 days) was instituted. Slow but clear recuperation was noted. Because of the remaining debilitating signs (gait ataxia and neurocognitive symptoms), additional intravenous immunoglobulin infusion (0.4 g/kg) was administered for 3 days starting at day 15. Further improvement was observed, although with remaining nondisabling signs (mild ataxia and concentration problems) for which outpatient rehabilitation was started, with full recovery within 2 months.
Discussion
This case provides further evidence of a COVID‐19‐associated myoclonic syndrome characterized by a generalized stimulus‐sensitive and action myoclonus. The subacute onset after SARS‐CoV‐2 infection, in addition with the characteristic symptomatology and clear positive response to immunotherapy are, similar to the previously reported cases, 3 , 4 , 5 pointing toward a postinfectious autoimmune encephalitis. Besides the myoclonus, our patient presented with prominent limb ataxia, stuttering speech, and neuropsychiatric symptoms, which is most frequently described in the context of the opsoclonus‐myoclonus‐ataxia (OMA) syndrome. 6 Although the patient showed mild oculomotor abnormalities, he never developed an opsoclonus and neither did the previously reported COVID‐19 cases. 2 , 3 , 4 , 5 However, because the overall majority of his symptoms and disease course are typical for OMA, our case might have developed an OMA‐like syndrome that occurs as a myoclonus/ataxia dominant variant without opsoclonus.
The etiology of OMA is in the majority of cases either parainfectious or paraneoplastic, highlighting the relevance of a neoplastic workup, especially in children. 6 The parainfectious variant of OMA is usually responsive to immunotherapy, although symptoms may last for several months. We started, in line with the current literature, treatment with corticosteroids with a modest effect followed by immunoglobulin infusion with a good response and no worsening of respiratory COVID‐19 symptoms.
Parainfectious neurological complications of COVID‐19 in the peripheral nervous system (Guillain‐Barré syndrome) and central nervous system (encephalitis, myelitis, and cerebrovascular diseases) have recently been reported. 2 A common mechanism of autoimmune cross‐reactivity, which might be involved in some COVID‐19‐associated neurological manifestations, could also be implicated in the postinfectious syndrome of our patient. Further research and meticulous data collection in clinical registries will contribute to increasing our knowledge on the prevalence of neurological symptoms in patients with COVID‐19 and will hopefully clarify the causal relationship between SARS‐CoV‐2 infection and the post‐COVID‐19 myoclonic syndrome either as new phenomenon or as part of the OMA spectrum.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
F.D.: 1A, 1B, 1C, 2A, 2B
T.V.B.: 1A, 1B, 1C, 2A, 2B
B.W.: 1A, 1B, 1C, 2B
P.C.: 1A, 1B, 1C, 2B
D.C.: 1A, 1B, 1C, 2A, 2B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed consent from the patient was obtained for the inclusion of clinical and video data in scientific publications. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Supporting information
Video S1. This video was taken on day 9 after the onset of symptoms, after methylprednisolone 1 g intravenously was started, with already a clear effect on ataxia and modest effect on the myoclonic jerks. The video shows an extensive generalized (predominantly action) myoclonus, with gait instability attributed to action myoclonus and subtle gait ataxia. The finger‐to‐nose and heel‐to‐chin tests show subtle ataxia, most pronounced in the right leg.
Femke Dijkstra and Tobi Van den Bossche contributed equally.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Bio Med Atenei Parmensis 2020;91:157–160. 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767–783. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS‐CoV‐2. J Neurol Neurosurg Psychiatry 2020;91(9):1013–1014. 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 4. Beach SR, Praschan NC, Hogan C, et al. Delirium in COVID‐19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry 2020;65:47–53. 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rábano‐Suárez P, Bermejo‐Guerrero L, Méndez‐Guerrero A, et al. Generalized myoclonus in COVID‐19. Neurology 2020;95(6):e767–e772. 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oh S‐Y, Kim J‐S, Dieterich M. Update on opsoclonus‐myoclonus syndrome in adults. J Neurol 2019;266:1541–1548. 10.1007/s00415-018-9138-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. This video was taken on day 9 after the onset of symptoms, after methylprednisolone 1 g intravenously was started, with already a clear effect on ataxia and modest effect on the myoclonic jerks. The video shows an extensive generalized (predominantly action) myoclonus, with gait instability attributed to action myoclonus and subtle gait ataxia. The finger‐to‐nose and heel‐to‐chin tests show subtle ataxia, most pronounced in the right leg.


