Abstract
Background
During the course of COVID‐19, the disease caused by the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), thrombotic phenomena and/or diffuse vascular damage are frequent, and viral elements have been observed within endothelial cells.
Objectives
CD146 + circulating endothelial cells (CD146 + CECs) and their progenitors (CEPs) are increased in cardiovascular, thrombotic, infectious, and cancer diseases. The present study was designed to investigate their kinetics in novel coronavirus (COVID‐19) patients.
Methods
We used a validated flow cytometry procedure to enumerate viable and apoptotic CD146 + CECs and CEPs in COVID‐19 patients during the course of the disease and in patients who recovered.
Results
Viable CEPs per milliliter were significantly increased in COVID‐19 patients compared with healthy controls. This increase was observed in patients with mild symptoms and not further augmented in patients with severe symptoms. In patients who recovered, CEPs decreased, but were in a range still significantly higher than normal controls. Regarding mature CD146 + CECs, in COVID‐19 patients, their absolute number was similar to those observed in healthy controls, but the viable/apoptotic CD146 + CEC ratio was significantly different. Both mild and severe COVID‐19 patients had significantly less apoptotic CD146 + CECs compared with healthy controls. Patients who recovered had significantly less CD146 + CECs per milliliter when compared with controls as well as to mild and severe COVID‐19 patients. A positive correlation was found between the copies of SARS‐CoV‐2 RNA in the cellular fraction and apoptotic CEPs per milliliter in severe COVID‐19 patients.
Conclusions
CD146 + CECs and CEPs might be investigated as candidate biomarkers of endothelial damage in COVID‐19 patients.
Keywords: circulating endothelial cells, circulating endothelial progenitors, Covid‐19, endothelial cells, SARS‐CoV‐2
Essentials
-
•
Thrombotic phenomena and/or diffuse vascular damage are frequent in Covid‐19, and viral elements have been observed within endothelial cells.
-
•
We found that viable circulating endothelial progenitors (CEPs) were significantly increased in Covid‐19 patients compared to healthy controls.
-
•
A positive correlation was found between the copies of SARS‐CoV‐2 RNA in the cellular fraction and apoptotic CEPs/mL in severe Covid‐19 patients.
-
•
CEPs might be investigated as candidate biomarkers of endothelial damage in Covid‐19 patients.
Alt-text: Unlabelled Box
1. INTRODUCTION
The virus causing the 2020 coronavirus epidemic has been called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The disease caused by the new coronavirus has been called COVID‐19.1 During the course of the disease, and particularly in the advanced phase, thrombotic phenomena and/or diffuse vascular damage are frequently observed.2., 3., 4. Moreover, viral elements have been observed within endothelial cells in COVID‐19 patients.5 An early assessment of these phenomena is believed to potentially help in selecting patients to be treated more quickly.
Circulating endothelial cells (CD146 + CECs) and their progenitor counterparts (CEPs) are endothelial cells present in the peripheral blood of healthy subjects (where apoptotic CD146 + CECs deriving from the turnover of the vascular endothelium are observed) and increased in cardiovascular, thrombotic, infectious, and cancer diseases.6., 7. A validated and reproducible procedure8 allows to quantify CD146 + CECs and CEPs by multiparametric flow cytometry and to divide them into their viable and apoptotic fractions.
In the present work, we measured CD146 + CECs and CEPs in active COVID‐19 patients, subjects who had COVID‐19, recovered and tested negative in the previous week, and age‐ and gender‐matched healthy controls. SARS‐CoV‐2 RNA viremia was determined in the plasma and cellular fraction of every COVID‐19 patient with digital droplet PCR, an assay known to reduce the frequency of false negatives when comparted to RT‐PCR.9
2. PATIENTS AND METHODS
2.1. Patients and controls
During April 2020, we investigated 27 consecutive active COVID‐19 patients (13 severe and 14 mild according to C‐Reactive Protein values1, and 9 subjects who had COVID‐19, recovered, and tested negative in the previous week. There were 17 females and 19 males, median age was 52 (range 27‐92). Eight age‐ and gender‐matched healthy controls were also investigated.
Patient clinical and laboratory data were retrieved from the electronic clinical record.
Laboratory data included whole blood cell count, RCP, D‐dimer, creatinine, interleukin‐6 (IL‐6), and ferritin.
2.2. Flow cytometry
CD146 + CECs and CEPs were evaluated by flow cytometry according to Mancuso et al8 with little modifications. In brief, blood was collected in EDTA tubes as anticoagulant. After lysis of 7 mL of blood with ammonium chloride (NH4Cl), cells were incubated with monoclonal antibodies for 15 minutes, at 4°C, in the dark. CD146 + CECs and CEPs were evaluated by 10‐color flow cytometry (Navios EX, Beckman Coulter) using the nuclear staining Syto16 for DNA (Thermo Fisher Scientific, Eisai, Medipost), 7‐AAD (BD), and a panel of monoclonal antibodies including anti‐CD45 (to exclude hematopoietic cells), anti CD34, anti‐CD31 (both from Beckman Coulter), and anti‐CD146 (BD) as endothelial cell markers. After acquisition of at least 3 × 106 events, an appropriate gating strategy was used to enumerate viable and apoptotic CD146 + CECs and CEPs (Kaluza software, Beckman Coulter).
To the best of our knowledge, until now there is no a definitive consensus regarding mature CEC and CEP phenotype. According to some authors,10 CD146 is expressed on both CEPs and CECs. Some other authors6., 7., 8. 11 have restricted CEP clonogenic potential to DNA+, CD146‐negative, CD45‐negative, and CD34 + cells.
In this work, CD146 + CECs were identified as DNA+, CD45−, CD31+, CD34+, and CD146+, and CEPs as DNA+, CD45−, CD31+, CD34+, and CD146–. Viable and apoptotic cells were identified by 7‐AAD detection. The absolute number of the cells was calculated by a dual‐platform counting technique using the total number of leukocytes in peripheral blood obtained by hemocytometer, according to routine methods.
2.3. Digital droplet PCR
RNA was extracted from 1200 μL of plasma and 600 μL of packed cellular fraction by QIAamp circulating nucleic acid kit (Qiagen) and tested by SARS‐CoV‐2 RNA digital droplet polymerase chain reaction (QX200, Bio‐Rad) with CDC primers and probes (Integrated DNA Technologies, 2019 nCOV‐KIT9).
2.4. Ethical approval
The “Studio virologico, immunologico, clinico e genetico sui pazienti con infezione da nuovo coronavirus SARS‐CoV‐2” was approved with the registration number 2020/ST/049 at the “Sacco” Hospital in Milan.
2.5. Statistical analyses
Normal distribution of the data was assessed using Shapiro Wilk's normality test and the homogeneity of the variance was checked with the F‐test for single comparison while using Bartlett’s test for pairwise comparisons. Single and pairwise comparisons were performed using either the Student t test or the Wilcoxon's rank sum test according on normal or not normal distributions, respectively; eventually, the pairwise comparison P values were adjusted by the Benjamini‐Hochberg method.
For the correlations, Pearson or Spearman tests were used based on normal or not normal distributions assessed again using Shapiro Wilk's normality test.
Only significant differences (P < .05) were plotted. Statistical analyses and plots were carried out using ggpubr and ggplot packages on R. All the data and the scripts used for data analysis are available from Github (https://github.com/raveancic/endo_cells_COVID19).
3. RESULTS AND DISCUSSION
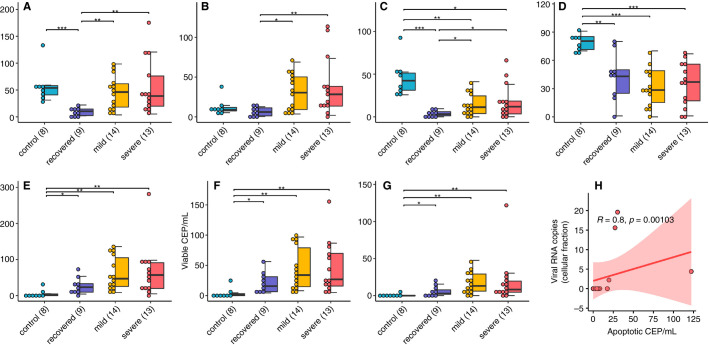
As shown in Figure 1A‐G , viable and apoptotic CEPs per milliliter were significantly increased in COVID‐19 patients compared with healthy controls (P < .001). This increase was larger than those usually observed in other neoplastic or vascular diseases.6., 7., 8. Interestingly, the increase was observed in patients with mild symptoms and not further augmented in patients with severe symptoms. Patients who recovered had less viable and apoptotic CEPs per milliliter, but in a range still significantly higher than normal controls (P < .05).
FIGURE 1.
Features of CEP and CD146 + CEC in COVID‐19 patients. A‐G, Distributions of CEP and CD146 + CEC values in the patient groups (*P < .05, **P < .01, ***P < .001). H, Significative correlation of apoptotic CEP and viral RNA copies in the cellular fraction
Regarding mature CD146 + CECs, in COVID‐19 patients their absolute number was similar to those observed in healthy controls, but the viable/apoptotic CD146 + CEC ratio was significantly different, as patients had less apoptotic CD146 + CECs (P < .001). Again, this difference was larger than those usually observed in other neoplastic or vascular diseases.6., 7., 8. Patients who recovered had significantly less apoptotic CD146 + CECs per milliliter when compared with controls (P < .001) as well as to mild and severe COVID‐19 patients (P < .01).
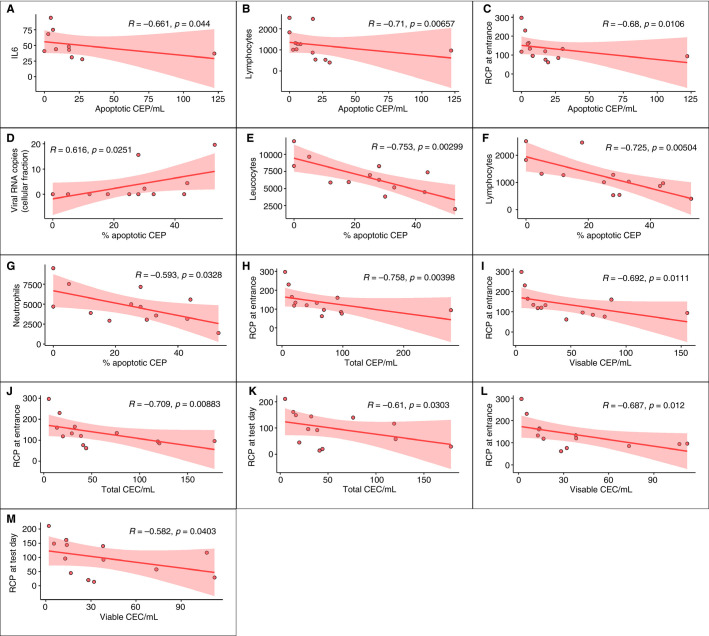
SARS‐CoV‐2 RNA was found in the plasma and/or in the cellular fraction of 8 of 13 severe COVID‐19 patients (61%), and of 3 of 14 mild COVID‐19 patients (21%, P = .034 by chi‐squared test). An interesting positive correlation (R = .80, P = .001) was found between the copies of SARS‐CoV‐2 RNA in the cellular fraction and apoptotic CEPs/mL in severe COVID‐19 patients (Figure 1H). Along a similar line, as shown in Figure 2D , a positive correlation was found between viral RNA copies and the percentage of apoptotic CEPs (R = .6, P = .02). As also shown in Figure 2, in severe COVID‐19 patients other significant correlations were found between apoptotic CEPs and IL‐6, lymphocytes, RCP at entrance, total leukocyte count, lymphocytes, and monocytes. In this subgroup of patients, viable and/or apoptotic CD146 + CECs were found to correlate with RCP at enrollment and/or at the time of test.
FIGURE 2.
Significant correlations among CEP and CD146 + CEC categories and hematological parameters in severe COVID‐19 patients
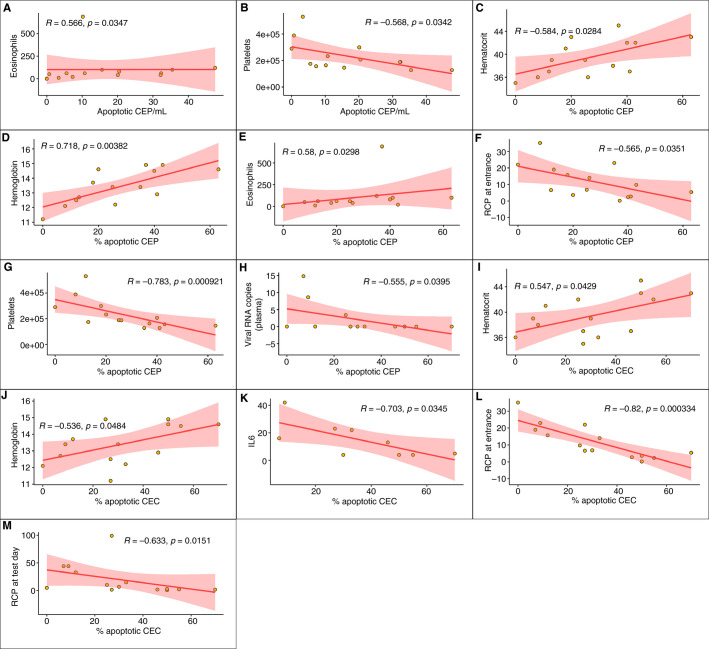
As shown in Figure 3 , in patients affected by mild COVID‐19, significant correlations were found between apoptotic CEPs and eosinophils, platelets, hematocrit, hemoglobin, IL‐6, and RCP at enrollment. Apoptotic CD146 + CECs correlated with viral RNA copies in plasma, hematocrit, hemoglobin, IL‐6, and RCP at enrollment and the time of test.
FIGURE 3.
Significant correlations among CEP and CD146 + CEC categories and hematological parameters for the in mild COVID‐19 patients
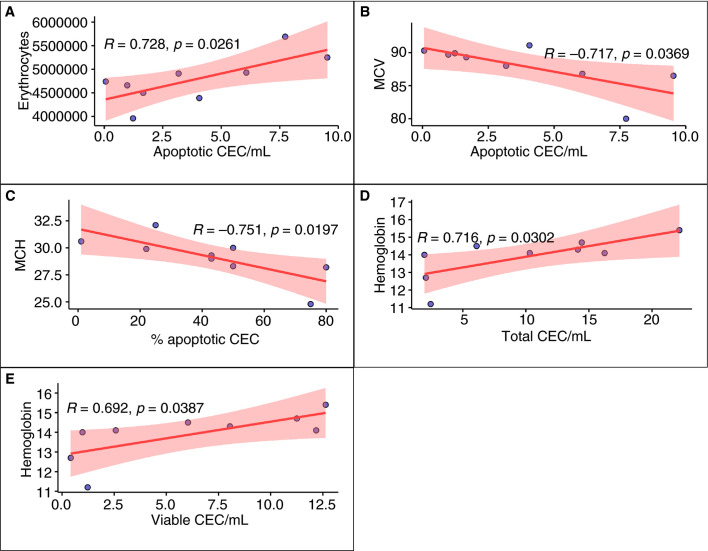
As shown in Figure 4 , in patients who recovered after COVID‐19, and tested negative in the week before, significant correlations were found between CD146 + CECs and red blood cell count, hemoglobin, mean corpuscolar volume, and mean cell hemoglobin.
FIGURE 4.
Significant correlations among CEP and CD146 + CEC categories and hematological parameters in recovered COVID‐19 patients
There is emerging evidence that cardiovascular complications are frequently a crucial step in COVID‐19 progression and related deaths.1., 2., 3., 4. The SARS‐CoV‐2 virus infects patients by mean of the angiotensin‐converting enzyme 2 (ACE2) receptor, which is widely expressed in organs in the respiratory tract, as well as in the kidney, intestine, and cardiovascular system. Endothelial cells express high levels of the ACE2 receptor, and a recent report has shown evidence of direct viral infection of endothelial cells and diffuse endothelial inflammation in COVID‐19 patients.5 To our knowledge, this is the first report showing that CEPs are significantly unbalanced in COVID‐19 patients with a pattern that was not previously reported in cancer, infectious, and/or cardiovascular diseases. Nizzoli et al12 reported an increase in CD146 + CECs in COVID‐19 patients. However, in their work, they did not present separate data about viable and apoptotic CD146 + CECs, and did not separate patients according to severity of their disease.
Data reported here suggest that already in patients affected by mild COVID‐19 disease CD146 + CECs are significantly less apoptotic than in healthy controls, and that this unbalance is present also in severe COVID‐19 patients. Both viable and apoptotic CEPs are significantly increased in mild and severe COVID‐19 patients. Interestingly, COVID‐19 patients who recovered from symptoms and tested negative in the week before had significantly less apoptotic CD146 + CECs when compared with controls and SARS‐CoV‐2‐positive patients, whereas CEPs were still increased when compared with controls.
The mechanisms causing these unbalances deserve further translational investigation. Teuwen et al13 and Atkermann et al14 report that the lungs from patients with COVID‐19 show distinctive vascular abnormalities which included severe endothelial injury associated with the presence of intracellular virus. Pulmonary vessels in such patients also showed widespread thrombosis and microangiopathy. Interestingly, in the lungs of patients with COVID‐19, there was evidence of new blood vessel growth (ie, angiogenesis) but not as a result of sprouting angiogenesis, but through a process known as “intussusceptive” angiogenesis. The authors speculate that this burst in intussusceptive angiogenesis may be the result of an adaptive response to the hypoxia associated with acute respiratory distress syndrome in such COVID patients.
The correlation found between copies of SARS‐CoV‐2 RNA and apoptotic CEPs, if confirmed by cell sorting, suggest the hypothesis that also these progenitors, in addition to mature endothelial cells, might be a direct target of this pathogen. According to Monteil et al,15 SARS‐COV‐2 virus can directly infect blood vessels in three‐dimensional kidney organoids in cell culture. Damage inflicted on the pulmonary endothelium would result in an increase in apoptotic CECs, whereas the adaptive angiogenesis response may do the opposite and result in an increase in CEPs. SARS‐CoV‐2‐related endothelial damage might have triggered CEP mobilization from the bone marrow (or other reservoirs such as the white adipose tissue16., 17.) in an attempt to generate a newly functional vessel lining. These hypotheses should be investigated in adequate preclinical models.
There is ongoing interest in undertaking clinical trials of antiangiogenic drugs such as anti‐vascular endothelial growth factor antibodies as a possible treatment strategy for COVID‐19 patients. The rationale is that vascular endothelial growth factor can act as a potent permeability factor that causes vessel leakage,12., 13. and hence may be responsible for the pulmonary edema associated with acute respiratory distress syndrome in COVID‐19 patients. Should, in the future, it be possible to pinpoint circulating endothelial cells originating from the lung or different organs, this piece of information will be of great interest to better understand their role in COVID‐19.
Taken together, our findings indicate that CD146 + CECs and CEPs deserve further study as possible candidate biomarkers of endothelial damage in COVID‐19 patients, particularly in the early phases of the disease. This hypothesis is currently under investigation in our institutions, and might pave the way to clinical trials using these biomarkers for patient selection and/or stratification.
CONFLICT OF INTEREST
Authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Patrizia Mancuso, Antonio Gidaro, and Francesco Bertolini designed the study. Patrizia Mancuso, Antonio Gidaro, Giuliana Gregato, Paola Cremonesi, Jessica Quarna, Sonia Caccia, Luca Gusso, Stefano Rusconi, and Andrea Giacomelli performed the study. Alessandro Raveane analyzed the data. Antonio Gidaro, Antonio Gidaro, Chiara Cogliati, and Francesco Bertolini wrote the manuscript.
ACKNOWLEDGMENTS
Supported in part by AIRC and Italian Ministry of Health.
Associazione Italiana per la Ricerca sul Cancro
Footnotes
P.M. and A.G. contributed equally to this work.
Manuscript handled by: Scott Diamond
Final decision: Scott Diamond, 31 July 2020
REFERENCES
- 1.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 2.South A.M., Diz D.I., Chappell M.C. COVID‐19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo M., Bertinato E.M., Birocchi S., et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blann A.D., Woywodt A., Bertolini F., et al. Circulating endothelial cells. Biomarker of vascular disease (review) Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 7.Bertolini F., Shaked Y., Mancuso P., Kerbel R.S. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 8.Mancuso P., Antoniotti P., Quarna J., et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry, molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- 9.Suo T., Liu X., Guo M., et al. ddPCR: a more sensitive and accurate tool for SARS‐CoV‐2 detection in low viral load specimens. Emerg Microbes Infect. 2020;9:1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delorme B., Basire A., Gentile C., et al. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–1279. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 11.Timmermans F., Plum J., Yoder M.C., Ingram D.A., Vandekerckhove B. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nizzoli N.E., Merati G., Tenore A., et al. Circulating endothelial cells in COVID‐19. Am J Hematol. 2020;95(8):187–188. doi: 10.1002/ajh.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID‐19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis and angiogenesis in Covid‐19. New Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteil V., Kwon H., Prado P., et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonin M.G. Progenitor cell mobilization from extramedullary organs (Review) Methods Mol Biol. 2012;904:243–252. doi: 10.1007/978-1-61779-943-3_20. [DOI] [PubMed] [Google Scholar]
- 17.Orecchioni S., Gregato G., Martin‐Padura I., et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73:5880–5891. doi: 10.1158/0008-5472.CAN-13-0821. [DOI] [PubMed] [Google Scholar]