Abstract
Diffuse alveolar hemorrhage is a severe respiratory complication of systemic lupus erythematosus. The illness develops over hours to a few days and is the systemic lupus erythematosus-associated syndrome with highest mortality. Although no specific symptoms have been identified, a number of features are associated with diffuse alveolar hemorrhage, with a drop in blood hemoglobin the most prominent. Dyspnea, blood-stained sputum, diffuse infiltrates identified by chest imaging, elevated single breath-diffusing capacity for monoxide, thrombocytopenia and C3 hypocomplementemia are other commonly reported signs of diffuse alveolar hemorrhage. The etiology is not completely understood but many patients develop diffuse alveolar hemorrhage concomitant with lupus nephritis, suggesting immune complex-driven pathology. Biopsy studies have identified both cases with capillaritis and a bland non-inflammatory phenotype. An animal model of diffuse alveolar hemorrhage has indicated requirement of B lymphocytes and complement receptor-mediated apoptotic body phagocytosis by monocytes as part of the pathogenesis. This review will discuss considerations when diagnosing the condition and available therapies. Infections and other causes of hemorrhage have to be excluded as these require different treatment strategies. Methylprednisolone and cyclophosphamide remain the most commonly used therapies. Plasmapheresis and rituximab are other beneficial treatment options. A few studies have also considered intrapulmonary Factor VII therapy, extracorporeal membrane oxygenation and mesenchymal stem cell therapy. There is an unmet need of better definition of diffuse alveolar hemorrhages etiology and pathology for development of improved treatment strategies.
Keywords: Anti-DNA antibodies, antiphospholipid syndrome, capillaritis, diffuse alveolar hemorrhage, nephritis, vasculitis
Systemic lupus erythematosus affects the respiratory tract
Systemic lupus erythematosus (SLE) is an autoimmune disease signified by a range of manifestations including skin rashes, chronic fatigue, arthritis, glomerulonephritis, neurological, cardiovascular involvement, stroke, and influences on the lungs. Several of these manifestations can lead to premature death.1 The disease affects 20–70 individuals per 100,000 people, 6–10 times more often women.1 Disease pathology is driven by a combination of environmental and genetic factors. A feature of SLE is defective phagocytosis and removal of apoptotic cells leading to the accumulation of cell debris of nuclear, cytosolic and membrane origin. Apoptotic debris activates auto-reactive B cells and T cell leading to production of auto-antibodies.1 Immune complexes (ICs) formed by antibodies (Abs), reactive to nuclear and cytosolic antigen, present in the circulation are responsible for several facets of the pathology. ICs can activate the classical pathway of the complement system, which can induce inflammation in kidneys and other organs. This persistent auto-Ab-mediated augmentation of the complement system leads to complement depletion and reduced ability of phagocytic removal of dead-cell debris.1
Involvement of the respiratory tract occurs in up to 50–70% of SLE patients and includes pleuritis, infiltrating pneumonia, bronchiolitis obliterans, muscular and diaphragmatic involvement, and vascular aberrations such as pulmonary hypertension, antiphospholipid syndrome (APS) and diffuse alveolar hemorrhage (DAH).2,3 Defect phagocytosis, ICs, complement depletion, and auto-Abs are responsible for these respiratory tract manifestations. ICs inducing inflammation in the alveolar capillaries might be the leading cause for DAH.
Diffuse Alveolar Hemorrhage
DAH was first described in 1904 by Dr William Osler and is one of the most devastating complications of SLE.4,5 Patients experiencing DAH present with dyspnea, cough and fever, blood-stained sputum and sometimes hemoptysis, with symptoms developing rapidly in hours or over a few days. Other autoimmune patient groups are also susceptible for developing DAH. Hence, the disorder is more common in ANCA-vasculitides and patients with APS can develop DAH.6 The exact cause of DAH pathology is unknown but the general view is that IC-induced pulmonary capillaritis or bland hemorrhage leads to damage to basement membranes and leakage of erythrocytes into the alveolar space (see pathology section below, and Figure 1).6 DAH incidence in SLE patients can range from 0.6% to 5.4%, which can be compared to 0.5% to 9% of all hospital admissions for the syndrome.7 However, autopsies of SLE patients have identified focal collections of red blood cells (RBCs) or more diffuse involvement in 30–66% of cases, suggesting both the presence of unidentified cases and the incidence of subclinical disease.7,8 DAH is most common in young women (mean age 27 years) and can occur at an early stage of the disease, at an average 35 months since onset (range 0–276 months).7,9 Affected patients may also have recurrent episodes.4 DAH is a very serious and potentially fatal condition, although with reduced mortality over recent years, reported rates remain between 0–92% (estimated average 50%).2,6 Factors such as development of acute catastrophic hemoptysis, requirement of mechanical ventilation, infections and thrombocytopenia are associated with increased risk of mortality.6
Figure 1.
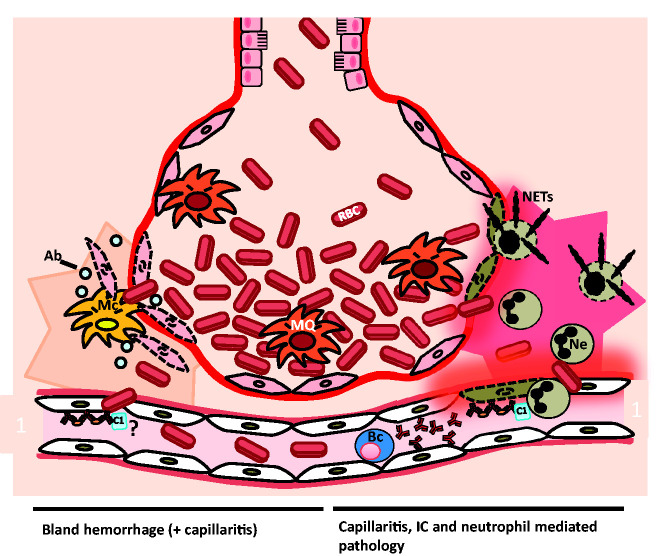
Diagram suggesting pathologies that lead to diffuse alveolar hemorrhage (DAH). Shown is an alveolus with an adjacent pulmonary capillary during DAH. Two reported mechanisms are outlined. The left-hand side shows the mechanism of bland hemorrhage, which involves apoptosis of cells associated with the alveoli. The cells undergoing apoptosis might be the epithelial cells. The cell destruction might be driven by immune complexes (ICs) or by another mechanism. Apoptosis or bland hemorrhage leads to leakage of red blood cells (RBC) into the alveolar space. Macrophages (MQ) in the alveolar lumen and monocytes (Mc) in the alveolar wall are phagocytosing apoptotic bodies (ab) and RBC. The right-hand side shows IC, complement, capillaritis and neutrophil (Ne) mediated DAH. ICs bind endothelial cells inducing an immune response. Tissue infiltrating neutrophils are undergoing necrosis, leading to neutrophil extracellular trap (NET) formation and destruction of capillary and alveolar basement membrane. The damage facilitates leakage of blood into the alveolar space. Hemosiderin-laden macrophages are present in the alveolar space. B cells (Bc) might be involved both by providing antibodies for IC formation and inflammatory cytokines driving the disease.
Typical for the condition is a drop in hemoglobin level and diffuse infiltrates visible by chest X-ray (CXR) or high-resolution chest computed tomography (CT, Figure 2).9 Elevated single breath-diffusing capacity for carbon monoxide reflects increased availability of hemoglobin within the alveoli, which is measured by diffusing capacity or transfer factor of the lung for carbon monoxide.10 Some information on the risk factors associated with the development of DAH in SLE patients is available. Active disease with a SLE Disease Activity Index above 10, serologically high titers of anti-dsDNA, thrombocytopenia, C3 hypocomplementemia, leucopenia, capillaritis, and ICs found in biopsies is associated with increased risk of DAH, but none of these factors are disease specific.6 Hemosiderin-laden macrophages found in bronchoalveolar lavage fluid (BALF) often appear only after several days of disease.6 An increased risk of DAH has been reported during active renal disease, especially when manifesting as lupus nephritis. Such an association has been described in up to 80% of cases.7 Histological analysis of kidney biopsies from such patients often describes class III or IV lupus nephritis.11
Figure 2.
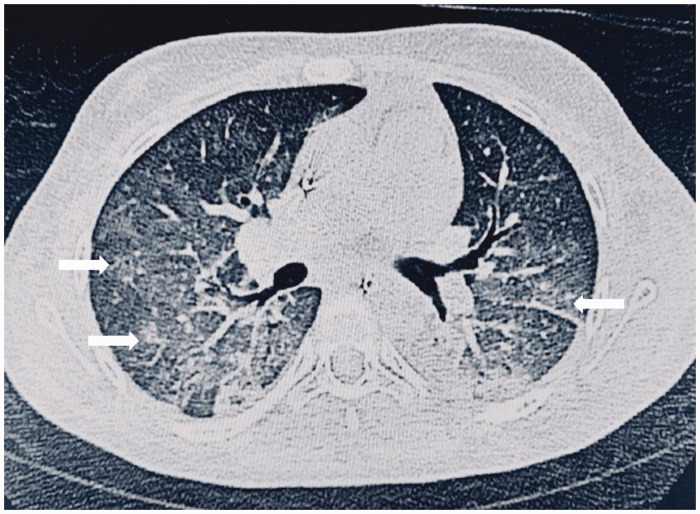
Computed tomography image of bilateral alveolar infiltrates suggestive of diffuse alveolar hemorrhage. The patient attended the Royal Hospital, Muscat, Oman. Diffuse, patchy infiltrates are visible in both lungs (arrows). The patient was treated with steroids, intravenous immunoglobulin (IVIG), plasmapheresis, and rituximab (RTX). The patient survived the episode.
Infections can be associated with DAH and should be ruled out as a cause during the differential diagnosis. DAH patients with concurrent infections generally show a poor prognosis.7,12 Some reports have also indicated that DAH can occur as a side effect of immune suppressive treatment.13 In our clinical practice we have experienced on four occasions that SLE patients developed DAH after pulse with steroids (unpublished observation). We speculate that this could have occurred either due to the already severe disease level of these patients and/or that subclinical DAH might have been present. It is not known in these cases whether initiation of steroid therapy could have been a factor for the pathology experienced.
Pathogenesis
Experimental studies have indicated that recruitment of macrophages and neutrophils in the lung occur a few days before the onset of alveolar hemorrhage.14 Associated with the onset of the disease is a drop in complement factors and hemoglobin. Development of DAH pathology has been described both as a result of inflammation and capillaritis and due to a non-inflammatory bland alveolar hemorrhage. For the occurrence of both types of hemorrhage, deposition of ICs in the alveolar capillaries seems to be required.11 A predominant neutrophil interstitial infiltration outside the capillaries, inflammation and necrosis of the alveolar and capillary walls are common during inflammatory capillaritis. One study identified capillaritis in 67% of available biopsy samples.15 Accumulated neutrophils undergo cytolysis with release of neutrophil extracellular traps (NETs) and cytotoxic proteins leading to loss of integrity of the alveolar-capillary basement membrane.16 This destruction result in leakage of RBCs into the alveolar space (Figure 1, right).9 The exact initiation factor for the capillaritis is not known. Antiphospholipid antibodies could be one initiating factor and have been reported in some cases of DAH.17,18
Non-inflammatory bland hemorrhage has also been described and has in some studies been suggested to be a more common cause of DAH.11 Bland hemorrhage is associated with predominant monocyte infiltration in the alveolar wall.11 Also, this form is associated with deposition of ICs on the arterial wall.11 A study reported increased apoptosis in the alveolar wall, which could explain the hemorrhage process. The origin of the apoptotic cells was unclear but could be alveolar epithelial cells. The apoptosis was accompanied by macrophages, presumably for removal of apoptotic debris and for prevention of inflammation (Figure 1, left).11 These macrophages had a high expression of myeloperoxidase probably as a result of phagocytosis of erythrocytes. No neutrophils were present in the lesions.11 An alternative suggestion has been proposed to explain the high prevalence of non-inflammatory DAH; bland hemorrhage might be associated with a widely scattered, difficult to detect capillaritis, hence indicating ongoing complement or IC-mediated inflammation in these cases.11,19 It is not known if the epithelial cell-derived apoptotic bodies are inefficiently removed due to the phagocytosis defect that has been reported for SLE.1 A third cause of DAH has been described; diffuse alveolar damage, involving edema of alveolar septa and formation of hyaline membranes.19
An animal model of pristane-induced lupus with DAH highlighted the importance of B cell-mediated ICs for pathology. Also, complement was important for disease development but neutrophils were dispensable for pathology in this model. DAH pathology occurred due to complement receptor-mediated apoptotic body phagocytosis by monocytes as in bland hemorrhage pathology. The anti-inflammatory cytokine interleukin (IL)-10 was found to dampen the symptoms in this model.20
Diagnosis
A careful history and physical examination are required to establish the diagnosis of DAH. A combination of physical examination, radiographic analysis of the lung and blood and BALF analysis will provide evidence for the diagnosis. On examination, the rapid onset of dyspnea, hypoxemia, occasionally with hemoptysis, could suggest DAH. In addition, symptoms such as cough, paleness, thoracic pain, hypotension, and pulmonary crackles might be early manifestations of the condition.21 It is recommended to perform bronchoscopy immediately and to retrieve consecutive BALF. BALF from patients with DAH is usually hemorrhagic. Equal to or more than 20% hemosiderin-laden macrophages in BALF is criterion for DAH but might take 48 to 72 hours to appear.9 Accumulation of these cells can occur due to other causes such as infections, acute exacerbations of interstitial pulmonary fibrosis and idiopathic pneumonia syndrome.22 Collected BALF samples should undergo cultures and analysis for bacterial, fungal (such as Pneumocystis jiroveci) and viral infections.21 Infections as a cause of the hemorrhage have to be excluded as they are the most common cause of lung disease in SLE.8 Infections with, for example, cytomegalovirus and legionella can lead to bland DAH and diffuse alveolar damage.19,23,24 It should be noted that fungi such as Candida albicans are present in lungs of healthy individuals.
Blood analysis will provide information of whether a drop in hemoglobin levels has occurred (drop by 1.5–2 g/dL), suggestive of alveolar hemorrhage, and is important for diagnosis. Laboratory analysis can also provide information of thrombocytopenia and C3 hypocomplementemia, associated with worse SLE and reported during DAH.24 History of deficit in these two factors is also associated with an increased risk of DAH.18
Radiological imaging including CXR and high-resolution chest CT scans provide important information to establish the diagnosis. The radiograph may show atypical findings with focal asymmetric bilateral areas of consolidation (Figure 2) or ground glass opacities.25 Findings consisting of bilateral patchy infiltrates may, however, also be found in infectious etiologies and acute respiratory distress syndrome.19,25 Ground glass opacities might occur during pulmonary edema, infections, interstitial pulmonary fibrosis or by other causes.26 Lupus DAH patients' CXRs might also have a normal or close to normal appearance. Due to the instability of the patient, procedures to obtaining lung biopsies are risky. Biopsy of the lung may be considered in cases where the patient condition is stable and the cause of DAH is unclear.
The combination of dyspnea, drop in hemoglobin, elevated single-breath diffusing capacity for carbon monoxide, and pulmonary interstitial or alveolar infiltrates will alert for the possibility of DAH. This should particularly be considered for patients with active SLE.21
There are a number of conditions with similar presentation that have to be excluded. Clinical signs may be similar to heart failure, infections or acute lupus pneumonitis.8 Left ventricular failure due to myocarditis or non-bacterial thrombotic endocarditis can cause acute pulmonary syndrome associated with hemoptysis and new pulmonary infiltrates in SLE patients.27 However, it should be emphasised that diastolic dysfunction, hemodynamic alterations, pulmonary overload and hyperazotemia can be a result of the rapid accumulation of RBCs within alveoli in lupus DAH.28 Pulmonary-renal syndrome, associated with SLE or other autoimmune diseases, is another cause of DAH-like pathology. Uremia due to lupus nephritis can lead to pneumonitis and diffuse alveolar damage. Localized pulmonary hemorrhage can occur unrelated to SLE due to chronic bronchitis, bronchiectasis, congestive heart failure with acute pulmonary edema, tumors, blunt trauma, or drugs.29
Management of DAH in SLE
There is a paucity in randomized clinical trials to better treat patients with SLE-associated DAH and management remains individualized across different medical centers.18 The most frequently used therapies are methylprednisolone, cyclophosphamide, and plasmapheresis.30 One study analyzing 140 patients (172 episodes) found that corticosteroids were most frequently used (98%) followed by cyclophosphamide (54%), plasmapheresis (31%), azathioprine (7%), intravenous immunoglobulin (IVIG, 5%), mycophenolate (3%), the B cell-targeting therapy rituximab (RTX, 6%), and stem cell transplantation (2%).15
The usage of methylprednisolone is recommended until cessation of the hemorrhage. Empirical studies have indicated the survival rate is higher for patients receiving a dose of methylprednisolone above what is conventionally used (4–8 g instead of 3 g).31 Also, treatment using cyclophosphamide has been shown to improve survival.15 In another study, cyclophosphamide was given to patients that required mechanical ventilation. In this study, the mortality rate was high (70%) and likely due to the severity of the cases.7 The combination of methylprednisolone and cyclophosphamide is associated with increased survival rate.24 Cyclophosphamide treatment is associated with better survival rates than other treatment options such as plasmapheresis (see below).15 In addition to therapies mentioned, several studies have included antibiotics empirically (see the next section).13
Treatment of infections
As immunosuppressive therapy is the preferred treatment for DAH and pulmonary symptoms in SLE often are due to infections, antibiotic therapy is highly recommended until microbial cause of the disease has been excluded.32 Infections are responsible for 22–25% of SLE patients deaths, which has in part been attributed to the therapies used.33 Hence, untimely usage of immunosuppressants can facilitate microbial growth leading to disease exacerbation.19,21,34 When infection is suspected, broad-spectrum antibiotics should be considered as they might reduce mortality.2
Rojas-Serrano et al. performed a study to determine the infection rate in 13 SLE patients diagnosed with DAH.35 Evaluation by bronchoscopy and cultures for bacteria, fungi, and mycobacteria was performed during 14 episodes. The assessment was performed during the first 48 hours of admission and infections were demonstrated in 57% of cases, including Pseudomonas aeruginosa, Serratia marcescens, Citrobacter freundii, and Aspergillus fumigatus.35 Martínez-Martínez et al. evaluated factors associated with infections in patients with DAH and SLE, and found such an association with hypocomplementemia and mechanical ventilation.12 These findings support initiation of broad spectrum antibiotics early and that patients should undergo continuous surveillance for possible emerging infections. Therapies targeting viruses, P. jiroveci pneumonia, other fungal infections and tuberculosis should be considered based on findings from the initial evaluation and from the BALF result.34
IVIG has been considered in a few cases for patients with active infection. This would be as an adjuvant therapy while waiting for response to antibiotic treatment. In some studies, IVIG did not increase survival rate.15 Such therapy should be considered on an individual case basis, dependent on clinical scenario and disease manifestations.
Plasmapheresis
Plasmapheresis is an efficacious therapy for causes of DAH such as ANCA-associated vasculitis, cryoglobulinemic vasculitis, anti-glomerular basement membrane disease and APS.21,36 The therapy is thought to remove the pathogenic ICs that are responsible for vascular inflammation.21 ICs and pathological antibodies are probably responsible for capillaritis in lupus DAH. DAH due to potentially IC-independent bland hemorrhage has also been described. One study found IC deposition in lung tissue in five of six of SLE patients with DAH whereas others have suggested this deposition is less frequent.15,17,19
Plasmapheresis as a treatment strategy has been chosen for cases where patients responded inadequately to high doses of corticosteroid and cyclophosphamide therapy.37 A recent large study analyzed 66 patients with autoimmune diseases subjected to plasmapheresis. Eleven of these were cases of lupus with DAH. Of all patients with DAH who were treated (total 20), 55% showed improvement.38 In two other studies plasmapheresis showed similar survival rates as in patients that did not undergo this intervention.15,18,30 The authors reported the presence of anti-dsDNA antibodies in plasma in 75% of cases, suggesting plasmapheresis could have been a suitable option. The authors speculated the lack of efficacy could be either because anti-dsDNA is not the cause of DAH or that the antibodies were already bound to the alveolar membrane and could not be removed by the treatment.15 Another study reported plasmapheresis treatment in 16 of 47 lupus DAH patients, with a resulting mortality of 29.2% of the 47 cases. In this study plasmapheresis treatment was associated with death (odds ratio, 7.62). Patients subjected to plasmapheresis did, however, have more severe disease.38 Other small studies have described efficacy and increased survival rates when patients were treated with plasmapheresis.37
Plasmapheresis is therefore a good treatment option for lupus DAH patients. There seems to be some variability in treatment response, which might be related to the severity of the disease.38 Further studies to gain knowledge of the underlying etiology for DAH in SLE would be helpful to identify whether there might be subgroups that are more suitable for this intervention.
Rituximab
RTX is a chimeric monoclonal Ab that recognizes CD20, a surface receptor on B cells. B cell death is triggered when the Ab binds the receptor. RTX is used in treatment of certain autoimmune diseases including cases of SLE.39 The therapy reduces levels of anti-nuclear Abs, ICs, and cytokines produced by the B cells. RTX has been described in several case reports to successfully treat DAH. The biologic has been used without addition of cyclophosphamide but never without corticosteroids.30 RTX treatment was reported to result in superior survival rates compared to plasmapheresis.30 However, in one study of three cases treated with RTX, none of the patients survived.12
As mentioned before, B cell activation is an important factor in SLE pathology. In some, but perhaps not all, DAH cases, the disease might be mediated the deposition of ICs in the alveolar capillaries.19 During acute DAH there might not be sufficient time for the RTX therapy to suppress IC generation. B cells include, however, different subsets and can present antigens and produce and release inflammatory cytokines, which might explain RTX's efficacy for some patients.30,40 B cell depletion therapy is a good option, with a late-action mechanism, but it must be used in combination with other therapies.
Experimental treatment strategies for DAH
A few approaches have been described for treatment of lupus DAH that are yet to be considered in general practice. These include delivery of recombinant Factor VIIa (rFVIIa) by the intrapulmonary route, utility of extracorporeal membrane oxygenation support (ECMO) and mesenchymal stem cells (MSC) transplantation. Cases reported with these treatment strategies are summarized in Table 1.
Table 1.
Reported SLE cases with DAH treated by recombinant activated clotting Factor VII, extracorporeal membrane oxygenation or umbilical cord mesenchymal stem cells transplantation
| Author | Case (age/gender) | Received therapy | Outcome | |
|---|---|---|---|---|
| rFVIIa | Esper et al., 20143 | 37 F | MTPx3, RTX, rFVIIa | Survived |
| Alabed, 201442 | 37 F | MTPx3, CPM, rFVIIa | Survived | |
| Pathak et al., 201541 | 2 cases | Steroids, plasmapheresis, rFVIIa | Both Survived | |
| ECMO | Wang et al., 201830 | 3 cases | MTP, CPM, plasmapheresis, ECMO | All three died |
| Pais F et al., 201728 | 38 F | MTP, plasmapheresis, ECMO | Survived | |
| Pacheco Claudio et al., 201448 | 33 F | MTP, CPM, ECMO | Died | |
| 36 M | MTP, CPM, ECMO | Survived | ||
| Kimura et al., 201549 | 14 F | MTP, CPM, plasmapheresis, IVIG, ECMO | Survived | |
| Patel et al., 201450 | 28 F | MTP, CPM, RTX ECMO | Survived | |
| MSCT | Liang J et al., 201243 | 19 F | MTP, IVIG, UC-MSCT | Survived |
| Shi D et al., 201244 | 4 cases | Several regimens + UC-MSCT | All four improved |
F: female; M: male; rFVIIa: Recombinant activated clotting factor VII; MTP: methylprednisolone; RTX: rituximab; CPM: cyclophosphamide; ECMO: extracorporeal membrane oxygenation; IVIG: Intravenous immunoglobulin; UC-MSCT: umbilical cord mesenchymal stem cell transplantation.
Intrapulmonary rFVIIa
Tissue factor (TF) is an important regulator of the extrinsic coagulation cascade.29 The factor is expressed on smooth muscle cells and other cells present on the abluminal side of blood vessels. When damage or leakage occurs to the blood vessel, FVII/VIIa, either prebound or delivered from plasma) activates TF, resulting in conversion and activation of factors of the coagulation cascade, leading to blood clotting. Patients with the bleeding disorder hemophilia are successfully treated with rFVIIa to prevent uncontrolled bleeding.41 As the lung contains high levels of TF, usage of rFVIIa has been considered in individual SLE DAH cases with severe uncontrolled bleeding.36,41,42 The protein has been administered both intravenously and by intrapulmonary delivery using a bronchoscope or a nebulizer. Intrapulmonary delivery was considered as it would increase the chance of rFVIIa to meet TF expressed in the alveolar interstitium and reduce chance of leakage of the factor systemically.29 Treatment of patients with rFVIIa, particularly if administered intravenously, implicates the risk of development of harmful thrombosis that has to be carefully monitored. In small case series, this therapy has been successful.3,42 Certain patient groups should be excluded such as those with liver disease, history of myocardial infarction, stroke, or with thrombophilic conditions.41
Extracorporeal membrane oxygenation support
During DAH, despite the patient being supported by mechanical ventilation, the rapid accumulation of RBCs within alveoli can result in refractory hypoxemia. The resulting increase in lung compliance and worsening pulmonary hypertension can potentiate cardiogenic shock from acute right ventricular failure.28 ECMO might be considered to provide adequate amount of alveolar gas exchange to sustain life for such deteriorating patients. ECMO provide oxygenation of the blood outside the body. Blood can either be led from a common femoral vein to the oxygenator and be returned to the femoral artery (arterial-venous ECMO) or to the right internal jugular vein (venous-venous ECMO), with the latter method not providing cardiac support. A complication when using ECMO is that patients should be treated with intravenous heparin to prevent thrombus formation from clotting of the oxygenator, which might result in increased alveolar bleeding. However, recent technical advances in the extracorporeal circuit have allowed for the reduction or omission of systemic anticoagulation in this sub-group of patients.28 During extraordinary circumstances, such as cardiac compromise, ECMO might therefore be considered to maintain life support. ECMO has been used successfully in some DAH cases, but in others it has not increased the survival rate of patients.28,30,48-50 A further evaluation of the safety and efficacy of ECMO for SLE DAH, perhaps in the form of a controlled trial, should be appreciated.
MSC transplantation as therapy for DAH
MSCs are multipotent cells able to differentiate into various mesenchymal-derived lineages. These cells show low immunogenicity and can reduce immune responses. Although with some controversy, application of MSCs has been considered for treatment of various conditions including graft-versus-host and autoimmune diseases.43 In the aim to reduce inflammation for DAH patients unresponsive to other therapies, umbilical cord-derived MSCs as single bolus intravenous infusion (1–2 x 106 cells/kg bodyweight) have been used in experimental series. The therapy was given in additions of daily doses of 40 mg methyl prednisolone and resulted in a good response. Oxygen saturation was improved, and complete resolution of lung infiltrates was seen after 2–3 weeks. However, blood hemoglobin did not return to normal levels until several months after the MSC infusions.43,44
Exactly how MSCs promote DAH resolution is unknown. The cells have been suggested to augment various regulatory immune cells including regulatory T and B cells, thereby facilitating an anti-inflammatory environment.43 It has also been shown the MSCs have the ability to differentiate into novel alveolar epithelial cells.43 MSC therapy as a treatment option for DAH in SLE remains in the experimental stage in small case series.
Conclusion
The mortality rates of DAH in SLE has been reduced over recent years. This is likely due to increasing knowledge of the disorder and the application of novel technologies. However, mortality rates remain unacceptably high. Patients under suspicion of DAH require a prompt investigation to exclude unrelated causes with similar presentation. There is, for example, a delicate balance between initiating early treatment with immunosuppression or treating potential underlying infections with similar pathology.19 Alveolar hemorrhage caused by infection but treated with immune suppression can worsened the status of the patient.
Studies, often in the form of single case studies, are gathering incremental knowledge of this disorder. Hence, the underlying cause for DAH concomitant to SLE seems to be slightly different from the same pathology in other autoimmune diseases. Plasmapheresis is often efficacious and in cases when not, might be dependent on severity of the disease. It is not clear whether different causes to the pathology exist, as suggested in Figure 1, involving different cell types that could explain cases when plasmapheresis is not helpful.1,11 An animal model of SLE DAH has pointed to the importance of IC formation and B cells in the pathology.20 The experimental study showed the importance of IL-10 in prevention of DAH. Regulatory B cells are known producers of IL-10 that are decreased in SLE.45 The fact that RTX can be a viable treatment option is shining further light on B cells.
To gain a better view of DAH etiologies, an organized multicenter study is warranted to recruit a sufficient number of patients. When possible, biopsies would give an insight of the relation between capillaritis and bland hemorrhage that has been reported (Figure 1). Also, information on immune cells, including B cells, T cells, neutrophils and monocytes/macrophages, in blood and biopsies could be assessed in such a study (Figure 1).
Evaluating therapies such as RTX and MSCs should be undertaken in randomized trials.18 For SLE in general and particularly for SLE DAH, there is an unmet need for novel therapies.46 In DAH, a DNA degrading enzyme, DNase, delivered bronchially to remove neutrophil NETs and for removal of ICs, an humanized effector-null FcγRIIA Ab, might be novel treatment strategies to consider.16,47,48
For the benefit of patients and to establish improved guidelines, the formation of a committee of individuals from diverse specialties such as rheumatology, intensive care, nephrology, pulmonology, hematology, and infectious diseases would be helpful.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
J Bystrom https://orcid.org/0000-0002-6458-3028
References
- 1.Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014; 384: 1878–1888. [DOI] [PubMed] [Google Scholar]
- 2.Pego-Reigosa JM, Medeiros DA, Isenberg DA. Respiratory manifestations of systemic lupus erythematosus: Old and new concepts. Best Pract Res Clin Rheumatol 2009; 23: 469–480. [DOI] [PubMed] [Google Scholar]
- 3.Esper RC, Estrada IE, de la Torre Leon T, Gutierrez AO, Lopez JA. Treatment of diffuse alveolar hemorrhage secondary to lupus erythematosus with recombinant activated factor VII administered with a jet nebulizer. J Intensive Care 2014; 2: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade C, Mendonca T, Farinha F, et al. Alveolar hemorrhage in systemic lupus erythematosus: A cohort review. Lupus 2016; 25: 75–80. [DOI] [PubMed] [Google Scholar]
- 5.Osler W. On the visceral manifestations of the erythema group of skin diseases [Third Paper.] 1904. Am J Med Sci 2009; 338: 396–408. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Martinez MU, Oostdam DAH, Abud-Mendoza C. Diffuse alveolar hemorrhage in autoimmune diseases. Curr Rheumatol Rep 2017; 19: 27. [DOI] [PubMed] [Google Scholar]
- 7.Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997; 76: 192–202. [DOI] [PubMed] [Google Scholar]
- 8.Quadrelli SA, Alvarez C, Arce SC, et al. Pulmonary involvement of systemic lupus erythematosus: Analysis of 90 necropsies. Lupus 2009; 18: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 9.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 2010; 137: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 10.Greening AP, Hughes JM. Serial estimations of carbon monoxide diffusing capacity in intrapulmonary haemorrhage. Clin Sci (Lond) 1981; 60: 507–512. [DOI] [PubMed] [Google Scholar]
- 11.Hughson MD, He Z, Henegar J, McMurray R. Alveolar hemorrhage and renal microangiopathy in systemic lupus erythematosus. Arch Pathol Lab Med 2001; 125: 475–483. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Martinez MU, Sturbaum AK, Alcocer-Varela J, et al. Factors associated with mortality and infections in patients with systemic lupus erythematosus with diffuse alveolar hemorrhage. J Rheumatol 2014; 41: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Martinez MU, Abud-Mendoza C. Diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Clinical manifestations, treatment, and prognosis. Reumatologia clinica 2014; 10: 248–253. [DOI] [PubMed] [Google Scholar]
- 14.Barker TT, Lee PY, Kelly-Scumpia KM, et al. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab Invest 2011; 91: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ednalino C, Yip J, Carsons SE. Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus: Focus on outcome and therapy. J Clin Rheumatol 2015; 21: 305–310. [DOI] [PubMed] [Google Scholar]
- 16.Jarrot PA, Tellier E, Plantureux L, et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J Autoimmun 2019; 100: 120–130. [DOI] [PubMed] [Google Scholar]
- 17.Deane KD, West SG. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: A case series and literature review. Seminars Arthritis Rheum 2005; 35: 154–165. [DOI] [PubMed] [Google Scholar]
- 18.Kazzaz NM, Coit P, Lewis EE, McCune WJ, Sawalha AH, Knight JS. Systemic lupus erythematosus complicated by diffuse alveolar haemorrhage: risk factors, therapy and survival. Lupus Sci Med 2015; 2: e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ta R, Celli R, West AB. Diffuse alveolar hemorrhage in systemic lupus erythematosus: Histopathologic features and clinical correlations. Case Rep Pathol 2017; 2017: 1936282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang H, Han S, Lee PY, et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol 2017; 69: 1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Holanda BA, Barreto IG, de Araujo IS, de Araujo DB. Alveolar hemorrhage as the initial presentation of systemic lupus erythematosus. Reumatologia 2016; 54: 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maldonado F, Parambil JG, Yi ES, Decker PA, Ryu JH. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur Respir J 2009; 33: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 23.Kwong YL, Wong KL, Kung IT, Chan PC, Lam WK. Concomitant alveolar haemorrhage and cytomegalovirus infection in a patient with systemic lupus erythematosus. Postgrad Med J 1988; 64: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virdi RP, Bashir A, Shahzad G, Iqbal J, Mejia JO. Diffuse alveolar hemorrhage: A rare life-threatening condition in systemic lupus erythematosus. Case Rep Pulmonol 2012; 2012: 836017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primack SL, Miller RR, Muller NL. Diffuse pulmonary hemorrhage: Clinical, pathologic, and imaging features. AJR Am J Roentgenol 1995; 164: 295–300. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt MG, Miller WT, Jr., Reilly TJ, Simpson S. The relative frequencies of causes of widespread ground-glass opacity: A retrospective cohort. Eur J Radiol 2014; 83: 1970–1976. [DOI] [PubMed] [Google Scholar]
- 27.Carette S, Macher AM, Nussbaum A, Plotz PH. Severe, acute pulmonary disease in patients with systemic lupus erythematosus: Ten years of experience at the National Institutes of Health. Seminars Arthritis Rheum 1984; 14: 52–59. [DOI] [PubMed] [Google Scholar]
- 28.Pais F, Fayed M, Evans T. The successful use of extracorporeal membrane oxygenation in systemic lupus erythematosus-induced diffuse alveolar haemorrhage. Eur J Case Rep Intern Med 2017; 4: 000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heslet L, Nielsen JD, Nepper-Christensen S. Local pulmonary administration of Factor VIIa (rFVIIa) in diffuse alveolar hemorrhage (DAH): A review of a new treatment paradigm. Biologics 2012; 6: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CR, Liu MF, Weng CT, Lin WC, Li WT, Tsai HW. Systemic lupus erythematosus-associated diffuse alveolar haemorrhage: A single-centre experience in Han Chinese patients. Scand J Rheumatol 2018; 47: 392–399. [DOI] [PubMed] [Google Scholar]
- 31.Barile LA, Jara LJ, Medina-Rodriguez F, Garcia-Figueroa JL, Miranda-Limon JM. Pulmonary hemorrhage in systemic lupus erythematosus. Lupus 1997; 6: 445–448. [DOI] [PubMed] [Google Scholar]
- 32.Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: Presentation and management. Chest 2000; 118: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 33.Ward MM, Pyun E, Studenski S. Causes of death in systemic lupus erythematosus: Long-term followup of an inception cohort. Arthritis Rheum 1995; 38: 1492–1499. [DOI] [PubMed] [Google Scholar]
- 34.Demoruelle MK, Kahr A, Verilhac K, Deane K, Fischer A, West S. Recent-onset systemic lupus erythematosus complicated by acute respiratory failure. Arthritis Care Res (Hoboken) 2013; 65: 314–323. [DOI] [PubMed] [Google Scholar]
- 35.Rojas-Serrano J, Pedroza J, Regalado J, et al. High prevalence of infections in patients with systemic lupus erythematosus and pulmonary haemorrhage. Lupus 2008; 17: 295–299. [DOI] [PubMed] [Google Scholar]
- 36.Pazzola G, Zuily S, Erkan D. The challenge of bleeding in antiphospholipid antibody-positive patients. Curr Rheumatol Rep 2015; 17: 7. [DOI] [PubMed] [Google Scholar]
- 37.Claridge S, Das P, Dorling A, Robson MG. Plasmapheresis as rescue therapy for systemic lupus erthyematosus-associated diffuse alveolar haemorrhage. BMJ Case Rep 2011, pp. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguirre-Valencia D, Naranjo-Escobar J, Posso-Osorio I, et al. Therapeutic plasma exchange as management of complicated systemic lupus erythematosus and other autoimmune diseases. Autoimmune Dis 2019; 2019: 5350960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tse JR, Schwab KE, McMahon M, Simon W. Rituximab: An emerging treatment for recurrent diffuse alveolar hemorrhage in systemic lupus erythematosus. Lupus 2015; 24: 756–759. [DOI] [PubMed] [Google Scholar]
- 40.Taher TE, Ong VH, Bystrom J, et al. Association of defective regulation of autoreactive interleukin-6-producing transitional B lymphocytes with disease in patients with systemic sclerosis. Arthritis Rheumatol 2018; 70: 450–461. [DOI] [PubMed] [Google Scholar]
- 41.Pathak V, Kuhn J, Gabriel D, Barrow J, Jennette JC, Henke DC. Use of activated Factor VII in patients with diffuse alveolar hemorrhage: A 10 years institutional experience. Lung 2015; 193: 375–379. [DOI] [PubMed] [Google Scholar]
- 42.Alabed IB. Treatment of diffuse alveolar hemorrhage in systemic lupus erythematosus patient with local pulmonary administration of Factor VIIa (rFVIIa): A case report. Medicine (Baltimore) 2014; 93: e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang J, Gu F, Wang H, et al. Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol 2010; 6: 486–489. [DOI] [PubMed] [Google Scholar]
- 44.Shi D, Wang D, Li X, et al. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol 2012; 31: 841–846. [DOI] [PubMed] [Google Scholar]
- 45.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32: 129–140. [DOI] [PubMed] [Google Scholar]
- 46.Murphy G, Isenberg DA. New therapies for systemic lupus erythematosus: Past imperfect, future tense. Nat Rev Rheumatol 2019; 15: 403–412. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Vousden KA, Naiman B, et al. Humanised effector-null FcgammaRIIA antibody inhibits immune complex-mediated proinflammatory responses. Ann Rheum Dis 2019; 78: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacheco CC, Charbonney E, Durand M, Kolan C, Laskine M. Extracorporeal membrane oxygenation in diffuse alveolar hemorrhage secondary to systemic lupus erythematosus. J Clin Med Res 2014; 6: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura D, Shah S, Briceno-Medina M, et al. Management of massive diffuse alveolar hemorrhage in a child with systemic lupus erythematosus. J Intensive Care 2015; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel JJ, Lipchik RJ. Systemic lupus-induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: A case report and review of the literature. J Intensive Care Med 2014; 29: 104–109. [DOI] [PubMed] [Google Scholar]